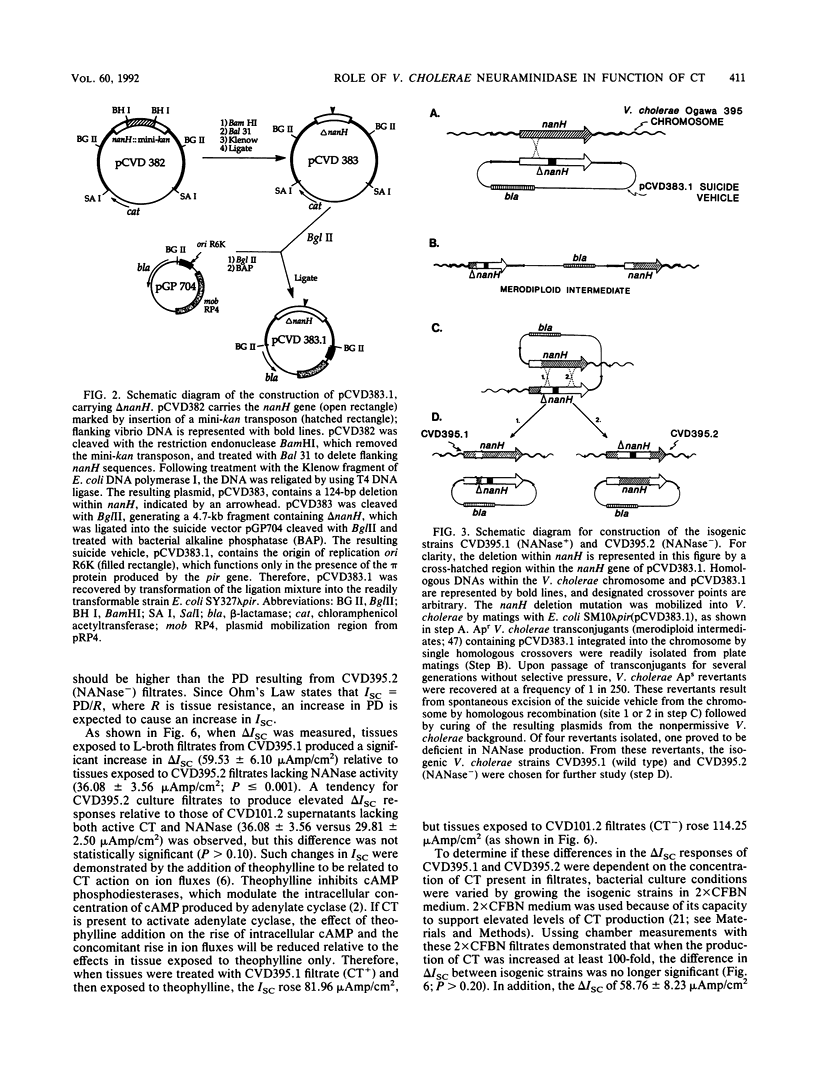
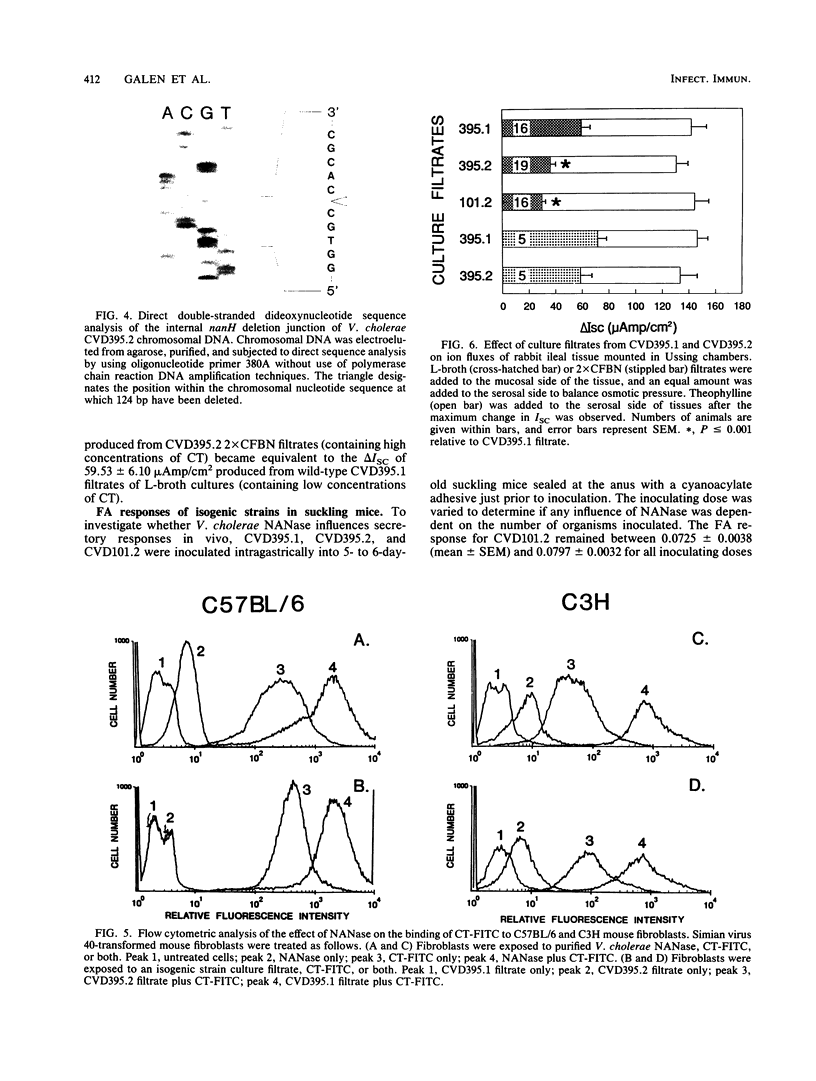
Abstract
Vibrio cholerae neuraminidase (NANase) is hypothesized to act synergistically with cholera toxin (CT) and increase the severity of a secretory response by increasing the binding and penetration of CT to enterocytes. To test this hypothesis, the NANase gene (nanH) from V. cholerae Ogawa 395 was first cloned and sequenced. Isogenic wild-type and NANase- V. cholerae 395 strains were then constructed by using suicide vector-mediated mutagenesis. The influence of NANase on CT binding and penetration was examined in vitro by using culture filtrates from these isogenic strains. Fluorescence due to binding of fluorescein-conjugated CT to C57BL/6 and C3H mouse fibroblasts exposed to NANase+ filtrates increased five- and eightfold, respectively, relative to that with NANase- filtrates. In addition, NANase+ filtrates increased the short-circuit current measured in Ussing chambers 65% relative to that with NANase- filtrates, although this difference decreased as production of CT increased. The role of NANase in V. cholerae pathogenesis was examined in vivo by intragastric inoculation of the isogenic strains into CD1 suckling mice. No difference in fluid accumulation ratios was seen at doses of 10(4) to 10(8) CFU, but NANase+ strains produced 18% higher fluid accumulation ratios at 10(9) CFU than NANase- strains when inoculated into nonfasted suckling mice. It is concluded that NANase plays a subtle but significant role in the binding and uptake of CT by susceptible cells under defined conditions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Dwyer J. D., Bloomfield V. A. Subunit arrangement of cholera toxin in solution and bound to receptor-containing model membranes. Biochemistry. 1982 Jun 22;21(13):3227–3231. doi: 10.1021/bi00256a030. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by cytotoxic lymphocytes generated in vivo and in vitro to syngeneic SV40 transformed cells. J Immunol. 1977 Mar;118(3):920–927. [PubMed] [Google Scholar]
- Griffiths S. L., Finkelstein R. A., Critchley D. R. Characterization of the receptor for cholera toxin and Escherichia coli heat-labile toxin in rabbit intestinal brush borders. Biochem J. 1986 Sep 1;238(2):313–322. doi: 10.1042/bj2380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R., Rappuoli R. Pertussis toxin promoter sequences involved in modulation. J Bacteriol. 1989 Jul;171(7):4026–4030. doi: 10.1128/jb.171.7.4026-4030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guandalini S., Fasano A., Migliavacca M., Marchesano G., Ferola A., Rubino A. Effects of berberine on basal and secretagogue-modified ion transport in the rabbit ileum in vitro. J Pediatr Gastroenterol Nutr. 1987 Nov-Dec;6(6):953–960. doi: 10.1097/00005176-198711000-00023. [DOI] [PubMed] [Google Scholar]
- Haksar A., Maudsley D. V., Peron F. G. Neuraminidase treatment of adrenal cells increases their response to cholera enterotoxin. Nature. 1974 Oct 11;251(5475):514–515. doi: 10.1038/251514a0. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981 Jul 30;292(5822):413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S., Ahmad N., Ali S. Neuraminidase production by Vibrio cholerae O1 and other diarrheagenic bacteria. Infect Immun. 1984 Jun;44(3):747–749. doi: 10.1128/iai.44.3.747-749.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Kothary M. H., Richardson S. H. Fluid accumulation in infant mice caused by Vibrio hollisae and its extracellular enterotoxin. Infect Immun. 1987 Mar;55(3):626–630. doi: 10.1128/iai.55.3.626-630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman H. A., Galen J. E., Kaper J. B. Vibrio cholerae enterotoxin genes: nucleotide sequence analysis of DNA encoding ADP-ribosyltransferase. J Bacteriol. 1984 Sep;159(3):1086–1089. doi: 10.1128/jb.159.3.1086-1089.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Miller-Podraza H., Bradley R. M., Fishman P. H. Biosynthesis and localization of gangliosides in cultured cells. Biochemistry. 1982 Jul 6;21(14):3260–3265. doi: 10.1021/bi00257a002. [DOI] [PubMed] [Google Scholar]
- Miller V. L., Mekalanos J. J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988 Jun;170(6):2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Misra T. K. DNA sequencing: a new strategy to create ordered deletions, modified M13 vector, and improved reaction conditions for sequencing by dideoxy chain termination method. Methods Enzymol. 1987;155:119–139. doi: 10.1016/0076-6879(87)55012-1. [DOI] [PubMed] [Google Scholar]
- Myers R. W., Lee R. T., Lee Y. C., Thomas G. H., Reynolds L. W., Uchida Y. The synthesis of 4-methylumbelliferyl alpha-ketoside of N-acetylneuraminic acid and its use in a fluorometric assay for neuraminidase. Anal Biochem. 1980 Jan 1;101(1):166–174. doi: 10.1016/0003-2697(80)90056-1. [DOI] [PubMed] [Google Scholar]
- Pacuszka T., Fishman P. H. Generation of cell surface neoganglioproteins. GM1-neoganglioproteins are non-functional receptors for cholera toxin. J Biol Chem. 1990 May 5;265(13):7673–7678. [PubMed] [Google Scholar]
- Richardson S. H., Kuhn R. E. Studies on the genetic and cellular control of sensitivity to enterotoxins in the sealed adult mouse model. Infect Immun. 1986 Nov;54(2):522–528. doi: 10.1128/iai.54.2.522-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristaino P. A., Levine M. M., Young C. R. Improved GM1-enzyme-linked immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1983 Oct;18(4):808–815. doi: 10.1128/jcm.18.4.808-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Kaper J. B., Galen J., Lawrisuk L., Vimr E. R., Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Thompson J. S., Godoy V. G., Malamy M. H. Cloning and expression of the Bacteroides fragilis TAL2480 neuraminidase gene, nanH, in Escherichia coli. J Bacteriol. 1990 May;172(5):2594–2600. doi: 10.1128/jb.172.5.2594-2600.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. The Myxococcus xanthus FprA protein causes increased flavin biosynthesis in Escherichia coli. J Bacteriol. 1990 Jan;172(1):24–30. doi: 10.1128/jb.172.1.24-30.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Lawrisuk L., Galen J., Kaper J. B. Cloning and expression of the Vibrio cholerae neuraminidase gene nanH in Escherichia coli. J Bacteriol. 1988 Apr;170(4):1495–1504. doi: 10.1128/jb.170.4.1495-1504.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Broadbent D. A. Biochemical characterization of extracellular proteases from Vibrio cholerae. Infect Immun. 1982 Sep;37(3):875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]




