Abstract
This study compared the rewarding and aversive effects of nicotine in adolescent, adult, and adult rats pre-exposed to nicotine during adolescence. Prior to conditioning, the rats were tested for their initial preference for either of 2 distinct compartments. Adolescent and adult rats then received various nicotine doses in their initially non-preferred side on one day and saline in the other side on alternate days. This 2-day procedure was repeated over 8 consecutive days. Following conditioning, rats were re-tested for their preference. Another cohort of adolescent and adult rats were conditioned with various doses of d-amphetamine. Nicotine produced CPP in an inverted U-shaped manner in both age groups. However, adolescents displayed a larger upward shift in CPP that was significant across a wider dose range relative to adults. There were no developmental differences to CPP produced by d-amphetamine. In a final study, adolescents were prepared with pumps that delivered nicotine for 14 days. These rats were conditioned later as adults using the same procedures used previously. Pre-exposure to nicotine during adolescence diminished the aversive effects produced by the highest nicotine dose in naïve adults. Taken together, these studies provide a basis for enhanced vulnerability to nicotine during adolescence.
Keywords: adolescence, conditioning, conditioned place preference, aversion, rat, pre-exposure, reward, dependence, amphetamine, abuse
1. Introduction
Epidemiological studies suggest that there is a heightened vulnerability to nicotine dependence during the adolescent period of development. Although the prevalence of smoking has generally decreased over recent years, there has been a steady rise in the rate of smoking behavior among adolescents (National Institute on Drug Abuse [NIDA], 2006). It has also been suggested that exposure to nicotine during adolescence may enhance vulnerability to nicotine abuse later in adulthood. For example, it is estimated that 90% of adult smokers begin smoking before the age of 20 (United States Department of Health and Human Services, 1994). Furthermore, individuals who initiate smoking during adolescence consume more tobacco products as adults relative to individuals who initiate smoking during adulthood (NIDA, 2006). Collectively, these reports suggest that adolescence is a period of enhanced vulnerability to nicotine dependence.
Animal studies have examined the rewarding properties of nicotine using the conditioned place preference (CPP) paradigm. The CPP paradigm assesses the motivational properties of a drug using Pavlovian conditioning procedures (see Tzschentke, 1998). This paradigm typically employs a conditioning apparatus that consists of 2 distinct compartments that differ in various sensory modalities such as color, tactile and odor cues. In CPP procedures, a drug is repeatedly administered on one side of the conditioning apparatus, and on alternate days saline is administered on the adjacent side. This 2-day procedure constitutes a conditioning trial. Following several conditioning trials, the animals form an association between the environmental cues and the affective properties of the drug. The animals are then tested for preference by allowing them free access to both compartments simultaneously in the absence of any drug injections. The nature of the affective properties of the drug is evident on the test day when the environmental cues elicit either preference (i.e., CPP) or avoidance (conditioned place aversion; CPA) to the drug-paired side relative to the neutral side. The ability of this procedure to detect preference or aversion is advantageous when comparing the affective properties of various doses of a drug that produces both rewarding and aversive effects.
Studies using place-conditioning procedures vary depending on how the drug-paired compartment is assigned (i.e., biased versus unbiased). In a biased procedure, a pre-test is conducted in order to determine the animal's initial preference. During conditioning, the animals receive drug in their initially non-preferred side. After conditioning, an additional preference test is conducted. On the test day, preference is defined as a significant increase in the amount of time spent in the initially non-preferred side after conditioning minus before conditioning. In an unbiased approach, animals are randomly assigned to receive drug in either compartment. Following conditioning, preference is defined as an increase in the amount of time spent in the drug-paired compartment relative to control animals that spend equal time in both compartments on the test day.
The results from place-conditioning studies using nicotine can vary depending on whether a biased or unbiased procedure is used. For example, some laboratories are unable to detect the rewarding effects of nicotine using unbiased place-conditioning procedures, and this is likely due to nicotine's weak reinforcing effects (see Clark and Fibiger, 1987; Jorenby et al., 1990). Although some studies do report nicotine CPP using unbiased procedures, a recent review suggests that biased procedures are more sensitive to detect small shifts in preference produced by nicotine (see Le Foll and Goldberg, 2005). Furthermore, several laboratories using biased place-conditioning procedures report developmental differences to the rewarding effects of nicotine (Belluzzi et al., 2004; Kota et al., 2007; Torrella et al., 2004; Vastola et al., 2002). Thus, the current study employed a biased method of conditioning to address our experimental questions regarding developmental differences to nicotine.
Previous studies have used CPP procedures to compare the rewarding properties of nicotine in adolescent and adult rats. These reports demonstrate that intermediate doses of nicotine (0.5−0.8 mg/kg) produce CPP that is enhanced in adolescent versus adult rats (Belluzzi et al., 2004; Shram et al., 2006; Vastola et al., 2002). In addition, a single injection of nicotine (0.5 mg/kg) produces CPP in adolescent but not adult rats (Brielmaier et al., 2007). Furthermore, adolescent mice display nicotine-induced CPP at lower doses of nicotine relative to adult mice (Kota et al., 2007). The findings from CPP studies are also consistent with self-administration studies showing that operant responding for nicotine is enhanced in adolescent versus adult rats (Levin et al., 2003; 2007). Collectively, these studies suggest that the rewarding effects of nicotine are enhanced during the adolescent period of development. However, a characterization of both the rewarding and aversive effects of various nicotine doses has not been compared across different stages of development. This is important to address in light of the biphasic behavioral effects of nicotine with low doses typically producing reward and high doses producing aversive effects (Laviolette and Van der Kooy, 2004).
Our laboratory is interested in investigating developmental differences to both the rewarding and aversive effects of nicotine in rats. Recently, we demonstrated that the aversive effects of nicotine withdrawal are lower in adolescent versus adult rats (O'Dell et al., 2006; 2007). The present study expands our previous work by characterizing developmental differences to the rewarding and/or aversive effects of nicotine in adolescent and adult rats. Furthermore, we are interested in examining whether the behavioral effects of nicotine are enhanced in adult rats pre-exposed to nicotine during adolescence. Therefore, this study compared nicotine–induced CPP using various doses of nicotine in adolescent, adult, and adult rats pre-exposed to nicotine during adolescence.
Overall, we hypothesize that adolescence is a period of enhanced vulnerability to the rewarding effects of nicotine. Therefore, we expect to observe enhanced nicotine-induced CPP in adolescent rats and adults pre-exposed to nicotine during adolescence relative to naïve adult rats. The possibility exists that adolescent rats may be more susceptible to rewarding effects in general. To address this issue, we compared CPP produced by d-amphetamine, another psychostimulant compound with strong reinforcing properties. It is not expected that adolescent and adult rats will differ with respect to CPP produced by d-amphetamine, since a previous report demonstrated that the magnitude of amphetamine-induced CPP is similar across these age groups (Mathews and McCormick, 2007). However, inclusion of the d-amphetamine study will provide a positive control group since this drug consistently produces robust CPP. Furthermore, these data will also enable us to determine whether or not our developmental differences are specific to nicotine.
2. Methods
2.1. Subjects
Male Wistar rats were bred and housed in the animal vivarium of the Psychology Department of the University of Texas at El Paso (UTEP). All rats were fully out bred with each experimental group consisting of animals from different litters. Specifically, we assigned one animal per litter to a given experimental group, thereby distributing animals from a given litter across different experimental groups. Rats were weaned on PND 21 and then group housed with 2−3 rats per cage. All rats were handled for 3 days prior to experimentation. Rats had free access to food and water, except during the 30-min conditioning sessions. All rats were housed in a humidity- and temperature-controlled (22°C) vivarium on a 12-hr light/dark cycle (lights on at 8 am and off at 8 pm). Animals were group-housed with same sex littermates. Testing was conducted during the light phase of the animal's light/dark cycle. At the end of each experiment, the animals were euthanized using CO2 inhalation. All procedures were approved by UTEP's Institutional Animal Care and Use Committee and were conducted in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.2. Drugs
The drugs used in these experiments were (-) nicotine hydrogen tartrate and d-amphetamine (Sigma Aldrich, Inc). The nicotine doses refer to the base of the compound and the d-amphetamine doses refer to the salt. Both drugs were dissolved in 0.9% sterile saline and made fresh every 2 days. All compounds were injected subcutaneously (sc) in a volume of 1 ml/kg. Pre-exposure to nicotine during adolescence was done via subcutaneous delivery of nicotine in osmotic mini-pumps. The pumps were purchased from Alzet (model 2ml2) and delivered a constant dose of nicotine (4.7 mg/kg/day; base) continuously for 14 days. The rationale for the nicotine dose is based on previous work showing that the physical signs of withdrawal are evident in adolescent rats that received 14 days of exposure to this dose of nicotine (O'Dell et al., 2006).
2.3. Apparatus
Our conditioning apparatus consisted of 2 rectangular stainless steel chambers (76 × 24 × 30 cm) with one-way mirrors on the front walls to allow for behavioral observations. Each chamber was divided into 2 distinct compartments of equal proportions that were separated by a removable solid stainless steel partition. One compartment had pine bedding beneath a perforated Plexiglas® floor with small holes. The other compartment had green-tinted pine bedding beneath a metal bar floor. Both compartments were equally illuminated during conditioning and testing procedures.
2.4. General conditioning procedures
These studies employed a biased CPP procedure consisting of 3 phases: an initial preference test, 8 conditioning days, and a final preference test. During the initial preference test, the solid partition separating the 2 compartments was removed and replaced by a similar partition with an opening in the center (8 × 8 cm high) to allow rats free access to both sides simultaneously. Rats were allowed to shuttle freely between the 2 compartments for 15 min. Rats were considered to have entered a compartment when both of their front paws were placed on the floor of that compartment. Thirteen animals with an initial preference of greater than 65% for one of the compartments were eliminated from the study. This criterion was employed based on previous work in our laboratory and others demonstrating that it is difficult to detect a shift in time spent in a compartment where an animal had a strong initial bias prior to conditioning. This is particularly important for studies employing place-conditioning procedures using a drug with weak reinforcing properties such as nicotine.
Conditioning began 6 days after the initial preference test in order to minimize latent inhibition that could weaken the association between the drug and the external environmental cues. During conditioning, a solid partition separated the chambers so that the rats could be confined to only 1 side of the conditioning apparatus. Separate groups of rats received various doses of nicotine (0, 0.2, 0.4, 0.6, 0.8 or 1.2 mg/kg, base, sc) or d-amphetamine (0, 0.5, 1.0 or 2.0 mg/kg, salt, sc) and were placed immediately into their initially non-preferred side for 30 min. An additional group of adolescent rats were conditioned with 1.8 mg/kg of nicotine. On alternate days, all rats received saline and were confined to their initially preferred side for 30 min. This 2-day procedure was repeated over 8 consecutive days. The order of drug treatment was counterbalanced within treatment groups such that some rats received drug on the first day of conditioning and the other half of the rats received drug on the second day of conditioning. Each animal was assigned to only 1 treatment condition (e.g., saline or a single dose of nicotine or d-amphetamine). Control groups received saline in both compartments during conditioning. All experiments were conducted in a dark room to allow for behavioral observations via the two-way mirrors on the front of the chambers, and to minimize day-to-day differences in the conditioning room. Continuous white noise was also used to minimize any external disturbances during conditioning.
The day after the last conditioning session, rats were re-tested for their preference for 15 min. Since the order of exposure to the paired vs. unpaired chambers was counterbalanced, some animals were tested 24 hrs after their last nicotine administration, whereas others were tested 48 hrs after the last nicotine injection. An analysis was conducted to determine whether there were any differences in test performance between the animals tested at these 2 intervals following the last nicotine administration. This analysis revealed that the magnitude of CPP produced by nicotine or d-amphetamine was similar in adolescent and adult rats that received drug 1 or 2 days before the final preference test.
When assessing the boundaries of the adolescent period in rodents, most researchers agree that the prototypic age range for adolescence conservatively ranges from postnatal day 28 to 45 (for a review see Spear, 2000). Adolescence reflects a period during which age-specific behavioral discontinuities from younger and older animals are most evident, and most behavioral and physiological systems reach maximal maturation by postnatal day (PND) 60 in rats, and are considered adults beyond this age. Therefore, in the present study adolescent rats received the initial preference test on PND 28, then six days later the conditioning procedures began on PND 34. The final preference test was conducted in adolescents on PND 42. Adult rats received the initial preference test on PND 60, then six days later the conditioning procedures began on PND 66. The final preference test was conducted in adults on PND 74.
2.5. Study 1
This study compared nicotine-induced CPP in naïve adolescent and adult rats (n=7−14 per condition). Separate groups of rats received various doses of nicotine (0, 0.2, 0.4, 0.6, 0.8 or 1.2 mg/kg, base, sc) and were conditioned as previously described. An additional group of adolescent rats (n=8). received 1.8 mg/kg of nicotine. A higher dose of nicotine was included for the adolescent rats to account for possible group differences that may be due to higher metabolic rates of nicotine in young rats. Various doses of nicotine were used in order to establish a CPP dose-response curve that could be compared across age groups.
An additional experiment was conducted to compare d-amphetamine-induced CPP in naïve adolescent and adult rats (n=7−14 per group). Rats received various doses of d-amphetamine (0, 0.5, 1.0 or 2.0 mg/kg, salt, sc) and were conditioned as previously described. The purpose of this experiment was to examine potential developmental differences to another psychostimulant compound with strong reinforcing properties.
2.6. Study 2
This study compared nicotine-induced CPP in adult rats pre-exposed to nicotine during adolescence (n=5−14 per group) to their naïve counterparts from Study 1. A group of adolescent rats was anesthetized with an isoflurane/oxygen mixture (1−2% isoflurane) and surgically prepared with Alzet osmotic mini-pumps [model 2ml2; Durect Corporation] that delivered nicotine continuously (4.7 mg/kg/day, base) for 14 days. The nicotine concentration in the pump was adjusted based on the rats weight on the surgery day. The nicotine pump dose was based on observations from our laboratory and others (Trauth et al., 1999, 2000) demonstrating that this dose regimen produces nicotine dependence and neurochemical changes in adolescent rats. The adolescents were prepared with the pumps on PND 28 and they were removed on PND 42. Conditioning procedures began later when the rats were adults on PND 60. Separate groups of adult rats pre-exposed to nicotine during adolescence were conditioned using various doses of nicotine (0, 0.2, 0.4, 0.6, 0.8 or 1.2 mg/kg, base, sc), as previously described.
2.7. Statistical Analyses
Data were analyzed using difference scores, which reflect the amount of time spent in the initially non-preferred compartment after conditioning minus before conditioning such that positive values reflect a shift in preference for the drug-paired compartment. CPP was operationally defined as a significant increase in the difference score obtained from drug-treated rats relative to controls that received saline during conditioning.
Our statistical analyses include overall ANOVAs followed by post-hoc tests where appropriate. Specifically, the ability of various doses to produce CPP or CPA was compared across age (adolescent versus adult) or treatment groups (naïve adults versus pre-exposed adults). In our experience with place conditioning procedures, significant overall interactions are not usually observed, and group differences are most evident at individual doses rather than overall shifts in dose-response curves (O'Dell et al. 1996; 2007). This is because of the “all-or-none” nature of the results with certain doses producing CPP or CPA in some but not all experimental groups. Further, it is difficult to compare the magnitude of preference across groups because ceiling effects may conceal group differences. Although the present studies did not reveal any significant interaction effects, robust main effects of age and treatment were observed. These significant effects were followed by ANOVA analyses that compared dose effects in a particular age or treatment group.
The goal of our study was to compare the rewarding and/or aversive effects of nicotine in rats of different ages (adolescent versus adult) and treatment conditions (naïve adults versus pre-exposed adults). Thus, individual planned comparisons were also conducted between age and treatment groups at individual doses using the Fisher's LSD test (p ≤ 0.05). The type of analysis that was performed is denoted by different symbols on the figures. Asterisks (*) denote a significant difference relative to their respective saline controls and daggers (†) reflect a significant difference between age or treatment groups at each individual drug dose.
3. Results
Study 1 compared the ability of various doses of nicotine to produce CPP in naïve adolescent and adult rats (see Figure 1). Overall, the results revealed that nicotine produced preference or aversion in an inverted U-shaped dose-response manner. The statistical analyses revealed that both age groups displayed preference at lower doses and aversive effects at higher doses of nicotine [F(6,112)=7.2, p=0.001]. Regarding developmental differences, the results revealed enhanced positive shifts in preference in adolescent relative to adult rats. First, an examination of the area under the curve from 0 to 0.8 mg/kg of nicotine revealed that the mean total area under the curve is approximately 2-fold larger in adolescent (513.74 sec) relative to adult (285.23 sec) rats. Second, adolescent rats displayed CPP over a wider range of nicotine doses than adults. The statistical analyses revealed an overall main effect of age [F(1,112)=8.35, p=0.005], with adolescents receiving 0.2, 0.4 and 0.6 mg/kg of nicotine displaying enhanced positive shifts in preference relative to controls (p≤0.05). In contrast, CPP was only observed in adult rats that received 0.2 mg/kg of nicotine during conditioning (p≤0.05). Third, planned comparisons examining developmental differences revealed a significant difference between adolescent and adult rats conditioned with 0.4, 0.6, and 1.2 mg/kg (p≤ 0.05).
Fig 1.
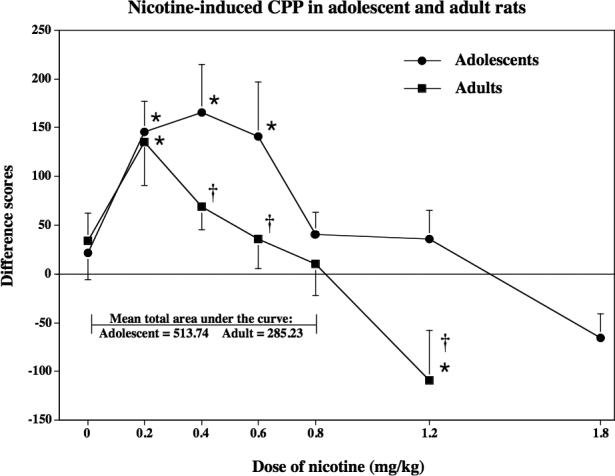
This graph reflects nicotine-induced CPP in naïve adolescent versus adult rats (n=7−14) conditioned with various nicotine doses (0, 0.2, 0.4, 0.6, 0.8 or 1.2 mg/kg, base, sc). An additional group of adolescent rats (n=8) received 1.8 mg/kg of nicotine during conditioning. This dose was included to address higher metabolic rates of nicotine in adolescent versus adult rats. These data are presented as difference scores (±SEM), which reflect time spent in the initially non-preferred side after conditioning minus before conditioning such that values above “0” reflect a positive shift in preference (i.e., CPP). Mean total area under the curve reflects the sum of the averages for each age group from the 0 to 0.8 mg/kg dose of nicotine. The asterisks (*) denote a significant difference from their respective saline control group (p ≤ 0.05). Daggers (†) denote a significant difference between age groups at each dose of nicotine (p ≤ 0.05).
The place conditioning results also revealed that high doses of nicotine produced aversion in adult rats that were conditioned with 1.2 mg/kg of nicotine (p≤0.05). However, this aversive effect was not observed in adolescent rats, even when the dose of nicotine was increased to a high dose (1.8 mg/kg) that appeared to produce toxic effects in these young rats.
A subsequent study compared the ability of d-amphetamine to produce CPP in naïve adolescent and adult rats (see Figure 2). D-amphetamine produced preference in an inverted U-shaped dose-response manner. Both age groups displayed preference at a lower dose range of d-amphetamine [F(3,59)=8.4, p=0.001]. This effect was significant across both adolescent and adult rats receiving 0.5 and 1.0 mg/kg of d-amphetamine relative to controls (p≤0.05). However, there were no developmental differences in the magnitude of CPP produced by any dose of d-amphetamine in both age groups [F(1,59)=0.12, p=0.72].
Fig 2.
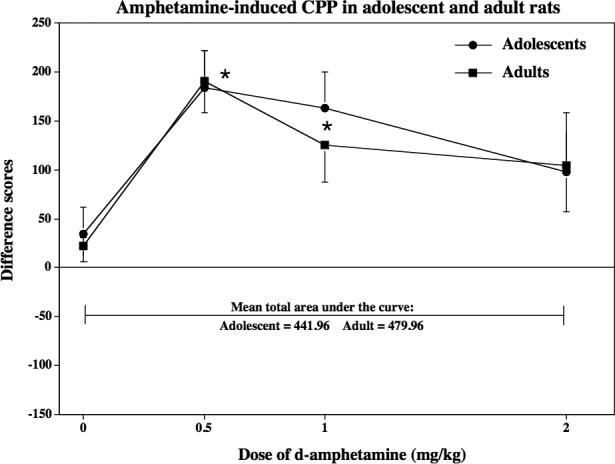
This graph reflects amphetamine-induced CPP in naïve adolescent and adult rats (n=7−14) that were conditioned with various d-amphetamine doses (0, 0.5, 1.0 or 2.0 mg/kg, sc). These data are presented as difference scores (±SEM), which reflect time spent in the initially non-preferred side after conditioning minus before conditioning such that values above “0” reflect a positive shift in preference (i.e., CPP). Mean total area under the curve reflects the sum of the averages for each age group from the 0 to 2.0 mg/kg dose of d-amphetamine. The asterisks (*) denote a significant difference from their respective saline control group (p ≤ 0.05).
Study 2 compared the ability of nicotine to produce CPP in adult rats pre-exposed to nicotine during adolescence relative to the CPP effects observed in naïve adults from Study 1 (see Figure 3). The analyses from Study 1 revealed that naive adults conditioned with 0.2 mg/kg of nicotine displayed CPP, whereas naïve adults conditioned with 1.2 mg/kg displayed CPA. In contrast, the curve in the adult rats that were pre-exposed to nicotine during adolescence was relatively flat. The aversive effects produced by the highest dose of nicotine in naïve adults were absent in adults that were pre-exposed to nicotine during adolescence (p ≤ 0.05). This suggests that the aversive effects of high nicotine doses are reduced in animals that were exposed to nicotine during adolescence.
Fig 3.
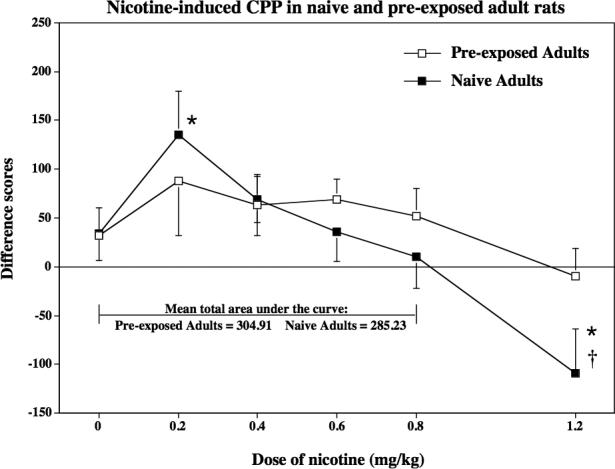
This graph reflects nicotine-induced CPP in naïve adult rats and adults that were pre-exposed to nicotine during adolescence (n=5−14). Rats were conditioned using various nicotine doses (0, 0.2, 0.4, 0.6, 0.8 or 1.2 mg/kg, base, sc). These data are presented as difference scores (±SEM), which reflect time spent in the initially non-preferred side after conditioning minus before conditioning such that values above “0” reflect a positive shift in preference (i.e., CPP). Mean total area under the curve reflects the sum of the averages for each age group from the 0 to 0.8 mg/kg dose of nicotine. The asterisks (*) denote a significant difference from their respective saline control group (p ≤ 0.05). The dagger (†) denotes a significant difference between treatment groups (p ≤ 0.05).
Given the lack of CPP in adult rats that were pre-exposed to nicotine via pumps, we included an additional group of adult rats that were pre-exposed to lower nicotine doses during adolescence (data not shown). Specifically, two additional groups of adolescent rats received either 1 (n=6) or 4 (n=6) injections 0.4 mg/kg, sc for 14 consecutive days. These rats were conditioned later as adults using the 0.8 mg/kg, sc. These procedures were chosen based on Adriani et al., (2006) who demonstrated that adult rats that were pre-exposed to nicotine during adolescence (10 daily injections of 0.4 mg/kg) display enhanced nicotine-induced CPP later in adulthood. However, our results revealed that this treatment regiment did not facilitate CPP in adult rats that received nicotine once a day (11.8±34.8 sec) or 4 times a day (10.5±19.7 sec) relative to naïve adult rats (10.5±32.7 sec). Thus, treatment regimens involving lower doses of nicotine during adolescence did not facilitate CPP later in adulthood relative to naive adult rats.
4. Discussion
The present study supports our hypothesis that adolescence is a period of enhanced vulnerability to the rewarding effects of nicotine. This is based on our finding that adolescent rats displayed a larger shift in preference that was significant across a wider range of nicotine doses relative to their adult counterparts. These results appear to be specific to nicotine, as adolescent and adult rats displayed a similar magnitude of CPP produced by various doses of d-amphetamine.
Our dose-response curve for nicotine-induced CPP was inverted and U-shaped across both age groups of rats. Intermediate doses of nicotine produced CPP; however, this effect was not observed at low or high doses. The shape of the curve is presumably due to a lack of nicotine reward at low doses and the emergence of aversive effects produced by high nicotine doses. Our dose-response curve is consistent with previous studies using various doses of nicotine to produce CPP in adult rats (Belluzzi et al., 2004; Fudala et al., 1984; Fudala and Iwamoto, 1986; Janhunen et al., 2005; Kashkin and De Witte, 2005; Le Foll and Goldberg, 2005; Spina et al., 2006). In general, previous studies using a dose of nicotine within a low range (0.2−0.6 mg/kg) typically report CPP, whereas studies using a dose within a high range (0.8−1.2 mg/kg) report CPA, and this is consistent with the behavioral profile observed in the present study.
A major finding of this report is that the rewarding effects of nicotine are enhanced during adolescence. Specifically, CPP was observed across a wider range of nicotine doses in adolescent versus adult rats. Also, adolescent rats display a greater shift in preference relative to adults as evidenced by a 2-fold larger area under the curve in adolescent relative to adult rats (see Figures 1). Lastly, we did not observe significant aversive effects of nicotine in adolescent rats, even when the dose of nicotine was increased to 1.8 mg/kg, which appeared to produce overt toxic effects.
Our interpretation of enhanced rewarding effects of nicotine during adolescence is supported by reports from other laboratories. Most relevant to our study is a report by Vastola et al. (2002) demonstrating that CPP is enhanced in adolescent versus adult rats conditioned with a similar dose (0.6 mg/kg), age range (adolescent=PND 28−40 and adult=PND 58−70) and conditioning regimen (4 trials) as the current study. Two other studies have also demonstrated that nicotine-induced CPP is enhanced in adolescent versus adult rats receiving a single conditioning trial (Belluzzi et al., 2004; Brielmaier et al., 2007). The latter studies observed enhanced rewarding effects of nicotine in adolescent rats conditioned with a single trial that was shorter (less than 15 min) than the length of the trials employed in the present study (30 min). In addition, it has been demonstrated that adolescent mice display enhanced nicotine-induced CPP relative to adult mice (Kota et al., 2007). However, Shram et al. (2006) demonstrated that nicotine-induced CPP is enhanced in adolescent rats conditioned with a high nicotine dose (0.8 mg/kg) that did not produce CPP in the present study. This difference may be due to the fact that Shram et al. (2006) used younger adolescent rats on the test day (PND 35) that may be more sensitive to the rewarding effects of nicotine relative to the adolescents that were tested in the present study (PND 44).
Our findings are also consistent with intravenous self-administration (IVSA) studies demonstrating that adolescent rats generally display enhanced rewarding effects of nicotine relative to adult animals. For example, both male and female adolescent rats acquire nicotine IVSA more readily and display higher levels of nicotine intake relative to their adult counterparts under both limited (Levin et al., 2003; 2007) and extended (Chen et al., 2007) access conditions. However, we also acknowledge that Shram et al., (2008a, 2008b) recently reported that adolescent rats displayed reduced nicotine IVSA under both progressive and fixed ratio schedules of reinforcement. Taken together, the CPP and IVSA studies suggest that the rewarding effects of nicotine are enhanced during the adolescent period of development.
Our finding regarding enhanced behavioral effects of nicotine during adolescence is also consistent with previous studies comparing the stimulant effects of nicotine in adolescent and adult rats. For example, Faraday et al. (2003) demonstrated that adolescent rats display enhanced locomotor activity following acute and repeated administration of nicotine relative to adult rats. Furthermore, Cruz et al. (2005) demonstrated that adolescent rats display enhanced locomotor activity following acute nicotine exposure whereas adult rats require repeated nicotine to produce stimulant behavior. Lastly, Schochet et al. (2004) demonstrated that adolescent rats display sensitized stimulant behavior at lower doses of nicotine relative to adult rats. Taken together, these reports provide converging lines of evidence that the stimulant and rewarding effects of nicotine are enhanced during the adolescent period of development.
The present study also provided evidence that our observed developmental differences are specific to nicotine. This is based on our finding that adolescent and adult rats display a similar magnitude of CPP produced by d-amphetamine. A lack of developmental differences to the behavioral effects of d-amphetamine is consistent with reports demonstrating that adolescent and adult rats display the same magnitude of CPP (Mathews and McCormick, 2007) and startle responses (Brunell and Spear, 2006) produced by amphetamine.
A unique contribution of this report to the literature is a characterization of the affective properties of various doses of nicotine in adult rats that were pre-exposed to nicotine during adolescence. Our findings demonstrated that adult rats pre-exposed to nicotine during adolescence do not display preference produced by low doses or aversion produced by the highest dose of nicotine. In fact, the dose-response curve for these rats was relatively flat. It was originally hypothesized that adolescent exposure to nicotine would facilitate the rewarding effects of nicotine later in adulthood. Our hypothesis was based on previous studies demonstrating that exposure to nicotine during adolescence facilitates the rewarding effects of amphetamine (Collins et al., 2004) and cocaine (Kelly and Rowan, 2004; McQuown et al., 2007). Furthermore, Adriani et al. (2006) demonstrated that nicotine-induced CPP is enhanced in adult rats that were pre-exposed to nicotine during adolescence. However, a major difference between our study and Adriani et al. (2006) is that they exposed their adolescent rats to a lower dose of nicotine (0.4 mg/kg) for fewer days (10 days) relative to our study that employed continuous infusion of a higher dose of nicotine (4.7 mg/kg/day) for more days (14 days).
It may be argued that the flat dose-response curve in adult rats that were exposed to nicotine during adolescence may be due to tolerance produced by pre-exposure to high nicotine doses. To address this issue, we conducted an additional study whereby separate groups of adolescent rats received 1 or 4 injections of nicotine (0.4 mg/kg, sc) for 14 days (n=6 per group). These rats were conditioned later as adults using a 0.8 mg/kg, sc dose of nicotine. The rationale for our treatment regimen is based on the findings of Adriani et al. 2006. The results revealed that this treatment regimen did not alter nicotine-induced CPP in adult rats that received adolescent nicotine exposure 1 (11.8±34.8) or 4 times a day (10.5±19.7) relative to naïve adult rats (10.5±32.7). Thus, the inability of adolescent nicotine exposure to facilitate CPP later in adulthood does not appear to be due to tolerance produced by high nicotine doses. Furthermore, it should be noted that the interval between chronic continuous exposure to nicotine and the administration of nicotine for CPP testing was 24−25 days. Therefore, it is unlikely that tolerance can explain this pattern of results.
In the adolescent pre-exposure study, the largest behavioral difference that we observed was at the highest dose of nicotine that produced aversive effects in naïve adults but not in adults that were pre-exposed to nicotine during adolescence. This finding suggests that adolescent nicotine exposure may attenuate the aversive effects of nicotine later in adulthood. Clinical reports suggest that adolescent nicotine exposure leads to higher rates of smoking behavior in adulthood (Chen and Millar, 1998). Although it is unclear whether a reduction in the aversive effects of nicotine would facilitate smoking behavior in adults, our findings suggest this may be an important issue to address in future studies.
In conclusion, our results suggest that adolescence is a period of development during which there is a heightened vulnerability to the rewarding effects of nicotine. Previous work in our laboratory has demonstrated that adolescent rats display less nicotine withdrawal relative to their adult counterparts (O'Dell et al., 2006; 2007). Taken together, our work suggests that adolescent rats are more sensitive to the positive effects of nicotine reward and less sensitive to the negative aspects of nicotine withdrawal. Therefore, we suggest that during adolescence nicotine produces strong reinforcing effects that play a primary role in driving smoking behavior in young persons.
Acknowledgements
This research was supported by start up funds from The University of Texas at El Paso, and a grant from the National Institute of Health (R01-DA021274), the National Science Foundation (0245071), and the UTEP BBRC-RCMI (5G12RR008124). The authors would like to acknowledge Drs. Wendy Francis, Christine Sobin, Louis Irwin, and Donald Moss for their helpful comments on this manuscript. The authors also thank Arturo Orona and Sofia Beas for their technical assistance.
References
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-reward properties in adult rats. Psychopharmacol. 2006;184:382–90. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacol. 2004;174:389–95. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol and Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effects of acute ethanol or amphetamine administration on the acoustic startle response and pre-pulse inhibition in adolescent and adult rats. Psychopharmacol. 2006;186:579–86. doi: 10.1007/s00213-006-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacol. 2007;32:700–09. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar MJ. Age of smoking initiation: Implications for quitting. Health Reports. 1998;9:39–46. [PubMed] [Google Scholar]
- Clarke PD, Fibiger HC. Apparent absence of nicotine-induced conditioned place preference in rats. Psychopharmacol. 1987;92:84–8. doi: 10.1007/BF00215484. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Dev Brain Res. 2004;153:175–87. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Cruz FC, DeLucia R, Planeta CS. Differential behavioral and neuroendocrine effects of repeated nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2005;80:411–17. doi: 10.1016/j.pbb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav. 2003;74:917–31. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto E. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1984;22:237–41. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Iwamoto ET. Further studies on nicotine-induced conditioned place preference in the rat. Pharmacol Biochem Behav. 1986;25:1041–49. doi: 10.1016/0091-3057(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Janhunen S, Linnervuo A, Svensk M, Ahtee L. Effects of nicotine and epibatidine on locomotor activity and conditioned place preference in rats. Pharmacol Biochem Behav. 2005;82:758–65. doi: 10.1016/j.pbb.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Joreny DE, Steinpreis RE, Sherman JE, Baker TB. Aversion instead of preference learning indicated by nicotine place conditioning in rats. Psychopharmacol. 1990;101:533–38. doi: 10.1007/BF02244233. [DOI] [PubMed] [Google Scholar]
- Kashkin VA, De Witte P. Nicotine increases microdialysate brain amino acid concentrations and induces conditioned place preference. Euro Neuropsychopharmacol. 2005;15:625–32. doi: 10.1016/j.euroneuro.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kelly BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. Int J Dev Neurosci. 2004;22:339–48. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preference over a large range of doses in rats. Psychopharmacol. 2005;178:481–92. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani A, Montoya R, Rose J, Swartrzwelder H. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacol. 2003;169:141–9. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: Duration of effects and differential nicotinic receptors correlates. Neurotox and Teratol. 2007:29–458−65. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews IZ, McCormick CM. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Beh Pharmacol. 2007;18:641–50. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotox and Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse Monitoring the future, National results on adolescent drug use, overview of key findings, 2006. NIH Publication No. 01−4923.
- O'Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacol. 2006;186:612–19. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to nicotine exposure in adult male rats. Neurotox and Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochet TL, Kelly AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacol. 2004;175:265–73. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacol. 2006;186:201–08. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacol. 2008a;33:739–48. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Li Z, Lê AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacol Ber. 2008b;197:45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G. Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacol. 2006;184:447–55. doi: 10.1007/s00213-005-0211-4. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Res. 1999;851:9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–80. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Torrella TA, Badanich KA, Philpot RM, Kirstein CL, Wecker L. Developmental differences in nicotine place conditioning. Ann N Y Acad Sci. 2004;1021:399–403. doi: 10.1196/annals.1308.052. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effect, recent progress and new issues. Progress in Neurobio. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Atlanta, Georgia: U.S. Department of Health and Human Services, Public Health Services, Centers for Disease Control and Prevention and Health promotion, Office on Smoking and Health. U. S. Government Printing Office; Washington, D.C.: 1994. Preventing tobacco use among young people: a report of the Surgeon General. [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Beh. 2002;77:107–14. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]


