Summary
Native liver glycine N-methyltransferase is N-acetylated while the recombinant enzyme is not. We show here that acetylation of the N-terminal valine affects several of kinetic parameters of the enzyme. Glycine N-methyltransferase is a regulatory enzyme mediating the availability of methyl groups by virtue of being inhibited by folate. N-acetylation does not affect the overall structure of the protein and does not affect basal enzyme activity of GNMT. Binding of both the mono- and pentaglutamate forms of 5-methyltetrahydrofolate is the same for the acetylated and non-acetylated forms of the enzyme, however the pentaglutamate form is bound more tightly than the monoglutamate form in both cases. Although binding of the folates are similar for the acetylated and non-acetylated forms of the enzyme, inhibition of enzyme activity differs significantly. The native, N-acetylated form of the enzyme shows 50% inhibition at 1.3 µM concentration of the pentaglutamate while the recombinant non-acetylated form shows 50% inhibition at 590 µM. In addition, the binding of folate results in cooperativity of the substrate, AdoMet, with a Hill coefficient of 1.5 for 5-methyltetrahydrofolate pentaglutamate.
Keywords: Glycine N-methyltransferase, Folate, Binding, Inhibition, Cooperativity, N-terminal acetylation
1. Introduction
Rat liver cytosolic methyltransferase, glycine N-methyltransferase (GNMT) (EC 2.1.1.20), is an important regulatory enzyme, which serves to remove excess S-adenosylmethionine, the universal methyl donor in the cell, by the methylation of glycine. This reaction results in production of S-adenosylhomocysteine (AdoHcy) and N-methyl glycine (sarcosine).
GNMT is an abundant liver enzyme [1, 2] that was subsequently shown to be a major folate binding protein [3.4]. This enzyme binds and is inhibited by 5-methyltetrahydrofolate (5-CH3-THF) and forms part of a regulatory mechanism to maintain a constant level of AdoMet [5]. The regulatory role of GNMT was proven by studies of in human patients with mutated GNMT [6–8] and in two mouse models with GNMT knocked out [9,10]. In all cases inactivation of GNMT or complete absence of the enzyme results in very high concentrations of AdoMet in liver and plasma and in liver disease.
GNMT has been isolated and studied from a variety of sources [11–13]; inhibition of enzyme activity by folate was described in some detail [14] and several crystal structures were solved [15–18]. Recently the crystal structure of the GNMT-folate complex was reported [19]. All of the GNMT proteins studied to date have amino acid sequence similarity of about 90%. All are tetrameric enzymes consisting of four identical subunits with active centers on each subunit. Two molecules of folate inhibitor, 5-CH3-THF, are bound in intersubunits areas of the rat enzyme [19].
Detailed knowledge of the structure and kinetics of GNMT is needed in order to understand the role this enzyme plays in the metabolism of methionine, folate and one-carbon metabolism. This is especially true because of the fact that mathematical models of methionine and AdoMet metabolism have been developed based on published kinetic data [20–22]. An important fact is that all of the structural studies have been carried out using recombinant enzyme while most of the kinetic studies used native liver enzyme. Previous studies have shown that liver and recombinant GNMTs differ kinetically in at least one feature; the dependence of reaction velocity on AdoMet concentration is highly cooperative in the case of native liver enzyme but not in the case of recombinant GNMT [23]. This difference was attributed to the fact that the N-terminal valine is N-acetylated in the native enzyme but is not in the recombinant enzyme.
Our crystallographic studies showed that the N-terminal fragments of all subunits in the tetrameric structure of GNMT participate in folate binding [19]. The question we address in these studies is whether the acetyl group on the N-terminal valine of the native liver enzyme is important in folate-GNMT interactions.
2. Materials and Methods
2.1. Chemicals
All chemicals for buffers and microbiological media, S-adenosylmethionine, S-adenosylhomocysteine, activated charcoal and Sephacryl S-200 resin were from Sigma. Ceramic hydroxylapatite and Bio-Gels were from Bio-Rad. [3H-CH3]-S-Adenosylmethionine was from NEN Radiochemicals. (6S)-5-CH3-THF-Glu1 was a gift from EPROVA (Switzerland). To prepare (6S)-5-CH3-THF-Glu5 the mixture of (6R/6S)-5-CH3-THF-Glu5 purchased from Schircks Laboratories (Switzerland) was separated by binding to GNMT, removal of non-bound folate by using Spin-Columns and release of free folate by heating the GNMT-folate complex at +80 °C in presence of 60 mM β-mercaptoethanol
2.2. Protein preparation
Rat liver GNMT
Rat liver (frozen, from Pel-Freez Biologicals) was homogenized in a teflon/glass homogenizer in Homogenization Buffer (10 ml buffer for 1 g tissue) in which concentration of the components was as follows: 20 mM Tris-HCl pH 7.8, 1 mM EDTA, 2 mM PMSF, 14 mM β-mercaptoethanol. To 100 ml of Homogenization Buffer was added 500 µl of Protease Inhibitor Cocktail (Sigma). The homogenate was centrifuged 20 min at 45 000 × g and GNMT was precipitated from the supernatant between 35–50% of ammonium sulfate saturation. The pellet after 50% ammonium sulfate precipitation was dissolved in 20 mM Tris-HCl, pH 7.8 containing 14 mM β-mercaptoethanol and desalted on a 60 ml Bio-Gel P-30 column. After desalting the protein solution was loaded on an ion-exchange column (DE-52, Beckman) equilibrated with 20 mM Tris-HCl pH 7.8 containing 14 mM β-mercaptoethanol. GNMT was eluted using the same buffer containing 30 mM NaCl. The eluted GNMT was concentrated and the buffer changed to 2 mM Na-phosphate, pH 7.5 by chromatography on a Bio-Gel P-10 (Bio-Rad) column. The GNMT was applied to a ceramic hydroxylapatite Type I (Bio-Rad) column equilibrated with 2 mM Na-phosphate, pH 7.5 containing 14 mM β-mercaptoethanol. GNMT of about 95–97% purity was eluted from the column by 30 mM Na-phosphate, pH 8.0. The protein purity was further increased to at least 98% by size-exclusion chromatography on a Sephacryl S-200 column equilibrated with 50 mM Tris pH 7.5, 200 mM NaCl, 1 mM EDTA and 14 mM β-mercaptoethanol. All purification steps were performed at +3° C.
Recombinant GNMT
Cloning of wild type rat GNMT cDNA in pET17-b expression vector, protein expression and purification was as described earlier [24]. In the recombinant protein the N-terminal residue is valine as in native liver enzyme but it is not acetylated [25].
2.3. Activity assay
GNMT activity was assayed using the charcoal adsorption method described earlier [4]. Briefly, the reaction mixture contained the following components in a final volume of 100 µl; 200 mM Tris-HCl, pH 7.5, 5 mM DTT, 0.1–0.2 µM GNMT (tetramer), and various concentrations of [3H-CH3]-S-adenosylmethionine and glycine. The reaction was started by addition of AdoMet and continued for the desired time. In most experiments the reaction was performed at 25° C for 30 min, during which the time course of reaction was linear. The reaction was stopped by addition of 50 µL 10% trichloroacetic acid and 250 µL of a charcoal suspension in 0.1 M acetic acid (3.8 g/100 ml). After 15 min incubation on ice, the mixture was centrifuged to remove charcoal and the presence of radioactive sarcosine was measured in the supernatant by a scintillation counter. In folate inhibition experiments the reaction mixture was pre-incubated with folate in the absence of AdoMet for 1h on ice. All experiments were done in duplicate and presented as mean values.
2.4. Folate binding
Binding to GNMT was measured by a filtration method. The proteins were incubated with different concentrations of folate in 300 µl of 20 mM Tris pH 7.5, 25 mM NaCl and 5 mM β-mercaptoethanol (or Tris (2-carboxyethyl) phosphine hydrochloride), for 1 h on ice. The solution was placed into a Microcon YM-10 concentrator (Amicon Bioseparation) or Nanosep 10K Omega (Pall Life Sciences) and a small volume of the solution, containing unbound folate was quickly separated by centrifugation (2 min at 3000 × g). Concentration of folate in the filtrate (Ffree) was measured by absorbance at 290 nm (maximum for folate at neutral pH) using a molar extinction coefficient of 3.17×104 [26]. Subtracting the free concentration of folate from the total concentration gave the concentration of bound folate (Fbound). When binding of folate pentaglutamate was studied the concentration of folate in the filtrate was measured by a fluorescence method with excitation at 297 nm and emission at 359 nm at pH 2.5 [14]. Fluorescence measurement was done on a Cary Eclipse Fluorescence Spectrophotometer (Varian, Inc.).
2.5. Folate assay
All samples of GNMT were analyzed for the presence of folate. The folate content was assayed by using Lactobacillus casei method as described [27]. The protein samples were mixed with 10 volumes of extraction buffer (50 mM HEPES, 50 mM CHES, pH 7.85, 18 mM β-mercaptoethanol, 2% ascorbate) and heated for 10 min in a boiling water bath. Precipitated proteins were separated by centrifugation and the supernatant was treated with conjugase prepared from rat for 3 hours at +37° C [27]. Reaction mixtures were boiled again, centrifuged and the supernatant was used for analysis.
2.6. Protein assay
The concentration of protein in samples was determined by the BCA method (BCA Protein Assay kit, Pierce) using bovine serum albumin as a standard or by UV-absorbance using a value of 1.46 for a 0.1% solution of GNMT at 278 nm as [19]. Protein purity was determined by SDS electrophoresis.
3. Results
3.1. Protein preparation
Native GNMT was prepared from rat liver and recombinant GNMT expressed in E.coli using the same initial steps: preparation of crude extracts, precipitation by ammonium sulfate and ion-exchange chromatography on DE-52 resin as previously published for purification of recombinant GNMT [24]. Purification of liver enzyme required an additional step using a ceramic hydroxylapatite column. The last step in all proteins samples used purification size-exclusion chromatography on a Sephacryl S-200 column. This was done to remove any aggregated material and collect only correctly folded tetrameric form of GNMT with a molecular mass of 130 kDa. Purity of the final protein samples was at least 98% as determined by scanning of the gel after SDS electrophoresis and Coomassie staining. As reported previously, the N-terminal residue in liver GNMT was acetylated valine but in recombinant protein the N-terminal valine was not acetylated [23, 25].
The folate content of the two GNMT preparations was measured. In the liver enzyme only a small amount of folate was measured amounting to a folate/GNMT ratio of less than 1 mole/200 moles of GNMT tetramer. Interestingly, a very small amount of folate (less than 1 mole/200 mole of GNMT tetramer) was found also in recombinant GNMT. We believe that the small amount of folate found in both GNMT samples was not enough to affect the protein characteristics we studied.
In an earlier study we showed that both purified native liver and recombinant GNMT proteins contain small amounts of phosphorylated serine residues [25]. Only a single species with a mass corresponding to the N-terminal acetylated form was found by LC-MS/MS analysis of the native liver enzyme. Very small amounts of phosphorylated serine residues were detected after tryptic digestion and mass spectrometric analysis of both native liver and recombinant proteins. The overall properties are very similar for both preparations of GNMT. Both eluted from the size exclusion column at very similar volumes indicating similar quaternary structures. The intrinsic fluorescence emission spectra are the same for both proteins indicating similar tertiary structures.
3.2. Enzyme kinetics
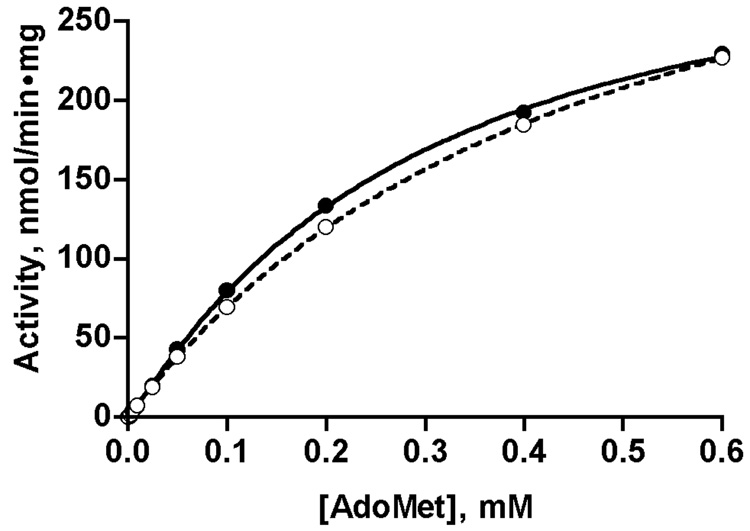
We measured the dependence of reaction velocity on AdoMet and glycine concentrations in order to detect any differences in kinetic parameters of liver and recombinant GNMTs. The values are presented in Fig. 1 and Table 1. Experimental data were fitted using the Hill and the Michaelis-Menten equations and the results were compared using GraphPad Prism. For AdoMet dependence it was found that the data fit better using the Hill equation. Table 1 shows that cooperativity with respect to AdoMet concentration is very small in both cases with a Hill coefficient of 1.10 for the liver enzyme and 1.05 for the recombinant protein. Dependence of reaction velocity on glycine concentration is purely hyperbolic for both proteins and the data are best fitted using the Michaelis-Menten equation. Vmax and kcat are very similar for both preparations. Table 1 shows a difference between the K0.5 (AdoMet) but this is only apparent as shown by the similarity of AdoMet dependence for the two enzymes in Fig. 1. GNMT is inhibited by AdoHcy, the product of the reaction but values were essentially the same for the liver and recombinant enzymes, 97 and 95 µM, respectively. The conclusion to be drawn from these kinetic data is that rat liver and recombinant GNMT perform the enzymatic reaction with the same efficiency and there is no major effect of acetylation of the N-terminal valine on these parameters.
Fig. 1. Dependence of enzyme reaction velocity of liver and recombinant GNMTs on AdoMet concentration.
Reaction velocity was measured as described in Methods with a constant concentration of glycine (8 mM) and different concentrations of AdoMet in the reaction mixture. Data for liver and recombinant enzymes are shown by closed and open circles respectively and were fitted by the Hill equation.
Table 1.
Kinetic parameters of liver and recombinant rat GNMT.
| Analysis methods | Parameters | Proteins | |
|---|---|---|---|
| Liver GNMT | Recombinant WT GNMT | ||
| Hill | Vmax, nmol/min·mg | 326 | 400 |
| K0.5 (AdoMet), µM | 280 | 460 | |
| Hill coefficient | 1.10 | 1.02 | |
| kcat, min−1 | 42.3 | 51.9 | |
| Michaelis-Menten | Km (Gly), mM | 1.9 | 1.2 |
3.3. Folate binding
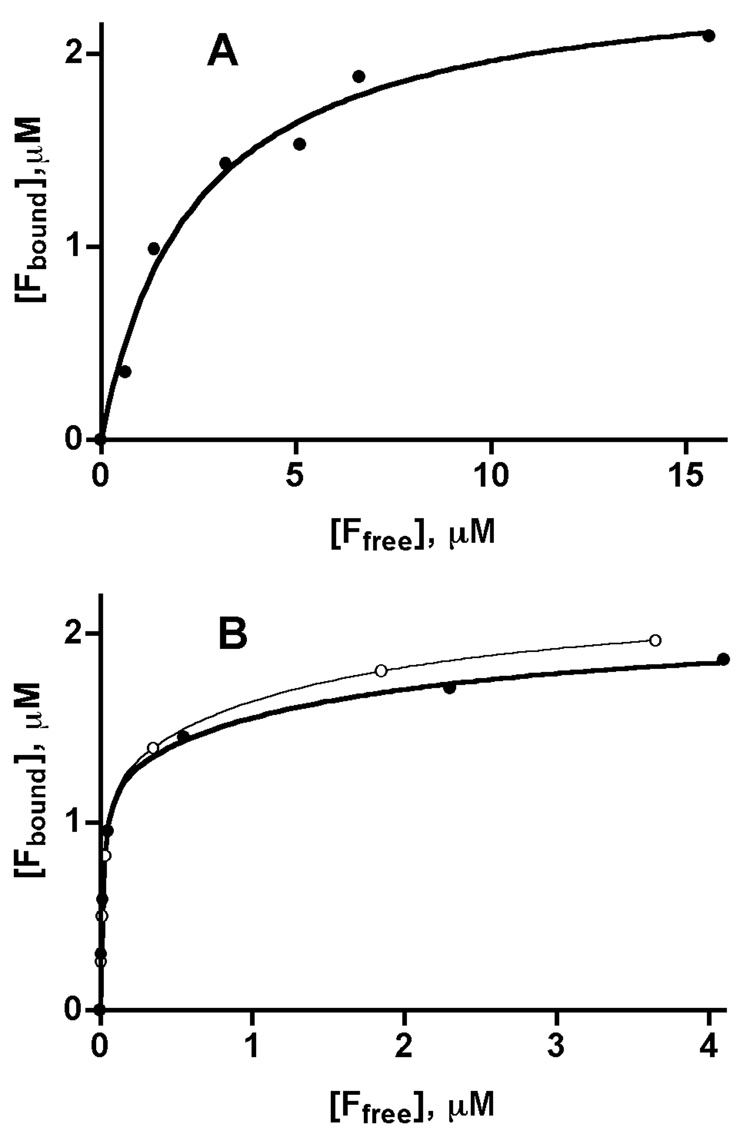
To determine whether differences in folate binding and inhibition are influenced by N-terminal acetylation we studied folate binding to rat liver and recombinant GNMTs by methods previously used [19]. Earlier we showed that that recombinant GNMT binds two molecules of 5-CH3-THF-monoglutamate to one molecule of tetrameric protein with a KD value of 2.92 µM. In this work we found that rat liver enzyme also binds two moles of 5-CH3-THF-monoglutamate per GNMT tetramer (Fig.2A) with a slightly lower KD (2.43 µM). Binding of both folate molecules occurs with the same dissociation constants and fitting of experimental data by using one-site- or two-site approaches give the same KD values.
Fig. 2. Binding of folates by GNMT.
(A) Binding of 5-CH3-THF-monoglutamate by liver GNMT. Data were fitted by using a one-site binding algorithm. Using a two-sites binding equation results with the same binding parameters for both sites. (B) Binding of (6S)-5-CH3-THF-pentaglutamate by liver and WT recombinant GNMT. Data for liver (closed circles) and recombinant (open circles) enzymes were fitted by using a two-sites binding algorithm. Binding of folate to GNMT was measured by analysis of folate in filtrate after removing of non-bound ligand from the mixture of folate and GNMT by Nanosep concentrators. The GNMT concentration in all cases was 1µM (tetramer).
Binding of the natural form of folate, 5-CH3-THF-pentaglutamate, was significantly different, however, from the monoglutamate form. The pentaglutamate form was bound by both liver and recombinant GNMTs, with much higher affinities than the monoglutamate. As shown in Fig. 2 B, two molecules of 5-CH3-THF-Glu5 are bound at much lower folate concentrations than the monoglutamate form. The binding of the two molecules of 5-CH3-THF-Glu5 was described by different KD values. When binding data were fitted by GraphPad Prism, the KD for one of the binding sites was about 0.02 µM and for the second binding site KD was about 1.6–1.7 µM for both liver and recombinant GNMTs (Table 2).
Table 2.
Folate binding and inhibition of rat liver and recombinant GNMTs.
| Analysis | Parameter | Protein | |
|---|---|---|---|
| Liver GNMT | Recombinant GNMT | ||
| Binding of 5-CH3-THF-pentaglutamate | KD, µM | 2.43 | 2.92* |
| Mol folate/mole GNMT (tetramer) | 2.4 | 1.99* | |
| Binding of 5-CH3-THF-pentaglutamate | KD, µM | KD1= 0.018 KD2= 1.68 |
KD1= 0.019 KD2= 1.59 |
| Mol folate/mole GNMT (tetramer) | 2.1 | 1.9 | |
| Inhibition by 5-CH3-THF-monoglutamate | K50%, mM | 0.05 | 1.89 |
| Inhibition by 5-CH3-THF-pentaglutamate | K50%, mM | 0.00126 | 0.59 |
From [19].
3.4. Folate inhibition
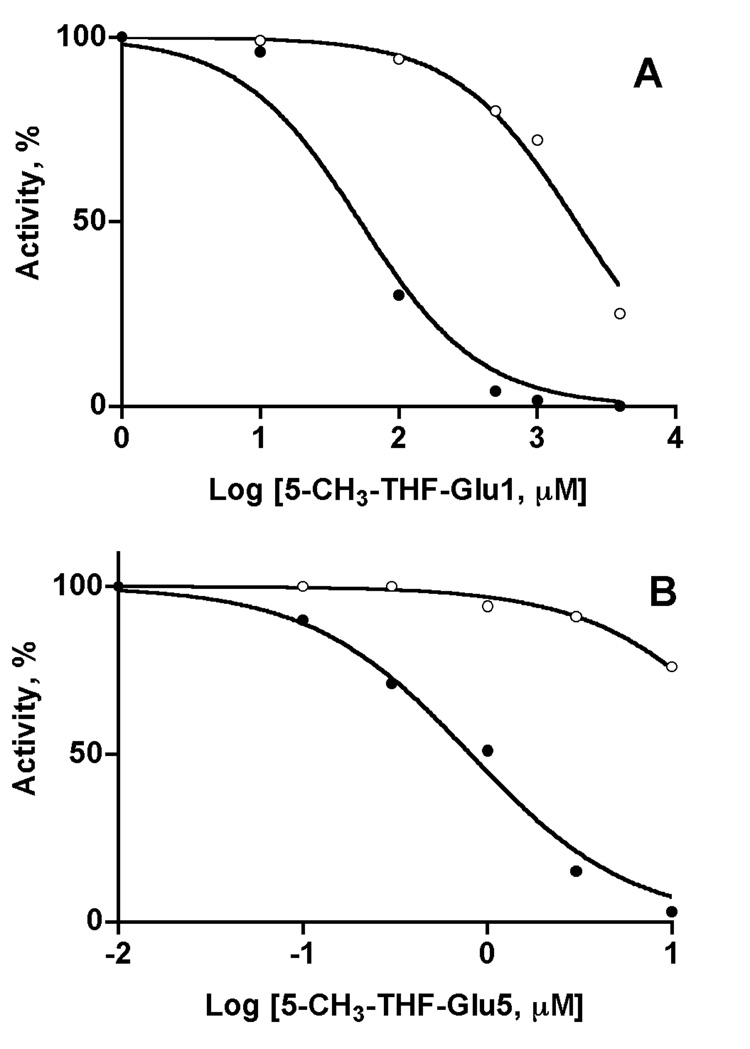
Despite the similarities of the properties of liver and recombinant GNMTs, inhibition by both 5-CH3-THF, mono- and pentaglutamate is significantly different. As shown in Fig. 3A, inhibition by 5-CH3-THF-monoglutamate of rat liver GNMTs is much more sensitive than that of the recombinant enzyme. The value for 50% inhibition of the rat liver enzyme (50 µM) is at least 30-times lower than for the recombinant enzyme (1.89 mM).
Fig. 3. Inhibition of GNMT by (6S)-5-CH3-THF-mono- (A) and pentaglutamate (B).
Enzyme activity of liver (closed circles) and recombinant (open circles) GNMTs was measured at 400 µM concentration of AdoMet after pre-incubation with different concentrations of folates. Data are presented as percent of activity of enzymes in the absence of folate against the logarithm of folate concentrations. The activity of GNMT in 1 µM monoglutamate and 0.01 µM pentaglutamate was equal to the activity without folate.
An even greater difference in inhibition was found in inhibition by 5-CH3-THF-pentaglutamate. As shown in Fig. 3B, at a folate concentration of 1 µM the liver enzyme was about 90% inhibited, while the recombinant enzyme was only inhibited about 15%. Fifty per cent inhibition for liver and recombinant GNMTs was seen at 1.26 and 590 µM respectively, i.e. 5-CH3-THF-pentaglutamate is 467 times as potent an inhibitor for the liver enzyme than for the recombinant enzyme.
3.5. Allosteric Properties
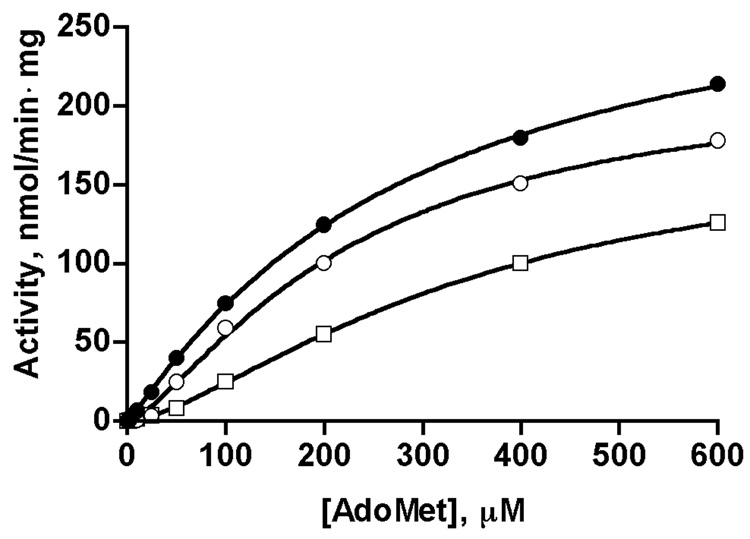
A number of previous studies have noted that GNMT from various sources display cooperativity with respect to AdoMet concentration in kinetic studies [12–14]. This cooperativity is increased in the presence of folate suggesting that some of the preparations that showed cooperativity may have had bound folate as a contaminant. We measured velocity of the reaction with increasing concentrations of AdoMet in the presence of 1 µM 5-CH3-THF-pentaglutamate (at which the liver enzyme is about 40% inhibited). The data obtained were fitted to the Hill equation. As shown in Fig. 4, liver GNMT (open squares) exhibits clear cooperativity toward AdoMet with a Hill coefficient of 1.50. In the absence of folate (solid circles) this value remained at 1.10. In the presence of folate the Km value increased but Vmax value decreased compared to those values obtained in the absence of inhibitor, which suggests that folate inhibition is non-competitive with AdoMet.
Fig. 4. Cooperativity of dependence of reaction velocity of rat liver GNMT on AdoMet concentrations in presence of 5-CH3-THF-pentaglutamate.
Dependence of velocity on AdoMet concentrations is shown for GNMT apo-protein (closed circles, taken from Fig.1), GNMT-5-CH3-THF-Glu5 complex after incubation with 1 mM 5-CH3-THF-Glu5 followed by passage through Spin-Columns to remove excess folate, as described in the text (open circles), and in the presence of 1 µM 5-CH3-THF-Glu5 in the reaction mixture (open squares). Data fitted with the Hill equation.
3.6. Presence of folate during GNMT purification
The fact that reaction velocity with respect to AdoMet concentration is seen in the presence of folate raises the question of whether any folate possibly present in numerous GNMT samples after purification would be enough to cause cooperativity. We tested this in an experiment where folates (both monoglutamate and pentaglutamate forms) were first bound with GNMT at 1 mM folate concentration and non-bound folates were then removed by double desalting on spin-columns. Samples of GNMT were analyzed for folate content and dependence of activity on AdoMet concentrations. We found that, indeed, after two passages through desalting columns, GNMT folate was still bound with ratios of moles folate/moles GNMT tetramer of 0.26 for monoglutamate and 1.47 for pentaglutamate. The difference in the amount of folate that remained bound results from different binding constants for the mono and pentaglutamate forms. The cooperativity in this sample with respect to AdoMet concentration is clearly demonstrated in Fig. 4 where a Hill coefficient of 1.37 was found (open circles).
4. Discussion
The data presented here show that rat GNMT purified from two sources, from liver or expressed in E.coli, have very similar kinetic characteristics. There is also almost no difference in binding of the folate inhibitor. However, the liver and recombinant enzymes are significantly different in susceptibility to inhibition by folate: the 5-CH3-THF-pentaglutamate concentration for 50% inhibition of recombinant enzyme is about 500-times higher than for liver enzyme. Moreover, in the presence of the same concentration of 5-CH3-THF-pentaglutamate, the liver enzyme exhibits distinct cooperativity toward AdoMet with a Hill coefficient of 1.4–1.5 but the recombinant GNMT does not. This suggests that previous reports of cooperativity of rat liver GNMT toward AdoMet might be caused by presence of folate in GNMT preparations. This assumption is in line with an absence of such cooperativity in rabbit liver GNMT [11, 28], which might be explained by the absence of folate in these GNMT preparations.
A possible explanation for the difference in inhibition between the liver and the recombinant enzymes may be the structural configuration around the N-terminal valine, which is the same in all subunits, as shown in the apo-enzyme and the GNMT folate complex (Fig. 5) [16, 19]. The closest amino acid residues to the Val1 (B) are Pro144 (C), Ser146 (C), Ile17 (D) and Leu143 (C) in the crystal structure of the apo-protein and GNMT-folate complex. The NH3-group of valine is directed toward Ile17, Leu143 and Pro144, i.e. toward hydrophobic residues. Acetylation of the NH3-group of valine results not only in removing the positive charge but also in introduction of an additional hydrophobic methyl group directed to hydrophobic residues within which AdoMet is bound. While this did not result in a higher binding constant for folate it could result in a stronger interaction of the acetylated valine with the hydrophobic globular part of the protein in the GNMT-folate complex. The mechanism of GNMT action has been proposed to involve the movement of the interacting N-termini to expose entry of AdoMet to the active site [17]. Binding of folate to this region would diminish the flexibility of the N-termini and explain the inhibition. The stronger interaction of the acetylated residue with the surrounding hydrophobic groups could then account for the difference between the acetylated liver enzyme and the non-acetylated recombinant enzyme. In addition, this could also account for the allosteric behavior with respect to AdoMet that is seen in the presence of folate (Fig. 4) since removal of a single molecule of folate could then participate in the access of AdoMet to several active sites.
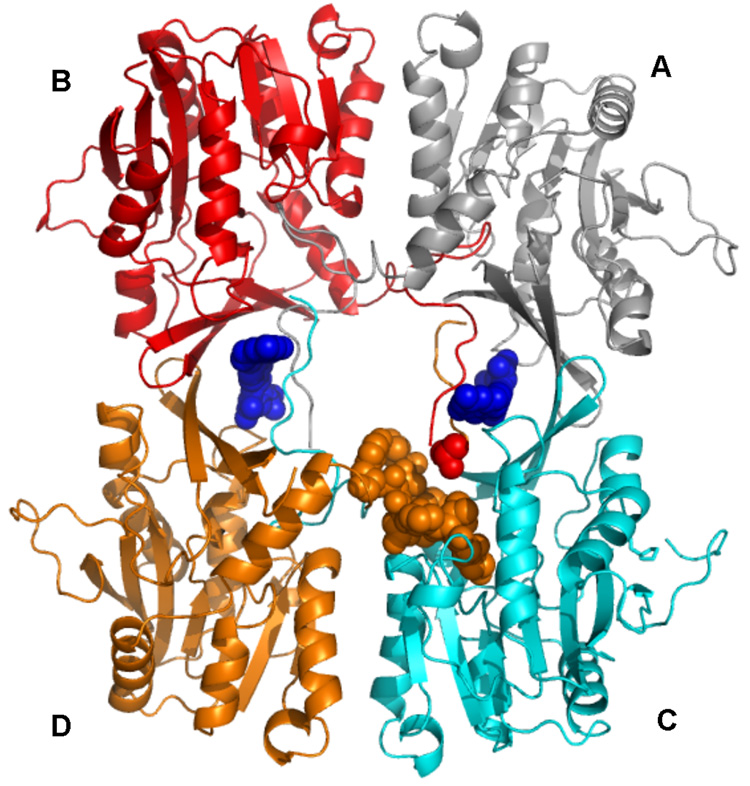
Fig. 5. Interactions of Val1 of rat GNMT apo-protein.
Position of N-terminal valine (from subunit B, red spheres) relative to N-terminal 10–20 amino acid loop of subunit D and globular part of subunit C is shown. N-terminal loop of subunit D is shown as orange spheres. The protein coordinates are those from PDB code 2IDK.
ACKNOWLEDGMENTS
Work was supported by NIH Grant DK15289 to CW.
Abbreviations
- GNMT
Glycine N-methyltransferase
- 5-CH3-THF-Glu1
5-methyltetrahydrofolate monoglutamate
- 5-CH3-THF-Glu5
5-methyltetrahydrofolate pentaglutamate
- AdoMet
S-adenosylmethionine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blumenstein J, Williams GR. The enzymatic N-methylation of glycine. Biochem. Biophys. Res. Comm. 1960;3:259–263. [Google Scholar]
- 2.Kerr SJ. Competing methyltransferase systems. J. Biol. Chem. 1972;247:4248–4252. [PubMed] [Google Scholar]
- 3.Zamierowski MM, Wagner C. Identification of folate binding proteins of rat liver. J. Biol. Chem. 1977;252:933–938. [PubMed] [Google Scholar]
- 4.Cook RJ, Wagner C. Glycine N-methyltransferase is a folate binding protein of rat liver cytosol. Proc. Natl. Acad. Sci. U S A. 1984;81:3631–3634. doi: 10.1073/pnas.81.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem. Biophys. Res. Comm. 1985;127:746–752. doi: 10.1016/s0006-291x(85)80006-1. [DOI] [PubMed] [Google Scholar]
- 6.Mudd SH, Cerone R, Schiaffino MC, Fantasia AR, Minniti G, Caruso U, Lorini R, Watkins D, Matiaszuk N, Rosenblatt DS, Schwahn B, Rozen R, LeGros L, Kotb M, Capdevila A, Luka Z, Finkelstein JD, Tangerman A, Stabler SP, Allen RH, Wagner C. Glycine N-methyltransferase deficiency: a novel inborn error causing persistent isolated hypermethioninaemia. J. Inherit. Metab. Dis. 2001;24:448–464. doi: 10.1023/a:1010577512912. [DOI] [PubMed] [Google Scholar]
- 7.Luka Z, Cerone R, Phillips J, Mudd HS, Wagner C. Mutations in human glycine N-methyltransferase give insights into its role in methionine metabolism. Hum. Genet. 2002;110:68–74. doi: 10.1007/s00439-001-0648-4. [DOI] [PubMed] [Google Scholar]
- 8.Augoustides-Savvopoulou P, Luka Z, Karyda S, Stabler SP, Allen RH, Patsiaoura K, Wagner C, Mudd SH. Glycine N -methyltransferase deficiency: a new patient with a novel mutation. J. Inherit. Metab. Dis. 2003;26:745–759. doi: 10.1023/B:BOLI.0000009978.17777.33. [DOI] [PubMed] [Google Scholar]
- 9.Luka Z, Capdevila A, Mato JM, Wagner C. A glycine N-methyltransferase knockout mouse model for humans with deficiency of this enzyme. Transgenic Res. 2006;15:393–397. doi: 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SP, Li YS, Chen YJ, Chiang EP, Li AF, Lee YH, Tsai TF, Hsiao M, Hwang SF, Chen YM. Glycine N-methyltransferase−/− mice develop chronic hepatitis and glycogen storage disease in the liver. Hepatology. 2007;46:1413–1425. doi: 10.1002/hep.21863. [DOI] [PubMed] [Google Scholar]
- 11.Heady JE, Kerr SJ. Purification and characterization of glycine N-methyltransferase. J. Biol. Chem. 1973;248:69–72. [PubMed] [Google Scholar]
- 12.Ogawa H, Fujioka M. Purification and properties of glycine N-methyltransferase from rat liver. J. Biol. Chem. 1982;257:3447–3452. [PubMed] [Google Scholar]
- 13.Ogawa H, Gomi T, Fujioka M. Mammalian glycine N-methyltransferases. Comparative kinetic and structural properties of the enzymes from human, rat, rabbit and pig livers. Comp. Biochem. Physiol. -: Comp. Biochem. 1993;106B:601–611. doi: 10.1016/0305-0491(93)90137-t. [DOI] [PubMed] [Google Scholar]
- 14.Yeo EJ, Briggs WT, Wagner C. Inhibition of glycine N-methyltransferase by 5-methyltetrahydrofolate pentaglutamate. J. Biol. Chem. 1999;274:37559–37564. doi: 10.1074/jbc.274.53.37559. [DOI] [PubMed] [Google Scholar]
- 15.Fu Z, Hu Y, Konishi K, Takata Y, Ogawa H, Gomi T, Fujioka M, Takusagawa F. Crystal structure of glycine N-methyltransferase from rat liver. Biochemistry. 1996;35:11985–11993. doi: 10.1021/bi961068n. [DOI] [PubMed] [Google Scholar]
- 16.Pattanayek R, Newcomer ME, Wagner C. Crystal structure of apo-glycine N-methyltransferase (GNMT) Protein Sci. 1998;7:1326–1331. doi: 10.1002/pro.5560070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takata Y, Huang Y, Komoto J, Yamada T, Konishi K, Ogawa H, Gomi T, Fujioka M, Takusagawa F. Catalytic mechanism of glycine N-methyltransferase. Biochemistry. 2003;42:8394–8402. doi: 10.1021/bi034245a. [DOI] [PubMed] [Google Scholar]
- 18.Pakhomova S, Luka Z, Grohmann S, Wagner C, Newcomer ME. Glycine N-methyltransferases: a comparison of the crystal structures and kinetic properties of recombinant human, mouse and rat enzymes. Proteins. 2004;57:331–337. doi: 10.1002/prot.20209. [DOI] [PubMed] [Google Scholar]
- 19.Luka Z, Pakhomova S, Loukachevitch LV, Egli M, Newcomer ME, Wagner C. 5-methyltetrahydrofolate is bound in intersubunit areas of rat liver folate-binding protein glycine N-methyltransferase. J. Biol. Chem. 2007;282:4069–4075. doi: 10.1074/jbc.M610384200. [DOI] [PubMed] [Google Scholar]
- 20.Martinov MV, Vitvitsky VM, Mosharov EV, Banerjee R, Ataullakhanov FI. A substrate switch: a new mode of regulation in the methionine metabolic pathway. J. Theor. Biol. 2000;204:521–532. doi: 10.1006/jtbi.2000.2035. [DOI] [PubMed] [Google Scholar]
- 21.Prudova A, Martinov MV, Vitvitsky VM, Ataullakhanov FI, Banerjee R. Analysis of pathological defects in methionine metabolism using a simple mathematical model. Biochim. Biophys. Acta. 2005;1741:331–338. doi: 10.1016/j.bbadis.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Reed MC, Nijhout HF, Neuhouser ML, Gregory JF, 3rd, Shane B, James SJ, Boynton A, Ulrich CM. A mathematical model gives insights into nutritional and genetic aspects of folate-mediated one-carbon metabolism. J. Nutr. 2006;136:2653–2661. doi: 10.1093/jn/136.10.2653. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa H, Gomi T, Takata Y, Date T, Fujioka M. Recombinant expression of rat glycine N-methyltransferase and evidence for contribution of N-terminal acetylation to co-operative binding of S- adenosylmethionine. Biochem. J. 1997;327:407–412. doi: 10.1042/bj3270407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luka Z, Wagner C. Expression and purification of glycine N-methyltransferases in Escherichia coli. Protein Expr. Purif. 2002;20:280–286. doi: 10.1016/s1046-5928(02)00710-6. [DOI] [PubMed] [Google Scholar]
- 25.Luka Z, Ham AJ, Norris JL, Yeo EJ, Yermalitsky V, Glenn B, Caprioli RM, Liebler DC, Wagner C. Identification of phosphorylation sites in glycine N-methyltransferase from rat liver. Protein Sci. 2006;15:785–794. doi: 10.1110/ps.051906706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta VS, Huennekens FM. Preparation and properties of crystalline 5-methyltetrahydrofolate and related compounds. Arch. Biochem. Biophys. 1967;120:712–718. [Google Scholar]
- 27.Horne DW, Patterson D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem. 1988;34:2357–2359. [PubMed] [Google Scholar]
- 28.Kloor D, Karnahl K, Kompf J. Characterization of glycine N-methyltransferase from rabbit liver. Biochem. Cell Biol. 2004;82:369–374. doi: 10.1139/o04-007. [DOI] [PubMed] [Google Scholar]