Abstract
This study investigated the TNF-α-induced ICAM-1 and VCAM-1 expression on mouse lingual lymphatic vessels. All podoplanin-positive lymphatic vessels expressed PECAM-1. In the lamina propria mucosae of TNF-α-treated tongue, almost all initial lymphatics expressed ICAM-1. There were initial lymphatics with the VCAM-1 expression and also the vessels without the expression. In the tunica muscularis of TNF-α-treated tongue, collecting lymphatic vessels expressed ICAM-1, but rarely expressed VCAM-1 whereas blood vessels simultaneously expressed ICAM-1 and VCAM-1. The ICAM-1-positive rate increased with TNF-α to 75% from 10% on initial lymphatics, and to 40% from 0% on collecting lymphatic vessels while it increased to 90% from 45% on blood vessels. The VCAM-1-positive rate increased with TNF-α to 30% from 0% on initial lymphatics, and to 5% from 0% on collecting lymphatic vessels while it increased to 75% from 5% on blood vessels. These findings suggest that the lingual lymphatic endothelium has the ability to express ICAM-1, and VCAM-1 to a lesser extent than the ICAM-1 induction with TNF-α, and that the ICAM-1 and VCAM-1 induction predominantly occurs in the initial lymphatics compared with collecting lymphatic vessels.
Keywords: lymphatic endothelium, podoplanin, PECAM-1, ICAM-1, VCAM-1
I. Introduction
The lymphatic vascular system maintains tissue fluid homeostasis and mediates the afferent immune response. Although the mechanisms that control the blood vascular and immunocyte interaction system have been well studied, those of the lymphatic vessels are poorly understood. In blood vessels, the endothelium and immunocyte interaction through leukocyte adhesion molecules contributes to leukocyte extravasation at the inflammatory sites [20]. The platelet-endothelial cell adhesion molecule-1 (PECAM-1) which binds to PECAM-1 itself, heparan sulfate proteoglycans, and αvβ3 integrin, functions to promote leukocyte transendothelial cell migrationv[2, 4–6, 14]. The intercellular adhesion molecule-1 (ICAM1) which binds to lymphocyte function-associated antigen-1 (LFA-1), and vascular cell adhesion molecule-1 (VCAM-1) which binds to the very late antigen 4 (α4β1), assist the migration and antigen-specific response of lymphocytes [3, 12, 15]. Expression of these molecules on blood endothelium increases with the inflammatory cytokines [5]. It was previously reported that the lymphatic endothelium of the human small intestine usually expresses PECAM-1, but does not express endothelial cell-selectin or ICAM-1 [16]. However in the inflamed human small intestine, the lymphatic endothelium expresses ICAM-1 and VCAM-1 [17]. It is possible that lymphatic endothelium expresses leukocyte adhesion molecules under immunologically activated conditions.
Recently, podoplanin has been reported as a new specific marker for a lymphatic endothelium. Normal lymphatic vasculature formation requires podoplanin, and its deficiency causes lymphedema [1, 9, 19]. This study examined the expression of PECAM-1, ICAM-1, and VCAM-1 on the lymphatic endothelium of the mouse tongue in the presence of TNF-α.
II. Materials and Methods
Immunohistochemistry
The tongue of eight-week-old closed colony ICR mice (male, n=5; Charles River Japan Inc., Yokohama, Japan) was obtained 24 hr after injections of a 0.01-ml volume of saline containing 10 ng/ml of mouse TNF-α (DakoCytomation Co. Ltd., Copenhagen, Denmark) into the region of the apex linguae, and of saline without TNF-α (sham) into the region of the radix linguae. The collection of the tissue was conducted after euthanasia by intraperitoneal injection with sodium pentobarbital (10 ml/kg, Nembutal, Abbott Laboratories, North Chicago, IL). Mice were perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The protocol for animal use was reviewed and approved by the animal experiment committee of Fukuoka Dental College, Fukuoka, Japan. Immediately after trimming off the injected regions, frozen 10 µm serial sections were cut in a cryostat and fixed in 30% acetone in 10 mM phosphate-buffered saline (PBS, pH 7.2) for 10 min at 4°C. To identify the lymphatic vessels, this study used a hamster monoclonal antibody specific to mouse podoplanin (AngioBio Co., Del Mar, CA), and Alexa Fluor 488 (A488)-conjugated goat anti-hamster IgG (Invitrogen Com., Eugene, OR). The expression of leukocyte adhesion molecules was examined by rat anti-mouse PECAM-1, ICAM-1, and VCAM-1 (R&D Systems Inc., Minneapolis, MN), and Alexa Fluor 568 (A568)-conjugated goat anti-rat IgG (Molecular Probes). The sections were treated for 10 min at 25°C with 1% normal goat serum diluted with PBS, exposed by a first antibody cocktail of 1 µg/ml of anti-podoplanin, and anti-PECAM-1, ICAM-1, or VCAM-1, and treated with 100 ng/ml of A488- or A568-conjugated second antibodies (Invitrogen) at 25°C for 1 hr. The sections were examined by laser-scanning microscopy (Axiovert 135M, Carl Zeiss, Jena, Germany) with a ×40 oil planapochromatic lens (numerical aperture ×1.4). Confocal fluorescence images of optical slices on the Z-axis were acquired at 0.5-µm intervals within a depth of 4 to 9 µm below the cut surface. The digital images were processed by a Zeiss LSM Image Browser version 3.0 (Carl Zeiss).
Lymphatic and blood vessels usually express PECAM-1 and podoplanin is a well-known lymphatic marker [10, 18]. This study investigated the ratio of lymphatic vessels expressing ICAM-1, and VCAM-1 in mouse tongue with TNF-α treatment. Adhesion molecule expression units were expressed as percentages: number of vessels expressing ICAM-1 or VCAM-1/total number of blood vessels expressing PECAM-1 or total number of lymphatic vessels expressing PECAM-1 and podoplanin. Examination was performed about ten visual fields in ×200. The statistical significance of differences (p<0.001) was determined by the unpaired two-tailed Student’s t test with STATVIEW 4.51 software (Abacus Concepts, Calabasas, CA).
III. Results
In the mouse apex linguae all lymphatic vessels were immunostained with both anti-podoplanin and anti-PECAM-1, and all blood vessels were immunostained with only anti-PECAM-1. The glandula lingualis was immunostained with anti-podoplanin but not with anti-PECAM-1 (Fig. 1). The inflammatory cell infiltration and vasodilation were observed in the lamina propria mucosae and in the lingual tunica muscularis, but not observed in the sham-injected tissue (Fig. 2).
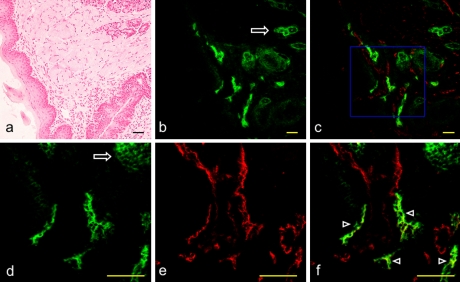
Fig. 1.
Expression of PECAM-1 and podoplanin on the mouse lingual lymphatic vessels. (a) H-E stained section of the mouse apex linguae. (b) Immunostaining with anti-podoplanin of the section adjacent to a. Lymphatic vessels expressing podoplanin in the tunica muscularis are visualized in green. The glandula lingualis is also immunostained with anti-podoplanin (arrow). (c) Merged image of immunostaining with anti-podoplanin and anti-PECAM-1. Lymphatic vessels expressing podoplanin and PECAM-1 are visualized in yellow green while blood vessels expressing only PECAM-1 are visualized in red. (d) High-magnification image of the region highlighted in a box in c. Lymphatic vessels expressing podoplanin are visualized in green. The glandula lingualis is also immunostained with anti-podoplanin (arrow). (e) High-magnification image of the region highlighted in a box in c. Lymphatic and blood vessels expressing PECAM-1 are visualized in red. The expression of PECAM-1 is not observed in the glandula lingualis. (f) Merged image of d and e. Lymphatic vessels expressing both podoplanin and PECAM-1 are visualized in yellow green (arrowheads) while blood vessels expressing only PECAM-1 are visualized in red. Bar=100 µm.
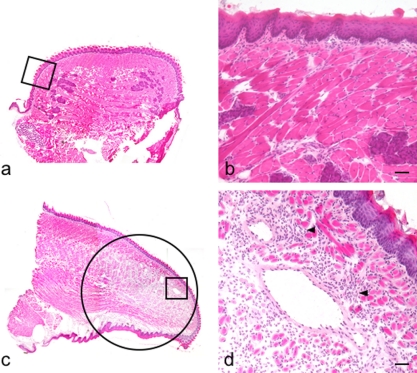
Fig. 2.
Induction of inflammatory reaction with TNF-α in the mouse tongue. H-E stained section (original magnification ×40). (a) Coronal section of the sham-injected radix linguae. (b) Region highlighted in a box in a. There is no inflammatory cell infiltration. (c) Sagittal section of the TNF-α-injected apex linguae. Inflammatory cell infiltration and vasodilation are observed in a circle. (d) Region highlighted in a box in c. Inflammatory cell infiltration and vasodilation are observed in the lamina propria mucosae and in the lingual tunica muscularis (arrowheads). Bar=100 µm.
In the lamina propria mucosae there were initial lymphatics immunostained with anti-PECAM-1, and with anti-ICAM-1 (Fig. 3). There were initial lymphatics with the immunoreaction with anti-VCAM-1 and also the vessels without the reaction (Figs. 3, 4). In the lingual tunica muscularis collecting lymphatic vessels were immunostained with anti-PECAM-1, and with anti-ICAM-1, but rarely with anti-VCAM-1. It is also observed that there are blood vessels simultaneously immunostained with anti-PECAM-1, anti-ICAM-1, and anti-VCAM-1 (Fig. 5).
Fig. 3.
Expression of PECAM-1, ICAM-1, and VCAM-1 on the mouse initial lymphatics of the radix linguae. (a) Immunostaining with anti-podoplanin and anti-PECAM-1 of the section adjacent to Figure 2b. (b) Immunostaining with anti-podoplanin and anti-ICAM-1 of the section adjacent to a. (c) Immunostaining with anti-podoplanin and anti-VCAM-1 of the section adjacent to b. (d, e, f) High-magnification images of regions highlighted in boxes in a, b, and c. Initial lymphatics expressing podoplanin, and PECAM-1 (d, arrowheads) or ICAM-1 (e, arrowheads) in the lamina propria mucosae are visualized in yellow green. Blood vessels are visualized in red with the immunoreaction to anti-PECAM-1 (d, arrows) or anti-ICAM-1 (e, arrows), but not to anti-podoplanin. There are no initial lymphatics immunostained with anti-VCAM-1. Bar=100 µm.
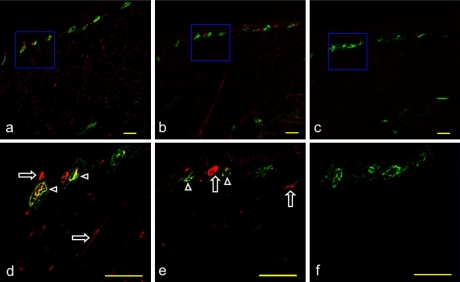
Fig. 4.
Expression of VCAM-1 on the mouse initial lymphatics of the radix linguae. (a) H-E stained section. (b) Immunostaining with anti-podoplanin of the section adjacent to a. Initial lymphatics expressing podoplanin in the lamina propria mucosae are visualized in green. (c) Immunostaining with anti-VCAM-1 of the section b. Vessels expressing VCAM-1 in the lamina propria mucosae are visualized in red. (d) Merged image of immunostaining with anti-podoplanin and anti-VCAM-1. There are initial lymphatics with the immunoreaction to anti-VCAM-1 (arrows) and also the vessels without the reaction (arrowheads). Bar=100 µm.
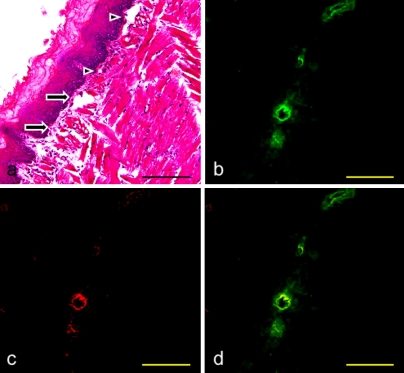
Fig. 5.
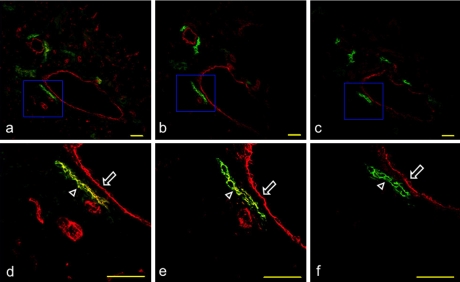
Expression of PECAM-1, ICAM-1, and VCAM-1 on the mouse collecting lymphatic vessels of the lingual tunica muscularis. (a) Immunostaining with anti-podoplanin and anti-PECAM-1 of the section adjacent to Figure 2d. (b) Immunostaining with anti-podoplanin and anti-ICAM-1 of the section adjacent to a. (c) Immunostaining with anti-podoplanin and anti-VCAM-1 of the section adjacent to b. (d, e, f) High-magnification images of regions highlighted in boxes in a, b, and c. The collecting lymphatic vessels expressing podoplanin, PECAM-1, and ICAM-1, but not VCAM-1 are visualized in yellow green (arrowhead). It is also observed that the blood vessel simultaneously expressing PECAM-1, ICAM-1, and VCAM-1 is visualized in red (arrow). Bar=100 µm.
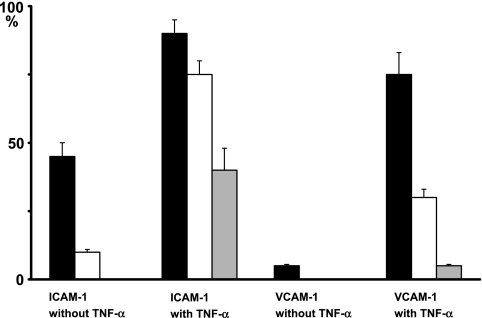
In the tissue without TNF-α, 45% of the blood vessels and 10% of the initial lymphatics expressed ICAM-1, and 5% of the blood vessels but none of the lymphatic vessels expressed VCAM-1. With TNF-α 90% of the blood vessels, 75% of the initial lymphatics, and 40% of the collecting lymphatic vessels expressed ICAM-1 while 75% of the blood vessels, 30% of the initial lymphatics, and 5% of the collecting lymphatic vessels expressed VCAM-1 (Fig. 6).
Fig. 6.
Ratio of lymphatic vessels expressing ICAM-1 and VCAM-1 in mouse tongue with TNF-α. Without TNF-α 45% of blood vessels (closed bar) and 10% of initial lymphatics (open bar) expressed ICAM-1 while no collecting lymphatic vessels (grey bar) expressed ICAM-1. With TNF-α 90% of blood vessels, 75% of initial lymphatics, and 40% of collecting lymphatic vessels expressed ICAM-1. Without TNF-α 5% of blood vessels expressed VCAM-1 while no lymphatic vessels expressed VCAM-1. With TNF-α 75% of blood vessels, 30% of initial lymphatics, and 5% of collecting lymphatic vessels expressed VCAM-1. In all cases the ICAM-1 and VCAM-1 expression was significantly higher in blood vessels than lymphatic vessels. Data are expressed as mean±SEM.
IV. Discussion
Podoplanin, a 38-kDa membrane glycoprotein, is one of the most highly expressed lymphatic-specific genes. Podoplanin is expressed on the lymphatic endothelium with the predominant localization to the luminal plasma membrane of lymphatic vessels, but not on quiescent or proliferating blood endothelium. Podoplanin −/− mice have defects in lymphatic vessel, but not blood vessel, pattern formation [7, 13, 19], and was further characterized as podoplanin because of its low level of expression in kidney podocytes [1]. In the mouse tongue tissue, there was cross reaction of anti-podoplanin to the glandula lingualis (Fig. 1), and some somatic tissue cells including the epithelia of glands may express the podoplanin gene. However in this study, the antibody used to mouse podoplanin showed no reaction to blood vessels expressing PECAM-1, ICAM-1, and VCAM-1, with open-circled and regularly-outlined thick walls which were easily distinguished from the podoplanin and PECAM-1 positive lymphatic vessels (Figs. 1–4). These results corresponded to studies of podoplanin expression in the lymphatic vascular system [7–9, 11, 13]. Since all lymphatic vessels expressed podoplanin and PECAM-1, all blood vessels expressed PECAM-1, and glandula lingualis was immunostained with anti-podoplanin but not with anti-PECAM-1, it is thought that the double immunostaining technique with anti-podoplanin and anti-PECAM-1 is useful in the discrimination of blood and lymphatic vessels with regard to the tissue having cross reaction to anti-podoplanin like salivary glands.
We have previously reported that the lymphatic endothelium expresses multiple leukocyte adhesion molecules including PECAM-1, ICAM-1, and VCAM-1, in inflamed human small intestine [16, 17]. This study showed that the inflammatory cell infiltration and vasodilation in the tongue tissue injected with TNF-α, suggesting that the tissue treated with TNF-α is useful as a inflammatory model (Fig. 2). In the lamina propria mucosae of the TNF-α-treated tongue, almost all initial lymphatics expressed ICAM-1. There were initial lymphatics with the VCAM-1 expression and also the vessels without the expression (Figs. 3, 4). In the tunica muscularis of TNF-α-treated tongue, collecting lymphatic vessels expressed PECAM-1 and ICAM-1, but rarely expressed VCAM-1 whereas blood vessels simultaneously expressed PECAM-1, ICAM-1, and VCAM-1 (Fig. 5). The ICAM-1-positive rate increased with TNF-α to 75% from 10% on initial lymphatics, and to 40% from 0% on collecting lymphatic vessels while it increased to 90% from 45% on blood vessels. The VCAM-1-positive rate increased with TNF-α to 30% from 0% on initial lymphatics, and to 5% from 0% on collecting lymphatic vessels while it increased to 75% from 5% on blood vessels (Fig. 6). These findings suggest that the lingual lymphatic endothelium has the ability to express ICAM-1, and VCAM-1 to a lesser extent than the ICAM-1 induction with TNF-α, and that the ICAM-1 and VCAM-1 induction predominantly occurs in the initial lymphatics compared with collecting lymphatic vessels. The expression of adhesion molecules on the lymphatic endothelium may aid immunocyte migration from tissue into the lymphatic vessels.
V. References
- 1.Breiteneder-Geleff S., Matsui K., Soleiman A., Meraner P., Poczewski H., Kalt R., Schaffner G., Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley C. D., Doyonnas R., Newton J. P., Blystone S. D., Brown E. J., Watt S. M. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J. Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- 3.Cook-Mills J. M. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol. Immunol. 2002;39:499–508. doi: 10.1016/s0161-5890(02)00206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLisser H. M., Yan H. C., Newman P. J., Muller W. A., Buck C. A., Albelda S. M. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J. Biol. Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- 5.Dustin M. L., Bivona T. G., Philips M. R. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 2004;5:363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- 6.Fawcett J., Buckley C., Holness C. L., Bird I. N., Spragg J. H., Saunders J., Harris A., Simmons D. L. Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J. Cell Biol. 1995;128:1229–1241. doi: 10.1083/jcb.128.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirakawa S., Hong Y. K., Harvey N., Schacht V., Matsuda K., Libermann T., Detmar M. Transcriptional profiling of isolated human lymphatic versus blood vascular endothelial cells. Am. J. Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong Y. K., Harvey N., Noh Y. H., Schacht V., Hirakawa S., Detmar M., Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev. Dyn. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 9.Kriehuber E., Breiteneder G. S., Groeger M., Soleiman A., Schoppmann S. F., Stingl G., Kerjaschki D., Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroshima S., Sawa Y., Kawamoto T., Yamaoka Y., Notani K., Yoshida S., Inoue N. Expression of Toll-like receptors 2 and 4 on human intestinal lymphatic vessels. Microvasc. Res. 2004;67:90–95. doi: 10.1016/j.mvr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Maekinen T., Veikkola T., Mustjoki S., Karpanen T., Catimel B., Nice E. C., Wise L., Mercer A., Kowalski H., Kerjaschki D., Stacker S. A., Achen M. G., Alitalo K. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishibori M., Takahashi H. K., Mori S. The regulation of ICAM-1 and LFA-1 interaction by autacoids and statins: a novel strategy for controlling inflammation and immune responses. J. Pharmacol. Sci. 2003;92:7–12. doi: 10.1254/jphs.92.7. [DOI] [PubMed] [Google Scholar]
- 13.Petrova T. V., Makinen T., Makela T. P., Saarela J., Virtanen I., Ferrell R. E., Finegold D. N., Kerjaschki D., Yla-Herttuala S., Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piali L., Hammel P., Uherek C., Bachmann F., Gisler R. H., Dunon D., Imhof B. A. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J. Cell Biol. 1996;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J. Cell Biol. 1992;117:179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sawa Y., Yamaoka Y., Ebata N., Ashikaga Y., Kim T., Suzuki M., Yoshida S. Immunohistochemical study on leukocyte adhesion molecules expressed on lymphatic endothelium. Microvasc. Res. 1999;57:292–297. doi: 10.1006/mvre.1998.2137. [DOI] [PubMed] [Google Scholar]
- 17.Sawa Y., Shibata K., Braithwaite M. W., Suzuki M., Yoshida S. Expression of immunoglobulin superfamily members on the lymphatic endothelium of inflamed human small intestine. Microvasc. Res. 1999;57:100–106. doi: 10.1006/mvre.1998.2132. [DOI] [PubMed] [Google Scholar]
- 18.Sawa Y., Ueki T., Hata M., Iwasawa K., Tsuruga E., Kojima H., Ishikawa H., Yoshida S. The LPS-induced IL-6, IL-8, VCAM-1, and ICAM-1 expression in human lymphatic endothelium. J. Histochem. Cytochem. 2008;56:97–109. doi: 10.1369/jhc.7A7299.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schacht V., Ramirez M. I., Hong Y. K., Hirakawa S., Feng D., Harvey N., Williams M., Dvorak A. M., Dvorak H. F., Oliver G., Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]