Abstract
Purpose
Klebsiella pneumoniae is a common cause of endogenous bacterial endophthalmitis, a disease that frequently results in a poor visual outcome. Hypermucoviscosity has been identified as a virulence factor among clinical bacteremia isolates of K. pneumoniae. In this study, we established an experimental murine model of K. pneumoniae endophthalmitis and analyzed the role of hypermucoviscosity in pathogenesis.
Methods
C57BL/6J mice were intravitreally injected with 100 CFU of hypermucoviscous (HMV+) or non-hypermucoviscous (HMV−) K. pneumoniae. Intraocular bacterial growth, retinal function, gross pathology, and inflammatory responses were monitored every 3 h until the eyes lost significant (>90%) retinal function or appeared to clear the infection.
Results
The HMV+ strain grew logarithmically in eyes until approximately 15 h postinfection, reaching a stationary phase of growth at approximately 8.0 log10 CFU/eye. The HMV− strain grew logarithmically to approximately 7.6 log10 by 18 h, but bacterial numbers declined to approximately 6.4 log10 CFU/eye by 21 h postinfection. Eyes infected with the HMV+ strain retained approximately 35% A-wave and <10% B-wave function by 18 h postinfection. These eyes also had a cumulative clinical score of 14+ by 18 h and underwent phthisis between 21–24 h. Eyes infected with the HMV− strain had a cumulative clinical score of <6 and retained >60% A-wave and >50% B-wave function throughout 21 h. Five of 7 eyes had <100 CFU HMV− K. pneumoniae at 27 h postinfection.
Conclusions
Our findings demonstrate the site-threatening consequences of K. pneumoniae endophthalmitis and the importance of the hypermucoviscosity phenotype in the pathogenesis of experimental K. pneumoniae endophthalmitis.
Keywords: Klebsiella pneumoniae, endophthalmitis, inflammation, mouse, retina
INTRODUCTION
Bacterial endophthalmitis is an infection in the posterior segment of the eye which frequently results in significant vision loss, if not loss of the eye itself. Bacteria can infect the eye following intraocular surgery or a penetrating eye injury, with infection probabilities ranging between 0.1–0.2% and 3–17%, respectively.1–5 Endogenous endophthalmitis results from bacterial spread from a distant focus of infection into the eye, and accounts for approximately 2–8% of all endophthalmitis cases.6–8 Although endogenous endophthalmitis is rare, the potential for vision loss, including bilateral blindness, makes this type of infection a potential threat to patients with underlying systemic bacterial infections.6,9
Within the past 20 years, Klebsiella pneumoniae has become the most commonly reported bacterial species responsible for endogenous endophthalmitis.6,10 K. pneumoniae is an opportunistic, Gram-negative bacterium responsible for both communal and hospital acquired infections.6,11,12 Mortality rates of nosocomial K. pneumoniae bacteremia range from 20%–50%, with no improvement in outcomes within the last decade.11–15 K. pneumoniae is known worldwide for its rapid acquisition of resistance to traditional and new antimicrobial drugs, including resistance to third-generation cephalosporins, fluoroquinolones, carbapenem, and extended-spectrum β-lactamases.12–19 K. pneumoniae endophthalmitis has been strongly associated with the presence of primary liver abscesses and an underlying diabetic condition.6,20 Despite surgical and/or therapeutic intervention, K. pneumoniae endophthalmitis commonly results in a poor visual outcome and often the need for evisceration or enucleation of the eye.6,10 As the number of communal and hospital acquired K. pneumoniae infections continues to rise with the drastic increase in the incidence of diabetes worldwide, the number of cases of endogenous K. pneumoniae endophthalmitis may also increase.
Numerous studies have identified virulence determinants of K. pneumoniae in clinical isolates. Of importance are clinical isolates which possess serotype K1 or K2 capsules and/or the presence of genes associated with a capsule-associated mucopolysaccharide web, also known as the hypermucoviscous (HMV) phenotype.21–26 When a standard bacteriological loop is passed through a colony and forms a mucoviscous string greater than 5mm, the strain is characterized as having the HMV phenotype.21 Capsular serotypes K1 and K2 have been shown to perturb phagocytosis resistance to neutrophils obtained from type II diabetic patients25,27 and increased lethality in mice intraperitoneally injected with K. pneumoniae25. K. pneumoniae clinical isolates with the HMV phenotype are commonly associated with bacteremia and distinct invasive syndromes such as primary liver abscesses, meningitis, and endophthalmitis.28 Both magA (mucoviscosity-associated gene A) and rmpA (regulator of the mucoid phenotype) have been associated with K. pneumoniae strains with the HMV phenotype.21,22,24–26,28–30 The magA gene has been found to be restricted to K1 isolates, whereas the rmpA gene is found in K1, K2, and other serotypes.24–26,29,30 The specific role of these genes in association with endophthalmitis has not been experimentally analyzed.
The purpose of this study was two-fold: 1) develop an experimental K. pneumoniae endophthalmitis murine model amenable to the analysis of pathogenesis, and 2) test the hypothesis that the HMV phenotype contributes to the intraocular virulence of experimental K. pneumoniae endophthalmitis. Using the experimental murine endophthalmitis model, we compared the intraocular virulence of hypermucoviscous and non-hypermucoviscous strains of K. pneumoniae and determined that the HMV phenotype was important to the pathogenesis of infection.
METHODS
Bacteria and Growth Conditions
The non-hypermucoviscous (HMV−) K. pneumoniae strain was a clinical isolate from the vitreous of an endophthalmitis patient and a generous gift of Dr. Darlene Miller, Bascom Palmer Eye Institute (Miami, FL). The hypermucoviscous (HMV+) strain was a clinical isolate from a primary liver abscess patient and a generous gift from Dr. Steve Libby, University of Washington (Seattle, WA).22 Single colonies of each K. pneumoniae strain were cultured for 18 h in brain heart infusion media (BHI; EMD/Merck KGaA, Darmstadt, Germany) at 37°C with aeration. Overnight cultures were subcultured into BHI, grown to logarithmic phase, and serially diluted in BHI to approximately 100 colony-forming units (CFU)/0.5 μl for intravitreal injection, as described below.
Preparation of Supernatants and Metabolically-Inactive K. pneumoniae
Single colonies of each K. pneumoniae strain were cultured for 18 h in BHI at 37°C with aeration. For preparation of supernatants, cultures were centrifuged, filter-sterilized with a 0.22 μm MILLEX GV filter unit (Carrigtwahill Co., Cork, Ireland), and kept on ice until intravitreal injection as described below. For preparation of metabolically-inactive K. pneumoniae, overnight cultures were subcultured, grown to 8.0 log10 CFU/ml, centrifuged, and the pellets were resuspended to the same concentration in sterile PBS. Three ml of the suspension was removed and subjected to UV light (999.9 mJ/cm2, 40 m). One ml of UV-treated K. pneumoniae was inoculated onto BHI agar and incubated overnight at 37°C. Lack of bacterial growth on agar plates verified that the UV-treated K. pneumoniae suspensions lacked viable organisms.31
Modified String Test for Hypermucoviscosity
A variation of the string test was used to determine the HMV phenotype.21,22,28 K. pneumoniae strains were inoculated on BHI plates and incubated overnight at 37°C. A standard bacteriological loop was used to vertically stretch a mucoviscous string from a single colony. The formation of a mucoid string >5 mm was regarded as a HMV+ phenotype.
DNA Isolation and PCR
Genomic DNA was extracted from cultures of each K. pneumoniae strain using a previously described protocol.32 Briefly, 1.5 ml of an overnight of bacteria was harvested by centrifugation. The pellet was suspended directly in lysis buffer (40 mM Tris-acetate pH 7.8, 20 mM sodium acetate, 1 mM EDTA, and 1% SDS). To precipitate cellular debris, 66 μl of 5M NaCl was added. Debris was removed by centrifugation at 16,000 × g for 10 min at 4°C. The supernatant was extracted with an equal volume of chloroform, ethanol precipitated, and washed with 70% ethanol before resuspending the pellet in 50 μl of water. Each PCR reaction consisted of 0.6 mM of each forward and reverse primer, 0.05 mM of each dNTP, 2 mM MgCl2, 1 U Taq polymerase, and GoTaq buffer (Promega, Madison, WI). Fragments were amplified with primers specific for magA, rmpA, or a 16s ribosomal subunit specific for K. pneumoniae (Table 1). PCR was carried out under the following conditions: 94°C initial denature for 3 min followed by 25 cycles of 95°C for 30 sec, 55°C for 30 sec, 74°C for 1 min 30 sec and final extension for 5 min at 74°C. DNA fragments were separated and visualized on a 1.2% agarose gel with sodium borate buffer containing 0.2 μg/ml ethidium bromide.33
Table 1.
PCR primers.
| Primer | Sequence | Reference |
|---|---|---|
| magA forward | 5′-GAGAGGATCCGGTGCTCTTTACATCATTGC-3′ | 21 |
| magA reverse | 5′-GAGAGGATCCGCAATGGCCATTTGCGTTAG-3′ | 21 |
| rmpA forward | 5′-ACTGGGCTACCTCTGCTTCA-3′ | 26 |
| rmpA reverse | 5′-GCGGTAATACGGAGGGTGC-3′ | 26 |
| 16s rRNA forward | 5′-GCGGTAATACGGAGGGTGC-3′ | 22 |
| 16s rRNA reverse | 5′-CACATCCGACTTGACAGACC-3′ | 22 |
Experimental K. pneumoniae Endophthalmitis
C57BL/6J mice (male, 6–8 weeks old, Jackson Laboratories, Bar Harbor, ME) were obtained and maintained in accordance with institutional guidelines and the Association for Research in Vision and Ophthalmology Statement on the Use of Laboratory Animals in Ophthalmic Research. Mice were anesthetized with a cocktail of ketamine (85 mg/kg of body weight; Phoenix Scientific, Inc., St. Joseph, MO) and xylaxine (14 mg/kg of body weight; Bayer Corp., Shawnee Mission, KS). A single drop of topical anesthetic (0.5% Proparacaine HCl, Allergan, Hormigueros, Puerto Rico) was placed on each eye before injection. Eyes were intravitreally injected as previously described.34 The contralateral eye was left undisturbed (absolute control). Throughout the course of infection, eyes were analyzed by biomicroscopy, electroretinography, histology, and whole eye quantitation of bacterial growth, as described below.
Electroretinography (ERG)
Retinal function was analyzed as previously described.34 Mice were dark-adapted for a minimum of 6 h. Mice were anesthetized and eyes were topically anesthetized as described above. Pupils were dilated with topical phenylephrine (10%; Acorn, Inc., Buffalo Grove, IL). Gold-wire electrodes (99.95%, 0.25mm, Alfa Aesar, Ward Hill, MA) were placed on each cornea along with a small drop of Gonioscopic Prism Solution (Hypromeliose Ophthalmic Demulscent Solution 2.5%, Wilson Ophthalmic Corp., Mustang, OK) between the cornea and electrode to facilitate maximum conductance.
Full-field scotopic ERGs were recorded for each eye (UTAS-E 3000; LKC Technologies, Inc., Gaithersburg, MD). The amplitude of the A-wave (a measure of photoreceptor cell function) was measured from the pre-stimulus baseline to the A-wave trough. The amplitude of the B-wave (a measure of Muller, biopolar, and amacrine cell function) was measured from the A-wave trough to the B-wave peak. Percent retinal function retained was calculated by using the following equations: (i) 100 − {[1− (experimental A-wave amplitude/absolute control A-wave amplitude)] × 100} or (ii) 100 − {[1− (experimental B-wave amplitude/absolute control B-wave amplitude)] × 100}.34
Bacterial Quantitation
Quantitation of bacteria in ocular tissues has been previously described.34 Briefly, eyes were enucleated, placed into 400 μl of sterile PBS and homogenized (60 s, 5,000 rpm; Mini-BeadBeater, Biospec Products Inc., Bartlesville, OK) using sterile glass beads (1.0 mm; Biospec Products, Inc.). Intraocular bacteria were quantified by plating serial 10-fold dilutions in triplicate on BHI agar.
Biomicroscopy and Histology
Eyes were photographed with an operating biomicroscope (Zeiss S7; Zeiss Inc, Thornwood, NY) prior to harvest. A rating scale was used to score the degree of change in anterior and posterior segment inflammation and retinal architecture, based on a scale from 0 (no change) to 4+ (significant inflammation and retinal architectural damage).34 Eyes used for histology were enucleated and fixed in Z-fix (Anatech Ltd., Detroit, MI) for 24 h. The eyes were embedded in paraffin, sectioned, and stained with hematoxylin and eosin by standard procedures.35
Analysis of Intraocular Inflammation
Myeloperoxidase (MPO) concentrations of infiltrating polymorphonuclear leukocytes (PMN) were assayed in whole eyes as previously described.34 Eyes were removed and homogenized with glass beads in 400 μl lysis buffer (20 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycine [vol/vol], 1 mM PMSF, 1 μg/ml leupeptin, 28 μg/ml aprotinin). Absolute controls were included. Supernatants were further diluted 1:4 and analyzed for MPO by ELISA according to manufacturer’s instructions (Mouse MPO ELISA Test Kit, Cell Sciences, Canton, MA). Results are reported as ng MPO/eye.
Statistics
All values represent the mean ± standard error of the mean (SEM) for ≥ 4 eyes per time point unless otherwise specified. Descriptive statistics and two-tailed, two-sample t-tests were used for statistical comparisons between groups. A P-value of ≤ 0.05 was considered significant.
RESULTS
Phenotypic and Genetic Analysis of K. pneumoniae Strains
The hypermucoviscous phenotype of the strains used in this study was determined by a modified string test (Figure 1). The HMV+ mucoid string was approximately 12 mm, indicating a hypermucoviscous phenotype. The HMV− string was approximately 1 mm, indicating a non-hypermucoviscous phenotype. The presence of the magA and rmpA genes was verified by PCR (Figure 2, 1,282-bp and 585-bp fragments, respectively). PCR analysis verified that the HMV+ strain used in this study possessed magA and rmpA, whereas the HMV− strain did not. Both strains were PCR positive for the K. pneumoniae 16s rRNA fragment.
Figure 1. Modified string test for determination of the hypermucoviscocity phenotype of K. pneumoniae.
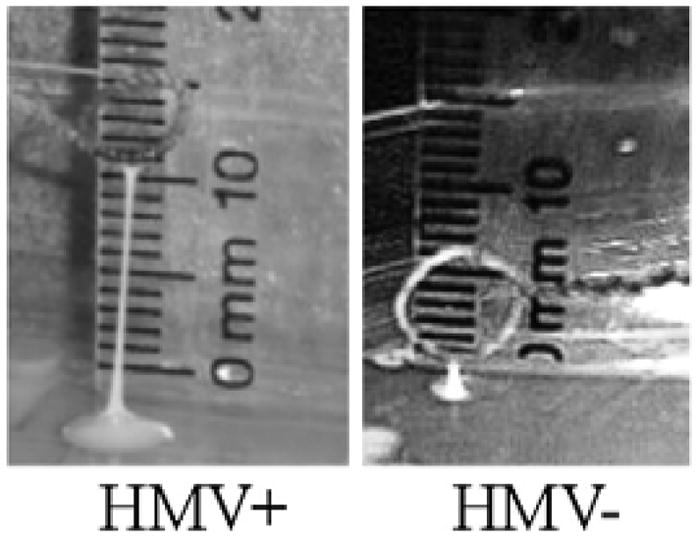
Representative photographs of stretched, mucoviscous strings from a single colony of HMV+ or HMV− K. pneumoniae. The formation of a mucoid string >5 mm is regarded as a hypermucoviscous phenotype.22
Figure 2. PCR verification of magA and rmpA genes in clinical isolates of K. pneumoniae.
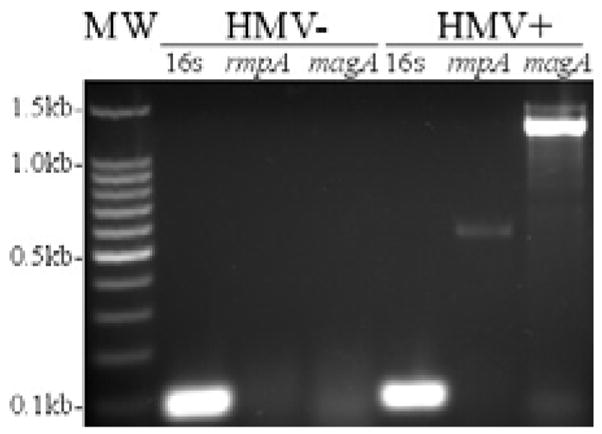
PCR analysis verified the presence of magA (1,282 kb) and rmpA (585 kb) in the HMV+ K. pneumoniae isolate and the absence of both genes in the HMV− K. pneumoniae isolate.
Clinical Progression of Experimental Murine K. pneumoniae Endophthalmitis
Photographs and clinical scores of eyes infected with K. pneumoniae are summarized in Figure 3. Preoperative clinical scores were similar to that of absolute control eyes scored throughout the experiment (data not shown). Clinical scores of absolute control eyes were similar to that of infected eyes until 12 h postinfection. At 12 h postinfection, eyes infected with either strain had cumulative clinical scores of ≤ 3. By 15 h postinfection, a mild vitreous haze and slightly reduced red reflex were observed in eyes infected with either HMV− or HMV+ strains, resulting in clinical scores of 4.25 ± 0.06 or 6.75 ± 1.88, respectively (P<0.05). Eyes infected with the HMV− strain maintained a clinical score of ≤ 6 throughout 21 h postinfection. In contrast, eyes infected with the HMV+ strain presented with a clinical score of 15.75 ± 0.06 (P<0.001) at 21 h postinfection. At this time, red reflexes were absent and a dense vitreous haze was present in eyes infected with the HMV+ strain.
Figure 3. Clinical progression of experimental murine K. pneumoniae endophthalmitis.
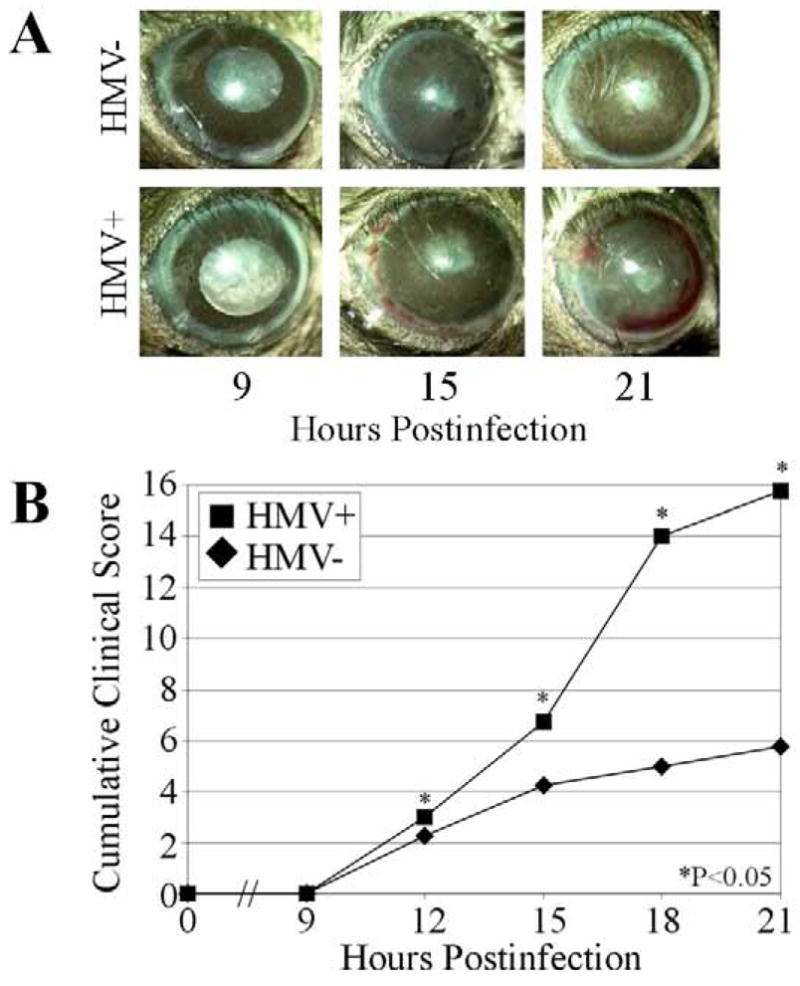
(A) Representative photographs of eyes following intravitreal injection of 100 CFU of HMV+ or HMV− K. pneumoniae. Infection and inflammation were more severe in eyes infected with the HMV+ strain than the HMV− strain. (B) Infected eyes were scored34 and each inflammatory parameter summed to give a cumulative clinical score. Cumulative clinical scores of eyes infected with HMV+ K. pneumoniae were generally higher than that of eyes infected with HMV− K. pneumoniae. Values represent the mean ± SEM of N≥4 eyes per group.
Retinal Function
Retinal function analysis of K. pneumoniae infected eyes is summarized in Figure 4. Retinal function of absolute control eyes was similar to that of preoperative eyes measured throughout the experiment (data not shown). In eyes infected with the HMV+ strain, significant A-wave function declines began at 15 h postinfection, decreasing to approximately 35% by 18 h postinfection. B-wave function declines began at 12 h postinfection, decreasing to <10% function by 18 h postinfection. Eyes infected with HMV− K. pneumoniae had significantly greater A-wave function (P≤0.03) and B-wave function (P≤0.001) at 18 and 21 h postinfection than eyes infected with the HMV+ strain. When compared to eyes infected with the HMV+ strain, eyes infected with the HMV− strain experienced slower declines in A- and B-wave function until 21 h postinfection. Retinal function of eyes infected with the HMV− strain was also measured at 27 h postinfection. At this time, A-wave and B-wave amplitudes had recovered to approximately 100% and 80%, respectively.
Figure 4. Retinal function analysis of experimental K. pneumoniae endophthalmitis.
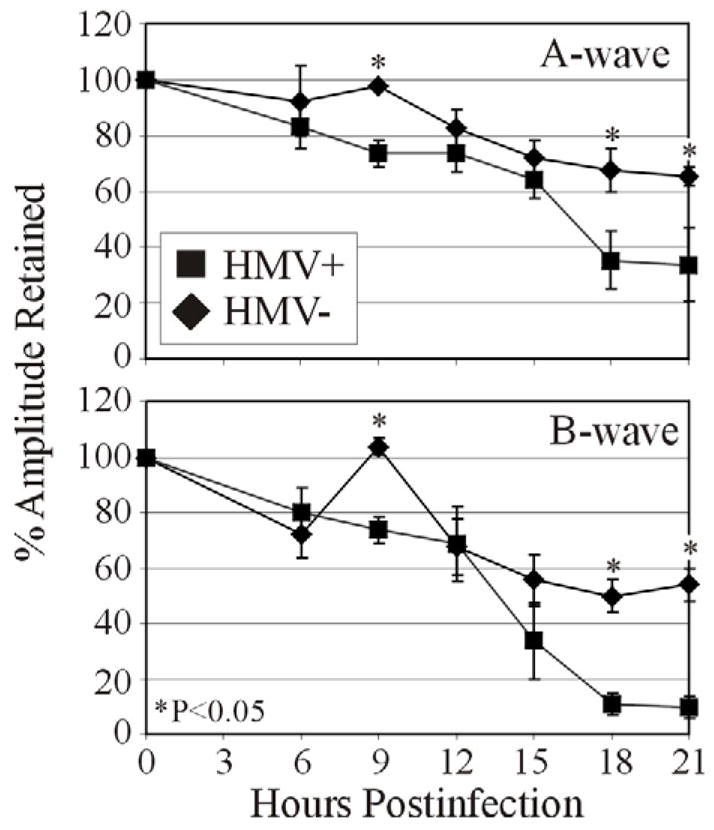
Approximately 100 CFU of either HMV+ or HMV− K. pneumoniae were injected into the mid-vitreous and scotopic ERGs were performed to determine retinal function. A- and B-wave amplitudes were recorded at 6 h postinfection and every 3 h thereafter until 21 h postinfection. In general, retinal function of eyes infected with the HMV+ K. pneumoniae strain declined more rapidly than in eyes infected with the HMV− strain. Values represent the mean ± SEM of N≥4 eyes per group.
Intraocular Bacterial Growth
The intravitreal growth rates of HMV+ and HMV− strains are summarized in Figure 5. Both strains grew at similar rates (P≥0.168) until 15 h postinfection. A stationary phase of growth was reached by 15 h postinfection at approximately 8.5 log10 CFU/eye for all eyes infected with the HMV+ strain, and intraocular numbers remained constant throughout 21 h postinfection. Eyes infected with HMV− strain maintained logarithmic growth until 18 h postinfection (approximately 7.6 log10 CFU/eye), but declined to approximately 6.4 log10 CFU/eye by 21 h postinfection. Bacterial quantities of HMV− infected eyes were also measured at 27 h postinfection. In this group, no bacteria were recovered from 2 of 7 eyes, <100 bacteria were recovered from 3 of 7 eyes, and the remaining 2 eyes had 1.05 × 106 CFU and 1.13 × 105 CFU.
Figure 5. Intraocular growth during experimental K. pneumoniae endophthalmitis.
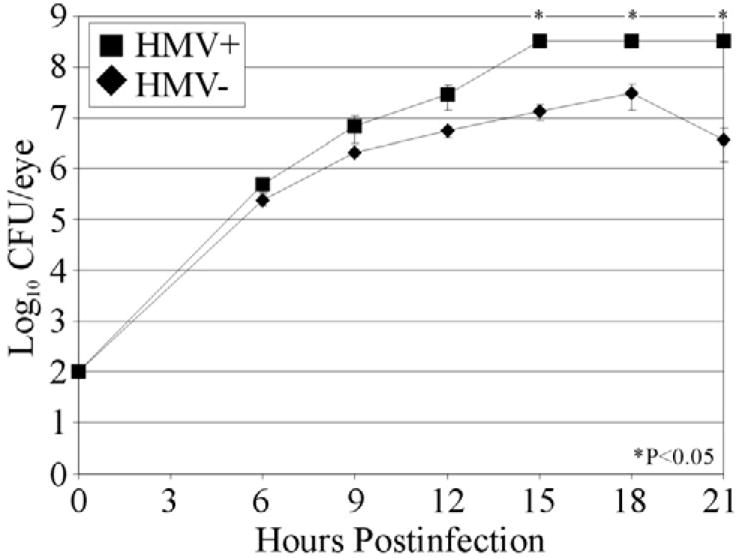
Approximately 100 CFU of either HMV+ or HMV− K. pneumoniae were injected into the mid-vitreous. Bacteria were quantified from whole eyes at 6 h postinfection and every 3 h thereafter until HMV+ infected eyes underwent phthisis. The HMV+ and HMV− strains grew similarly until 12 h postinfection. After that time, the HMV+ strain grew more rapidly than did the HMV− strain. All values represent the mean ± SEM of N≥4 eyes per group.
MPO Assay
MPO analysis of eyes infected with K. pneumoniae is summarized in Figure 6. MPO was not detected in control eyes. MPO concentrations in eyes infected with the HMV+ strain steadily increased between 9 and 21 h postinfection (P≤0.005). MPO concentrations of eyes infected with the HMV− strain steadily increased until 15 h postinfection, with no significant change in MPO concentrations in these eyes between 15 and 21 h postinfection (P>0.05). Significantly greater concentrations of MPO were detected in eyes infected with the HMV+ strain compared to that of eyes infected with HMV− K. pneumoniae at all times except 15 h postinfection (P<0.05).
Figure 6. MPO kinetics in whole eyes during experimental K. pneumoniae endophthalmitis.
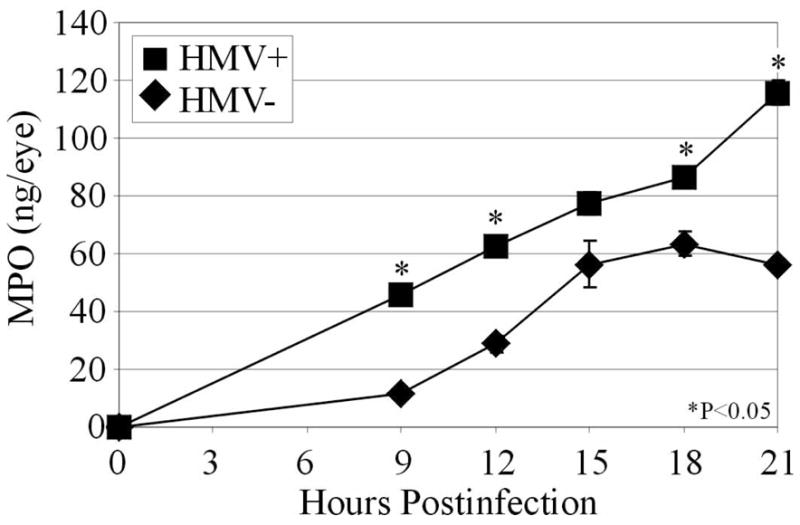
Approximately 100 CFU of either HMV+ or HMV− K. pneumoniae were injected into the mid-vitreous. Whole eyes were homogenized and supernatants analyzed for MPO by ELISA. In general, the MPO concentrations of eyes infected with the HMV+ strain were greater than that of eyes infected with the HMV− strain, indicating greater inflammation in these eyes. Values represent the mean ± SEM for N≥4 eyes per time point.
Histological Analysis
Histology sections of evolving K. pneumoniae endophthalmitis are presented in Figure 7. Eyes infected with the HMV− strain contained inflammatory cells in the posterior chamber, minimal amounts of fibrin, and no disruption of retinal layers at 15 or 21 h postinfection. In contrast, eyes infected with the HMV+ strain had inflammatory cells in both anterior and posterior segments, fibrin in the anterior chamber, and retinal folds in <25% of the retinal area in all eyes at 15 h postinfection. At 21 h postinfection, eyes infected with the HMV+ strain presented with inflammatory cells and fibrin in the anterior and posterior segments, and retinal folds and detachments were observed in 50–75% of the retinal area in all eyes.
Figure 7. Histology of experimental K. pneumoniae endophthalmitis.
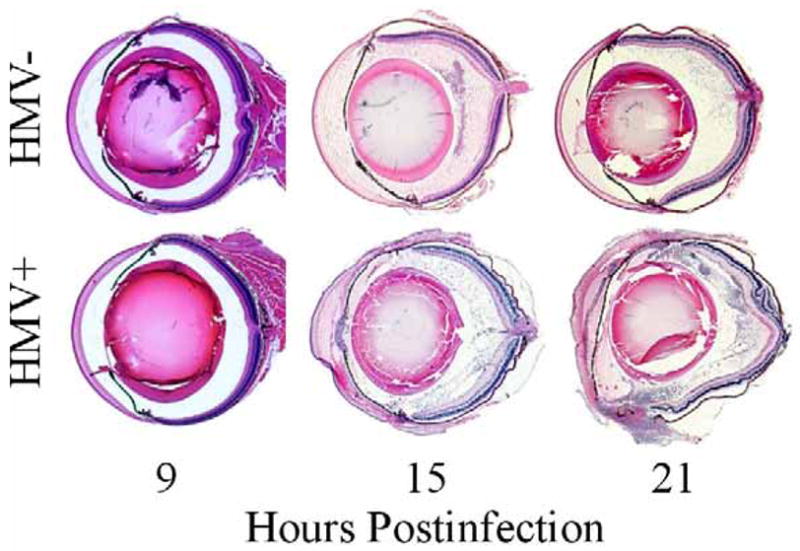
Approximately 100 CFU of either HMV+ or HMV− K. pneumoniae were injected into the mid-vitreous and whole eyes harvested for histology. Eyes were sectioned and stained with hematoxylin and eosin by standard procedures. In general, eyes infected with the HMV+ K. pneumoniae strain had greater inflammation and retinal detachments than did eyes infected with the HMV− strain. Histology sections are representative of N=3 eyes per group.
K. pneumoniae Supernatant and Metabolically Inactive K. pneumoniae
To determine whether differences during infection were due to products secreted by K. pneumoniae or by K. pneumoniae cells themselves, we analyzed ocular changes following intravitreal injection of supernatants or metabolically inactive K. pneumoniae of each phenotype, respectively (Figure 8).
Figure 8. Histology and retinal function analysis of eyes injected with K. pneumoniae supernatant or metabolically inactive K. pneumoniae.
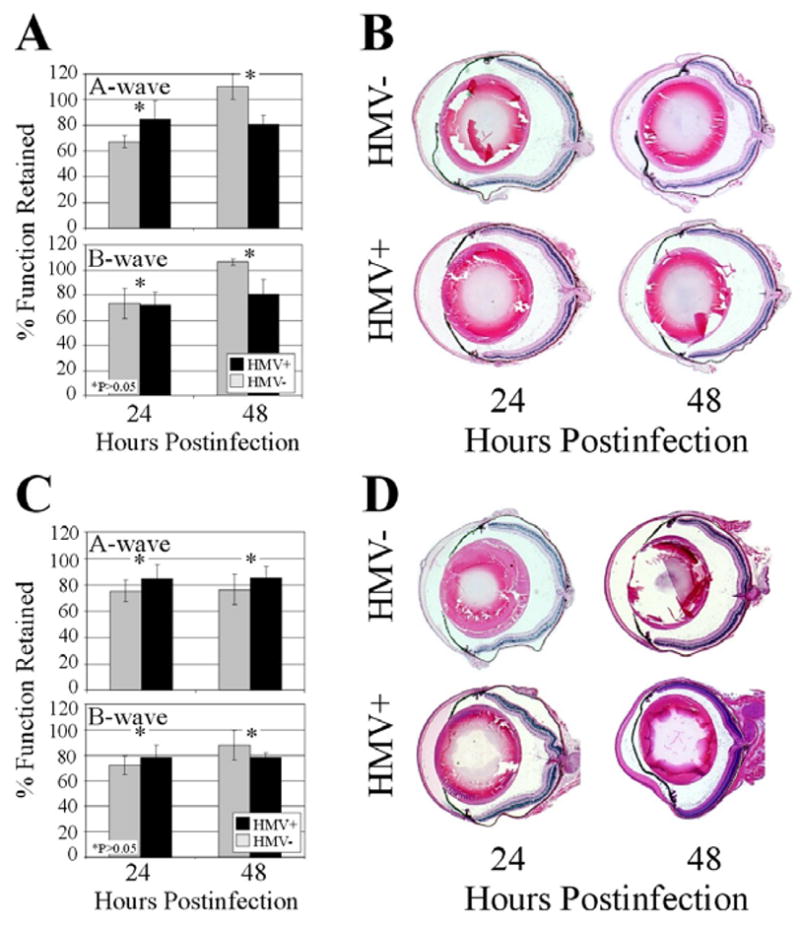
(A,B) Eyes were intravitreally injected with 0.5 μl of sterile-filtered supernatant of cultures of HMV+ or HMV− K. pneumoniae. Minimal retinal function loss (A) and inflammation (B) were observed with both groups at 24 and 48 h postinfection. (C,D) Eyes were intravitreally injected with 108 CFU equivalents of HMV+ or HMV− K. pneumoniae. Again, minimal retinal function loss (C) and inflammation (D) were observed with both groups at 24 and 48 h postinfection. Values represent the mean ± SEM for N≥4 eyes per time point and sections are representative of N=3 eyes per group.
Filter-sterilized supernatants from HMV− or HMV+ cultures were intravitreally injected and eyes were analyzed at 24 and 48 h postinjection. Eyes injected with HMV− or HMV+ supernatants retained approximately 70% retinal function at 24 h (P≥0.056, A- and B-wave) and >80% retinal function at 48 h (P≥0.083, A- and B-wave). At 48 h, eyes injected with the HMV− supernatant had higher levels of retinal response than did eyes injected with the HMV+ supernatant, but this difference was not significant (Figure 8A). Histological analysis of eyes injected with either supernatant demonstrated minimal inflammatory cell influx into the posterior segment and no retinal folding after 24 or 48 h (Figure 8B)
Metabolically inactive HMV+ or HMV− K. pneumoniae (108 CFU equivalents) were intravitreally injected and eyes were analyzed at 24 and 48 h postinjection. Eyes injected with metabolically inactive HMV− or HMV+ retained >70% retinal function at 24 h (P≥0.30, A- and B-wave) and approximately 80% retinal function at 48 h (P≥0.46, A- and B-wave), with no significant differences observed between eyes infected with the two strains at either time point (Figure 8C). Histology showed minimal inflammatory cell influx into the posterior chamber at 24 and 48 h, but no retinal folding or detachment in any eyes (Figure 8D).
DISCUSSION
Numerous reports have attributed the virulence of K. pneumoniae infections to capsular K1/K2 serotypes,24,25,27,29,30 the presence of a putative virulence gene magA,21,22 and/or a hypermucoviscous phenotype.26–28 The HMV+ strain used in this study contained magA (a part of the capsular polysaccharide gene cluster of serotype K1), rmpA (a gene associated with the hypermucoviscous phenotype), and tested positive for the hypermucoviscous phenotype on a string test. The HMV− strain used in this study lacked magA and rmpA, and was not hypermucoviscous. The two strains used in these studies were clinical isolates from invasive infections, but were not isogenic. Important genetic and phenotypic differences in these two strains involved factors associated with the hypermucoviscous phenotype, but may also have involved differences in capsule production. In addition to the lack of rmpA and magA in the HMV− K. pneumoniae strain, two genes (uge and wabG) involved in capsular biosynthesis were also not detected by PCR (data not shown). The HMV− strain did produce a capsule, but perhaps not the same capsular type as did the HMV+ strain. Bacterial capsule is known to be an important virulence factor in numerous infection models. The importance of K. pneumoniae capsule in intraocular infection is being carefully anayzed, since the contribution of K. pneumoniae capsule to infection virulence has been shown to be model-dependent.29 Aerobactin, a siderophore, has also been reported to be a virulence factor for K. pneumoniae isolates.7,36 The presence of aerobactin was not tested in the strains used in this study. In clinical isolates, the presence of rmpA correlated closely with the production of aerobactin7, so our HMV+ isolate may have also produced aerobactin.
Although K. pneumoniae is the most common Gram-negative species responsible for endogenous endophthalmitis6–10, the intraocular mechanisms involved in infection and vision loss caused by this pathogen have not been experimentally determined. Rabbit and mouse models have been used to analyze the pathogenesis of endophthalmitis caused by many common types of bacteria.34,37–39 The K. pneumoniae model in the present study was initiated by direct injection of bacteria into the vitreous, which does not mimic the route by which K. pneumoniae infects the eye during endogenous endophthalmitis. The model used herein bypasses the bloodstream-to-eye route of endogenous infection, but is relevant in that pathogenesis can be analyzed once the organisms reach the eye. To our knowledge, neither the mechanisms of bacterial spread into the eye from the bloodstream, nor the pathogenic mechanisms of K. pneumoniae in the eye have been addressed.
In this model, the majority of eyes injected with 100 CFU of the HMV− K. pneumoniae strain appeared to clear the infection within 27 h, but injection of 100 CFU of HMV+ K. pneumoniae resulted in phthisis within 24 h postinfection. The clearance of HMV− K. pneumoniae from infected eyes after 18 h postinfection is similar to that reported in an experimental Klebsiella oxytoca endophthalmitis rabbit model induced with intravitreal infection of 500–1000 CFU.40 In that study, reductions in viable K. oxytoca recovered from infected eyes began at 24 h postinfection. In experimental S. aureus endophthalmitis, C57BL/6 mice intravitreally infected with 500 CFU were able to clear the infection within 96 h, but were unable to clear an infection initiated with 5000 CFU infection, suggesting differences in outcome based on initial infection dose.37 In the present study, eyes injected with 100 CFU of the HMV+ strain were unable to clear the infection, suggesting differences in infection outcome based on differences in the hypermucoviscous phenotype. Genotypic differences in capsular biosynthesis genes were also noted, which may have also contributed to differences in the virulence of these strains.
In this model, K. pneumoniae with the HMV+ phenotype caused rapid retinal function decline and a significantly greater cumulative clinical score after 15 h postinfection when compared to eyes infected with the HMV− strain. The inflammatory changes observed between 15 and 21 h postinfection in eyes infected with the HMV+ strain are comparable to that of experimental Bacillus endophthalmitis at 6 and 8 h postinfection.34 However, eyes infected with either K. pneumoniae strain failed to exhibit the significant retinal damage observed during experimental B. cereus, Staphylococcus aureus, or Enterococcus faecalis endophthalmitis.31,34,37–39 Retinal destruction in these infections has been attributed to toxins and other excreted factors in the eye during infection. Classic virulence factors, such as cytolysins, hemolysins and enterotoxins, have been described infrequently for K. pneumoniae and have not been analyzed in terms of their contributions to virulence.12 Both K. pneumoniae isolates were non-hemolytic on blood agar, non-proteolytic on skim milk agar, and non-cytolytic when incubated with cultured retinal pigment epithelial cells (data not shown), indicating that classic lytic toxins or proteases were not produced by either strain. In the absence of toxin production by K. pneumoniae, the retinal function loss observed in eyes infected with the HMV+ strain may have been a consequence of bystander damage caused by the immune response. Compared to the intraocular response to infection with the HMV− strain, intraocular inflammation in response to infection with the HMV+ strain was quite robust, suggesting that factors associated with the hypermucoviscous phenotype were highly inflammogenic.
Of interest was the recovery of retinal function in eyes injected with the HMV− strain. These eyes had less inflammation than eyes infected with the HMV+ strain, and most eyes appeared to clear the infection. A less robust inflammatory response may have resulted in less bystander damage to the retina. On the other hand, the mere presence of numerous inflammatory cells in the vitreous may have, in part, altered the light path to the retina, resulting in a muted retinal response. Once the infection began to clear in eyes infected with the HMV− strain, inflammation subsided, and vitreous clarity was eventually restored.
Although the strains used in this study did not produce classic toxins, some strains of K. pneumoniae have been shown to produce proteases, toxins, and hemolysins.12,41–43 To confirm that the intraocular virulence associated with the HMV+ strain was not due to excreted factors, 18 h supernatants were intravitreally injected, and the resulting ocular changes were compared by ERG and histology. Only minimal inflammation and retinal function declines were observed, indicating that secreted factors did not contribute significantly to these changes during infection. To analyze whether K. pneumoniae cells themselves contributed to inflammation and retinal function loss, 108 CFU equivalents of metabolically-inactive HMV+ or HMV− K. pneumoniae were intravitreally injected. Again, inflammation and retinal function loss was minimal. Surprisingly, differences in inflammation and retinal function loss between eyes injected with metabolically inactive HMV+ or HMV− strains were not observed. During intraocular infection, HMV+ K. pneumoniae was significantly more virulent. If the mucopolysaccharide web of the HMV+ strain contributed to virulence, one would expect that the presence of this phenotype would confer greater protection from the immune response, increasing the potential for bystander damage caused by a futile attempt by the host to clear the infection, whether the organism be alive or dead. However, these results suggested that factors involved in the hypermucoviscous phenotype were important to intraocular virulence, but these factors may only have been synthesized during active replication.
To our knowledge, this is the first study examining the intraocular pathogenicity of K. pneumoniae, an organism that causes the vast majority of blinding cases of endogenous endophthalmitis. These results demonstrate the potentially devastating, site-threatening consequences of K. pneumoniae endophthalmitis and establish the hypermucoviscous phenotype as important to intraocular virulence in this model. Several questions remain in terms of the mechanisms of migration of K. pneumoniae (and other organisms) into the eye from a distant site of infection, resulting in endogenous endophthalmitis. Future studies will focus on analyzing the mechanisms involved in metastatic spread of bacteria into the eye during systemic infection and therapeutics aimed at preventing the progression to endogenous endophthalmitis.
Acknowledgments
This research was presented at the Association for Research in Vision and Ophthalmology Annual Meeting 2007 and was funded by a Lew R. Wasserman Award from Research to Prevent Blindness (M.C.C). This work was also supported, in part, by the National Institutes of Health R01EY12985 (M.C.C.), P30EY12191 (NIH CORE grant for Dr. Robert E. Anderson, OUHSC), P20RR17703 (NCRR COBRE grant for Dr. Robert E. Anderson, OUHSC), and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness. We thank Mark Dittmar and Nanette Wheatley (DMEI Animal Research Facility) for their technical assistance and Paula Pierce (Excalibur Pathology, Oklahoma City OK) for histology assistance. We also appreciate the helpful comments of Andrea Moyer and Drs. John Ash, James Chodosh, Jian-Xing Ma, H. Anne Pereira and Jody Summers-Rada (OUHSC).
Footnotes
Commercial Relationships: N for Wiskur, Hunt, and Callegan.
LITERATURE CITED
- 1.Jonas JB, Knorr HL, Budde WM. Prognostic factors in ocular injuries caused by intraocular or retrobulbar foreign bodies. Ophthalmology. 2000;107(5):823–828. doi: 10.1016/s0161-6420(00)00079-8. [DOI] [PubMed] [Google Scholar]
- 2.Meredith TA. Posttraumatic endophthalmitis. Arch Ophthalmol. 1999;117(4):520–521. doi: 10.1001/archopht.117.4.520. [DOI] [PubMed] [Google Scholar]
- 3.Danis RP. Endophthalmitis. Ophthalmol Clin North Am. 2002;15(2):243–248. doi: 10.1016/s0896-1549(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 4.Thompson ST, Parver LM, Enger CL, Meiler WF, Ligget PE. Infectious endophthalmitis after penetrating injuries with retained intraocular foreign bodies. National Eye Trauma System. Ophthalmology. 1993;100(10):1468–1464. doi: 10.1016/s0161-6420(93)31454-5. [DOI] [PubMed] [Google Scholar]
- 5.West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112(8):1388–1394. doi: 10.1016/j.ophtha.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Jackson TL, Eykyn SJ, Graham EM, Stanford MR. Endogenous bacterial endophthalmitis: A 17-year prospective series and review of 267 reported cases. Surv Ophthalmol. 2003;48(4):403–423. doi: 10.1016/s0039-6257(03)00054-7. 2003. [DOI] [PubMed] [Google Scholar]
- 7.Okada AA, Johnson RP, Liles WC, D’Amico DJ, Baker AS. Endogenous bacterial endophthalmitis: Report of a ten-year retrospective study. Ophthalmology. 1994;101(5):832–838. [PubMed] [Google Scholar]
- 8.Romero CF, Rai MK, Lowder CY, Adal KA. Endogenous endophthalmitis: Case report and brief review. Am Fam Phys. 1999;60(2):510–514. [PubMed] [Google Scholar]
- 9.Christensen SR, Hansen AB, LaCour M, Fledelius HC. Bilateral endogenous bacterial endophthalmitis: a report of four cases. Acta Ophthalmol Scand. 2004;82(3):306–310. doi: 10.1111/j.1600-0420.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald MJ, Wohl LG, Sell CH. Metastatic bacterial endophthalmitis: A contemporary reappraisal. Surv Ophthalmol. 1986;31(2):81–101. doi: 10.1016/0039-6257(86)90076-7. [DOI] [PubMed] [Google Scholar]
- 11.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ International Klebsiella Study Group. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13(7):986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity Factors. Clin Microbiol. 1998;11(4):589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson DJ, Engemann JJ, Harrell LJ, Carmeli Y, Reller LB, Kaye KS. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2006;50(5):1715–1720. doi: 10.1128/AAC.50.5.1715-1720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes CC, Vormittag E, Santos CR, Levin AS. Nosocomial infection with cephalosporin-resistant Klebsiella pneumoniae is not associated with increased mortality. Infect Control Hosp Epidemiol. 2006;27(9):907–912. doi: 10.1086/507276. [DOI] [PubMed] [Google Scholar]
- 15.Tumbarello M, Sanguinetti M. Predictors of mortality in patient with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51(6):1987–1994. doi: 10.1128/AAC.01509-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bratu S, Landman D, Haag R, Recco R, Eramo A. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: A new threat to our antibiotic armamentarium. Arch Intern Med. 2005;165(12):1430–1435. doi: 10.1001/archinte.165.12.1430. [DOI] [PubMed] [Google Scholar]
- 17.Waites KB, Duffy LB, Dowzicky MJ. Antimicrobial susceptibility among pathogens collected from hospitalized patients in the United States and in vitro activity of tigecycline, a new glycylcycline antimicrobial. Antimicrob Agents Chemother. 2006;50(10):3479–3484. doi: 10.1128/AAC.00210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzariol A, Roelofsen E, Koncan R, Voss A, Cornaglia G. Detection of a new SHV-Type extended-spectrum β-lactamase, SHV-31, in a Klebsiella pneumoniae strain causing a large nosocomial outbreak in the Netherlands. Antimicrob Agents Chemother. 2007;51(3):1082–1084. doi: 10.1128/AAC.00909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keynan Y, Rubinstein E. The changing face of Klebsiella pneumoniae infections in the community. Int J Antimicrob Agents. 2007;30(5):385–389. doi: 10.1016/j.ijantimicag.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Inf Dis. 2002;8(2):160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang CT, Chuang YP, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2005;199(5):697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang FC, Sandler N, Libby SJ. Liver abscess caused by magA+ Klebsiella pneumoniae in North America. J Clin Micro. 2005;43(2):991–992. doi: 10.1128/JCM.43.2.991-992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung CP, Chang FY, Lee SC, Hu BS, juo GI, Liu CY, Ho M, Siu LK. A global emerging disease of Klebsiella pneumoniae liver abscess: Is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50(3):420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA. Investigation of putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiology. 2005;54(11):1111–1113. doi: 10.1099/jmm.0.46165-0. [DOI] [PubMed] [Google Scholar]
- 25.Yeh KM, Karup A, Siu LK, Koh YL, Fung CP, Lin JC, Chen TL, Chang FY, Koh TH. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulece determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol. 2007;45(2):466–471. doi: 10.1128/JCM.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC. Association between rmpA and magA genes and clincial syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42(10):1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 27.Lin JC, Siu LK, Fung CP, Tsou HH, Wang JJ, Chen CT, Wang SC, Chang FY. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor-glycemic control. J Clin Endocrin Metab. 2006;91(8):3084–3087. doi: 10.1210/jc.2005-2749. [DOI] [PubMed] [Google Scholar]
- 28.Lee HC, Chuang YC, Yu WL, Lee NY, Change CM, Ko NY, Wang LR, Ko WC. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates in patients with community-acquired bacteraemia. J Int Med. 2006;259:606–614. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 29.Struve C, Krogfelt KA. Role of capsule in Klebsiella pneumoniae virulence: Lack of correlation between in vitro and in vivo studies. FEMS Microb Lett. 2003;218:149–154. doi: 10.1111/j.1574-6968.2003.tb11511.x. [DOI] [PubMed] [Google Scholar]
- 30.Yeh KM, Chang FY, Fung CP, Lin JC, Siu LK. magA is not a specific virulence gene for Klebsiella pneumoniae strains causing liver abscess but is part of the capsular polysaccharide gene cluster of K. pneumoniae serotype K1. J Med Microbiol. 2006;55(6):803–804. doi: 10.1099/jmm.0.46368-0. [DOI] [PubMed] [Google Scholar]
- 31.Callegan MC, Booth MC, Jett BD, Gilmore MS. Pathogenesis of Gram-positive bacterial endophthalmitis. Infect Immun. 1999;67(7):3348–56. doi: 10.1128/iai.67.7.3348-3356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Kuo T. A simple and rapid method for the preparation of Gram-negative bacterial genomic DNA. Nucleic Acid research. 1993;21(9):2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brody JR, Calhoun ES, Gallmeier E, Creavalle TD, Kern SE. Ultra-fast high-resolution agarose electrophoresis of DNA and RNA using low-molarity conductive media. Biotechniques. 2004;37(4):598, 600, 602. doi: 10.2144/04374ST04. [DOI] [PubMed] [Google Scholar]
- 34.Ramadan RT, Ramirez R, Novosad BD, Callegan MC. Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr Eye Res. 2006;31:1–11. doi: 10.1080/02713680600976925. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. Columbus, OH: Batelle Press; 1987. [Google Scholar]
- 36.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis. 2008 May 15; doi: 10.1016/j.diagmicrobio.2008.04.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Englebert M, Gilmore MS. Fas ligand but not complement is critical for control of experimental Staphylococcus aureus Endophthalmitis. Investig Ophthalmol Vis Sci. 2005;46(7):2479–2486. doi: 10.1167/iovs.04-1139. [DOI] [PubMed] [Google Scholar]
- 38.Booth MC, Atkuri RV, Nanda SK, Iandolo JJ, Gilmore MS. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig Ophthalmol Vis Sci. 1995;36(9):1828–1836. [PubMed] [Google Scholar]
- 39.Jett BD, Jensen HG, Atkuri RV, Gilmore MS. Evaluation of therapeutic measures for treating endophthalmitis caused by isogenic toxin-producing and toxin-nonproducing Enterococcus faecalis strains. Investig Ophthalmol Vis Sci. 1995;36(1):9–15. [PubMed] [Google Scholar]
- 40.Meyers-Elliot RH, Dethlefs BA. Experimental Klebsiella-Induced Endophthalmitis in the Rabbit. Arch Ophthalmol. 1982;100:1959–1963. doi: 10.1001/archopht.1982.01030040939015. [DOI] [PubMed] [Google Scholar]
- 41.Trishin AV, Zhdanovich MI, Savvateeva LV, Toptygin Aiu, Donenko FV, kiselevskii MV, Kurbatova EA, Gruber IM, Elkina SI, Kalina NG. Protease activity of Klebsiella pneumoniae of different virulence. Zh Mikrobiol Epidemiol Immunobiol. 2004 Jul-Aug;(4):7–11. [PubMed] [Google Scholar]
- 42.Chavan M, Rafi H, Wertz J, Goldstone C, Riley Phage associated bacteriocins reveal a novel mechanism for bacteriocin diversification in Klebsiella. J Mol Evol. 2005;60(4):546–556. doi: 10.1007/s00239-004-0263-9. [DOI] [PubMed] [Google Scholar]
- 43.Sekowska A, Gospodarek E, Janickca G, Jachna-Sawicka K, Sawicki M. Hydrolytic and haemolytic activity of Klebsiella pneumoniae and Klebsiella oxytoca. Med Dosw Mikrobiol. 2006;58(2):135–41. [PubMed] [Google Scholar]


