Abstract
Whether cocaine locomotor conditioning represents a cocaine positive effect; i.e., a Pavlovian cocaine conditioned response; or, a cocaine negative effect; i.e., interference with habituation to the test environment, is a subject of some controversy. Three separate experiments were conducted to compare the behavior (locomotion and grooming) of separate groups of rats given 1, 9 or 14 cocaine (10 mg/kg) treatments paired/unpaired with placement into an open-field arena. The behavior of the cocaine groups on subsequent saline tests were compared with the habituation rates of saline treated rats. After one cocaine pairing with the test environment, the subsequent behavior of the cocaine-paired group on saline tests was similar to a non-habituated control group. In the two experiments with repeated cocaine pairings to the test environment, the subsequent behavior of the cocaine treated groups did not parallel that of the non-habituated saline control groups. These results were not explicable in terms of cocaine anti-habituation effects. It is suggested that cocaine contextual cues paired with cocaine treatment can activate cocaine memory traces which with subsequent cocaine treatments are reinforced and strengthened. In this way repeated cocaine use can forge conditioned stimulus connections to the cocaine behavioral response that are highly resistant to extinction.
Drug conditioning is well-recognized to be of considerable importance to the development of drug addiction [Childress et al 1988;Ehrman et al 1992; Newlin 1992]. In the Pavlovian drug conditioning formulation, the situational cues in which the drug effects are experienced constitute the conditioned stimulus (CS). The drug treatment is the unconditioned stimulus (UCS) and the drug induced effect is the unconditioned response (UCR). Following repeated drug taking, the associated situational stimuli acquire drug CS properties such that the situational cues by themselves are able to evoke drug like effects; i.e., the conditioned response (CR) [Ehrman et al 1992]. In fact, clinical studies of drug addiction have shown that exposure to stimuli previously associated with drug taking elicits drug-like experiences [Newlin 1992]. This process has substantial importance for drug treatment strategies because, even though one can achieve abstinence from drug usage, the exposure to conditioned stimuli can briefly activate the drug effects and, thereby elicit craving [Newlin 1992;O’Brien et al 1993]. Since diverse stimuli can become conditioned drug stimuli in individuals with a prolonged history of drug use, the difficulty of coping with a potentially large array of stimuli which can evoke the drug effects, undoubtedly, contributes to the serious problem of relapse in former drug addicts.
While the importance of conditioning processes to cocaine behavioral effects and to clinical phenomena such as cocaine abuse and cocaine addiction is widely accepted, the transformation of unconditioned cocaine effects into conditioned cocaine effects remains a formidable and complex issue. Clinical reports, which validate the importance of conditioning processes to addictive behavior, can only provide information regarding the outcome of repeated drug use and not the process by which drug associated stimuli acquire conditioned stimulus properties. Clinical studies using techniques such as f-MRI and PET scans have shown, however, that repeated cocaine use also leads to changes in brain activity which persist into the abstinence state [Franklin et al 2002;Goldstein et al 2001;2002;2004;Lee et al 2003]. Thus, long term use, not only can change the functional significance of stimuli but it can also changes brain activity. In studies using animal models, it has been known for some time that repeated cocaine treatments, not only lead to cocaine conditioned behavioral effects, but also changes in reactivity to cocaine (i.e., to sensitization effects) [Pert et al 1990;Post et al 1992].
While it is not altogether surprising that long term use of potent psychoactive drugs can lead to lasting changes in the brain, it now appears that even a single exposure to an addictive drug such as cocaine can have persistent effects. Recent studies have shown that a single cocaine treatment can induce neurobiological effects which are retained after the acute effects have worn off [Le Foll et al 2005;Grignaschi et al 2004;Kim et al 2004]. In addition, it has been reported that even a single cocaine self-administration session can generate conditioned response effects which can last for several weeks [Ciccocioppo et al 2004]. These findings appear to dovetail with the neurobiological formulation of drug abuse in which the use of certain psychoactive drugs is postulated to induce lasting changes in the brain [Leshner 1996;Leshner & Koob 1999]. Of course observations of a biochemical change in the brain does not necessarily imply functional significance or even uniqueness to a drug treatment such as cocaine. Such changes could simply be an activity-driven end-product. Furthermore, while it is impressive that a single self-administration session can lead to a sustained conditioned response effect, nonetheless, a single session includes multiple cocaine injections.
Although a single cocaine treatment can result in some sustained neurobiological effects, and, at a very high dose, can even alter reactivity to cocaine [Pert et al 1990], nonetheless, the question remains whether a single cocaine exposure is sufficient to induce a cocaine conditioned stimulus effect. Indeed, we have recently reported [Carey et al 2006] that cocaine conditioned locomotion stimulant effects occur after one treatment. These cocaine conditioned locomotion stimulant effects, however, appear explicable as an anti-habituation effects, possibly related to drug state dependent or cocaine induced inattention effects [Carey et al 2008] Thus, following a single cocaine experience in a novel environment, cocaine treated animals in a conditioning test appear to behave similar to animals placed in a novel environment for the first time. Such an equivalence between a cocaine treatment and the absence of habituation could mean either that cocaine interfered with habituation processes (e.g., inattention to cues) or that the cocaine conditioned effects evoke a level of behavioral activation similar to novel environment cues. In the present study, we examine this important issue in detail.
If cocaine simply blocks the acquisition of habituation during cocaine drug treatment trials, then, if non-drug trials are interspersed with drug treatment trials within a cocaine treatment regimen, the cocaine conditioned effects should decline substantially due to the acquisition of habituation effects on the non-drug trials. On the other hand, if the behavior which occurs in the interspersed non-drug trials represents a conditioned cocaine effect, then, this effect should be sustained by the additional pairings of cocaine to the environmental test cues. In addition, after a series of cocaine test environment pairings, the cocaine anti-habituation effect interpretation of a cocaine conditioned effect predicts a decline in behavior with repeated non-drug trials parallel to that of a non-cocaine control group tested for the first time in the same environment. Alternatively, if the cocaine treatment induces persistent neurobiological changes manifested in a conditioned behavioral response, then, the conditioned response should persist during the repeated non-drug trials and should not exhibit a typical habituation function. The present report, details the outcome of these manipulations to differentiate possible cocaine anti-habituation effects from cocaine induced effects which persist as transformational effects on the behavioral response to cocaine associated cues.
1. Materials and Methods
1.1. Animals
90 naïve male Sprague-Dawley rats from Taconic Farms (Germantown, NY), 4 months old and weighing approximately 400 g at the start of the experiments were used. Upon arrival, the animals were housed in individual 48×27×20 cm clear polycarbonate cages in a climate-controlled room at 22–24°C with a 12-h dark and 12 hr. light cycle. During the 1st week after arrival, all animals were handled and weighed daily for 7 days. During the second week the animals received three injections (i.p.) of 0.9% saline (1.0 ml/kg) in order to acclimate the animals to the injection procedure. All experiments occurred during the 12-h light cycle (6AM-6PM). This protocol (IACUC 4-E) was approved by the Veterans Administration Medical Center’s Subcommittee for Animal Studies.
1.2. Drugs
Cocaine hydrochloride (Sigma Chemical., St. Louis MO) was dissolved in sterile distilled H2O to a concentration of 10.0 mg/ml. Cocaine injections were administered i.p. in a volume of 1.0 ml/kg. Saline injections (0.9% sodium chloride) were administered in a volume of 1.0 ml/kg (i.p.).
1.3. Apparatus
Behavioral tests were conducted in two identical 60 cm square by 40 cm high open-field arenas. To reduce noise and control ambient light each arena was placed in one of two smaller rooms within the main laboratory. Though the use of separate rooms had previously been ruled out as a variable, this factor was equated across groups and treatments for each experiment to control for any potential effects on behavior. In the present experiments, test room was not a statistically significant variable on any behavioral measure (P>0.05).
The interior walls and floor of each arena were white. Two overhead 12 V projection lamps provided illumination. These were placed 50 cm above the arena floor adjacent to the video camera. Each lamp was fitted with a red filter since testing under red light conditions is less stressful, thus favoring locomotor activation as the rats are transferred from the ambient light of the vivarium to the red light of the testing room [Nasello et al 1998]. A white noise generator (San Diego Instruments, San Diego, CA), also placed 50 cm above the arena floor, provided masking sound (75 dB). It was turned on immediately prior to placement of the animal into the test arena and turned off upon removal from the arena.
Each arena was monitored by a closed-circuit video camera (Sanyo VCB-5123B) mounted 50 cm above the arena floor. Analog video signals were digitized and analyzed by an automated video tracking system (Ethovision, Noldus Information Technology, Inc, Leesburg, VA). The accuracy of the system for distance measurements was corroborated by moving objects a measured distance and confirming that the tracking system generated the same distance. To provide contrast, the animal’s head was blackened with a non-toxic marker, the camera tracked only this feature of the rat’s body. data were captured at a rate of six samples per second and the input filters were set to a minimum distance of 2 cm per sample. In addition to distance measurements, the locomotion paths in the arena were recorded and these were similar to those reported previously [Dai et al 1995]. The paths recorded by the software were used to identify small repetitive movements. Such movements occurred infrequently and idiosyncratically. After each rat was marked and placed in the arena and the behavior capture session was initiated the test proceeded without the experimenters in the room. A small TV screen connected to the video system was located outside of each room. It enabled experimenters to monitor the rats throughout each test trial. At the completion of each trial, the arenas were cleaned and dried.
A VHS VCR was connected to each camera to provide a complete record of an animal’s behavior during a test trial. The videotapes were reviewed following completion of experimental treatments at the end of each to validate or take into account any abnormalities shown by the automated records. In addition, the videotapes were evaluated to score behaviors such as grooming that cannot be distinguished by the Ethovision System. Two uninformed experimenters observed the videotapes to score these behaviors. Prior to viewing the videotapes from the current experiments, the experimenters were trained on other similar videotapes until they achieved inter and intra-individual reliability correlations of r > 0.9
1.4. Design and procedure
1.4.1. Experiment 1. Effect of a Single Cocaine Injection
Two groups (N=20) were used. One group received cocaine (Coc-P) (10 mg/kg) immediately prior to placement into the test environment for 20 min; and, the other group (Coc-UP) received saline. Two hr after testing, the Coc-P group received a saline injection in the homecage and the Coc-UP group received the cocaine (10 mg/kg) injection in the homecage. Both groups were administered 20 min tests on each of the next two days and both tests were saline tests to determine possible cocaine conditioned effects.
1.4.2. Experiment 2. Effect of Interspersed Cocaine Conditioning Tests
In this experiment, two groups (N=10) received either cocaine, Coc-P, (10 mg/kg) or saline, Coc-UP, immediately prior to 20 min test sessions. The Coc-P group received saline in the homecage 2 hr after testing; and, the Coc-UP group received cocaine (10 mg/kg). Before the start the cocaine treatment protocol, all animals received a single 20 min saline test and were subdivided into groups equated on distance scores (P>.05). Subsequently, the animals received 20 additional test sessions. There were 14 cocaine treatment sessions interspersed with a series of saline conditioning tests in which both groups received saline immediately prior to testing. The first of these saline conditioning tests occurred after 3 cocaine/saline sessions, the second after 4 cocaine/saline sessions and the third after another 5 cocaine/saline sessions. The final three tests in this series occurred after the completion of the fourteenth cocaine/saline test session.
1.4.2 Experiment 3. Habituation vs. Extinction
In the third experiment, two groups (N=10) received saline injections and were then given a 20 min pretest on the first test session. On the basis of the locomotion distance scores on this test, the animals were subdivided into two groups equated on distance scores (P>.05). Subsequently, the groups received nine 20 min test sessions in which one group received cocaine (Coc-P) (10 mg/kg) immediately prior to testing; and, the other group, saline (S). Following this conditioning induction phase, the animals received 4 saline conditioning tests. Another group (Coc-UP), which had received cocaine (10 mg/kg) injections in the home cage on the same days as the conditioning induction tests were conducted, but were not tested, also received four saline test sessions on the same days as the Coc-P group. The test environment was novel for this group but it had the same exposure to cocaine as the Coc-P group. This group served as the habituation control group.
1.5. Statistical Analysis
Two-way between-within-subject analyses of variance (ANOVA) were used to assess the cocaine drug treatment effects upon the behavioral responses. T-tests were used for specific between group comparison. P<0.05 was the statistical criterion for null hypothesis rejection.
Results
2.1 Experiment 1
Figure 1 presents the results of the single cocaine treatment conditioning experiment. As can be seen in Fig. 1A,1B. Cocaine increased locomotion and decreased grooming behavior. On the conditioning test, a similar pattern of effects occurred but diminished in magnitude. This is the expected result for a conditioned effect in that the CR is a partial UCR (Pavlov 1927). A two-way ANOVA of the results with cocaine treatment across test days indicated a significant effect on distance scores (F1,18 = 14.4; F1,18 =108.0; F1,18 =17.4, P<.01, for group, test day and group × test day interaction, respectively). The same analysis for grooming responses also yielded significant differences (F1,18 = 4.8; F1,18 = 5.1, P<.05, for group and test day). In terms of locomotion, the Coc-P group had higher scores on both test sessions (P<.05); but, for grooming only the difference on the cocaine test session was significant (P<.05). In order to assess the issue of whether the effect observed in the conditioning test may have been a cocaine blocking of habituation to the test environment, Fig. 2 shows a comparison of the behavior of the Coc-P group on the first and second saline conditioning tests vs. the Coc-UP group on the first and second saline tests in the test environment. Thus, the two groups were compared on the first two saline tests for each group. As can be seen in Fig. 2, the scores were virtually identical in the two groups. It is of importance, however, that the two way ANOVA conducted on the distance scores revealed a highly significant effect of day of treatment (F1,18 = 74.5, P<.001). No other differences were significant (P>.05). This result shows that there was a marked and equivalent habituation effect in both groups from the first to the second saline tests.
Fig. 1.
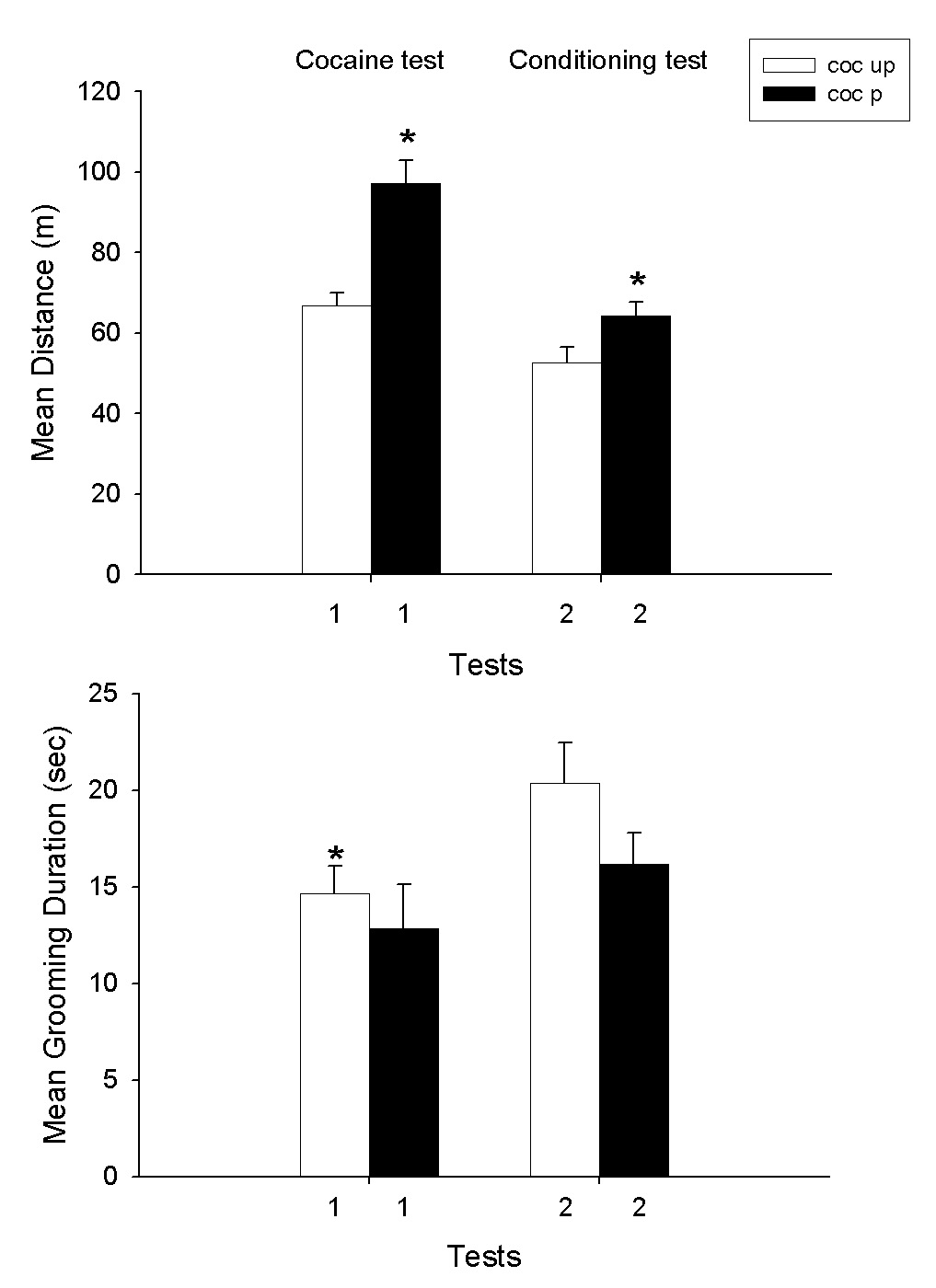
Means and SEMs of distance (m) and grooming duration scores (sec) on two 20 min test sessions. On Session 1, the Coc-P (cocaine paired) group received cocaine (10 mg/kg) immediately prior to testing. The Coc-UP (cocaine unpaired) group received saline but cocaine in the home cage. On test 2, both groups received saline. *P<.05, Coc-P vs. Coc-UP.
Fig. 2.
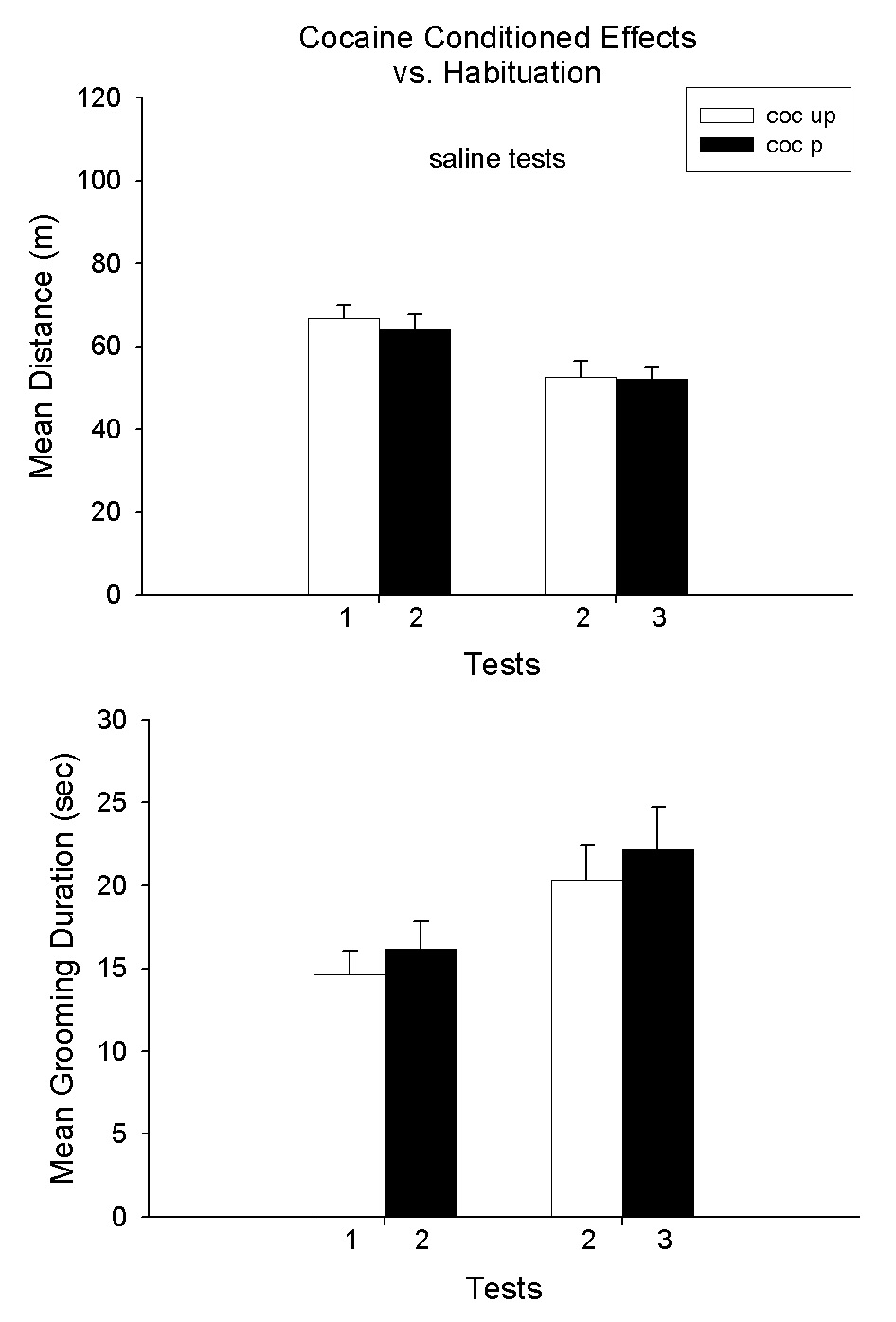
Means and SEMs of distance (m) and grooming duration scores (sec) on the first two saline tests for the Coc-P and Coc-UP groups. For the Coc-P group, the saline tests were the two tests after the cocaine test session. For the Coc-UP group, the tests were the first and second time the group was tested. *P<.05, Coc-P vs. Coc-UP.
2.2. Experiment 2
Fig. 3 shows the distance (A) and grooming (B) scores on the pre-test and on the subsequent 14 cocaine/saline tests. As can be seen in Fig. 3, the groups were closely matched in the pre-test but were differentially affected by the cocaine/saline treatments. Cocaine increased locomotor activity but suppressed grooming; whereas, with repeated saline treatments, locomotion decreased to a stable asymptote while grooming increased. Treatment effects were significant (F1,18 = 25.0, 71.4, P<.001, for distance and grooming, respectively); and, the group × day interactions were also significant (F14,252 = 2.7, 4.0, P<.001, for distance and grooming, respectively).
Fig. 3.
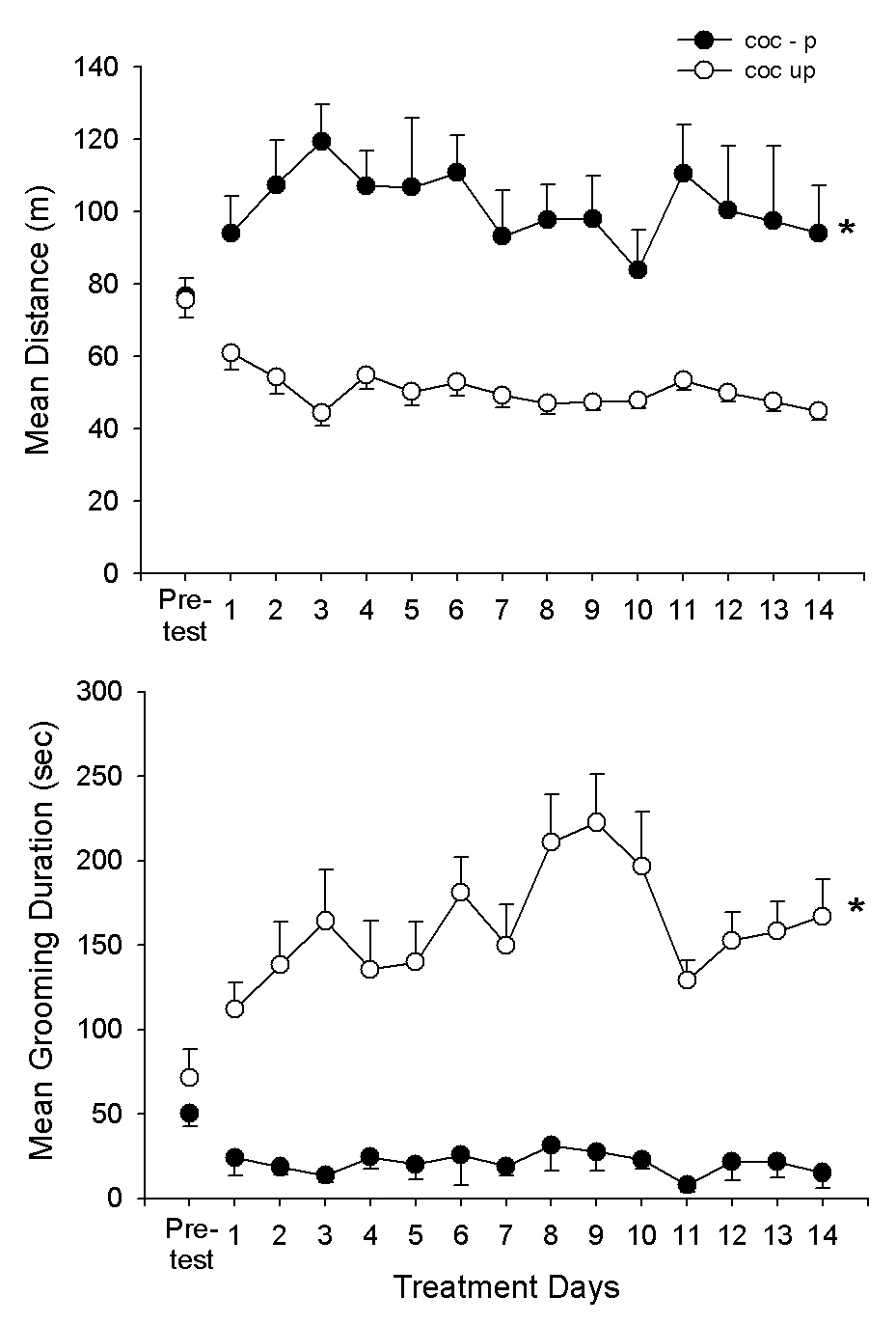
Means and SEMs of distance (m) and grooming duration scores (sec) for the Coc-P and Coc-UP groups in Experiment 2. The first test was saline pre-test for both groups. On the next 14 sessions, the Coc-P group received cocaine (10 mg/kg) immediately upon testing. The Coc-UP group received saline. *P<.05, Coc-P vs. Coc-UP.
The conditioning test results are shown in Fig. 4A, for distance and in Fig 4B, for grooming. As shown in Fig. 4A and 4B, the cocaine treated groups had higher distance scores and lower grooming scores across the 6 conditioning tests (F1,18 = 10.7, 11.2, P<.01, for distance and grooming, respectively). The treatment × day interactions were not significant (P>.05).
Fig. 4.
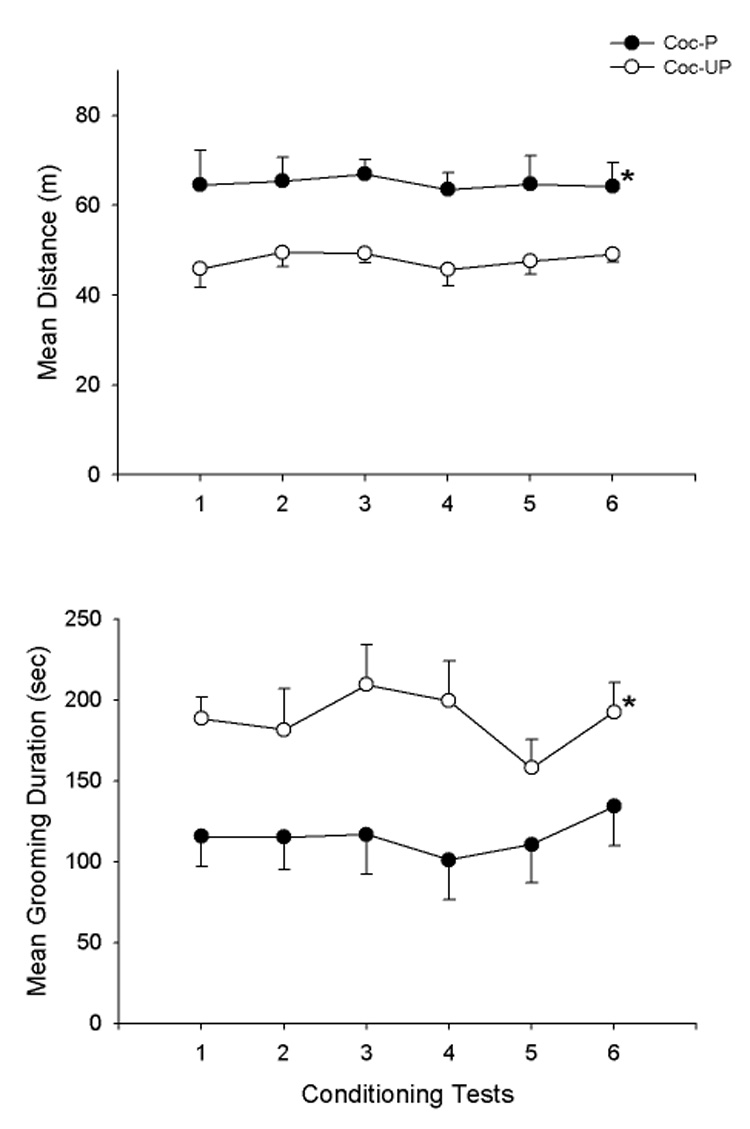
Means and SEMs of distance (m) and grooming duration scores (sec) for the Coc-P and Coc-UP groups on 6 conditioning tests in which both groups received saline immediately before testing. *P<.05, Coc-P vs. Coc-UP.
In order to evaluate the issue of habituation, Fig. 5A,B compares the Coc-P and Coc-UP groups on the pre-test and on subsequent saline tests for each group. For the Coc-UP group, the days were 2,3,4,5,6,7; whereas, for the Coc-P group, the saline test days were the 6 conditioning tests. As can be seen in Fig. 5A,B, the groups were closely matched on the pre-test and on the first subsequent saline test. After this test, however, the groups diverged. The Coc-UP group continued to decrease in locomotion and increase in grooming, a pattern consistent with the development of habituation to the test environment. The Coc-P group, however, remained at the same level across all saline tests. The Coc-P and Coc-UP differences were significant (F1,18 = 5.7, 4.5, P<.05, for distance and grooming, respectively); and, the group × day interaction was significant for distance (F6,108 = 3.0, P<.01) but not for grooming (P>.05).
Fig. 5.
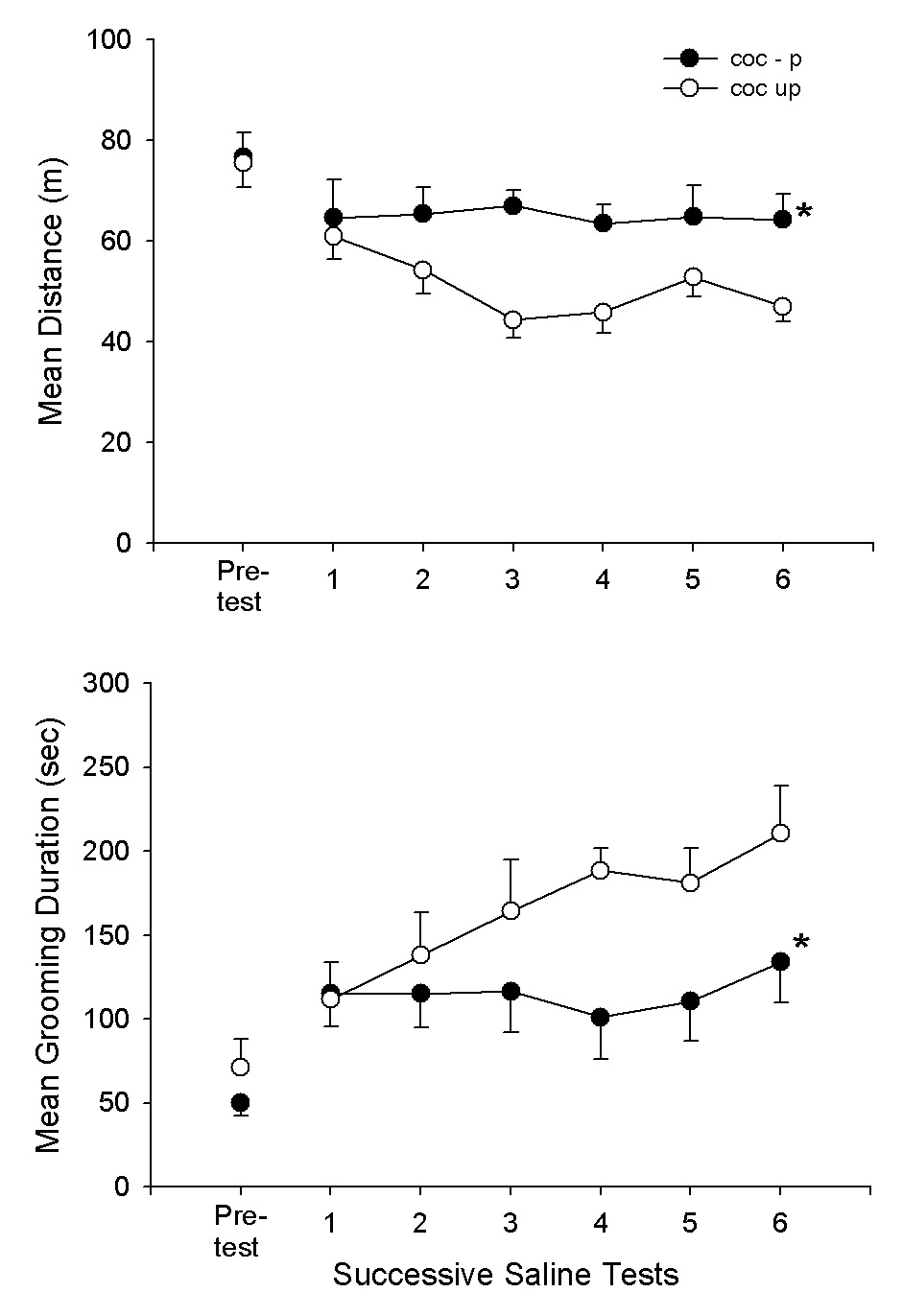
Means and SEMs of distance (m) and grooming duration scores (sec) for the Coc-P group on 6 saline conditioning tests and for the Coc-UP group on its first 6 test sessions. *P<.05, Coc-P vs. Coc-UP.
2.2. Experiment 3
Fig. 6A,B presents the findings of the third experiment for the cocaine and saline treatment groups on the pre-test and subsequent 9 cocaine/saline treatment sessions. The pre-test and treatment session results are comparable to those of experiment 2. Again, there were treatment effects (F1,18 = 14.8, 29.1, P<.001, for distance and grooming, respectively) as well as for treatment × day interactions (F9,162 = 4.9, 7.7, P<.001, for distance and grooming, respectively). At the completion of this treatment regimen, the groups were given 4 successive saline conditioning tests. The results are shown in Fig. 7A,B. There were significant group differences (F1,18 = 10.5, 37.8, P<.005, for distance and grooming, respectively). In order to assess the issue of habituation a third group which had received cocaine (10 mg/kg) in the homecage but without testing. This group (Coc-UP) was tested on 4 successive saline tests to provide a Coc-UP control group which received the same number of cocaine treatments as the Coc-P group but without exposure to the test environment. Thus, for this group, the environment was novel at the start of the saline tests. Fig. 8A,B compares the Coc-P with Coc-UP groups on the 4 saline tests. The treatment groups differed on distance (F1,18 = 19.1, P<.001); also the group × day interaction was significant (F3,54 = 11.6, P<.01). Subsequent specific comparisons with t-tests indicated that the groups differed on days 1, 3 and 4 (P<.05). For grooming duration, there was also a group difference (F1,18 = 6.8, P<.01) as well as a group × day interaction (F3,54 = 3.1, P<.05).
Fig. 6.
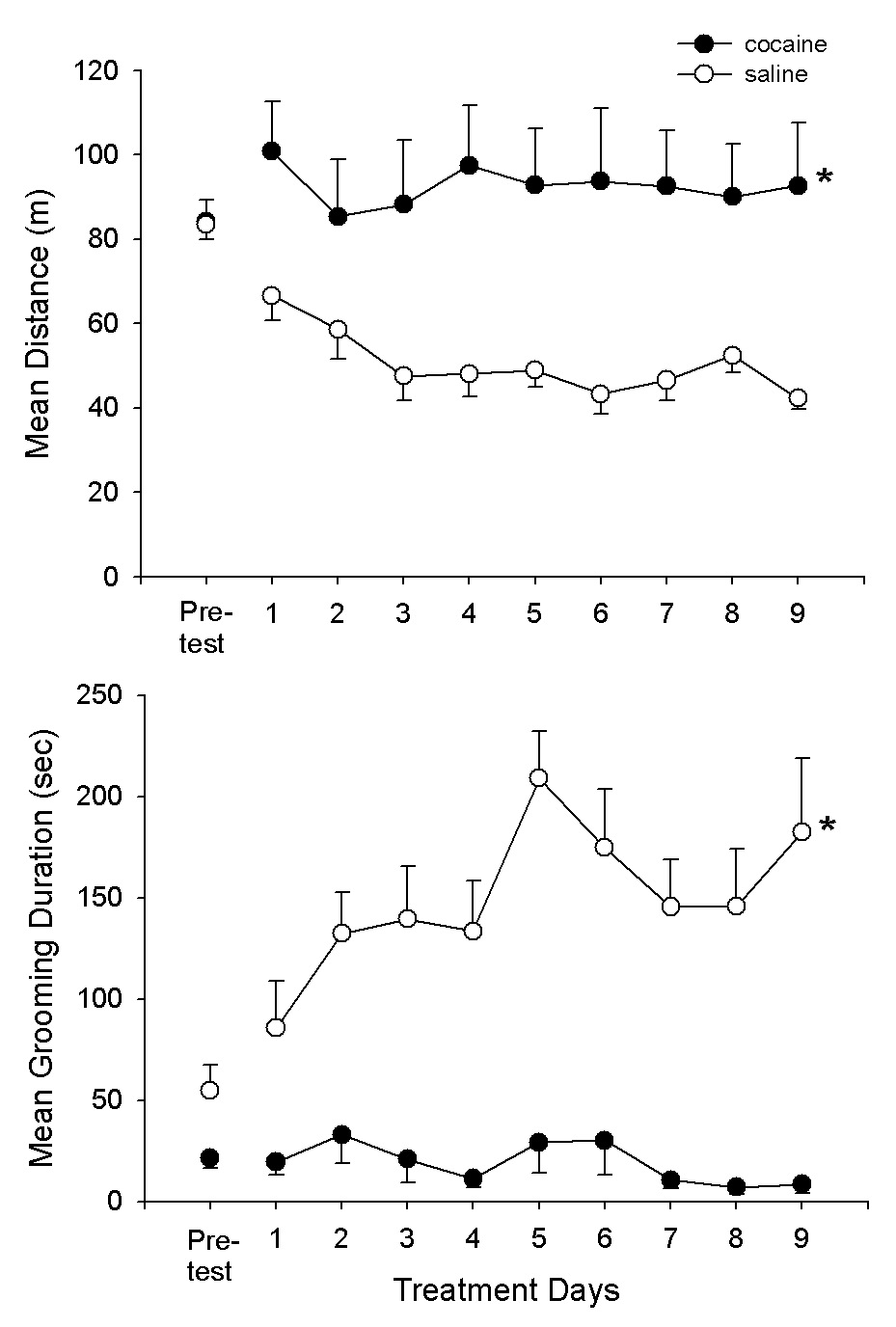
Means and SEMs of distance (m) and grooming duration scores (sec) for the Coc-P group and the saline (S) group on the pre-test and subsequent 9 cocaine/saline test sessions.*P<.05, Coc-P vs. S.
Fig. 7.
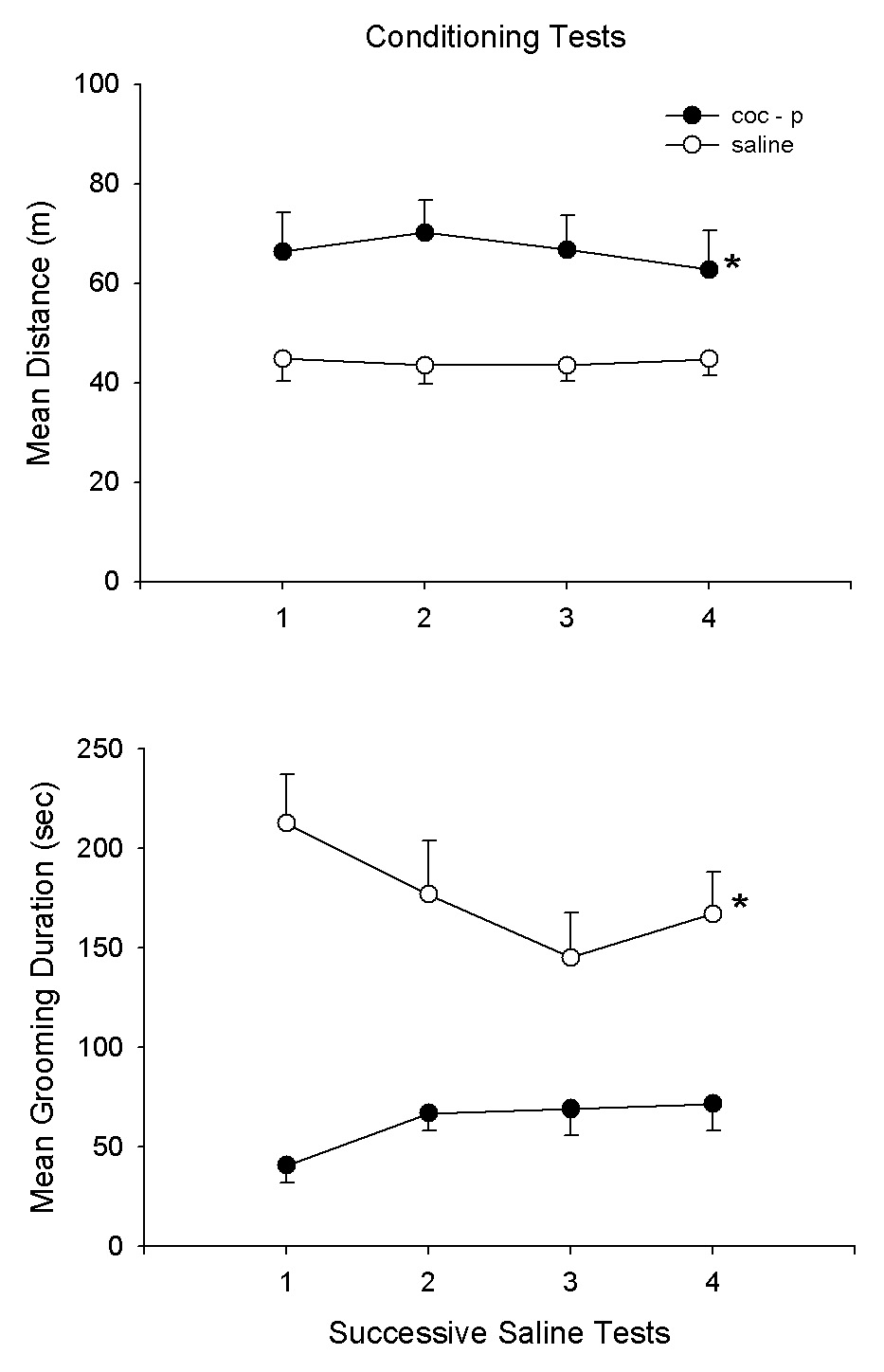
Means and SEMs of distance (m) and grooming duration scores (sec) for the Coc-P group and the saline (S) group on 4 saline tests conducted after completion of the 9 cocaine/saline test sessions.*P<.05, Coc-P vs. S.
Fig. 8.
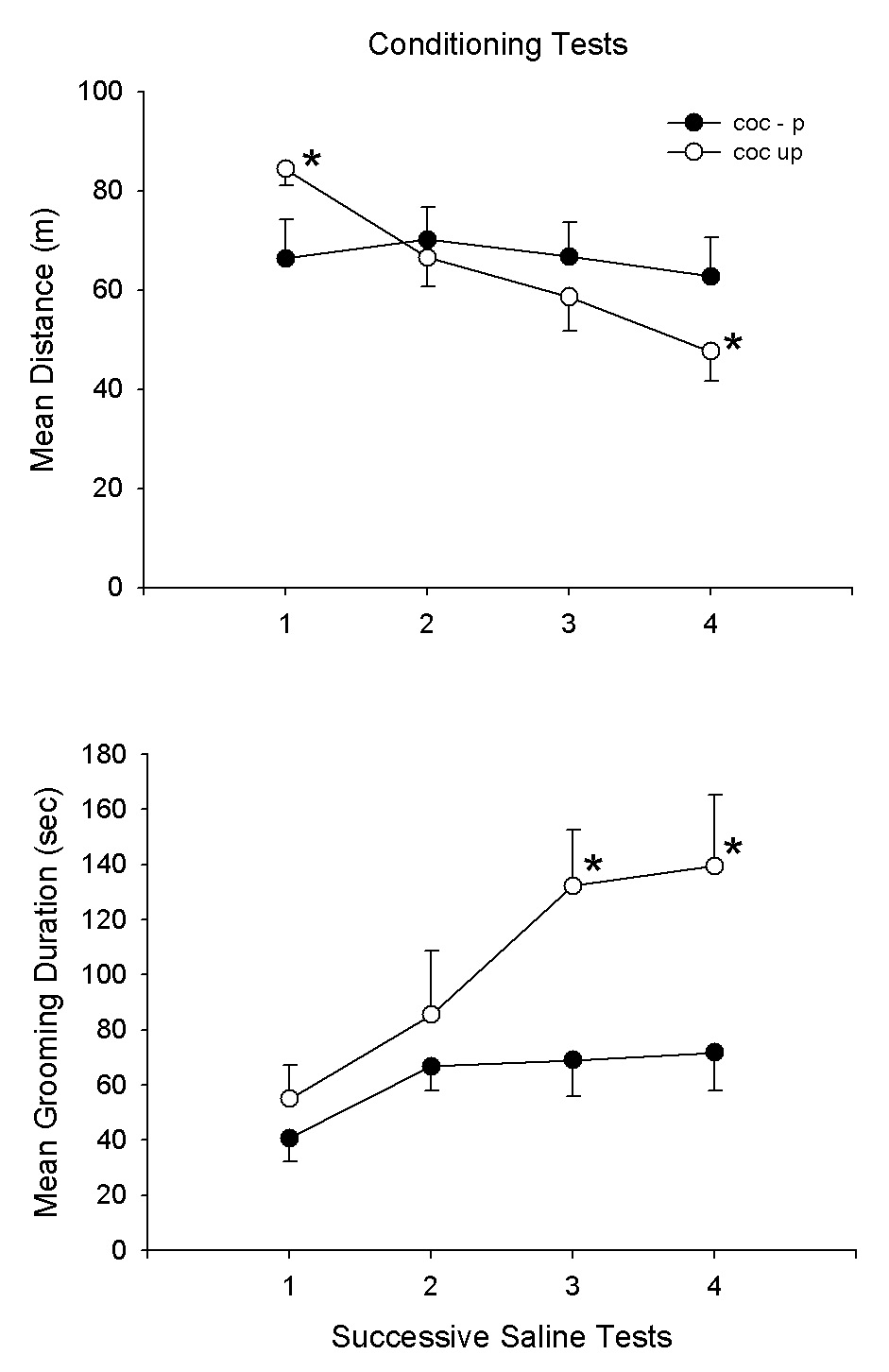
Means and SEMs of distance (m) and grooming duration scores (sec) for the Coc-P group and the Coc-UP group on 4 saline test sessions after completion of the 9 cocaine/saline sessions. The Coc-UP group received 9 cocaine (10 mg/kg) cocaine homecage treatments but no test environment exposures prior to these 4 saline test sessions.*P<.05, Coc-P vs. Coc-UP.
Discussion
We have reported [Carey et al 1998] that a brief 10 min exposure to a novel test environment can induce a persistent habituation effect. Consistent with this observation, other studies have shown [Sadile et al 1991] that the habituation mechanism activated by exposure to a novel environment induces a change in rat brain DNA synthesis and a potentiation of prefrontal cortex granule cell synthesis. Furthermore, the habituation response to a novel test environment has been used as a measurement of memory [Tomaz 1990]. As shown in the present study and in previous reports as well [Carey et al 2003; Carey & Damianopoulos 2006], we observed that an acute cocaine treatment appears to block the habituation process. Thus, if the cocaine conditioning effect is viewed simply as a habituation blocking factor, then, when animals are tested without cocaine, the animals would be expected to acquire habituation to the test environment since the tests are conducted without cocaine. In the present study, the anti-habituation interpretation of cocaine conditioning test effects appears to be supported by the one-trial conditioning tests. After one exposure to a novel test environment under the influence of cocaine, the behavior of the animals in the subsequent non-cocaine test closely matched the behavior of saline treated animals tested in a novel environment. Furthermore, when each group received a second non-drug test, the groups exhibited a parallel habituation effect. On the other hand, when the treatment/testing protocol was expanded to include multiple cocaine-test environment pairings, coupled with interspersed saline test to assess possible conditioned effects, there was no decline in the cocaine conditioned effect which would have been expected if this effect was simply a failure to habituate. While it might be argued that the intervals between the conditioning tests were of sufficient length to reverse the habituation effect acquired in the saline tests, it is also the case that habituation effects acquired in one saline trial can be carried over to a subsequent saline test. We have shown previously [Damianopoulos & Carey 1994] that habituation effects can persist undiminished after one week period of non-testing. The most definitive finding which argues against an anti-habituation interpretation of a cocaine conditioned effect is the observation that following a series of cocaine-test environment pairings, the behavior of the cocaine paired groups, given several repeat exposures to the test environment without cocaine, did not decline in parallel to an unpaired group tested without cocaine but with the test environment being novel. This cocaine unpaired novel environment group exhibited a rapid habituation function whereas the cocaine paired group did not exhibit a comparable habitation function when tested with saline. Thus, the cocaine paired group did not behave as if the environment was novel. Rather, the cocaine treatment seemed to have transformed the significance of the test environment cues (e.g., increased salience) (Robinson & Beninger 1983) or had changed the behavioral baseline. Seemingly, the use of cocaine unpaired treatment would also argue against a behavioral baseline effect. While cocaine homecage treatments rule out effects of exposure to cocaine per se, the adequacy of homecage administration of psychostimulant drugs need to be qualified in light of studies which demonstrate the profound differences in the neurobiological impact of psychostimulant drugs administered in the homecage vs. a novel environment (Klebar et al 2002; Li et al 2004;Uslaner et al 2001). Relevant to this consideration, is a previous study [Damianopoulos & Carey, 1994], in which we exposed animals to cocaine with a similar cocaine treatment and dose response regimen as the present study. We observed a conditioned drug effect but without a change in the behavioral baseline. In this latter study, we were able to exclude an upward behavioral baseline change as a possible contributing factor to the expression of a cocaine conditioned effect, by the use of another non-drug test environment to independently monitor behavioral baseline changes immediately prior to testing for conditioned drug effects. No differences were observed in locomotion in the non-cocaine associated environment but enhanced locomotion was observed selectively in the cocaine associated environment. This result demonstrated that cocaine can induce an enhanced locomotion response in paired cocaine environment that cannot be attributed to a behavioral baseline change.
In that a dual environment test protocol was not used in the present study, the question of change in the behavioral baseline cannot be answered unequivocally. Importantly, however, the present findings appear to rule out an anti-habituation interpretation of a cocaine effect upon subsequent non-cocaine behavior in the same test environment. These findings suggest that a persistent behavioral impact of cocaine upon non-cocaine behavior requires multiple cocaine exposures remain an open question. The rapid loss of an effect of cocaine upon non-drug behavior following a single cocaine treatment may indicate that, to be sustained, the initial cocaine effects need additional reinforcement by cocaine treatments. That is, the second time an animal is exposed to the test environment previously paired with cocaine, the cocaine memory is activated and this internal memory stimulus is reinforced by the second cocaine treatment. As the procedure is repeated, then, the cocaine memory would be progressively strengthened and, in this way, the bonding of the association of the contextual stimuli with cocaine effect on behavior would be increasingly stabilized and resistant to extinction. The exposure of animals to stimuli previously associated with drugs such as cocaine have become widely used as a method to evoke the memory of the drug experience and to introduce experimental manipulations to modify the drug-associated memory [Lee et al 2006]. From this perspective, then, the drug conditioning protocol involving repeated cocaine pairing can be seen as a procedure which elicits the cocaine memory and then enhances this memory by additional cocaine pairings.
While the neurochemical impact of drugs such as cocaine have long been associated with increased dopaminergic activation [Koob 1992; Koob 1998], in a number of recent reports, we have shown that cocaine also has substantial effects upon serotonin in brain structures important for sensory processes and memory including the hippocampus and neocortex [Müller et al 2002;2004;2007;Pum 2007]. Indeed, serotonergic increases induced by cocaine occur in the primary sensory cortex areas activated by contextual stimuli. This effect on cortical serotonin provides a direct mechanism for cocaine to interact with and enhance contextual stimuli [Müller et al 2007]. Possibly, with repeated cocaine treatments, the widespread serotonergic activation in the hippocampus and cortex in the presence of contextual stimuli is strengthened and reinforced by the concurrent increased activation of dopaminergic reward areas in the brain [Barke et al 2000;Koob 1992]. This combined serotonin/dopamine increase evoked by cocaine paired to contextual stimuli may facilitate the mnestic system to enable cocaine treatments to forge potent and lasting conditioned stimulus effects. In regard to the importance of the concurrent combined serotonin/dopamine overflow induced by cocaine, Yano and Steiner [2007], in a comprehensive review of methylphenidate effects, have pointed to the possible critical importance of combined dopaminergic and serotonergic activation in the development of addiction to dopaminergic drugs. In conclusion, the present findings suggest that cocaine can induce persistent behavioral changes that are not explicable as anti-habituation effects. Furthermore, placing Pavlovian drug conditioning into a framework of a memory trace model in which the contextual stimuli elicit memory traces of prior drug experiences which are reinforced by subsequent drug treatments, provides a new conceptualization of drug conditioning. Such a conceptualization makes simple Pavlovian drug conditioning models once again important for understanding the dynamics underlying the development and persistence of drug use leading to drug addiction.
Acknowledgements
This work was supported by a VA Merit Review Grant and by NIDA grant DA RO1 05366
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Dai H, Gui J. Effects of dizocilpine (MK-801) on motor activity and memory. Psychopharmacology (Berlin) 1998;137:241–246. doi: 10.1007/s002130050616. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN. Cocaine conditioning and sensitization: The habituation factor. Pharmacol Biochem Behav. 2006;84:178–183. doi: 10.1016/j.pbb.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Damianopoulos EN, Shanahan AB. Cocaine effects on behavioral responding to a novel object placed in a familiar environment. Pharmacol Biochem Behav. 2008;88:265–271. doi: 10.1016/j.pbb.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos EN. Cocaine conditioned behavioral effects: a role for habituation process. Pharmacol Biochem Behav. 2003;74:701–712. doi: 10.1016/s0091-3057(02)01072-9. [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? (Series 84).NIDA Res Mono. 1988:25–43. [PubMed] [Google Scholar]
- Ciccociopo R, Martin-Fardon R, Weiss F. Stimuli associated with a single cocaine exposure elicit long -lasting cocaine -seeking. Nature Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- Dai H, Gebhardt K, Carey RJ. Time course effects of MK-8012: the relationship between brain neurochemistry and behavior. Brain Res Bull. 1995;36:175–180. doi: 10.1016/0361-9230(94)00188-7. [DOI] [PubMed] [Google Scholar]
- Damianopoulos EN, Carey RJ. A new method to assess Pavlovian conditioning of psychostimulant drug effects. J Neurosci Methods. 1994;53:7–17. doi: 10.1016/0165-0270(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Ehrman R, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berlin) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiat. 2002;51:131–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gignaschi G, Burbasi S, Zennaro E, Bendotti C, Cervo L. A single high dose of cocaine induces behavioral sensitization and modifies mRNA encoding GluR1 and GAP-43 in rats. Eur J Neurosci. 2004;20:2833–2837. doi: 10.1111/j.1460-9568.2004.03712.x. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. NeuroReport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiat. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovian AC, Hoff AL, Hitzemann R, Basham F, Khalsa SS. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Nestler EJ. Initiation and adaptation: a paradigm for understanding psychotropic drug action. Am J Psychiatry. 1996;153:151–162. doi: 10.1176/ajp.153.2.151. [DOI] [PubMed] [Google Scholar]
- Kim JA, Pollak KA, Hjelmstad GO, Fields HL. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci USA. 2004;101:5664–5669. doi: 10.1073/pnas.0401373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebar JE, Ostrander MM, Norton CS, Akil H, Robinson TE. The ability of amphetamine to evoke arc (Arg.32) mRNA expression in the caudate nucleus accumbens and neocortex is modified by environmental context. Brain Res. 2002;930:30–36. doi: 10.1016/s0006-8993(01)03400-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology, and function of reward pathways. [Review] Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Lee JH, Telang FW, Springer CS, Jr, Volkow ND. Abnormal brain activation to visual stimulation in cocaine abusers. Life Sci. 2003;73:1953–1961. doi: 10.1016/s0024-3205(03)00548-4. [DOI] [PubMed] [Google Scholar]
- Lee JLC, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2006;26:5881–5887. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. Single cocaine exposure increases BDNF and D3 receptor expression: implications for drug conditioning. NeuroReport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Leshner AI. Understanding drug addiction: implication for treatment. Hospital Practice. 1996;31:57–59. doi: 10.1080/21548331.1996.11443361. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Acebo MJ, Robinson TE. The induction of behavioural sensitization is associated with context-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Nasselo AG, Machado C, Bastos JF, Felicio LI. Sudden darkness induces a high activity-low anxiety state in male and female rats. Physiol Behav. 1998;63:451–454. doi: 10.1016/s0031-9384(97)00462-9. [DOI] [PubMed] [Google Scholar]
- Newlin DB. A comparison of drug conditioning and craving for alcohol and cocaine. In: Galanter M, editor. Recent Developments in Alcoholism. Vol 10: Alcohol and Cocaine: Similarities and Differences. New York: Plenum Press; 1992. pp. 147–164. [DOI] [PubMed] [Google Scholar]
- Müller CP, Carey RJ, De Souza Silva MA, Joachim G, Huston JP. Cocaine increases serotonergic activity in the hippocampus and nucleus accumbens in vivo 5-HT1a-receptor antagonism blocks behavioral but potentiates serotonergic activation. Synapse. 2002;45:67–72. doi: 10.1002/syn.10083. [DOI] [PubMed] [Google Scholar]
- Müller CP, De Souza Silva MA, Huston JP. Double dissociation effects of sensory stimulation and cocaine on serotonin activity in the occipital and temporal cortices. Neuropharmacology. 2007;52:854–862. doi: 10.1016/j.neuropharm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Nasello AG, Machado C, Bastos JF, Felicio LF. Sudden darkness induces a high activity-low anxiety state in male and female rats. Physiol Behav. 1988;63:451–454. doi: 10.1016/s0031-9384(97)00462-9. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Developing treatments that address classical conditioning. (Series 135).NIDA Res Mono. 1993:71–91. [Review]. [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Pert A, Post RM, Weiss SRB. Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. (Series 97).NIDA Res Mono. 1990:208–240. [PubMed] [Google Scholar]
- Post RM, Weiss SRB, Fontana D, Pert A. Conditioned sensitization to the psychomotor stimulant cocaine. Ann NY Acad Sci. 1992;654:386–399. doi: 10.1111/j.1749-6632.1992.tb25983.x. [Review]. [DOI] [PubMed] [Google Scholar]
- Pum M, Carey RJ, Huston JP, Muller CP. Dissociating effects of cocaine and d-amphetamine on dopamine and serotonin in the perirhinal, entorhinal, and prefrontal cortex of freely moving rats. Psychopharmacology (Berl) 2007;193:375–390. doi: 10.1007/s00213-007-0791-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis for drug craving: An incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sadile AG, Neugebauer A, Morelli F, Horvath Z, Buzsáki G, Giuditta A. Distributed changes in rat brain DNA synthesis with long -term habituation and potentiation of the perforant path granule cell synapse. Behav Brain Res. 1991;46:83–94. doi: 10.1016/s0166-4328(05)80099-3. [DOI] [PubMed] [Google Scholar]
- Tomaz C, Aguilar MS, Nogueira PJ. Facilitation of memory by peripheral administration of substance P and naloxone using avoidance and habituation learning tasks. Neurosci Biobehav Rev. 1990;14:447–453. doi: 10.1016/s0149-7634(05)80067-3. [Review]. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani AM, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fus mRNA expression in the neocortex , caudate nucleus and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? TIPS. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]


