Abstract
The reaction mechanisms of two inhibitor TFK+ and TFK0 binding to H447I mutant mouse acetylcholinesterase (mAChE) have been investigated by using a combined ab initio quantum mechanical/molecular mechanical (QM/MM) approach and classical molecular dynamics (MD) simulations. TFK+ binding to the H447I mutant may proceed with a different reaction mechanism from the wild type. A water molecule takes over the role of His447 and participates in the bond breaking and forming as a “charge relayer”. Unlike in the wild-type mAChE case, Glu334, a conserved residue from the catalytic triad, acts as a catalytic base in the reaction. The calculated energy barrier for this reaction is about 8 kcal/mol. These predictions await experimental verification. In the case of the neutral ligand TFK0, however, multiple MD simulations on the TFK0/H447I complex reveal that none of the water molecules can be retained in the active site as a “catalytic” water. Taken together our computational studies confirm that TFK0 is almost inactive in the H447I mutant, and also provide detailed mechanistic insights into the experimental observations.
Keywords: ab initio QM/MM, molecular dynamics, free energy calculations, charge relayer
Introduction
Acetylcholinesterase (AChE, EC 3.1.1.7) is a hydrolytic enzyme that belongs to the serine hydrolase family. It plays important roles during the course of signal transmission at cholinergic synapses. The principal biological role of acetylcholinesterase is the termination of impulse transmissions by rapidly hydrolyzing the neurotransmitter, acetylcholine (ACh) [1–3]. Dysfunctions of AChE or other components of cholinergic synapses are involved in several human diseases, including myasthenia gravis, glaucoma, Alzheimer’s and Parkinson’s Diseases [4–9]. As a result, AChE has become an important target for rational drug design.
The crystal structure of AChE is characterized by a deep narrow gorge which penetrates halfway into the enzyme and contains the catalytic site located near the bottom, ca. 20 Å deep [10]. Kinetic studies have revealed that AChE possesses a remarkably high activity, with an ACh turnover rate of about 104 s−1 under physiological conditions, approaching the diffusion-controlled limit [11–13].
Similar to many other proteases, the catalytic triad in AChE consisting of Ser203(200) [14], His447(440) and Glu334(327) is believed to be essential to hydrolysis.
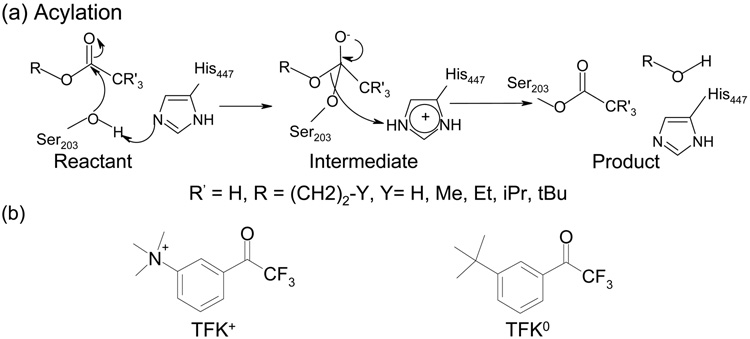
However, recent experimental mutagenesis studies have brought new challenges to all the above proposed reaction mechanisms. TFK+ (m-(N,N,N-trimethylammonio)trifluoroacetophenone (TMTFA)) (see Fig. 1 (b)), a common inhibitor to AChE, can still react with the mouse AChE (mAChE), even with the replacement of His447 by a hydrophobic Ile. In contrast, its neutral analog, TFK0, shows no apparent binding activity to H447I mutant, while it shows slightly reduced binding to the wild-type mAChE as compared to TFK+ (Table 1).
Figure 1.
(a) The acylation mechanism in the wild-type AChE enzyme; (b) The chemical structures of TFK+ and TFK0.
Table 1.
The rate constants for association and dissociation of inhibitors TFK+ and TFK0 with mouse AChEs measured by experiments [22–23]. The units of kon and ko f f are 109 M−1 min−1 and 10−3min−1, respectively.
| wild-type mAChE | H447I mutant mAChE | |||
|---|---|---|---|---|
| kon | ko f f | kon | ko f f | |
| TFK+ | 980±60 | 1.1±0.3 | ~ 10a | ~ 1.0a |
| TFK0 | 2.2±0.3 | 15±1 | N/Ab | N/Ab |
Unpublished data.
There is no apparent binding affinity detected.
In order to explore the enzymatic activity of H447I mutant mAChE, we have performed computational studies on both TFK+ and TFK0 binding to the wild-type and H447I mutant mAChEs, using a combined ab initio quantum mechanical and molecular mechanical (QM/MM) approach, as well as multiple MD simulations. In our study, a water molecule is found to play an essential catalytic role in place of His447 in the binding reaction of TFK+ to the H447I mutant. Along with this water molecule, Ser203 and Glu334 form a new stable catalytic triad. During the reaction, Ser203 delivers a proton to the water molecule while the water molecule serves as a charge relayer to pass one proton to Glu334. The QM/MM free energy barrier for the reaction is lower than 8.0 kcal/mol. On the other hand, the water triad was unable to be retained in the corresponding TFK0/H447I complexes from multiple MD simulations, indicating that TFK0 might not be able to stably bind to the H447I mutant. To further validate our QM/MM calculations, we also used thermodynamic integration (TI) calculations to investigate the binding energy differences between TFK0 and TFK+ in both the wild-type and H447 mutant enzymes. The TI calculations also suggest that the binding of TFK+ to both enzymes is much stronger than the neutral analog TFK0, which is consistent with experimental observations as well as the above QM/MM calculations.
Computational methods
Since there is no crystal structure of the H447I mutant so far, we tried two approaches to prepare the initial noncovalent complex structure of H447I and TFK+ (denoted as the [M•T+] model [21]). The first approach is to use the multiple docking approach introduced by Kua et al [15]. His447 in the apo mAChE crystal structure (PDB code: 1J06) was manually modified to Ile, and then TFK+ was docked into 1,000 snapshots evenly chosen from the last 1 ns trajectory of a 10 ns apo H447I mutant MD simulation. The Autodock 3.0 program [16] was used for all the docking studies. The search method used was the Lamarckian genetic algorithm (LGA) set at level 2 with the top 6 structures reported. Finally, according to the criteria suggested in Kua et al [15], the best 6 complex structures were selected and immersed into explicit water boxes , and subsequent MD simulations were set up to relax each system. To prevent TFK+ dislocation from the esteratic binding site, a 20.0 kcal/(mol·Å2) harmonic restraint between the carbonyl-C of TFK+ and Ser203-Oγ was applied during simulations. The second approach is to start from the noncovalent [W•T+] structure obtained from our QM/MM calculations of the wild-type enzyme with TFK+ (see below for details), and then manually replace HID447 (Note: The Nε proton originally in the [W-T+] complex is transferred to Ser203 in the [W•T+ ] model after the QM/MM run) with Ile. Another MD simulation of the resulting system was then set up by following the same procedure as outlined in the first approach. The total number of atoms in these 7 MD simulations is around 70,000–75,000. Similarly, 7 initial models of H447I and TFK0 ([M•T0]) were obtained with the same procedure. Additionally, an 8th [M•T0] model was obtained by directly modifying TFK+ to TFK0 in one of the [M•T+] models. Therefore, a total of 8 [M•T0] models were prepared and subjected to further theoretical investigations.
Results and Discussions
As mentioned in Methods section, 7 non-covalent complex [M•T+] models of the H447I mutant and TFK+ were obtained via docking and QM/MM calculations. During the subsequent MD simulations of these models, in 6 of 7 models, a water molecule was observed to diffuse into the center of the triangle formed by Ser203, Ser229 and Glu334 in the first 2 ns simulations; a representative snapshot from the simulation is shown in Fig. 2. Then we extended our MD simulations to 10 ns for each model. The average distance of the hydroxyl oxygen Oγ of Ser203 to the oxygen OW of the water is 2.72±0.12 Å, the carboxyl oxygen of the Ser229 backbone to OW of the water is 2.78±0.18 Å, and OW of the water to one of carboxyl oxygens Oδ1 of Glu334 is 2.66±0.12 Å in all of the 10 ns trajectories. Generally, an effective hydrogen bond can be characterized by the short distance between the heteroatoms (less than 3.50 Å), and the bond angle greater than 135°[17–18]. Using this criterion, the hydrogen bond between the Ser203 Oγ and OW is found in all the structures in the 10 ns simulations, while the hydrogen bond between OW and Oδ1 is ~99%, and between OW and Ser229-O is ~95%. The water molecule is observed to flip between Ser229-O and Oδ1, while the hydrogen bond between Oγ and OW stays stable.
Figure 2.
The “water” triad in the active site of the six [M·T+] models. The values of the distances in Å are averaged among six models.
Three snapshots from the above MD simulations were selected to explore the acylation reaction mechanism in the H447I mutant. The water molecules beyond a 27 Å solvent water sphere, centered on the active site (the hydroxyl oxygen Oγ of Ser203) were removed. The three prepared QM/MM models have 10059 atoms (562 water molecules), 10176 atoms (602 water molecules) and 10170 atoms (600 water molecules) respectively. Each snapshot was first relaxed by the MM method, and then optimized with B3LYP(6-31G*) QM/MM calculations using an iterative minimization approach [19], leading to an optimized structure for the reactant. The QM subsystem consists of the sidechains of Ser203 and Glu334, the water bridge and TFK+, with the broken Cβ -Cα bonds of Ser203 and Glu334 treated as two pseudobonds [20], resulting in a total of 48 QM atoms. All other atoms are treated by MM. The reaction coordinate (RC) is show in Fig. 3. Both forward and backward RC driving calculations yield consistent curves and all the reaction paths are smooth and continuous.
Figure 3.
Illustration of the reaction coordinate used for the H447I mutant and TFK+ reaction, which is dOγ−Hγ+dOw−H −dOγ−C −dOw−Hγ−dH−Oδ.
All the three QM/MM calculations led to very consistent results, in which the water molecule between Ser203 and Glu334 plays a “charge-relay” role during TFK+ binding reaction with the H447I mutant. Table 2 presents the relative potential energies in the reactant (non-covalent complex), transition state and product (covalent complex), respectively. The calculated potential energy barriers at the MP2(6-31+G*)/MM level are 8.2, 4.2, 10.6 kcal/mol.
Table 2.
The calculated QM/MM potential energy differences (kcal/mol) for three QM/MM models. The geometries are relaxed at the B3LYP(6-31G*)/MM level, and then single-point calculations are performed at three different levels.
| MP2(6-31+G*)/MM | B3LYP(6-31+G*)/MM | B3LYP(6-31G*)/MM | |
|---|---|---|---|
| The first snapshot | |||
| Reactant | 0.0 | 0.0 | 0.0 |
| Transition State | 8.2 | 7.4 | 8.2 |
| Product | −5.0 | −1.1 | 2.0 |
| The second snapshot | |||
| Reactant | 0.0 | 0.0 | 0.0 |
| Transition State | 4.2 | 5.8 | 7.5 |
| Product | −8.0 | −2.5 | 0.0 |
| The third snapshot | |||
| Reactant | 0.0 | 0.0 | 0.0 |
| Transition State | 10.6 | 10.3 | 10.4 |
| Product | 0.4 | 3.8 | 4.4 |
However, the “lock” can be broken easily or even never formed if TFK+ is replaced by TFK0. As compared to TFK+, larger RMSDs for TFK0 have been captured in MD simulations [24].
In conclusion, we explored the TFK+ and TFK0 inhibition mechanisms in the H447I mutant mAChE. Despite the replacement of the catalytic base His447, TFK+ still demonstrates high binding affinity to the H447I mutant. Our computational studies suggest that a water molecule might act as a “charge relayer” and facilitate the binding of the ligand to the enzyme while this is not true for the binding of the neutral analogue TFK0. We hope that these results will stimulate further experimental studies.
Acknowledgements
Y.H.C. thanks Prof. Palmer Taylor for helpful discussions and experimental data, and Prof. Yingkai Zhang for advice and access of his QM/MM code and his brilliant idea in simulating enzymatic reactions. This work has been supported in part by grants from the NSF and NIH. Additional support has been provided by NBCR, CTBP, HHMI, the W. M. Keck Foundation, and Accelrys, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenberry TL. Acetylcholinesterase. Adv. Enzymol. Relat. Areas Mol. Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- 2.Schumacher M, Camp S, Maulet Y, Newton M, Macpheequigley K, Taylor SS, Friedmann T, Taylor P. Primary structure of acetylcholinesterase - implications for regulation and function. Fed. Proc. 1986;45:2976–2981. [PubMed] [Google Scholar]
- 3.Taylor P. The Pharmacological Basis of Therapeutics. New York: MacMillan; 1985. [Google Scholar]
- 4.Contestabile A, Fila T, Bartesaghi R, Contestabile A, Ciani E. Choline acetyltransferase activity at different ages in brain of ts65dn mice, an animal model for down's syndrome and related neurodegenerative diseases. J. Neurochem. 2006;97:515–526. doi: 10.1111/j.1471-4159.2006.03769.x. [DOI] [PubMed] [Google Scholar]
- 5.Piazzi L, Rampa A, Bisi A, Gobbi S, Belluti F, Cavalli A, Bartolini M, Andrisano V, Valenti P, Recanatini M. 3-(4-{[benzyl(methyl)amino]methyl}-phenyl)-6,7-dimethoxy-2h-2-chromenone (ap2238) inhibits both acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation: a dual function lead for alzheimer's disease therapy. J. Med. Chem. 2003;46:2279–2282. doi: 10.1021/jm0340602. [DOI] [PubMed] [Google Scholar]
- 6.Bachurin SO. Medicinal chemistry approaches for the treatment and prevention of alzheimer disease. Med. Res. Rev. 2003;23:48–88. doi: 10.1002/med.10026. [DOI] [PubMed] [Google Scholar]
- 7.Sramek JJ, Zarotsky V, Cutler NR. Review of drug development and therapeutic role of cholinesterase inhibitors in alzheimer's disease. Drug Dev. Res. 2002;56:347–353. [Google Scholar]
- 8.Kryger G, Silman I, Sussman JL. Structure of acetylcholinesterase complexed with e2020 (aricept (r)): implications for the design of new anti-alzheimer drugs. Struct. Fold. Des. 1999;7:297–307. doi: 10.1016/s0969-2126(99)80040-9. [DOI] [PubMed] [Google Scholar]
- 9.Egan TM, North RA. Acetylcholine hyperpolarizes central neurons by acting on an m2 muscarinic receptor. Nature. 1986;319:405–407. doi: 10.1038/319405a0. [DOI] [PubMed] [Google Scholar]
- 10.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic-structure of acetylcholinesterase from torpedo-californica - a prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 11.Bazelyansky M, Robey E, Kirsch JF. Fractional diffusion-limited component of reactions catalyzed by acetylcholinesterase. Biochemistry. 1986;25:125–130. doi: 10.1021/bi00349a019. [DOI] [PubMed] [Google Scholar]
- 12.Hasinoff BB. Kinetics of acetylthiocholine binding to electric-eel acetylcholinesterase in glycerol water solvents of increased viscosity - evidence for a diffusion-controlled reaction. Biochim. Biophys. Acta. 1982;704:52–58. doi: 10.1016/0167-4838(82)90131-5. [DOI] [PubMed] [Google Scholar]
- 13.Nolte HJ, Rosenberry TL, Neumann E. Effective charge on acetylcholinesterase active-sites determined from the ionic-strength dependence of association rate constants with cationic ligands. Biochemistry. 1980;19:3705–3711. doi: 10.1021/bi00557a011. [DOI] [PubMed] [Google Scholar]
- 14.Amino acids and numbers refer to mouse or Human AChE, and the numbers in parentheses refer to the positions of analogous residues in TcAChE
- 15.Kua J, Zhang YK, McCammon JA. Studying enzyme binding specificity in acetylcholinesterase using a combined molecular dynamics and multiple docking approach. J. Am. Chem. Soc. 2002;124:8260–8267. doi: 10.1021/ja020429l. [DOI] [PubMed] [Google Scholar]
- 16.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a lamarckian genetic algorithm and an empirical binding free energy function. 1998;19:1639–1662. [Google Scholar]
- 17.Eriksson MAL, Nilsson L. Structural and dynamic differences of the estrogen receptor dna binding domain, binding as a dimer and as a monomer to DNA: molecular dynamics simulation studies. Eur. Biophys. J. Biophys. Lett. 1999;28:102–111. doi: 10.1007/s002490050189. [DOI] [PubMed] [Google Scholar]
- 18.Sheu SY, Yang DY, Selzle HL, Schlag EW. Energetics of hydrogen bonds in peptides. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12683–12687. doi: 10.1073/pnas.2133366100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YK, Liu HY, Yang WT. Free energy calculation on enzyme reactions with an efficient iterative procedure to determine minimum energy paths on a combined ab initio QM/MM potential energy surface. J. Chem. Phys. 2000;112:3483–3492. [Google Scholar]
- 20.Zhang YK, Lee TS, Yang WT. A pseudobond approach to combining quantum mechanical and molecular mechanical methods. J. Chem. Phys. 1999;110:46–54. [Google Scholar]
- 21.“M”, “T+” and “T0” represent H447I mutant mAChE, TFK+ and TFK0 respectively; [X-Y] and [X-Y] represent the covalent and noncovalent complexes of the X enzyme and Y ligand, respectively.
- 22.Radić Z, Quinn DM, McCammon JA, Taylor P. J. Biol. Chem. 1997;272:23265–23277. doi: 10.1074/jbc.272.37.23265. [DOI] [PubMed] [Google Scholar]
- 23.Radić Z, Taylor P. J. Biol. Chem. 2001;276:4622–4633. doi: 10.1074/jbc.M006855200. [DOI] [PubMed] [Google Scholar]
- 24.Cheng YH, Cheng XL, Radić Z, McCammon JA. J. Am. Chem. Soc. 2007;129:6562–6570. doi: 10.1021/ja070601r. [DOI] [PubMed] [Google Scholar]