Abstract
We studied the influence of rate of intravenous infusion of cocaine or amphetamine on drug-taking and seeking behavior. First, drug-naive rats were tested for acquisition of self-administration of increasing doses of amphetamine or cocaine infused over 5 or 100s. Second, self-administration of cocaine or amphetamine infused over 5–100 sec was assessed on fixed or progressive-ratio (PR) reinforcement schedules. Finally, the ability of a single 5 or 100 sec amphetamine or cocaine infusion to reinstate extinguished drug seeking was assessed. Although slower infusion rates produced a small effect on drug taking under continuous-reinforcement conditions, infusion rate did not alter drug taking on intermittent or PR reinforcement schedules, or the ability of cocaine or amphetamine to reinstate drug seeking. Taken together, our results suggest that variation in drug delivery rate over a range that we previously found alters the induction of behavioral sensitization, gene-expression and striatal dopamine activity, does not markedly alter drug-taking or seeking behavior.
Keywords: Reinforcement, reinstatement, schedules-of-reinforcement, acquisition, progressive-ratio
Addiction is characterized by compulsive patterns of drug seeking and taking behavior, and high rates of relapse even after prolonged periods of abstinence. Many factors are thought to contribute to the development and persistence of addiction, including individual, situational and drug-related factors. One factor that is believed to contribute to the susceptibility to addiction is the rate at which drugs enter the brain and reach their molecular targets. Thus, drugs, formulations and routes of administration that result in rapid delivery of a drug into the brain increase abuse liability and addictive potential (de Wit et al., 1992; Gorelick, 1998; Gossop et al., 1992; Mathias, 1997; Oldendorf, 1992; Sellers et al., 1989; Volkow et al., 2000; Winger et al., 1991). One reason for this could be that more rapid delivery increases the hedonic (euphorigenic) effects of drugs, which in turn increases their rewarding and reinforcing effects (de Wit et al., 1992; Fischman and Schuster, 1984). For instance, the rapid delivery of intravenous (i.v.) cocaine (2 versus 60 sec) produces greater self-reported ratings of euphoria (Abreu et al., 2001; Nelson et al., 2006, 2005). Alternatively, the rapid delivery of drugs to the brain may facilitate drug-induced neuroadaptations that promote addiction (Samaha et al., 2002; Samaha et al., 2004; Samaha and Robinson, 2005; Samaha et al., 2005).
Given that the rate of drug delivery is thought to play such an important role in susceptibility to addiction it is surprising that there are only few reports directly exploring whether the rate of drug delivery alters drug seeking and taking behavior and that the results have been inconsistent. Studies with monkeys suggest that the self-administration of cocaine (Balster and Schuster, 1973; Kato et al., 1987; Panlilio et al., 1998; Woolverton and Wang, 2004), pentobarbital (Kato et al., 1987) or nicotine (Wakasa et al., 1995) is facilitated by rapid drug administration. On the other hand, a number of reports have found no acute effects of varying infusion durations from 5–75 sec on the efficacy of cocaine (Liu et al., 2005; Pickens et al., 1969) or nicotine (Sorge and Clarke, 2007; Sorge et al., 2006) to reinforce instrumental responding in rats.
The present study was conducted, therefore, to more thoroughly explore the influence of rate of amphetamine or cocaine administration on drug taking and seeking behavior. In a series of experiments we examined whether variation in the rate of i.v. cocaine or amphetamine delivery between 5–100 s influences, 1) the acquisition of drug self-administration in drug-naive rats, 2) the maintenance of amphetamine or cocaine self-administration behavior under different fixed and progressive-ratio schedules of reinforcement, and 3) drug-prime induced reinstatement of extinguished drug seeking behavior.
This range of i.v. infusion rates was chosen for four reasons: First, drug-user self-reports indicate that a majority of cocaine users inject using a median rate of 5 sec (25–75% quartile of 3–10 sec Zernig et al., 2003). Second, as mentioned, variation over this range of infusion rates was found to affect the subjective effects of cocaine (Abreu et al., 2001; Nelson et al., 2005). Third, recent findings indicate that comparable variation affects the physiological effects (brain temperature) of cocaine in rats (Brown and Kiyatkin, 2005). Fourth, and most important, in rats variation over this range of infusion speeds was found to influence the ability of cocaine to, (1) produce psychomotor sensitization, (2) alter dopamine, but not glutamate, temporal dynamics in striatum, (3) evoke expression of the activity-regulated immediate early genes c-fos and Arc/Arg3.1 in the nucleus accumbens, caudate-putamen and prefrontal cortex (Ferrario et al., 2008; Samaha et al., 2002; Samaha et al., 2004; Samaha et al., 2005).
Experimental procedures
Subjects
Subjects were male Long-Evans rats (Harlan, Indianapolis, IN) weighing 275–325g at the start of the experiments. Rats were housed individually in a light, humidity and temperature controlled animal colony (14:10 hr light:dark cycle; lights on at 08:00) and food and water were always freely available. All procedures followed the “Guide for the Care and Use of Laboratory Animals” (PHS, 1996) and were approved by the Unit for Laboratory Medicine at The University of Michigan.
Surgery
One week after arrival in the colony, the rats were anaesthetized with Ketamine and Xylazine (100 mg/kg Ketamine + 10 mg/kg Xylazine, given IP) and prepared with i.v. catheters, as described previously (Crombag et al., 2005). Briefly, a silicone catheter was inserted into the right external jugular vein and was passed subcutaneously to exit on the back of the animal via a pedestal constructed from a 22 gauge cannula connected with dental cement to a piece of polyethylene mesh. Rats recovered from surgery for at least 5 days prior to testing. Throughout the experiments catheters were flushed daily with 0.1 ml sterile saline containing gentamicin (0.08 mg/ml).
Drugs and infusion procedures
Cocaine (The National Institute on Drug Abuse) and amphetamine (Sigma-Aldrich Corporation, St. Louis, MO) were dissolved in a 0.9% sterile saline solution. The concentration of drug in 10 cc volumes was adjusted every 4 days according to body weight for each rat to ensure correct dosing. Doses of amphetamine and cocaine used were based on previous published reports and/or pilot studies conducted in our laboratory.
All i.v. infusions were delivered in a bolus injection of 50 µl using syringe pumps with different motor speeds (Model R-E; Razel Scientific Instruments, St. Albans, VT). On training days, infusions were delivered over 2.8 sec (17.9 µl/sec) and each infusion was followed by a 20 sec timeout period during which additional responses were counted but did not result in drug infusions. On test days, amphetamine or cocaine were delivered over 5 (10 µl/sec), 25 (2 µl/sec), 50 (1 µl/sec) or 100 (0.2 µl/sec) sec in different groups. Importantly, on these days the time-out period for all subjects was set equal to the longest infusion duration (100 sec) such that the available time to earn drug infusions was always the same for all groups.
Apparatus
Behavioral testing was done in standard operant chambers (Med Associates, Inc., Georgia, VT) containing an acrylic front hinged loading door and back panel and stainless steel side panels (22 × 18 × 13 cm). The chambers were located inside sound- and light-attenuating cabinets equipped with fans providing constant ventilation and low level background noise. Two nose-poke (NP) ports, containing a recessed white cue-light, were located on one sidewall of the chamber at approximately 3 cm above a grid floor. Drug infusions were contingent on responses into one (the active) nose-poke port and responses into the alternate (the inactive) nose-poke port were recorded but had no programmed consequences.
General procedures
Each day rats were transported from their housing environment, their catheters were flushed with 0.1 ml saline, and they were placed into the operant chambers. Sessions commenced with the illumination of the house-light and the 20 sec illumination of the active nose-poke stimulus light. Except during the extinction training sessions (Exp. 5), rats were able to nosepoke for injections of amphetamine or cocaine such that, depending on the schedule of reinforcement, responses into the active nose-poke port activated an infusion pump delivering a single infusion of amphetamine or cocaine.
Experiment 1. Acquisition of cocaine or amphetamine self-administration
Experiment 1 examined the effects of rate of infusion on the acquisition of amphetamine or cocaine self-administration behavior. Drug-naive rats were able to self-administer, on a continuous (fixed ratio 1, FR1) schedule of reinforcement, amphetamine or cocaine delivered over 5 or 100 sec. Thus, there were 4 independent groups: COC-5 sec (n=10), COC-100 sec (n=9), AMPH-5 sec (n=10) and AMPH-100 sec (n=8). The animals were tested daily, and for the first 10 days a lower dose of cocaine (0.5 mg/kg/inf) or amphetamine (0.2 mg/kg/inf) was available. For the next for 5 days a medium dose of cocaine (0.6 mg/kg/inf) or amphetamine (0.25 mg/kg/inf) was available and for the last for 5 days a higher dose of cocaine (0.7 mg/kg/inf) or amphetamine (0.3 mg/kg/inf). This within-subjects (non-randomnized) escalating-dose procedure was used to minimize the number of groups needed to determine a cocaine or amphetamine dose-effect function whilst reducing the likelihood of carry-over effects across doses (e.g., due to the development of sensitization or acquisition at higher doses). Cocaine and amphetamine self-administration sessions were 1 and 2 hrs, respectively.
Experiment 2. Fixed-ratio schedule responding for cocaine or amphetamine
Experiment 2 examined the effects of rate of cocaine or amphetamine delivery on drug taking maintained on two different fixed-ratio (FR) schedules of reinforcement. Independent groups of rats were trained to self-administer 0.4 mg/kg/inf cocaine (n=15) or 0.2 mg/kg/inf amphetamine (n = 19) on a FR1 schedule of reinforcement. Once stable response rates were established (as indicated by less than 25% group variation across 3 consecutive sessions) each rat was tested on consecutive test sessions (one session/day; 1 and 2 hr sessions for cocaine and amphetamine, respectively) with each of 4 infusion durations (5, 25, 50 or 100 sec). Thus, the effect of infusion rate on schedule-controlled responding was tested using a within-subject design and test sessions were randomized using a Latin-Square design. Next, rats were trained to re-establish stable responding on a FR2 (cocaine group; n=13) or FR5 (amphetamine group; n=14) schedule of reinforcement and the effects of 4 infusion rates were again tested using the same procedures. These FR schedules were chosen with the expectation of producing comparable high baseline (5-sec infusion) levels of amphetamine and cocaine responding in both groups.
Experiment 3. Progressive-ratio (PR) schedule responding for cocaine or amphetamine
Experiment 3 examined the effects of rate of infusion on cocaine or amphetamine self-administration behavior on a progressive-ratio (PR) schedule of reinforcement. Rats were initially trained to self-administer 0.4 mg/kg/inf cocaine (n=10) or 0.2 mg/kg/inf amphetamine (n=13) on an FR1, FR2 and then FR5 schedule of reinforcement. Once reliable responding on the FR5 schedule was established, all rats were moved to a PR schedule using a ratio series derived from the following equation: response ratio = (5e(injections number × 0.25))-5 (Richardson and Roberts, 1996). Once stable responding was established on the PR schedule each rat was tested on consecutive 3 hr sessions with each of 4 infusion rates (1 infusion rate/session/day), again using a Latin Square design, to respond for 0.1 or 0.2 mg/kg/inf amphetamine or 0.4 or 0.8 mg/kg/inf cocaine.
Experiment 4. Effect of rate of infusion on drug-prime induced reinstatement of drug seeking
Experiment 4 examined the effects of rate of infusion on the reinstatement of drug seeking following extinction. Rats were initially trained to self-administer cocaine or amphetamine as described in Experiment 1. Next, rats underwent extinction training for 10 days. During these sessions all procedures were the same as during training except that nose-pokes in either nose-poke port had no programmed consequences. Following extinction training rats underwent a single 2 hr test session for reinstatement of drug seeking induced by a single i.v. priming injection of 2.0 mg/kg cocaine (n=18) or 1.0 mg/kg amphetamine (n=16) infused over either 5, 50 or 100 sec. Because of equipment limitations half as many rats were tested with the 50 (n = 5 and 4, for the cocaine and amphetamine groups, respectively) and the 100 sec infusion duration (n = 5 and 5, for the cocaine and amphetamine groups, respectively). Data from these groups were pooled to yield a single group (50–100 sec; n=9/10) for statistical comparison with the 5-sec infusion group (n = 8 and 7, for the cocaine and amphetamine groups respectively). Importantly, these groups were matched for pre-extinction and extinction response levels and infusion rates (5 or 100 sec) during acquisition.
Data analysis
The number of infusions, responses into the active and inactive nose-poke ports were each analyzed separately for differences as a function of rate of drug delivery using one-way repeated measures analysis of variance (ANOVA) followed by Bonferroni's Multiple Comparison tests when appropriate. P-values below 0.05 were considered statistically significant.
Results
Effects of infusion rate on the acquisition of self-administration in drug-naive rats
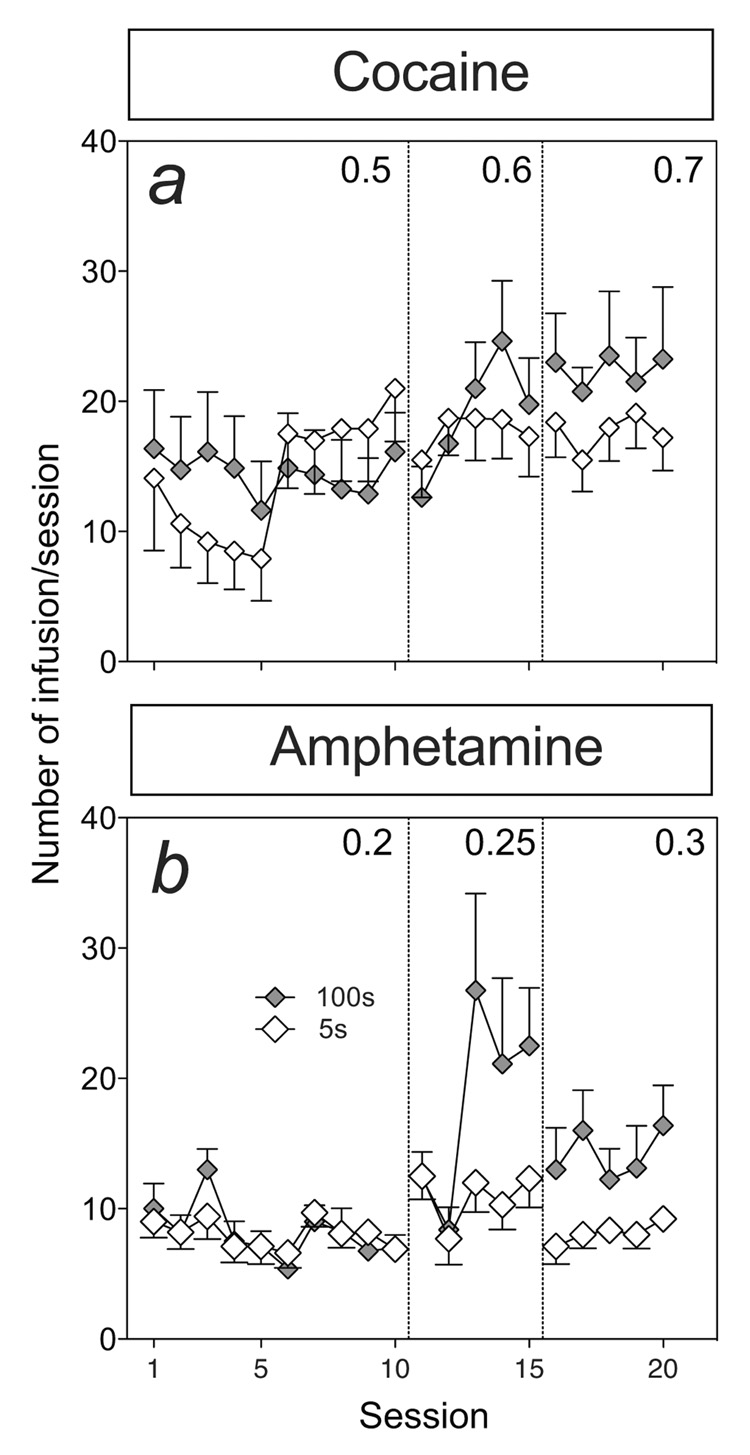
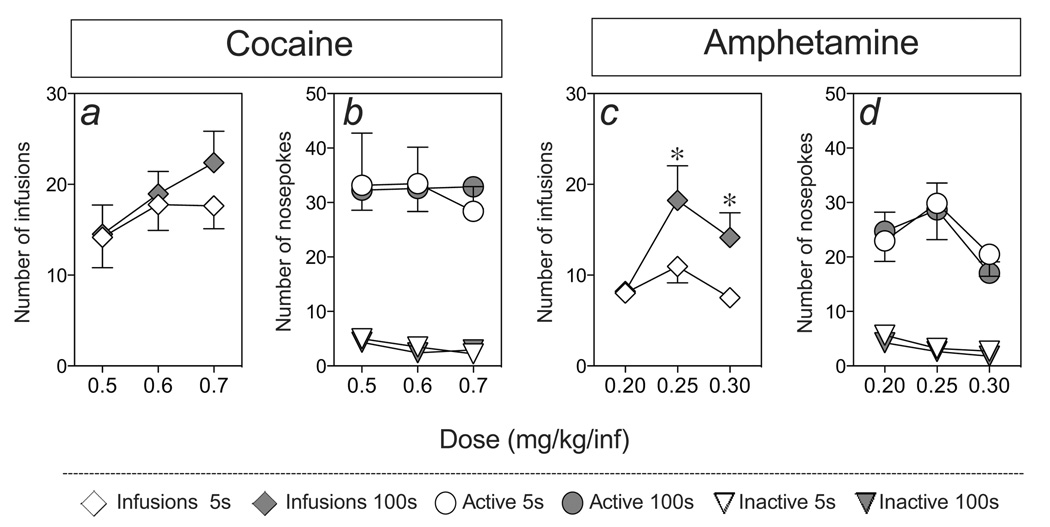
Figure 1 show the mean (±SEM) number of infusions earned for rats lever pressing to self-administer intravenous cocaine (panel a) or amphetamine (panel b) infused over 5 or 100 sec for each self-administration session. Initial inspection of these data indicates that infusion speed produced little effect on cocaine taking (panel a), and slower infusions produced greater amphetamine intake at higher doses. For statistical analysis, these data were collapsed by determining the average number of infusions earned per dose of cocaine or amphetamine. Thus, figure 2 shows the mean (±SEM) number drug infusions earned (panels a and c) and the number of nose-poke responses into the reinforced and non-reinforced ports (panels b and d) as a function of dose of cocaine (panels a and b) or amphetamine (panels c and d) and infusion duration (5 or 100 sec). There was a significant increase in the number of infusions earned with an increase in unit dose, and the number of training sessions, of cocaine or amphetamine (panels a and c; F(2,32) = 6.36, p < 0.005 and F(2,32) = 10.83, Ps < 0.001, respectively). Although, there were no significant main effects of infusion rate on the number of infusions earned, there was a significant interaction effect between dose and infusion rate in the amphetamine condition (F(2,32) = 3.938, p < 0.05), but not in the cocaine condition. As panel c shows, this interaction was due to a greater number of infusions earned at middle and higher doses of amphetamine when infused over 100 sec, compared to 5 sec (Ps < 0.5). The total number of responses into the active nose-poke port (i.e., number of drug reinforced nose-pokes + timeout responses) did not differ as a function of dose and/or infusion rate in either the cocaine or amphetamine conditions (panels b and d). Finally, there was a significant decrease in the number of non-reinforced port responses with dose (F(2,32) = 3.35, p < 0.05 and F(2,32) > 7.9, Ps < 0.005, respectively) but no significant main or interaction effects of infusion rate on responding into the inactive port. In summary, contrary to expectations, infusion rate failed to significantly alter acquisition of cocaine taking behavior and slower rates of i.v. infusion appeared to enhance amphetamine taking.
Figure 1.
The effect of varying rate of infusion (5 or 100 sec) on cocaine (n=10 and 9 for the 5 and 100 sec conditions, respectively) or amphetamine (n=10 and 8 for the 5 and 100 sec conditions, respectively) intake (number of infusions) across 20 self-administration sessions. Rats responded on an FR1 schedule or reinforcement for increasing doses of cocaine (0.5, 0.6, and 0.7 mg/kg/inf) or amphetamine (0.2, 0.25 and 0.3 mg/kg/inf), each infusion delivered over 5 sec (open symbols) or 100 sec (closed symbols). Stippled lines separate different dose/stages of the experiment.
Figure 2.
Dose-effect curves for the effect of varying rate of infusion (5 or 100 sec) on the acquisition of cocaine (n=10 and 9 for the 5 and 100 sec conditions, respectively) or amphetamine (n=10 and 8 for the 5 and 100 sec conditions, respectively) self-administration across increasing doses of cocaine (0.5, 0.6, and 0.7 mg/kg/inf) or amphetamine (0.2, 0.25 and 0.3 mg/kg/inf). Panels a and c show the number of infusions earned and panels b and d the number of responses into the drug reinforced (circles) and non-reinforced (triangles) nose poke ports. Asterisks indicate significant differences between the 5 and 100 sec infusion conditions (p < 0.05). Rate of infusion did not affect acquisition of cocaine taking and amphetamine self-administration was increased for rats earning self-infusions delivered over 100 sec versus 5 sec.
Effects of infusion rate on the maintenance of drug self-administration in drug-experienced rats
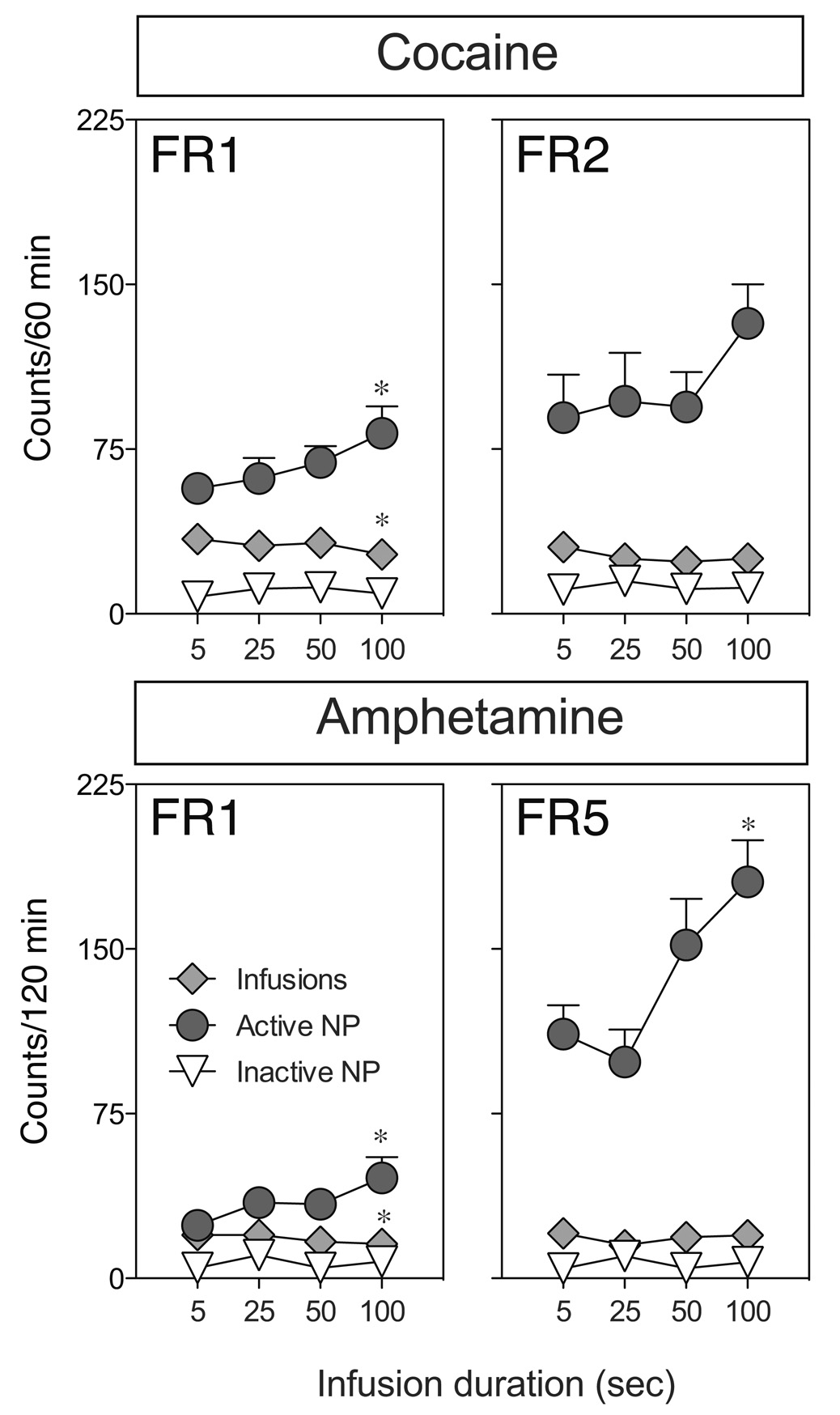
Figure 3 shows the mean (±SEM) number of infusions earned and the number of responses into the drug-reinforced and non-reinforced nose poke ports of rats responding under 2 different FR schedules for cocaine (top panels) or amphetamine (bottom panels) delivered over 5, 25, 50 or 100 sec. There was a small, but significant, decrease in the number of cocaine and amphetamine infusions received under the FR1 schedule with longer rates of infusion (for cocaine, F (3,42)= 4.632, p < 0.01 and for amphetamine, F(3,54)= 2.958, p < 0.05). This was not the case when rats responded for cocaine or amphetamine under the FR2 or FR5 schedules, respectively. Post-hoc analyses revealed that these effects of infusion rate were due to a significant decrease in the number of amphetamine or cocaine infusions when the drugs were delivered over 100 sec, relative to 5 sec (P < 0.05). Interestingly, responses into the active port (i.e., drug reinforced responses + time-out responses) increased as rate of infusion decreased. Specifically, rats responding for cocaine showed a significant increase in active port responding on the FR1 schedule (F(3,42)=4.702, p < 0.01), and a trend towards an increase on the FR2 schedule (F(3,36) = 1.909, p = 0.14). Rats responding for amphetamine showed the same increase on both the FR1 (F(3,54)= 4.632, p < 0.01) and FR5 (F(3,39)= 9.823, p < 0.001) schedules of reinforcement. Thus, as the number of infusions earned did not markedly change or slightly decreased with infusion rate, the increase in overall active port responses occurred during the 100 time-out period. Inactive nose-poke responses did not vary as a function of infusion rate.
Figure 3.
The effects of varying rate of infusion (5, 25, 50 and 100 sec infusion duration) on responding for cocaine of amphetamine maintained on different FR schedules of reinforcement. After acquisition of stable responding for 0.4 mg/kg/inf cocaine or 0.2 mg/kg/inf amphetamine, each rat was tested on 4 consecutive sessions with each of 4 infusion rates (in random order) for responding on, first an FR1 schedule (n=15 and 19 for the cocaine and amphetamine conditions, respectively), and subsequently on an FR2 (cocaine; n=13) or FR5 (amphetamine; n = 14) schedule. Asterisks’ indicate significant differences between the 5 and 100 sec infusion conditions (p < 0.05). Contrary to expectations, both cocaine and amphetamine intake decreased, by 20% and 21%, respectively, when infused over 100 sec versus 5 sec and no effects were evident on responding maintained on higher order schedules of drug reinforcement. At the same time responding into the cocaine or amphetamine reinforced nose-poke manipulandum significantly increases during the 100 sec infusion/time-out period (this effect only approached significance in rats responding for cocaine on an FR2 schedule).
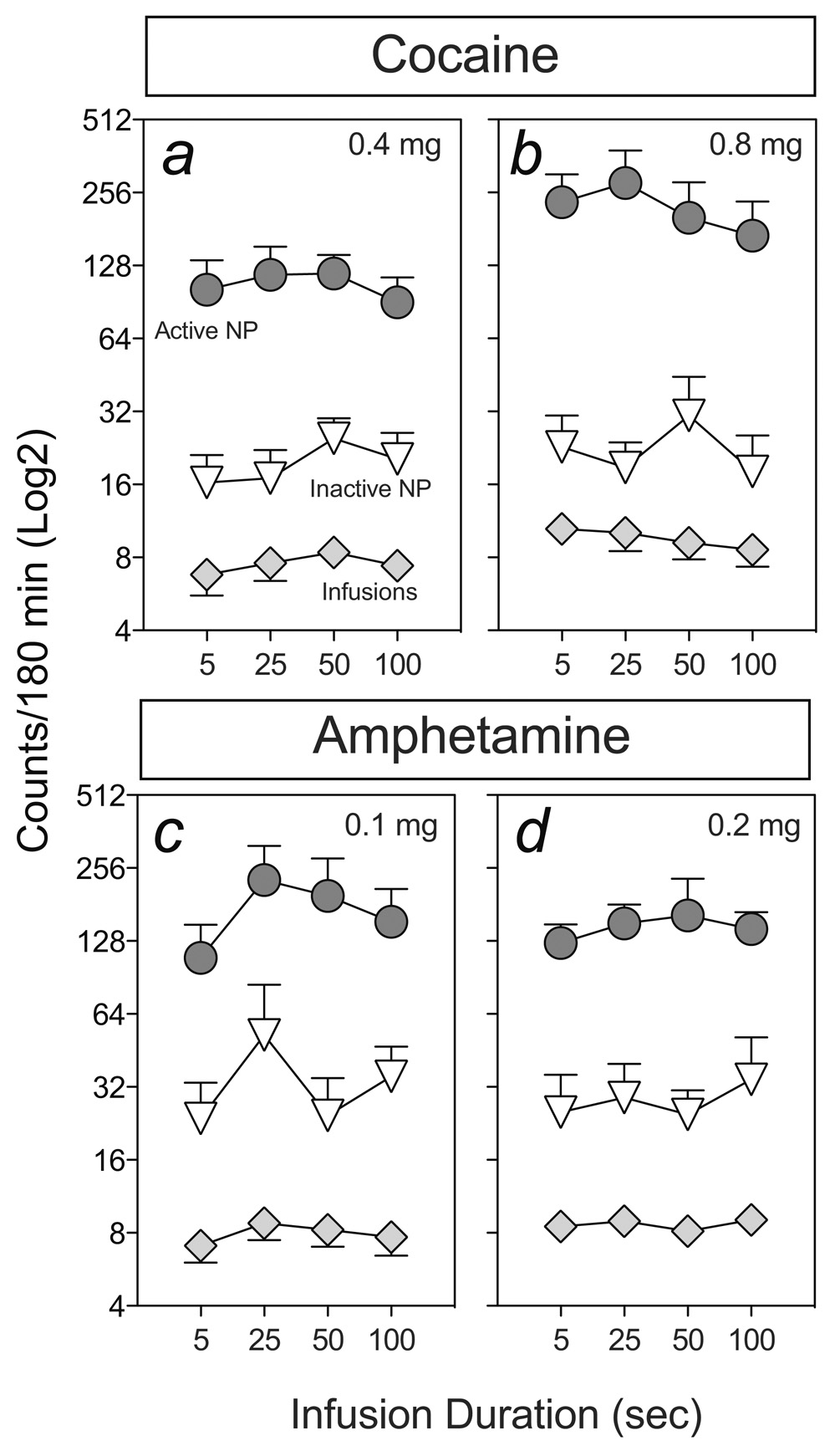
Figure 4 shows the mean (±SEM) number of infusions, and responses into the active and inactive nose-poke ports of rats responding on a progressive ratio schedule of reinforcement for cocaine (panels a and b) or amphetamine (panels c and d) delivered over 5–100 sec. Because few rats reached breaking point within the 3 hr test sessions, statistical analyses were conducted on the number of drug infusions earned and responses on the active and inactive nose-poke ports. In both the cocaine and amphetamine groups, infusion rate did not significantly affect the number of infusions earned or the number of responses into the active or inactive ports. Thus, as with fixed ratio schedules (Experiment 3), responding for cocaine or amphetamine under PR schedule conditions failed to reveal a marked effect of i.v. infusion duration. It should be noted, however, that whilst doubling the dose of cocaine increased progressive ratio responding for cocaine, responding for amphetamine was not markedly affected by the increase in dose. Thus, the PR schedule used may have been relatively insensitive to changes in the reinforcing magnitude of amphetamine.
Figure 4.
The effects of varying rate of infusion (5, 25, 50 and 100 sec infusion duration) on progressive ratio (PR) schedule responding for cocaine (panels a and b; n=10) or amphetamine (panels c and d; n=13). Once stable PR responding for established for 0.4 and then 0.8 mg/kg/inf cocaine or 0.1 and then 0.2 mg/kg/inf amphetamine, each rat was tested on 4 consecutive sessions with each of 4 infusion rates in random order. In both the cocaine and amphetamine groups, infusion rate did not significantly affect the number of infusions or responses into the active or inactive ports.
Effects of infusion rate on drug-induced reinstatement
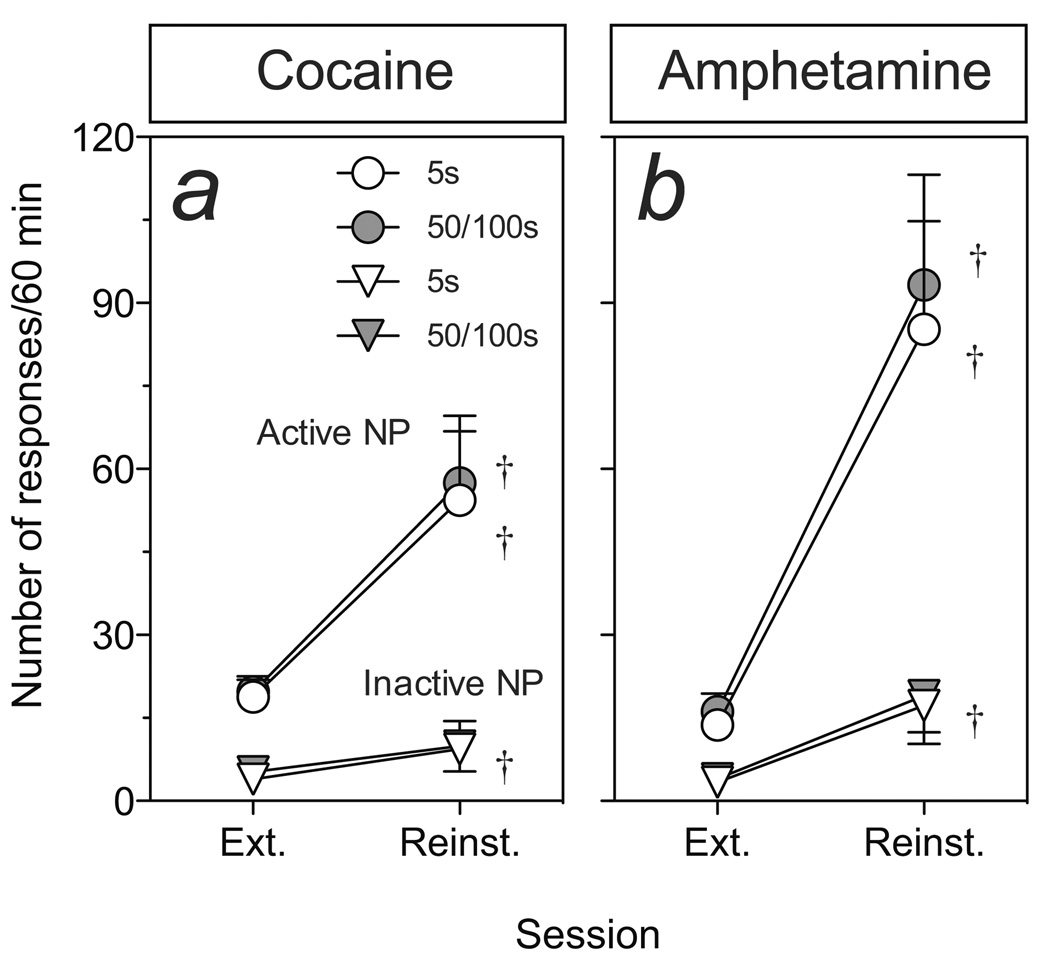
Figure 5 shows the mean (±SEM) number of responses into the port previously associated with cocaine or amphetamine infusions (active) or the control (inactive) port following a single i.v. priming infusion of amphetamine or cocaine delivered over 5, 50 or 100 sec period. Note that during this test, responses into neither the previously “active” nor “inactive” port resulted in drug delivery. There were no apparent differences between the 50 and 100 sec infusion rates (not shown) and, in order to yield a large enough group for statistical comparisons, these data were pooled to yield a single data point. Extinction training produced a gradual decrease in active port responding and, relative to the first extinction session, responding was reduced by 72% by the last session (data not shown). On the subsequent test for reinstatement, amphetamine and cocaine priming infusions produced a large and significant increase (relative to the mean response rate of the last 3 extinction sessions) in responding into the previously drug reinforced nose-poke port (AMPH-5 sec, t = 3.858, P < 0.01; COC-5 sec, t = 2.983, P < 0.05; AMPH-50/100 sec, t = 4.22, P < 0.01; COC-50/100 sec, t = 3.155, P < 0.05). There was no significant effect of infusion rate on the magnitude of reinstatement. Cocaine and amphetamine priming infusions delivered over 5 sec (but not 100 sec) also produced a small, but significant, increase in responding on the non-reinforced nose-poke port (for cocaine, t = 3.155, P < 0.05; for amphetamine, ts > 4.22, P < 0.01).
Figure 5.
The effects of varying rate of infusion (5 or 50/100 sec infusion duration) on amphetamine (n=7, 4 and 5 for the 5, 50 and 100 sec conditions, respectively) or cocaine-priming (n=8, 5 and 5 for the 5, 50 and 100 sec conditions, respectively) induced reinstatement of extinguished drug seeking. Data from the 50 and 100 sec conditions were pooled to yield a single group (50–100 sec) for comparison. A single i.v. infusion or cocaine (2.0 mg/kg) or amphetamine (1.0 mg/kg) triggered robust reinstatement relative to post-extinction levels of responding (average response during last 3 extinction sessions). Swords indicate a significant difference in responding between extinction and reinstatement test sessions. Rate of infusion did not affect the magnitude of cocaine or amphetamine-priming induced reinstatement.
Discussion
It is a common belief that more rapid delivery of drugs to the brain increases their abuse and addictive potential (de Wit et al., 1992; Gorelick, 1998; Mathias, 1997; Oldendorf, 1992; Sellers et al., 1989; Volkow et al., 2000). There are only very few studies, however, to have explored why this might be the case. One possibility is that the rate of drug delivery influences the reinforcing effects of drugs of abuse. Whereas there are number of reports consistent with this notion, showing that in monkeys rapid drug delivery facilitates cocaine and nicotine self-administration behavior (Balster and Schuster, 1973; Kato et al., 1987; Panlilio et al., 1998; Wakasa et al., 1995; Woolverton and Wang, 2004), this effect has often not been seen in rats (Liu et al., 2005; Pickens and Crowder, 1967; Sorge et al., 2006). The present results are, therefore, largely consistent with these latter reports. That is, other than a small decrease in the number of infusions earned when amphetamine or cocaine were delivered over 100 sec (vs. 5) on a FR1 schedule of reinforcement (see below), faster rates of i.v. amphetamine or cocaine delivery failed to significantly enhance the acquisition or maintenance of drug taking behavior. Furthermore, the ability of amphetamine or cocaine to reinstate extinguished drug seeking did not depend on the rate at which the i.v. priming infusion was delivered.
A number of factors can influence the rate at which drug-naive rats acquire drug self-administration behavior, including individual differences (Piazza et al., 1989), environmental influences (Goeders and Guerin, 1994; Piazza et al., 1990; Tidey and Miczek, 1997), past experience with the drug (Lett, 1989; Piazza et al., 1989) and dose. With respect to the latter, typically the probability to acquire amphetamine or cocaine self-administration increases, and the latency to acquisition decreases, as the unit injection dose of the drug increases (Carroll and Lac, 1997; Hu et al., 2004; van Ree et al., 1978). Consistent with this notion, in experiment 1 increasing doses of amphetamine (from 0.2 to 0.3 mg/kg/infusion) or cocaine (from 0.5 to 0.7 mg/kg/infusion) resulted in a progressively greater drug intake over sessions. Although the influence of rate of infusion on the acquisition of psychomotor stimulant drug self-administration had not been studied - in previous studies infusion rate was varied after stable schedule-controlled responding was established - we expected that, as with dose, acquisition of drug taking behavior would be positively correlated with rate of drug infusion. Contrary to this prediction we found that, over the range of doses that supported self-administration, cocaine taking was unaffected by infusion rate and acquisition of amphetamine self-administration was slightly enhanced when drug was infused over 100 sec.
Thus, if anything, our results suggest that slower rates of drug infusion facilitate the acquisition of amphetamine self-administration. It is possible that, when infused slower, higher doses of amphetamine were less likely to have aversive effects that could interfere with drug taking. Additionally, one could speculate that slower rates of i.v. infusion are less likely to have resulted in locomotor hyperactivity and/or stereotyped behaviors that could also have disrupted the rat's ability to respond (Yokel, 1987). Although we know that, depending on dose, infusion rate can alter the psychomotor response (locomotor hyperactivity and/or stereotyped behaviors) to a single, non-contingent i.v. injection of cocaine (Samaha et al., 2002) we do not know whether this is also the case for self-administered amphetamine.
A primary mechanism regulating drug intake in experienced rats is an adjustment of response rates to maintain stable and optimum brain levels of drug (Johanson and Fischman, 1989; Pickens and Thompson, 1968; Wise et al., 1995; Yokel, 1987). Thus, manipulations that reduce drug concentrations and/or binding to the target receptor - for instance, decreases in unit injection dose of the drug or co-administration of dopamine-receptor antagonists - typically result in increased drug taking to compensate for the reduced drug effectiveness. In turn, decreases in drug intake typically accompany increases in drug dose or pretreatments with agonists or could reflect (dose-dependent) response-interfering effects of the drug (e.g., locomotor hyperactivity or stereotyped behaviors). If slower infusion rates reduce the reinforcing effects of cocaine or amphetamine, we predicted that drug intake would increase with slower infusion rates. Contrary to this prediction, when drug-experienced rats were able to self-administer amphetamine or cocaine under continuous (FR1) reinforcement (experiment 2), the number of infusions slightly decreased when delivered over 100 sec (mean percent decrease relative to 5 sec infusion was 21% and 20% for amphetamine and cocaine respectively).
Interestingly, in these rats (but also in rats responding under higher FR schedules) 100 sec infusions also resulted in a marked increase in nose pokes into the active port during the period of infusion (i.e., the timeout period). It is not clear what caused this effect and/or whether the hyper-responsiveness during the slow ramping-up of drug levels somehow interfered with subsequent drug taking (at least under FR1 schedule of reinforcement). It is possible that the initial low concentrations of drug during the 100 sec infusion produced a priming effect and served as an interoceptive cue to elicit more responding (de Wit, 1996). Alternatively, the high rates of time-out responding may reflect what Amsel (1962) referred to as a frustration effect, which is often seen shortly after unexpected non-reward or partial reward. If so, this may suggest some reduction in reinforcement as a function of the rate of drug infusion after all.
Finally, we found that the ability of amphetamine or cocaine to elicit relapse or reinstatement if drug seeking was not influenced by the rate at which a priming-infusion of drug was delivered. That is, although a single i.v. priming injection of amphetamine and cocaine produced robust reinstatement of drug seeking following 10 days of extinction experience, the magnitude of reinstatement was insensitive to how rapidly the drug prime was delivered – at least over the range of i.v. priming doses used here.
Taken together, our results suggest that under the conditions tested here, and using infusion rates varying from 5 to 100 sec, slower infusions do not markedly alter drug taking or reinstatement of drug seeking. A number of recent studies in rats have found similar results (e.g., Liu et al., 2005). Indeed, rats trained to self-administer nicotine were found to prefer slower (30 sec i) compared to faster (3 sec) rates of infusion (Sorge et al., 2006). Interestingly, these investigators also found that the effects of dopamine D2 antagonism on nicotine self-administration varied depending whether drug taking was maintained under slow (low dose) or fast (high dose) infusion conditions – increasing intake under rapid infusion conditions and reducing intake under slow conditions. These finding suggest that different neural mechanisms may be engaged depending on infusion speed (Sorge and Clarke, 2007).
Of course, it is possible that infusion durations beyond 100 sec would have produced more pronounced and predictable effects on drug taking and seeking. For instance, others have reported significant effects of infusion rate in monkeys, but only when infusion times exceeded 100 sec (Panlilio et al., 1998; Woolverton and Wang, 2004). Although we cannot rule out this possibility, we note that in cocaine users variation in the rate of i.v. cocaine (but not hydromorphone) infusion between 2–60 sec is reported to significantly alter the subjective responses to the drug (Abreu et al., 2001; Nelson et al., 2005). More critically, our group has shown that variations in infusion rate over 5–100 sec alter cocaine-and nicotine-induced, 1) psychomotor sensitization (see also, Liu et al., 2005), 2) c-fos and arc mRNA expression in the nucleus accumbens, caudate nucleus and prefrontal cortex, and, 3) dopamine dynamics in striatum (Samaha et al., 2005). Additionally, similar variations in cocaine’s delivery rate affect its basic physiological actions on brain temperature (Brown and Kiyatkin, 2005).
Because dopamine-dependent neural activity in these regions is critical for the primary response reinforcing and reinstating effects of psychostimulant drugs, the question remains why variation in the rate of i.v. drug infusion over this same range did not markedly affect drug taking and seeking behavior. Although purely speculative, it is possible that both response contingent and non-contingent exposure to these drugs can produce response patterns that are relatively insensitive to changes in outcome (Dickinson et al., 1983; Tiffany, 1990). For instance, oral alcohol or cocaine seeking are relatively insensitive to changes in reward value induced by lithium chloride pairings (Dickinson et al., 2002; Miles et al., 2003). In addition, Vanderschuren and Everitt (2004) recently reported that rats with an extended cocaine-taking history (but not rats with limited access) were almost completely insensitive to the normal response-suppressing effects of a conditioned aversive stimulus. Finally, the propensity of drugs to promote habitual, reward-insensitive response patterns may, under some circumstances, generalize to responding maintained by natural rewards. That is, repeated non-contingent cocaine or amphetamine treatments, which produce robust behavioral sensitization, also make rats unable to use changes in outcome value to direct their behavior (Nelson et al., 2006; Schoenbaum et al., 2004; Schoenbaum and Setlow, 2005). Thus, if exposure to drugs makes response patterns more habitual and less sensitive to changes in outcome, and as a consequence increasingly dependent on antecedent stimuli, it may not be entirely surprising that outcome changes due to changing the rate of infusion had such little effect in the present study.
Finally, it is worth mentioning that species differences in pharmacokinetic and pharmacodynamic actions of psychomotor stimulants drugs, as well as in the neurobiological targets of these drugs, could have contributed to the apparent differences in results yielded by rodent versus non-human and human primate studies. With respect to the latter, for instance, there are substantial differences between rodents and primates in the anatomical distribution of dopamine inputs to prefrontal cortical areas as well as in the expression of different DA receptor subtypes (e.g., Berger et al., 1991). On the other hand, a number of reports indicate that, within regions of the basal ganglia, dopamine receptor distribution is remarkably preserved and shows similar patterns across different mammalian species, including rats and monkeys (e.g., Camps et al., 1990). Nonetheless, given the apparent variations seen within rodents in the pharmacokinetic, neurobiological and behavioral (including reinforcing) effects as a function of dispositional factors such as gender, strain, and age, one could easily expect species variations to have contributed to the differences seen between rats and monkeys in the influence of rate of drug infusion on the reinforcing effects of psychomotor stimulant.
Whatever the case, the present results suggest that, in rats, the rate of drug infusion has minimal effect on drug taking and seeking behavior directly, but it is possible that rate of infusion indirectly influences drug taking and seeking behavior. Samaha and colleagues (2002; 2004; 2005) reported in a series of studies that the rapid delivery of cocaine or nicotine promotes the development of drug-induced neuroplastic changes that underlie psychomotor sensitization, and alters patterns of drug-induced gene expression. That these findings are relevant for understanding drug taking and seeking was recently confirmed by a study showing that, although not affecting progressive ratio responding for cocaine directly (consistent with what we report here), the rate of cocaine infusion did influence sensitization to the reinforcing effects of cocaine on this same schedule (Liu et al., 2005). Thus, the reason drugs, formulations and routes of administration that result in the rapid delivery of drugs into the brain may preferentially promote the transition to addiction may not be because this makes the drugs more reinforcing per se, but because the rapid delivery of drugs to the brain facilitates their ability to induce forms of neurobehavioral plasticity that contribute to compulsive and excessive drug use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Amsel A. Frustrative nonreward in partial reinforcement and discrimination learning: some recent history and a theoretical extension. Psychol Rev. 1962;69:306–328. doi: 10.1037/h0046200. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psychopharmacology (Berl) 2005;181:299–308. doi: 10.1007/s00213-005-2244-0. [DOI] [PubMed] [Google Scholar]
- Camps M, Kelly PH, Palacios JM. Autoradiographic localization of dopamine D 1 and D 2 receptors in the brain of several mammalian species. Journal of neural transmission. 1990;80:105–127. doi: 10.1007/BF01257077. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- de Wit H. Priming effects of drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4:5–10. [Google Scholar]
- de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl) 1992;107:352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Nicholas DJ, Adams VI. The effect of instrumenal training contingency on suceptibility to reinforcer devaluation. Q J Exp Psychol. 1983;35B:35–51. [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? Q J Exp Psychol B. 2002;55:331–348. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Shou M, Samaha AN, Watson CJ, Kennedy RT, Robinson TE. The rate of intravenous cocaine administration alters c-fos mRNA expression and the temporal dynamics of dopamine, but not glutamate, overflow in the striatum. Brain Research. 2008 doi: 10.1016/j.brainres.2008.02.081. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR. Injection duration of cocaine in humans. Federation proceedings. 1984;43:570. [Google Scholar]
- Goeders NE, Guerin GF. Non-contingent electric footshock facilities the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to cocaine abuse treatment. Adv Pharmacol. 1998;42:995–997. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Fischman MW. The pharmacology of cocaine related to its abuse. Pharmacol Rev. 1989;41:3–52. [PubMed] [Google Scholar]
- Kato S, Wakasa Y, Yanagita T. Relationship between minimum reinforcing doses and injection speed in cocaine and pentobarbital self-administration in crab-eating monkeys. Pharmacol Biochem Behav. 1987;28:407–410. [PubMed] [Google Scholar]
- Lett BT. Repeated exposures intensify rather than diminish the rewarding effects of amphetamine, morphine, and cocaine. Psychopharmacology. 1989;98:357–362. doi: 10.1007/BF00451687. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. Rate and duration of drug activity play major roles in drug abuse, addiction and treatment. NIDA notes. 1997;12:8–11. [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: action or habit? Behav Neurosci. 2003;117:927–938. doi: 10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug and alcohol dependence. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug and alcohol dependence. 2005 doi: 10.1016/j.drugalcdep.2005.08.004. in press. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. Some relationship between addiction and drug delivery to the brain. US Government Printing Office; NIDA Research Monographs. 1992:13–25. [PubMed]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl) 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- PHS. Public Health Service Policy on Humane Care and Use of Laboratory Animals. WD: editor; Department of Health and Human Services. 1996:28.
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self- administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, le Moal M, Simon H. Stress-and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- Pickens R, Dougherty J, Thompson T. Minutes of the meeting of the committee on Problems of Drug Dependence. Palo Alto, CA: NAS-NRC; 1969. Effects of volume and duration of infusion on cocaine reinforcement, with concurrent activity recording. [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Pickens RW, Crowder WF. Effects of CS-US interval on conditioning of drug response, with assesment of speed of conditioning. Psychopharmacologia. 1967;11:88–94. doi: 10.1007/BF00401511. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Robinson TE. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Yau WY, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry. 2005;57:351–360. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2007. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- Sellers E, Busto U, Kaplan H. Pharmacokinetic and pharmacodynamic drug interactions: implicationas for abuse liability testing. NIDA Res Monogr. 1989;92:287–306. [PubMed] [Google Scholar]
- Sorge RE, Clarke PB. 2007 Neuroscience meeting. San Diego, CA: Society for Neuroscience; 2007. Slow/low intravenous infusions of nicotine in rats: a better model of smoking? [Google Scholar]
- Sorge RE, Stewart J, Clarke PB. Neuroscience meeting. Atlanta GA: Society for Neuroscience; 2006. Effect of infusion duration on intake during nicotine self administration in rats: Rapid infusions are not necessary. Online, 2006. [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- van Ree JM, Slangen JL, de Wied D. Intravenous self-administration of drugs in rats. J Pharmacol Exp Ther. 1978;204:547–557. [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;677:1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Takada K, Yanagita T. Reinforcing effect as a function of infusion speed in intravenous self-administration of nicotine in rhesus monkeys. Nihon Shinkei Seishin Yakurigaku Zasshi. 1995;15:53–59. [PubMed] [Google Scholar]
- Winger G, Woods JH, Patrick GA, Powell LH, Harris LS, Nader MA, Woolverton WL. Progress report from the testing program fot stimulant and depressant drugs. NIDA Res Monogr. 1991;119:625–639. [PubMed] [Google Scholar]
- Wise RA, Newton P, Leeb K, Brunette B, Pocock D, Justice JB. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Yokel R. Intravenous self-administration: response rates, the effects of of pharmacological challenges, and drug preference. In: Bozarth M, editor. Methods of assessing the reinforcing properties of drugs. New York: Springer-Verlag; 1987. [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A. Intravenous drug injection habits: drug users' self-reports versus researchers' perception. Pharmacology. 2003;68:49–52. doi: 10.1159/000068731. [DOI] [PubMed] [Google Scholar]