Abstract
Iron-sulfur clusters are one of the most ubiquitous redox centers in biology. Ironically, iron-sulfur clusters are highly sensitive to reactive oxygen species. Disruption of iron-sulfur clusters will not only change the activity of proteins that host iron-sulfur clusters, the iron released from the disrupted iron-sulfur clusters will further promote the production of deleterious hydroxyl free radicals via the Fenton reaction. Here, we report that ferritin A (FtnA), a major iron-storage protein in Escherichia coli, is able to scavenge the iron released from the disrupted iron-sulfur clusters and alleviates the production of hydroxyl free radicals. Furthermore, we find that the iron stored in ferritin A can be retrieved by an iron chaperon IscA for the re-assembly of the iron-sulfur cluster in a proposed scaffold IscU in the presence of the thioredoxin reductase system which emulates normal intracellular redox potential. The results suggest that E. coli ferritin A may act as an iron buffer to sequester the iron released from the disrupted iron-sulfur clusters under oxidative stress conditions and to facilitate the re-assembly of the disrupted iron-sulfur clusters under normal physiological conditions.
Keywords: Ferritin A, hydroxyl free radicals, iron-sulfur clusters, IscA, IscU
Introduction
Iron-sulfur clusters represent one of the major iron-containing cofactors in living organisms. Throughout evolution, iron-sulfur clusters have become integral parts of diverse physiological processes ranging from respiratory electron transfer, sugar metabolism, nitrogen fixation, photosynthesis, amino acids biosynthesis, heme and biotin biosynthesis, intracellular iron homeostasis, RNA modification, DNA synthesis and repair, and the regulation of gene expression (Beinert et al. 1997, Kiley and Beinert 2003, Johnson et al. 2005, Rouault and Tong 2005, Fontecave 2006, Lill and Muhlenhoff 2006). It is now clear that the biogenesis of iron-sulfur clusters is not a spontaneous process. Increasing evidence has suggested that the iron-sulfur cluster assembly requires multiple proteins which are highly conserved from bacteria to humans (Johnson et al. 2005, Rouault and Tong 2005, Lill and Muhlenhoff 2006). In Escherichia coli, at least two gene clusters iscSUA-hscBA-fdx (Zheng et al. 1998) and sufABCDSE (Takahashi and Tokumoto 2002, Outten et al. 2004) have been identified as critical for the biogenesis or repair of iron-sulfur clusters. Whereas deletion of iscSUA-hscBA-fdx severely decreases the iron-sulfur cluster assembly activity in E. coli, deletion of both iscSUA-hscBA-fdx and sufABCDSE results in an inviable phenotype (Takahashi and Tokumoto 2002). Among the proteins encoded by the gene clusters, IscS is a cysteine desulfurase containing a pyridoxal-5-phosphate (Flint 1996, Cupp-Vickery et al. 2003). IscS catalyzes desulfurization of L-cysteine and transfers sulfane sulfur for the iron-sulfur cluster assembly in a proposed scaffold IscU (Agar et al. 2000, Smith et al. 2001, Urbina et al. 2001, Kato et al. 2002, Smith et al. 2005) which eventually transfers the assembled clusters to target proteins (Wu et al. 2002, Unciuleac et al. 2007). Two heat shock cognate proteins, HscB and HscA, specifically interact with IscU (Silberg et al. 2004, Tapley and Vickery 2004) and stimulate the transfer of the assembled clusters from IscU to apo-ferredoxin in an ATP-dependent reaction (Chandramouli and Johnson 2006). SufS and IscS are paralogs with 30% sequence identity. The catalytic activity of SufS is relatively low, but can be stimulated by SufE and SufBCD complex (Loiseau et al. 2003, Outten et al. 2003). Recent studies further indicated that SufS transfers sulfane sulfur to SufB via SufE (Layer et al. 2007). IscA and SufA are also paralogs with 46% sequence identity. It was reported that both IscA and SufA can host transient iron-sulfur clusters, thus being proposed as alternative scaffold proteins (Krebs et al. 2001, Ollagnier-de-Choudens et al. 2001, Wollenberg et al. 2003, Sendra et al. 2007). However, unlike IscU (Agar et al. 2000), IscA and SufA show a strong iron binding affinity with an iron association constant of 2.0×1019M−1 in the presence of the thioredoxin reductase system (Ding and Clark 2004, Ding et al. 2005, Yang et al. 2006, Lu et al. 2008). Furthermore, the iron-bound IscA and SufA can efficiently provide iron for the iron-sulfur cluster assembly in IscU (Ding et al. 2004, Ding et al. 2005, Yang et al. 2006, Lu et al. 2008). These results suggested that the primary function of IscA/SufA may be to recruit intracellular iron and deliver iron for the biogenesis of iron-sulfur clusters. Based on these studies, we proposed that IscA/SufA (an iron donor) and IscS/SufS (a sulfur donor) may work in concert to coordinately deliver the iron and sulfur for the iron-sulfur cluster assembly in scaffold proteins IscU or SufBCD complex (Yang et al. 2006, Lu et al. 2008).
Ironically, iron-sulfur clusters are highly sensitive to reactive free radicals (Djaman et al. 2004, Jang and Imlay 2007). Disruption of iron-sulfur clusters will not only change the activity of proteins that host iron-sulfur clusters (Kiley and Beinert 2003), the iron released from the disrupted iron-sulfur clusters will further promote the production of deleterious hydroxyl free radicals via Fenton reaction (Keyer and Imlay 1996). The mechanisms underlying the sequestration of the iron released from the disrupted iron-sulfur clusters and the subsequent repair of the iron-sulfur clusters have not been fully understood. Here, we report that ferritin A (FtnA), a major iron storage protein in E. coli (Hudson et al. 1993, Abdul-Tehrani et al. 1999), is able to scavenge the iron released from the disrupted iron-sulfur clusters and alleviates the iron-mediated production of hydroxyl free radicals in the presence of hydrogen peroxide. Furthermore, we find that the iron stored in FtnA can be retrieved by IscA for the iron-sulfur cluster assembly in IscU in the presence of the thioredoxin reductase system. The physiological roles of ferritins in cellular response to oxidative stresses and in repair of the disrupted iron-sulfur clusters will be discussed.
Materials and Methods
Protein Preparation
The DNA fragment encoding ferritin A (FtnA) was amplified from E. coli genomic DNA. Two primers, FTN-1, 5’ GAGCACTACCATGGTGAAACCAGAAA-3’ and FTN-2, 5’-GAGCATTAGTTAAGCTTGTCGAGGGT-3’ were used for the PCR amplification. The PCR product was digested with two restriction enzymes HindIII and NcoI, and ligated into an expression vector pET28b+. The cloned DNA fragment was confirmed by direct sequencing using T7 primers. Recombinant FtnA was prepared following the procedures as previously described (Yang et al. 2002). The gel filtration analyses showed that recombinant FtnA was a homopolymer of 24 subunits with a molecular weight of ~ 440 kDa as reported previously by others (Hudson et al. 1993). The E. coli thioredoxin 1 (Veine et al. 1998) and thioredoxin reductase (Mulrooney 1997) were prepared as described in (Ding et al. 2005). The purity of purified proteins was greater than 95% as judged by the SDS electrophoresis analysis followed by staining with the Coomassie blue.
Measurements of hydroxyl free radicals
Hydroxyl free radicals were measured following the procedure described by Halliwell et al. (Halliwell et al. 1987). Briefly, hydroxyl free radicals generated in solution degrade 2-deoxyribose and form a malondialdehyde-like compound which reacts with thiobarbituric acid to generate a chromogen. In the experiments, the IscU [2Fe-2S] cluster or freshly prepared Fe(NH4)2(SO4)2 was incubated with potassium phosphate buffer K2PO4 (10 mM) (pH 7.4), NaCl (60 mM), 2-deoxyribose (4 mM) at 37°C for 10 min before hydrogen peroxide (0.5 mM) was added to initiate the Fenton reaction. The reactions were continued at 37°C for additional 25 min. A developing solution containing 1% thiobarbituric acid and 2.8% trichloroacetic acid (400 µl) was then mixed with the above incubation solutions (600 µL), and boiled for 15 min. The mixtures were centrifuged at 14,000 rpm for 15 min. The amounts of the chromogen in the supernatants were measured from the emission at a wavelength of 553 nm using an excitation wavelength of 532 nm in a Perkin-Elmer LS-3 Fluorometer.
Iron binding analyses
The iron binding in proteins was analyzed after the proteins were incubated with freshly prepared Fe(NH4)2(SO4)2 in the presence of the thioredoxin reductase system, followed by re-purification of the protein using a Mono-Q column (0.98 ml) as described previously (Ding et al. 2005). The re-purification procedure did not affect the iron binding in frataxin A, as over 95% of the total iron content remained in the protein after passing through the Mono-Q column. The total iron content in the eluted samples was determined using the Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) (Department of Geology and Geophysics/LSU) or an iron indicator ferrozine (Yang et al. 2006). The iron-ferrozine complex was measured at 564 nm using an extinction coefficient of 27.9 mM−1cm−1 (Cowart et al. 1993). Both iron analysis methods produced similar results.
Iron-sulfur cluster assembly in IscU
For the iron-sulfur cluster assembly reactions, IscU (50 µM) was pre-incubated with cysteine desulfurase IscS (1 µM) and Fe(NH4)2(SO4)2 (400 µM) in the presence of the thioredoxin reductase system (thioredoxin-1 (5 µM), thioredoxin reductase (0.5 µM) and NADPH (500 µM)) at 37°C for 5 min. If indicated, the iron-bound IscA or FtnA was used instead of Fe(NH4)2(SO4)2 as the iron donor. The iron-sulfur cluster assembly was initiated by adding L-cysteine (1 mM) and monitored in the Beckman DU640 spectrometer. After incubation, the IscU [2Fe-2S] cluster was re-purified using a Mono-Q column as described previously (Yang et al. 2006).
Results
Hydrogen peroxide disrupts the IscU [2Fe-2S] cluster and promotes the production of hydroxyl free radicals
IscU is a well characterized scaffold protein for the biogenesis of iron-sulfur clusters (Agar et al. 2000, Unciuleac et al. 2007). Purified E. coli IscU [2Fe-2S] cluster had a distinctive absorption peak at 456 nm (Agar et al. 2000, Yang et al. 2006). The [2Fe-2S] cluster in IscU was relatively stable in the presence of the thioredoxin reductase system. However, when hydrogen peroxide was added to the incubation solution, the absorption peak at 456 nm of the IscU [2Fe-2S] cluster was quickly eliminated (Figure 1). The total iron and sulfur content analyses of the re-purified IscU confirmed that the IscU [2Fe-2S] cluster was converted to apo-IscU by hydrogen peroxide.
Figure 1. Disruption of the IscU [2Fe-2S] cluster by H2O2.
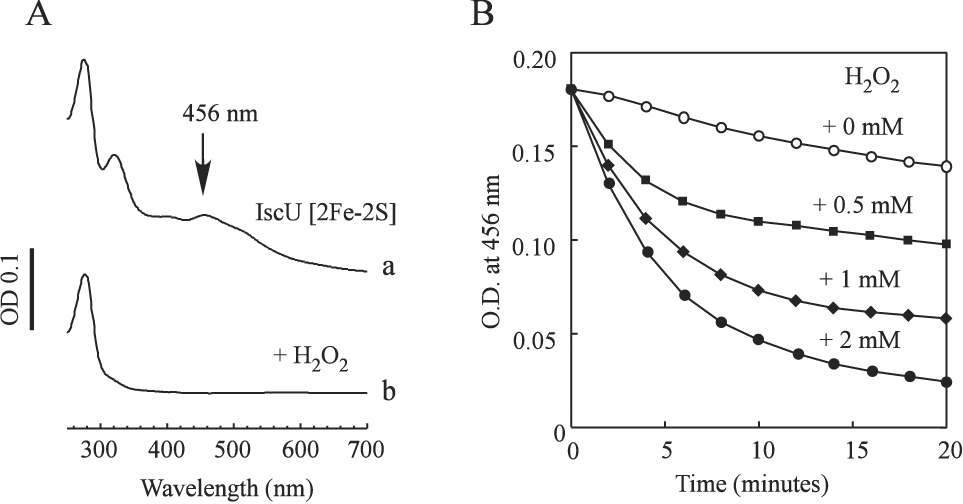
Purified IscU [2Fe-2S] cluster (50 µM) was incubated with different concentrations of H2O2 in the presence of the thioredoxin reductase system at 37°C. A), the UV-Vis absorption spectra of the IscU [2Fe-2S] cluster before (spectrum a) and after (spectrum b) incubation with 2 mM H2O2 for 20 min. B), the disruption kinetics of the IscU [2Fe-2S] cluster by H2O2. Disruption of the IscU [2Fe-2S] cluster by H2O2 was monitored at an absorption peak of 456 nm. The H2O2 concentrations in the incubation solutions were 0 mM (open circles), 0.5 mM (closed squares), 1 mM (closed diamonds), and 2 mM (closed circles), respectively.
The iron released from the disrupted iron-sulfur clusters could potentially promote the production of hydroxyl free radicals via the Fenton reaction (Keyer and Imlay 1996). To test this idea, we utilized the 2-deoxyribose method (Halliwell et al. 1987) to measure the production of hydroxyl free radicals in solutions as described in the Materials and Methods. Figure 2 shows that the production of hydroxyl free radicals was almost linearly proportional to the concentration of the IscU [2Fe-2S] cluster in the incubation solution. The amount of hydroxyl free radicals generated by the IscU [2Fe-2S] cluster was also close to that when an equivalent amount of ferrous iron were used in the incubation solutions, suggesting that both iron released from the IscU [2Fe-2S] cluster by hydrogen peroxide contributed to the production of hydroxyl free radicals. Taken together, these results showed that hydrogen peroxide disrupts the IscU [2Fe-2S] cluster and that the iron released from the disrupted iron-sulfur clusters promotes the production of hydroxyl free radicals in the presence of the thioredoxin reductase system.
Figure 2. The IscU [2Fe-2S] cluster promotes the production of hydroxyl free radicals in the presence of H2O2.
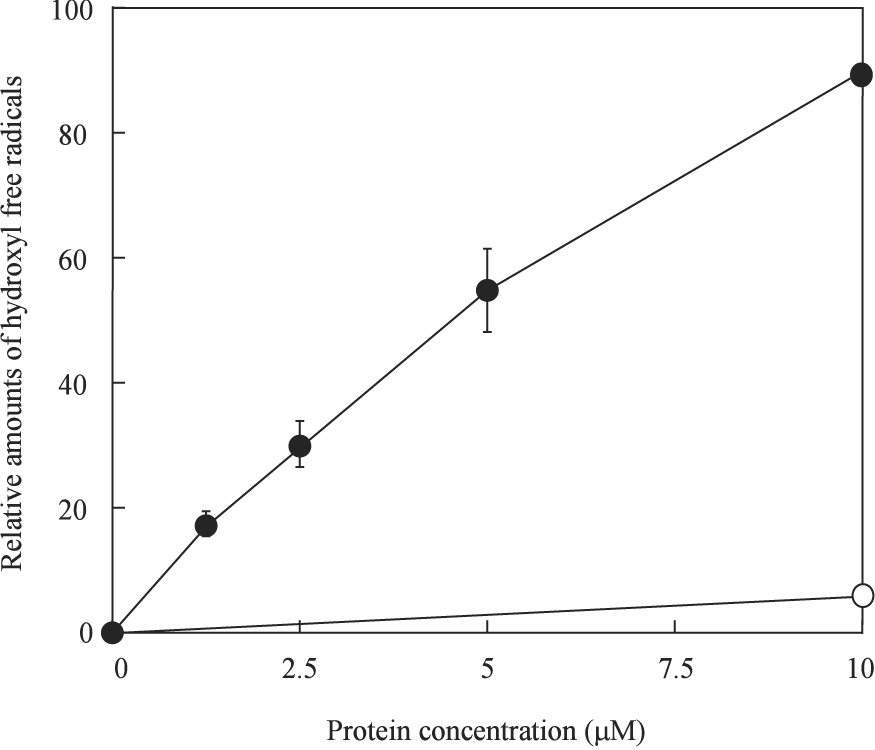
Purified IscU [2Fe-2S] cluster (0 to 10 µM) was mixed with 2-deoxyribose (4 mM), potassium phosphate buffer (10 mM) (pH 7.4) and NaCl (60 mM) at 37°C for 10 min before H2O2 (0.5 mM) was added to initiate Fenton reaction. The amounts of hydroxyl free radicals were measured as described in the Materials and Methods, and plotted as a function of the IscU [2Fe-2S] cluster concentration. The Y-axis at 100% represents the amount of hydroxyl free radicals produced when 20 µM freshly prepared Fe(NH4)2(SO4)2 was used in the incubation solution. Closed circles: the IscU [2Fe-2S] cluster; open circles: apo-IscU.
Ferritin A (FtnA) alleviates the production of hydroxyl free radicals by scavenging the iron released from the IscU [2Fe-2S] cluster
To avoid the excessive production of deleterious hydroxyl free radicals, the iron released from the disrupted iron-sulfur clusters must be efficiently sequestered. Although IscA is an iron binding protein and provides iron for the biogenesis of iron-sulfur clusters under physiologically relevant conditions (Ding and Clark 2004, Ding et al. 2005, Yang et al. 2006), IscA fails to bind any iron in the presence of hydrogen peroxide (Ding et al. 2007). Indeed, we found that addition of apo-IscA had no effect on the IscU [2Fe-2S] cluster-mediated production of hydroxyl free radicals in the presence of hydrogen peroxide (Figure 3). Thus, other cellular proteins likely exist to scavenge the iron released from the disrupted iron-sulfur clusters under oxidative stress conditions.
Figure 3. FtnA alleviates the iron-mediated production of hydroxyl free radicals.
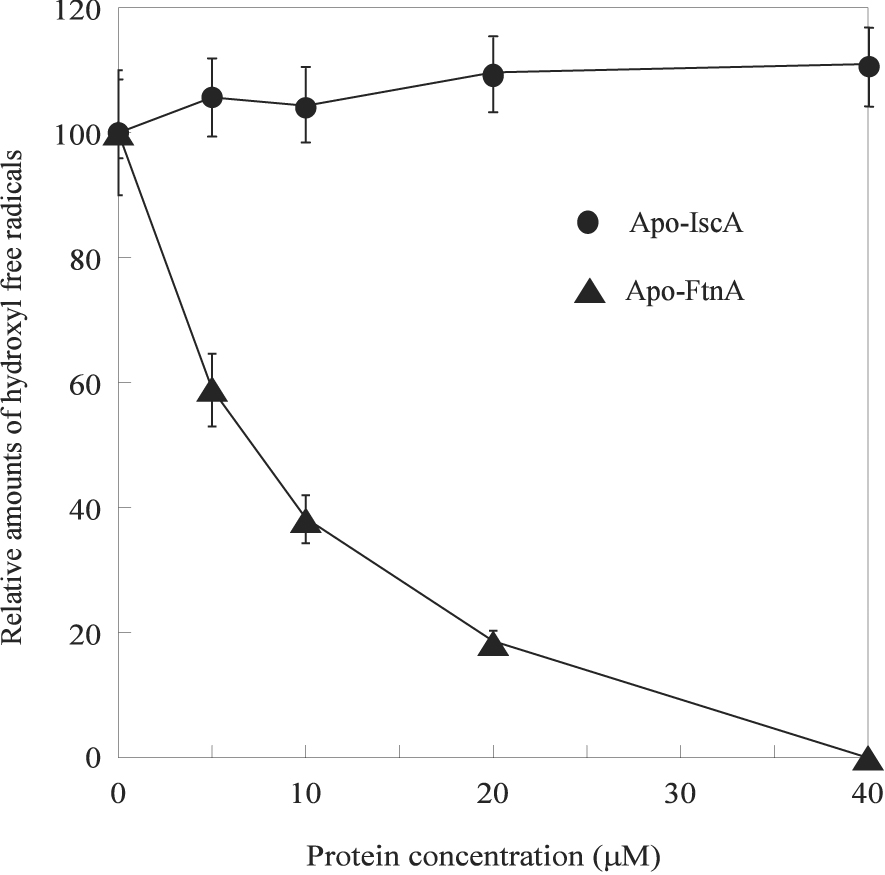
Different concentrations of apo-IscA and apo-FtnA were mixed with the IscU [2Fe-2S] cluster (5 µM), 2-deoxyribose (4 mM), potassium phosphate (10 mM) (pH 7.4), and NaCl (60 mM) at 37°C for 10 min before H2O2 (0.5 mM) was added to initiate the Fenton reaction. The relative amounts of hydroxyl free radicals were measured as described in the Materials and Methods.
Increasing evidence indicated that ferritins, a large family of iron-storage proteins found in bacteria and mammalian cells (Andrews et al. 2003, Carrondo 2003, Liu and Theil 2005, Sargent et al. 2005, Galatro and Puntarulo 2007), may have an important role in cellular defense against oxidative stresses (Abdul-Tehrani et al. 1999, Epsztejn et al. 1999, Cozzi et al. 2000, Bou-Abdallah et al. 2005, Zhao et al. 2006, Velayudhan et al. 2007). In E. coli, there are at least four ferritin-like proteins: ferritin A (FtnA), ferritin B (FtnB), bacterioferritin (Bft), and Dps (Andrews et al. 2003). To examine whether ferritins could scavenge the iron released from the disrupted iron-sulfur clusters under oxidative stress conditions, we have used ferritin A, a major iron storage protein in E. coli cells (Hudson et al. 1993, Abdul-Tehrani et al. 1999), as an example. Figure 3 shows that when apo-FtnA was incubated with the IscU [2Fe-2S] cluster and hydrogen peroxide in the presence of the thioredoxin reductase system, the production of hydroxyl free radicals was dramatically decreased.
To further test whether the iron released from the IscU [2Fe-2S] cluster was sequestered by apo-FtnA, both FtnA and IscU were re-purified after incubation with hydrogen peroxide using an anion exchange Mono-Q column. Figure 4A shows that the re-purified FtnA had a significant increase of the absorption peak at around 320 nm, indicative of iron binding in the protein. The total iron content analyses of the re-purified FtnA showed that over 90% of the iron in the IscU [2Fe-2S] cluster was transferred to apo-FtnA after incubation with hydrogen peroxide (Figure 4B). These results demonstrate that apo-FtnA is able to scavenge the iron released from the disrupted iron-sulfur clusters and alleviates the production of hydroxyl free radicals in the presence of hydrogen peroxide.
Figure 4. FtnA binds the iron released from the IscU [2Fe-2S] cluster in the presence of H2O2.
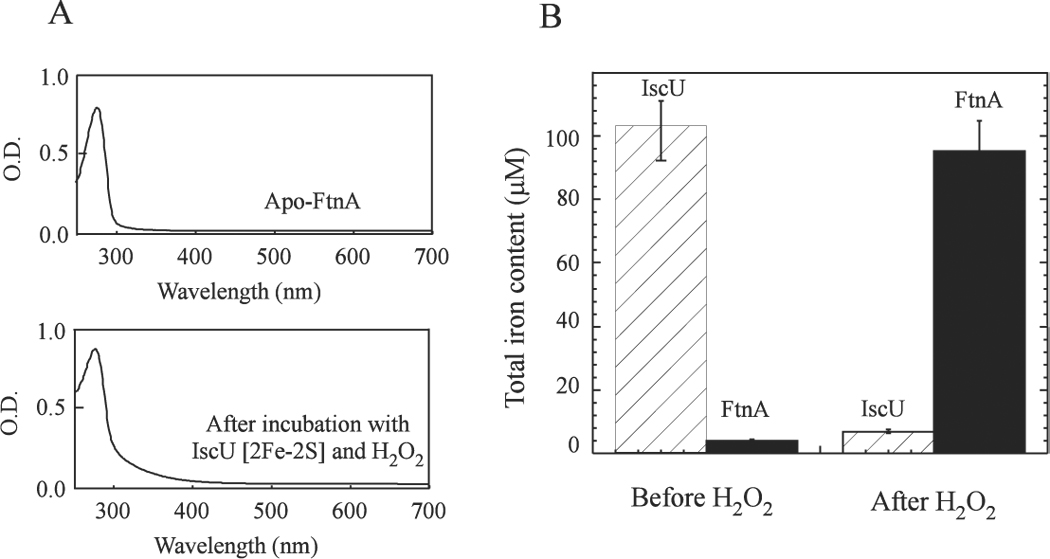
Purified IscU [2Fe-2S] cluster (50 µM) was incubated with apo-FtnA (100 mM) and H2O2 (2 mM) in the presence of the thioredoxin reductase system at 37°C for 20 min. FtnA and IscU were re-purified from the incubation solution using a gel filtration column (Superdex-200, Amersham Bioscience). A), the UV-Vis absorption spectra of the re-purified FtnA before and after incubation with the IscU [2Fe-2S] cluster and H2O2. B), the total iron contents in FtnA and IscU before and after incubation with H2O2.
The iron-bound FtnA is not an efficient iron donor for the iron-sulfur cluster assembly in IscU
As a major iron-storage protein in E. coli, it is possible that FtnA may act as an iron donor for the biogenesis of iron-sulfur clusters. To test this idea, the iron-bound FtnA (the ratio of iron to the FtnA monomer was about 4) was incubated with IscU, cysteine desulfurase IscS and L-cysteine in the presence of the thioredoxin reductase system at 37°C anaerobically. Figure 5A shows that little or no iron-sulfur clusters were assembled in IscU after 30 min incubation. Increase of the iron-bound FtnA concentration by 5 fold in the incubation solution did not significantly increase the iron-sulfur cluster assembly in IscU (data not shown). In contrast, when the iron-bound IscA was used as the iron donor, an absorption peak at 456 nm of the IscU [2Fe-2S] cluster quickly appeared as we reported previously (Yang et al. 2006). Thus, unlike the iron-bound IscA, the iron-bound FtnA is not an efficient iron donor for the iron-sulfur cluster assembly in IscU.
Figure 5. The iron-bound FtnA is not an efficient iron donor for the iron-sulfur cluster assembly in IscU.
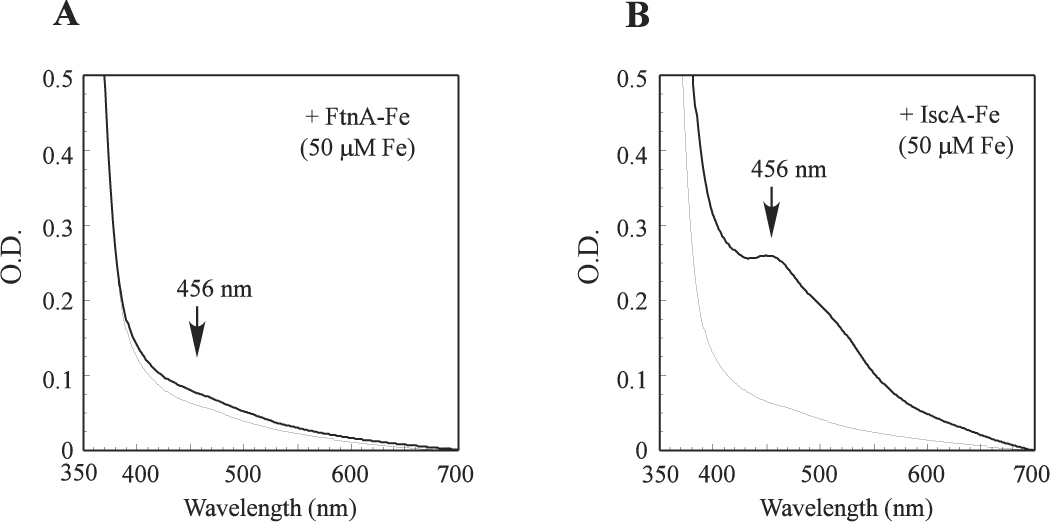
A), purified apo-IscU (50 µM) was pre-incubated with IscS (1 µM) and the iron-bound FtnA (containing 50 mM iron in 12.5 µM monomeric FtnA) in the presence of the thioredoxin reductase system at 37°C for 5 min before L-cysteine (1 mM) was added to initiate the iron-sulfur cluster assembly under anaerobic conditions. The UV-Vis absorption spectra were taken at 0 min (thin line) and 30 min (thick line) after addition of L-cysteine. B), as in A) except the iron-bound FtnA was replaced with the iron-bound IscA (containing 50 µM iron in 25 µM monomeric IscA) in the pre-incubation solution. The absorption peak at 456 nm indicates the assembly of the IscU [2Fe-2S] cluster.
IscA can retrieve iron from the iron-bound FtnA
In the presence of the thioredoxin reductase system IscA acts as a strong iron binding protein with an iron association constant of 2.0×1019M−1 (Ding and Clark 2004, Ding et al. 2005). This led us to speculate that IscA may be able to compete with FtnA for iron binding under normal physiological conditions.
In the experiments, apo-IscA was incubated with the iron-bound FtnA (the ratio of iron to the FtnA monomer was about 4) in the presence of the thioredoxin reductase system at 37°C for 1 hour to establish the iron binding equilibrium. IscA and FtnA were then re-purified using a Mono-Q column. The total iron content analyses showed that a significant amount of iron was transferred from the iron-bound FtnA to apo-IscA after the incubation (Figure 6B). Under the same experimental conditions, IscU failed to retrieve any iron from the iron-bound FtnA in the presence of the thioredoxin reductase system (data not shown), suggesting that IscA, but not IscU, can acquire the iron from the iron-bound FtnA under physiologically relevant conditions.
Figure 6. Apo-IscA can retrieve the iron from the iron-bound FtnA.
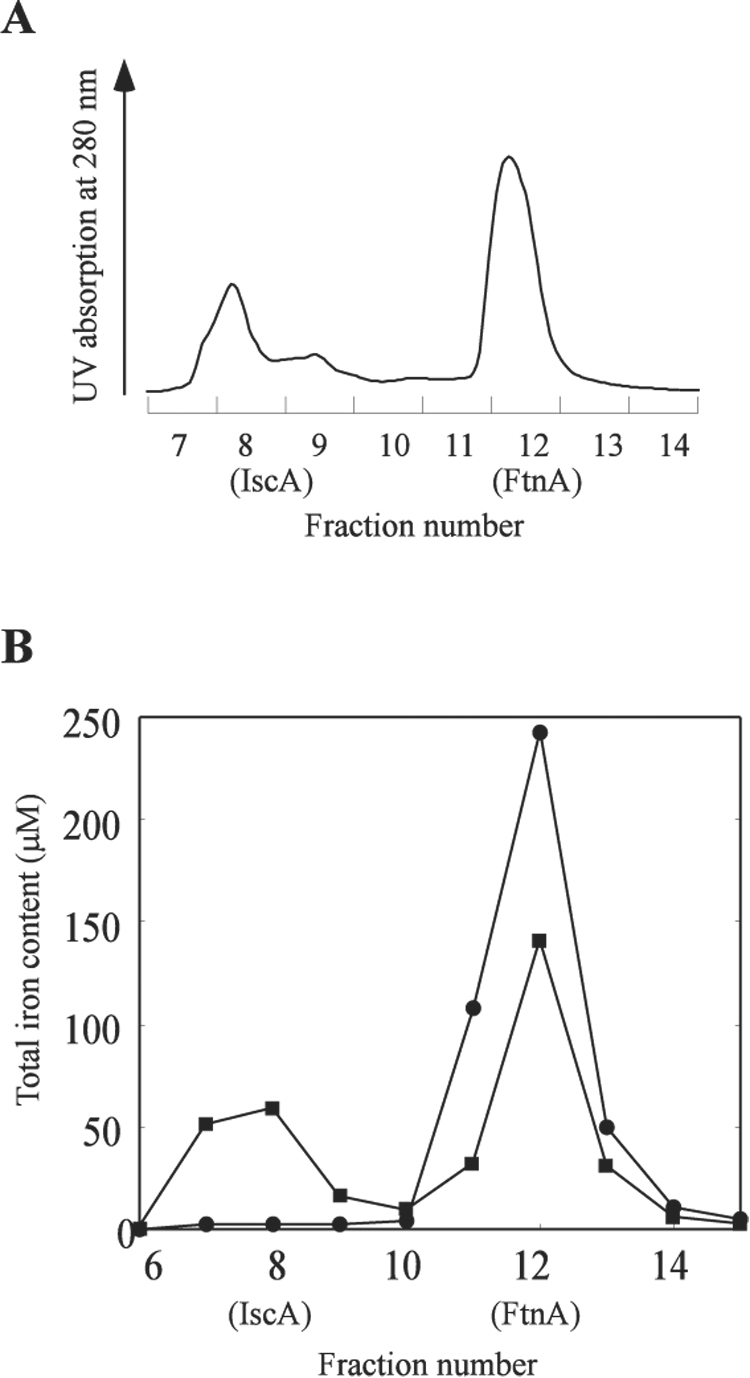
The iron-bound FtnA (containing 200 µM iron in 50 µM monomeric FtnA) was incubated with apo-IscA (50 µM) in the presence of the thioredoxin reductase system at 37°C for 1 hour, followed by re-purification of IscA and FtnA using a Mono-Q column. A), the elution profile of IscA and FtnA from the Mono-Q column. The SDS polyacrylamide gel electrophoresis showed that IscA was eluted in fraction 7 and 8, and FtnA in fraction 11 and 12. B), total iron contents in the eluted IscA and FtnA fractions after apo-IscA was incubated with the iron-bound FtnA in the presence of the thioredoxin reductase system (closed squares) or the thioredoxin reductase system missing thioredoxin reductase (closed circles).
The non-denaturing polyacrylamide electrophoresis and the gel filtration chromatography studies indicated that IscA and the iron-bound FtnA did not form any stable protein complexes (data not shown), indicating that the iron-bound FtnA and apo-IscA did not form a stable protein complex to facilitate the iron transfer. Because the iron mobilization from ferritins requires reduction of ferric iron to ferrous iron (Liu and Theil 2005), and IscA scavenges “free” ferrous iron only in the presence of the thioredoxin reductase system (Ding et al. 2005), we propose that the iron transfer from the iron-bound FtnA to apo-IscA is through the binding competition for ferrous iron in the presence of the thioredoxin reductase system.
Apo-IscA mediates the iron-sulfur cluster assembly in IscU when the iron-bound FtnA is used as the iron source in vitro
Since the iron-bound IscA can provide iron for the iron-sulfur cluster assembly in IscU (Figure 5), and apo-IscA can retrieve iron from the iron-bound FtnA (Figure 6), we reasoned that apo-IscA may promote the iron-sulfur cluster assembly in IscU when the iron-bound FtnA was used as the iron source. Indeed, addition of apo-IscA significantly increased the iron-sulfur cluster assembly in IscU under the experimental conditions (Figure 7). Re-purification of IscU from the incubation solution further revealed that the iron-sulfur clusters were assembled in IscU, but not in IscA (data not shown). Thus, the iron stored in FtnA can be mobilized by IscA for the iron-sulfur cluster assembly in IscU in the presence of the thioredoxin reductase system.
Figure 7. Apo-IscA promotes the FtnA-mediated iron-sulfur cluster assembly in IscU.
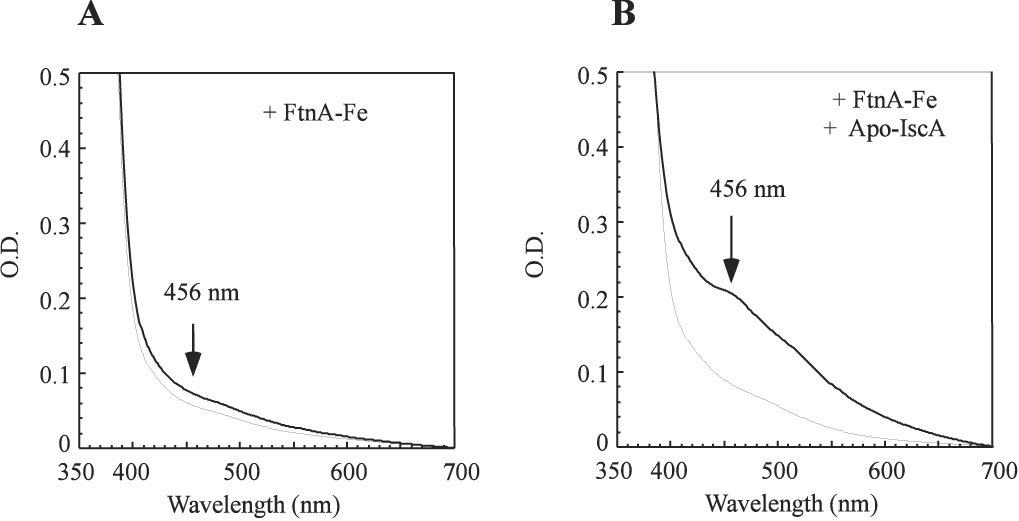
A) the iron-bound FtnA (containing 200 µM iron in 50 µM monomeric FtnA) was pre-incubated with apo-IscU (50 µM) and IscS (1 µM) in the presence of the thioredoxin reductase system at 37°C for 30 min. The UV-Vis spectra were taken 0 min (thin line) and 30 min (thick line) after L-cysteine (1 mM) was added under anaerobic conditions. B), as in A) except apo-IscA (50 µM) was included in the pre-incubation solution. The absorption peak at 456 nm indicates the formation of the IscU [2Fe-2S] cluster.
Discussion
Ferritins are a large group of iron-storage proteins found in diverse organisms (Andrews et al. 2003, Carrondo 2003, Liu and Theil 2005, Sargent et al. 2005, Galatro and Puntarulo 2007). In mammals, ferritins consist of 24 subunits of two types, H and L (Liu and Theil 2005). Under aerobic conditions, ferrous irons are rapidly oxidized to the mineral ferrihydrite by the built-in di-iron ferroxidase in ferritin H subunits (Sargent et al. 2005). Up to 4500 iron atoms can be stored inside an approximately 8 nm nanocage of the ferritin polymer (Andrews et al. 2003, Carrondo 2003, Liu and Theil 2005, Sargent et al. 2005). Over-expression of human H subunit ferritin in cultured HeLa cells and eryhtroid cells renders the cells resistive to hydrogen peroxide (Epsztejn et al. 1999, Cozzi et al. 2000). In vitro electron paramagnetic resonance (EPR) spin-trapping study also indicates that human H subunit ferritin can detoxify the iron-mediated production of hydroxyl free radicals (Zhao et al. 2006). In mitochondria (Galatro and Puntarulo 2007) and microbes (Andrews et al. 2003, Carrondo 2003), ferritins exist as a homopolymer composed of 24 identical “H-type” subunits. In bacteria, genetic studies suggest that ferritins also have an important role in the cellular defense against oxidative stresses (Abdul-Tehrani et al. 1999, Velayudhan et al. 2007). Here, we report that purified ferritin A (FtnA), a major iron-storage protein in E. coli (Hudson et al. 1993, Abdul-Tehrani et al. 1999), is able to scavenge the iron released from the disrupted iron-sulfur clusters and alleviates the iron-mediated production of hydroxyl free radicals in the presence of hydrogen peroxide in vitro (Figure 3 and Figure 4). The results provide biochemical evidence showing that ferritins can effectively prevent the production of hydroxyl free radicals due to the disruption of iron-sulfur clusters under hydrogen peroxide stress.
While the mechanism by which iron enters ferritins has been well characterized (Liu and Theil 2005), much less is known on how the iron is released from ferritins. At least two models have been proposed for the iron release from ferritins. In the first model, the iron-bound ferritins are degraded in lysosomes, resulting in iron release to cytoplasm (Radisky and Kaplan 1998, Kidane et al. 2006). However, the questions as how ferritins enter lysosomes and how iron is released to cytoplasm still remain to be addressed. Also, there are no lysosomes for possible degradation of ferritins in bacteria. In the second model, the ferritin pores formed in the interface of the subunits may reversibly unfold and fold to allow the iron release from ferritins (Liu et al. 2003, De Domenico et al. 2006). Recent studies further revealed that some specific peptides are able to promote the iron release from ferritins (Liu et al. 2007), supporting the notion that the iron release from ferritins may be regulated by other proteins. Here we show that in the presence of the thioredoxin reductase system which emulates normal intracellular redox potential (Aslund and Beckwith 1999), the iron bound in FtnA can be retrieved by an iron binding chaperon IscA without any degradation of FtnA (Figure 6). Since IscA only binds ferrous iron (Ding et al. 2005), we propose that ferric iron stored in FtnA is first reduced to ferrous iron by the thioredoxin reductase system, making the iron accessible for IscA to bind. Nevertheless, we were unable to mobilize all iron from FtnA for the iron binding in IscA under the experimental conditions. It is likely that additional protein partners are required to completely release iron from ferritins (Liu et al. 2007).
The finding that IscA can retrieve the iron from the iron-bound FtnA provides new evidence for the notion that IscA is a physiological iron donor for the assembly or repair of iron-sulfur clusters (Ding et al. 2005, Yang et al. 2006, Lu et al. 2008). We show here that IscA and FtnA have very different iron binding properties. Under normal physiological conditions (e.g. in the presence of the thioredoxin reductase system), IscA is a strong iron binding protein that can retrieve iron from the iron-storage protein FtnA (Figure 6). However, under oxidative stress conditions (e.g. in the presence of hydrogen peroxide), IscA fails to bind any iron (Ding et al. 2007) (Figure 3), whereas FtnA becomes a potent iron binding protein to scavenge the iron released from the disrupted iron-sulfur clusters. The iron binding in FtnA effectively prevents the production of hydroxyl free radicals (Figure 3), likely because the build-in ferroxidase of FtnA converts ferrous iron to mineral ferric iron and alleviates the Fenton reaction (Bou-Abdallah et al. 2005). When normal physiological conditions are re-established, IscA retrieves the iron from FtnA for the re-assembly of the disrupted iron-sulfur clusters in proteins. Figure 8 summarizes the interplay of IscA and FtnA for the iron binding under normal physiological and oxidative stress conditions. The dynamic iron binding equilibrium between IscA and ferritins is expected to prevent the iron-mediated production of hydroxyl free radicals under oxidative stress conditions and yet ensure the iron supply for the biogenesis and/or repair of iron-sulfur clusters under normal physiological conditions
Figure 8. A proposed model for the iron binding of IscA and FtnA under oxidative stress and normal physiological conditions.
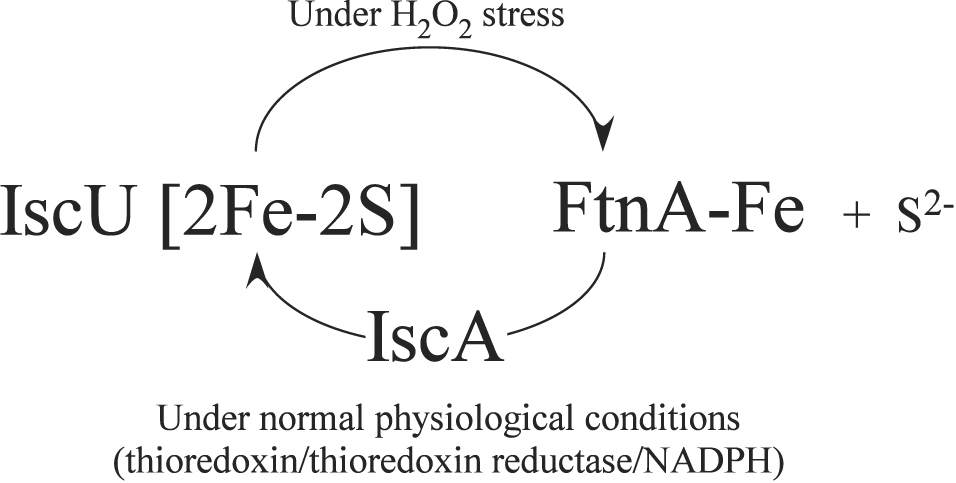
Under oxidative stress conditions, the IscU [2Fe-2S] cluster is disrupted, and the iron released from the disrupted iron-sulfur clusters is scavenged by FtnA. Under normal physical conditions, IscA retrieves iron from the iron-bound FtnA and transfers the iron for the re-assembly of iron-sulfur clusters in IscU.
Acknowledgement
This work was supported in part by the National Sciences Foundation grant (MCB-0416537) and the National Institutes of Health grant (CA107494) to H.D.
Abbreviations
- FtnA
E. coli ferritin A
- IscA
a proposed iron donor for the iron-sulfur cluster assembly
- IscS
cysteine desulfurase
- IscU
iron-sulfur cluster assembly scaffold protein
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- Abdul-Tehrani H, Hudson AJ, Chang YS, Timms AR, Hawkins C, Williams JM, Harrison PM, Guest JR, Andrews SC. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J Bacteriol. 1999;181:1415–1428. doi: 10.1128/jb.181.5.1415-1428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Aslund F, Beckwith J. The thioredoxin superfamily: redundancy, specificity, and gray-area genomics. J Bacteriol. 1999;181:1375–1379. doi: 10.1128/jb.181.5.1375-1379.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H, Holm RH, Munck E. Iron-sulfur clusters: nature's modular, multipurpose structures. Science. 1997;277:653–659. doi: 10.1126/science.277.5326.653. [DOI] [PubMed] [Google Scholar]
- Bou-Abdallah F, Woodhall MR, Velazquez-Campoy A, Andrews SC, Chasteen ND. Thermodynamic analysis of ferrous ion binding to Escherichia coli ferritin EcFtnA. Biochemistry. 2005;44:13837–13846. doi: 10.1021/bi0514212. [DOI] [PubMed] [Google Scholar]
- Carrondo MA. Ferritins, iron uptake and storage from the bacterioferritin viewpoint. Embo J. 2003;22:1959–1968. doi: 10.1093/emboj/cdg215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramouli K, Johnson MK. HscA and HscB stimulate [2Fe-2S] cluster transfer from IscU to apoferredoxin in an ATP-dependent reaction. Biochemistry. 2006;45:11087–11095. doi: 10.1021/bi061237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart RE, Singleton FL, Hind JS. A comparison of bathophenanthrolinedisulfonic acid and ferrozine as chelators of iron(II) in reduction reactions. Anal Biochem. 1993;211:151–155. doi: 10.1006/abio.1993.1246. [DOI] [PubMed] [Google Scholar]
- Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J Biol Chem. 2000;275:25122–25129. doi: 10.1074/jbc.M003797200. [DOI] [PubMed] [Google Scholar]
- Cupp-Vickery JR, Urbina H, Vickery LE. Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J Mol Biol. 2003;330:1049–1059. doi: 10.1016/s0022-2836(03)00690-9. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Vaughn MB, Li L, Bagley D, Musci G, Ward DM, Kaplan J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. Embo J. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B, Smith ES, Ding H. Mobilization of the iron centre in IscA for the iron-sulphur cluster assembly in IscU. Biochem J. 2005;389:797–802. doi: 10.1042/BJ20050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Clark RJ. Characterization of iron binding in IscA, an ancient iron-sulphur cluster assembly protein. Biochem J. 2004;379:433–440. doi: 10.1042/BJ20031702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Clark RJ, Ding B. IscA mediates iron delivery for assembly of iron-sulfur clusters in IscU under the limited accessible free iron conditions. J Biol Chem. 2004;279:37499–37504. doi: 10.1074/jbc.M404533200. [DOI] [PubMed] [Google Scholar]
- Ding H, Harrison K, Lu J. Thioredoxin reductase system mediates iron binding in IscA and iron delivery for the iron-sulfur cluster assembly in IscU. J Biol Chem. 2005;280:30432–30437. doi: 10.1074/jbc.M504638200. [DOI] [PubMed] [Google Scholar]
- Ding H, Yang J, Coleman LC, Yeung S. Distinct Iron Binding Property of Two Putative Iron Donors for the Iron-Sulfur Cluster Assembly: IscA AND THE BACTERIAL FRATAXIN ORTHOLOG CyaY UNDER PHYSIOLOGICAL AND OXIDATIVE STRESS CONDITIONS. J Biol Chem. 2007;282:7997–8004. doi: 10.1074/jbc.M609665200. [DOI] [PubMed] [Google Scholar]
- Djaman O, Outten FW, Imlay JA. Repair of oxidized iron-sulfur clusters in Escherichia coli. J Biol Chem. 2004;279:44590–44599. doi: 10.1074/jbc.M406487200. [DOI] [PubMed] [Google Scholar]
- Epsztejn S, Glickstein H, Picard V, Slotki IN, Breuer W, Beaumont C, Cabantchik ZI. H-ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood. 1999;94:3593–3603. [PubMed] [Google Scholar]
- Flint DH. Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase. J Biol Chem. 1996;271:16068–16074. [PubMed] [Google Scholar]
- Fontecave M. Iron-sulfur clusters: ever-expanding roles. Nat Chem Biol. 2006;2:171–174. doi: 10.1038/nchembio0406-171. [DOI] [PubMed] [Google Scholar]
- Galatro A, Puntarulo S. Mitochondrial ferritin in animals and plants. Front Biosci. 2007;12:1063–1071. doi: 10.2741/2126. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Hudson AJ, Andrews SC, Hawkins C, Williams JM, Izuhara M, Meldrum FC, Mann S, Harrison PM, Guest JR. Overproduction, purification and characterization of the Escherichia coli ferritin. Eur J Biochem. 1993;218:985–995. doi: 10.1111/j.1432-1033.1993.tb18457.x. [DOI] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Dean DR, Smith AD, Johnson MK. Structure, function, and formation of biological iron-sulfur clusters. Annu Rev Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- Kato S, Mihara H, Kurihara T, Takahashi Y, Tokumoto U, Yoshimura T, Esaki N. Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: Implications for the mechanism of iron-sulfur cluster assembly. Proc Natl Acad Sci U S A. 2002;99:5948–5952. doi: 10.1073/pnas.082123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol. 2006;291:C445–C455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- Kiley PJ, Beinert H. The role of Fe-S proteins in sensing and regulation in bacteria. Curr Opin Microbiol. 2003;6:181–185. doi: 10.1016/s1369-5274(03)00039-0. [DOI] [PubMed] [Google Scholar]
- Krebs C, Agar JN, Smith AD, Frazzon J, Dean DR, Huynh BH, Johnson MK. IscA, an alternate scaffold for Fe-S cluster biosynthesis. Biochemistry. 2001;40:14069–14080. doi: 10.1021/bi015656z. [DOI] [PubMed] [Google Scholar]
- Layer G, Gaddam SA, Ayala-Castro CN, Ollagnier-de Choudens S, Lascoux D, Fontecave M, Outten FW. SufE transfers sulfur from SufS to SufB for iron-sulfur cluster assembly. J Biol Chem. 2007;282:13342–13350. doi: 10.1074/jbc.M608555200. [DOI] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- Liu X, Jin W, Theil EC. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc Natl Acad Sci U S A. 2003;100:3653–3658. doi: 10.1073/pnas.0636928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Theil EC. Ferritins: dynamic management of biological iron and oxygen chemistry. Acc Chem Res. 2005;38:167–175. doi: 10.1021/ar0302336. [DOI] [PubMed] [Google Scholar]
- Liu XS, Patterson LD, Miller MJ, Theil EC. Peptides selected for the protein nanocage pores change the rate of iron recovery from the ferritin mineral. J Biol Chem. 2007;282:31821–31825. doi: 10.1074/jbc.C700153200. [DOI] [PubMed] [Google Scholar]
- Loiseau L, Ollagnier-de-Choudens S, Nachin L, Fontecave M, Barras F. Biogenesis of Fe-S cluster by the bacterial Suf system: SufS and SufE form a new type of cysteine desulfurase. J Biol Chem. 2003;278:38352–38359. doi: 10.1074/jbc.M305953200. [DOI] [PubMed] [Google Scholar]
- Lu J, Yang J, Tan G, Ding H. Complementary roles of SufA and IscA in the biogenesis of iron-sulfur clusters in Escherichia coli. Biochem J. 2008;409:535–543. doi: 10.1042/BJ20071166. [DOI] [PubMed] [Google Scholar]
- Mulrooney SB. Application of a single-plasmid vector for mutagenesis and high-level expression of thioredoxin reductase and its use to examine flavin cofactor incorporation. Protein Expr Purif. 1997;9:372–378. doi: 10.1006/prep.1996.0698. [DOI] [PubMed] [Google Scholar]
- Ollagnier-de-Choudens S, Mattioli T, Takahashi Y, Fontecave M. Iron-sulfur cluster assembly: characterization of IscA and evidence for a specific and functional complex with ferredoxin. J Biol Chem. 2001;276:22604–22607. doi: 10.1074/jbc.M102902200. [DOI] [PubMed] [Google Scholar]
- Outten FW, Wood MJ, Munoz FM, Storz G. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli. J Biol Chem. 2003;278:45713–45719. doi: 10.1074/jbc.M308004200. [DOI] [PubMed] [Google Scholar]
- Outten FW, Djaman O, Storz G. A <i>suf</i> operon requirement for Fe-S cluster assembly during iron starvation in <i>Escherichia coli</i>. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Kaplan J. Iron in cytosolic ferritin can be recycled through lysosomal degradation in human fibroblasts. Biochem J. 1998;336(Pt 1):201–205. doi: 10.1042/bj3360201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- Sargent PJ, Farnaud S, Evans RW. Structure/function overview of proteins involved in iron storage and transport. Curr Med Chem. 2005;12:2683–2693. doi: 10.2174/092986705774462969. [DOI] [PubMed] [Google Scholar]
- Sendra M, Ollagnier de Choudens S, Lascoux D, Sanakis Y, Fontecave M. The SUF iron-sulfur cluster biosynthetic machinery: sulfur transfer from the SUFS-SUFE complex to SUFA. FEBS Lett. 2001;581:1362–1368. doi: 10.1016/j.febslet.2007.02.058. [DOI] [PubMed] [Google Scholar]
- Silberg JJ, Tapley TL, Hoff KG, Vickery LE. Regulation of the HscA ATPase reaction cycle by the co-chaperone HscB and the iron-sulfur cluster assembly protein IscU. J Biol Chem. 2004;279:53924–59331. doi: 10.1074/jbc.M410117200. [DOI] [PubMed] [Google Scholar]
- Smith AD, Agar JN, Johnson KA, Frazzon J, Amster IJ, Dean DR, Johnson MK. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J Am Chem Soc. 2001;123:11103–11104. doi: 10.1021/ja016757n. [DOI] [PubMed] [Google Scholar]
- Smith AD, Frazzon J, Dean DR, Johnson MK. Role of conserved cysteines in mediating sulfur transfer from IscS to IscU. FEBS Lett. 2005;579:5236–5240. doi: 10.1016/j.febslet.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- Tapley TL, Vickery LE. Preferential substrate binding orientation by the molecular chaperone HscA. J Biol Chem. 2004;279:28435–28442. doi: 10.1074/jbc.M400803200. [DOI] [PubMed] [Google Scholar]
- Unciuleac MC, Chandramouli K, Naik S, Mayer S, Huynh BH, Johnson MK, Dean DR. In vitro activation of apo-aconitase using a [4Fe-4S] cluster-loaded form of the IscU [Fe-S] cluster scaffolding protein. Biochemistry. 2007;46:6812–6821. doi: 10.1021/bi6026665. [DOI] [PubMed] [Google Scholar]
- Urbina HD, Silberg JJ, Hoff KG, Vickery LE. Transfer of Sulfur from IscS to IscU during Fe/S Cluster Assembly. J Biol Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- Veine DM, Mulrooney SB, Wang PF, Williams CH., Jr Formation and properties of mixed disulfides between thioredoxin reductase from Escherichia coli and thioredoxin: evidence that cysteine-138 functions to initiate dithiol-disulfide interchange and to accept the reducing equivalent from reduced flavin. Protein Sci. 1998;7:1441–1450. doi: 10.1002/pro.5560070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol Microbiol. 2007;63:1495–1507. doi: 10.1111/j.1365-2958.2007.05600.x. [DOI] [PubMed] [Google Scholar]
- Wollenberg M, Berndt C, Bill E, Schwenn JD, Seidler A. A dimer of the FeS cluster biosynthesis protein IscA from cyanobacteria binds a [2Fe2S] cluster between two protomers and transfers it to [2Fe2S] and [4Fe4S] apo proteins. Eur J Biochem. 2003;270:1662–1671. doi: 10.1046/j.1432-1033.2003.03522.x. [DOI] [PubMed] [Google Scholar]
- Wu SP, Wu G, Surerus KK, Cowan JA. Iron-sulfur cluster biosynthesis. Kinetic analysis of [2Fe-2S] cluster transfer from holo ISU to apo Fd: role of redox chemistry and a conserved aspartate. Biochemistry. 2002;41:8876–8885. doi: 10.1021/bi0256781. [DOI] [PubMed] [Google Scholar]
- Yang J, Bitoun JP, Ding H. Interplay of IscA and IscU in biogenesis of iron-sulfur clusters. J Biol Chem. 2006;281:27956–27963. doi: 10.1074/jbc.M601356200. [DOI] [PubMed] [Google Scholar]
- Yang W, Rogers PA, Ding H. Repair of nitric oxide modified ferredoxin [2Fe-2S] cluster by cysteine desulfurase (IscS) J Biol Chem. 2002;277:12868–12873. doi: 10.1074/jbc.M109485200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Arosio P, Chasteen ND. Iron(II) and hydrogen peroxide detoxification by human H-chain ferritin. An EPR spin-trapping study. Biochemistry. 2006;45:3429–3436. doi: 10.1021/bi052443r. [DOI] [PubMed] [Google Scholar]
- Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273:13264–13272. doi: 10.1074/jbc.273.21.13264. [DOI] [PubMed] [Google Scholar]


