Abstract
This study was designed to investigate the distribution of cells expressing podoplanin in the mouse tooth bud. Podoplanin expression was detected in enamel epithelia of the cervical loop at cell-cell contacts strongly, and weakly on the loosely aggregated stellate reticulum in the center and the neighboring stratum intermedium. Odontoblasts exhibited intense podoplanin expression at the junction with predentin while no expression was detected in the enamel organ containing ameloblasts. These results suggest that proliferating inner and outer enamel epithelia express podoplanin but that the expression is suppressed in the differentiated epithelia containing ameloblasts. On the other hand the podoplanin expression occurs in the differentiating odontoblasts and the expression is sustained in differentiated odontoblasts, indicating that odontoblasts have the strong ability to express podoplanin. In cultured apical bud cells podoplanin was detected at cell-cell contacts. In real-time PCR analysis the amount of podoplanin mRNA of the apical buds was 2-fold compared with the amount of kidney used as a positive control. These findings indicate that apical bud cells have the strong ability to express the podoplanin gene. Podoplanin is a mucin-type glycoprotein negatively charged by extensive O-glycosylation and a high content of sialic acid, which expresses the adhesive property. The podoplanin may contribute to form odontoblastic fiber or function as the anchorage to the tooth development and in proliferating epithelial cells of cervical loop and apical bud.
Keywords: podoplanin, tooth germ, apical bud
I. Introduction
Podoplanin is a 43-kDa transmembrane glycoprotein which was first reported as E11 antigen and was further identified as podoplanin because of the expression in kidney glomerular epithelial cells, podocytes. Podoplanin is homologous to T1α which encodes an antigen expressed at alveolar type I cells and is also one of the most highly expressed lymphatic-specific genes [2, 3, 6, 10, 15, 20]. Podoplanin null mice die at birth because of respiratory defect and congenital lymphedema due to the failure in lymphatic pattern formation. The expression of podoplanin in the several epithelial cells, such as epidermis and alveolar epithelia, has been noticed [17, 22], and we have recently reported the podoplanin expression in mouse salivary glands [5]. Our study showed that podoplanin expression was rarely found in acini of the parotid gland but clearly found at the basal portion of ducts in the submandibular and sublingual glands, suggesting that podoplanin is a marker of myoepithelial cells of salivary glands.
Podoplanin is a mucin-type glycoprotein negatively charged by extensive O-glycosylation and a high content of sialic acid [2, 16]. Podoplanin possesses a platelet aggregation-stimulating (PLAG) domain, and the mutation of threonine residues in the PLAG domain reduces the platelet aggregation, suggesting that podoplanin could function as a ligand for proteins with lectin activity to sialic acid [7, 8]. The reports for the increased expression of podoplanin in almost all human intestinal tumors, and for the implication of podoplanin overexpression in oral cancer metastasis, also may provide a new physical role such as adhesion function in the proliferated cells. The up-regulation of podoplanin expression has been reported in squamous cell carcinomas of oral cavity, lung, and head and neck [9, 11, 23], and in central nervous system tumors [12, 18], and podoplanin promotes tumor cell invasion in vitro and in vivo [21]. These facts may suggest that the increased expression of podoplanin is associated with the ectodermal cell proliferation.
In our recent study to clarify the distribution of cells expressing podoplanin in mouse oral tissues, podoplanin expression was found in the mouse tooth bud. So far there has been no report about the expression of podoplanin about the cells in tooth bud, dental pulp, and periodontium. The study here was designed to investigate the distribution of cells expressing podoplanin in mouse tooth buds.
II. Materials and Methods
Cell culture
Pregnant mice (C57BL6/J, n=5) purchased from the Jackson Laboratory (Bar Harbor, ME, USA) were used. The collection of the tissue was conducted from mouse in 9 days after birth after euthanasia by intraperitoneal injection with sodium pentobarbital (10 ml/kg, Nembutal, Abbott Laboratories, North Chicago, IL, USA). The protocol for animal use was reviewed and approved by the animal experiment committee of Fukuoka Dental College, Fukuoka, Japan. The tissue of eternal tooth germ which formed at the apical end of lower incisors, referred to as the ‘apical bud’ [4, 14], and the periodontal ligament were cultured in Dulbecco’s modified Eagle medium (Gibco Life Technologies, Inc., Grand Island, NY, USA).
Immunohistochemistry
Pregnant mice (C57BL6/J, n=5) were used. The collection of the tissue was conducted from mouse in 9 days after birth as described above. Mice were perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The frozen 10 µm sections were cut in a cryostat and fixed in 5% formalin-PBS containing 0.1% glutaraldehyde for 10 min at 4°C. After the treatment with 0.1% rabbit serum the sections were treated with a cocktail of antibodies: 0.5 µg/ml of hamster anti-mouse podoplanin antibody (AngioBio Co., Del Mar, CA, USA) and rabbit anti-mouse nestin antibody (Abcam Inc., Cambridge, MA, USA) for 8 hr at 4°C. After reacting with first antibodies, the sections were immunostained for 1 hr at 20°C with a cocktail of second antibodies (0.1 µg/ml): Alexa Fluor (AF) 488 or AF568-conjugated goat anti-hamster or anti-rabbit IgGs (Probes Invitrogen, Eugene, OR, USA), and examined by laser-scanning microscopy (Axiovert 135M, Carl Zeiss, Jena, Germany) with a ×52 oil planapochromatic objective lens (numerical aperture ×1.3).
Reverse transcription (RT)-PCR and real-time PCR
The total RNA extraction from tissue of apical bud, kidney, and periodontal ligament of mice was achieved with a QIAshredder column and RNeasy kit (Qiagen). Contaminating genomic DNA was removed using DNAfree (Ambion, Huntingdon, UK), and the RT was performed on 30 ng of total RNA, followed by 25–30 cycles of PCR for amplification with 50 pM of primer sets using the Ex Taq hot start version (Takara Bio Inc., Otsu, Japan). We used primer sets of mouse β-actin (5-GTTCTACAAATGTGGCTGAGGA: upper, 5-ATTGGTCTCAAGTCAGTGTACAG: lower, 411 bp), and mouse podoplanin (upper: 5-CACCTCAGCAACCTCAGAC, lower: ACAGGGCAAGTTGGAAGC, 332 bp) where the specificities had been confirmed by the manufacturer (Sigma-Genosys Ltd., Cambridge, UK). The PCR products were separated on 2% agarose gel (NuSieve; FMC, Rockland, ME, USA) and visualized by Syber Green (Takara). The correct size of the amplified PCR products was confirmed by gel electrophoresis and amplification of accurate targets was confirmed by sequence analysis.
To quantify podoplanin mRNA generation, cDNA samples were analyzed by real-time quantitative PCR. A total of 1 ml of cDNA was amplified in 25 µl using PowerSYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in an 7500 Real-time PCR System (Applied Biosystems), and fluorescence was monitored at each cycle. Cycle parameters were 95°C for 15 min to activate Taq followed by 35 cycles of 95°C for 15 s, 58°C for 1 min, and 72°C for 1 min. For real-time analysis, a standard curve was constructed from amplicons for β-actin in three serial 4-fold dilutions of cDNA stock from LEC. The β-actin cDNA levels in each sample were quantified against a standard curve by allowing the software to accurately determine the sample β-actin units. The adhesion molecule cDNA levels in each sample were quantified against a standard curve as the sample adhesion molecule units and were normalized to the sample β-actin units. Thus, relative adhesion molecule production units were expressed as arbitrary units, calculated according to the following formula: relative adhesion molecule production units=sample adhesion molecule units/sample β-actin units.
Statistical analysis
All experiments were carried out five times, repeatedly, and data are expressed as mean±SEM. The statistical significance of differences (p<0.01) was determined by the paired two-tailed Student’s t test with StatView 4.51 software (Abacus Concepts, Calabasas, CA, USA).
III. Results and Discussion
Immunohistochemical analysis for mouse tooth germ
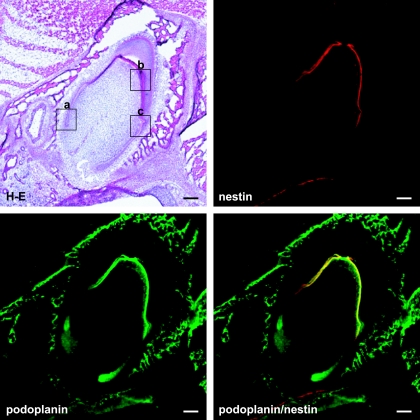
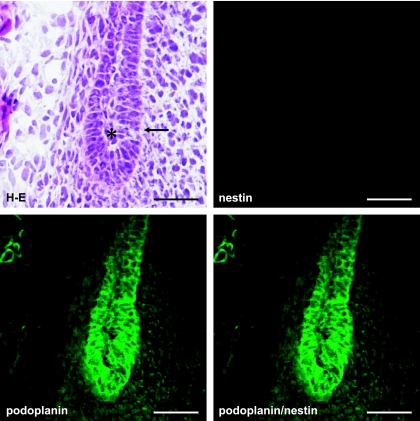
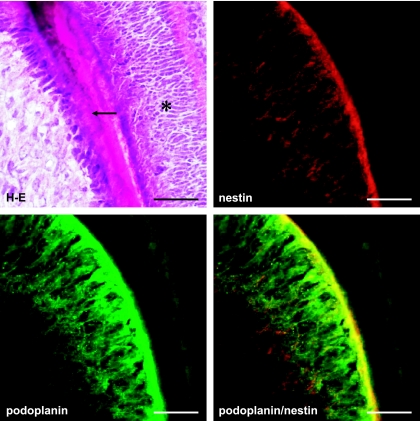
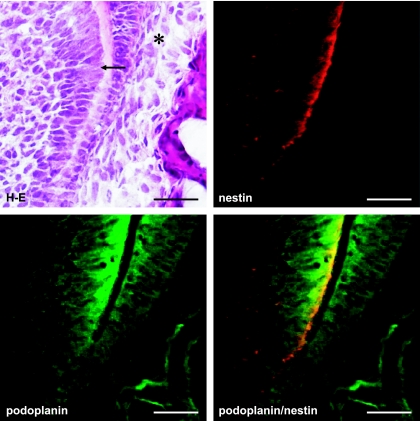
Figure 1 shows that a tooth germ at the bell stage in which the aggregated cells of the enamel organ lie above a condensation of ectomesenchymal cells, the dental papilla. The cervical loop where the outer enamel epithelium and the inner enamel epithelium join is observed at the apical side of the tooth germ. It has been recently reported that the intermediate filament nestin which is expressed in neural stem cells or progenitor cells is a specific marker for the odontoblast in the tooth germ from early to late developmental stages, and the nestin expression is sustained in differentiated odontoblasts [1, 13, 19]. In this study anti-nestin antibody was used to identify odontoblasts. Reaction products with anti-podoplanin antibody were detected in the cervical loop, and in the odontoblast layer immunoreacted with anti-nestin antibody (Fig. 1). In the cervical loop reaction products with anti-podoplanin antibody were detected strongly on the cell membrane of enamel epithelia at cell-cell contacts, and weakly on cells of the loosely aggregated stellate reticulum in the center and the neighboring stratum intermedium (Fig. 2). Odontoblasts exhibited intense reaction products with anti-nestin and anti-podoplanin antibodis at the junction with predentin while immunoreactivity with anti-podoplanin antibody was not detected in the enamel organ containing ameloblasts (Fig. 3). In the end of differentiating odontoblasts the reaction products with anti-podoplanin antibody were detected on the differentiating odontoblasts at the junction with predentin, and weakly detected on the inner enamel epithelium at cell-cell contacts (Fig. 4). These results indicate that proliferating inner and outer enamel epithelia express podoplanin but that the expression is suppressed in the differentiated epithelia of the enamel organ containing ameloblasts. On the other hand the podoplanin expression occurs in the differentiating odontoblasts and the expression is sustained in differentiated odontoblasts, indicating the strong ability to express podoplanin in odontoblasts.
Fig. 1.
Podoplanin expression in a tooth germ of mouse incisor at the bell stage. Aggregated cells of the enamel organ lie above a condensation of ectomesenchymal cells, the dental papilla. The cervical loop where the outer enamel epithelium and the inner enamel epithelium join is observed at the apical side of the tooth germ. Reaction products with anti-podoplanin antibody are observed in the cervical loop, and in the odontoblast layer with the immunoreactivity to anti-nestin. H-E, Hematoxylin-Eosin staining. Bar=100 µm.
Fig. 2.
Podoplanin expression in the cervical loop. Figure observed the area of (a) in Figure 1 at a high magnification. Reaction products with anti-podoplanin antibody are observed strongly on the cell membrane of enamel epithelia at cell-cell contacts (arrow), and weakly on cells of the stratum intermedium (asterisk). Bar=100 µm.
Fig. 3.
Podoplanin expression in the odontoblast layer. Figure shows the area of (b) in Figure 1 at a high magnification. Odontoblasts exhibited intense reaction products with anti-nestin and anti-podoplanin antibodies at the junction with predentin (arrow) while immunoreactivities with anti-podoplanin and anti-nestin antibodies are not observed in the enamel organ containing ameloblasts (asterisk). Bar=100 µm.
Fig. 4.
Podoplanin expression at the end of differentiating odontoblast layer. Figure shows the area of (c) in Figure 1 at a high magnification. Reaction products with anti-podoplanin antibody are observed on the differentiating odontoblasts at the junction with predentin (arrow), and weakly on the inner enamel epithelium at cell-cell contacts (asterisk). Bar=100 µm.
Analysis for a mouse apical bud
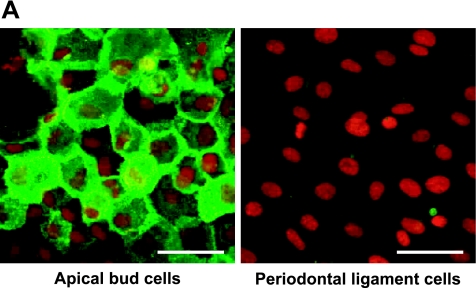
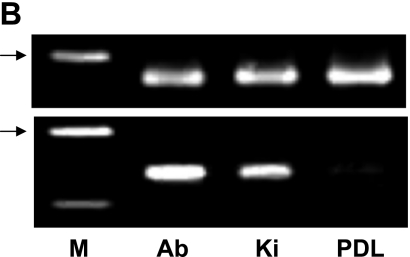
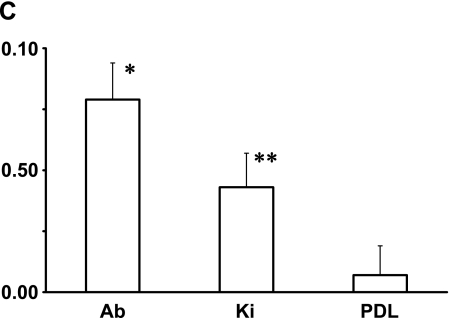
The specialized epithelial structure consists of dental epithelial stem cells, apical bud, and is formed at the region of the apical end in tooth development in continuously growing teeth. The region possesses a commonly specialized histological structure for the maintenance of adult stem cells and the production of various progenitor cells producing dental tissues [4, 14]. In cultured cells derived from the apical bud of the lower incisor, reaction products with anti-podoplanin antibody were detected at cell-cell contacts of primary cultured cells surrounding the apical bud tissue whereas no immunoreactivity with anti-podoplanin antibody was observed in cells derived from the periodontal ligament (Fig. 5A). In the RT-PCR total RNA extracts from the tissue of apical bud showed the PCR product for podoplanin mRNA as well as the tissue of kidney used as a positive control for the podoplanin expressing organ [5], whereas no products were detected in the tissue of periodontal ligament (Fig. 5B). In the real-time PCR analysis the amount of podoplanin mRNA in the total RNA extracts from the tissue of apical bud was 2-fold compared with the amount in the total RNA extracts from kidney, whereas little was detected in the tissue of periodontal ligament (Fig. 5C). The podoplanin expression of periodontal ligament may be ascribed to the lymphatic endothelial podoplanin mRNA in the periodontium [6, 10, 17]. These results indicate that apical bud cells have the strong ability to express podoplanin gene and that the expression occurs at cell-cell contacts.
Fig. 5.
Analysis for the mouse apical bud. (A) Immunohistochemical analysis for cultured cells derived from the apical bud of lower incisor. Reaction products with anti-podoplanin are observed at cell-cell contacts of primary cultured cells surrounding the apical bud tissue whereas no immunoreactivity with anti-podoplanin is observed in cells derived from the periodontal ligament. Nuclei are stained by propidium iodide. Bar=100 µm. (B) RT-PCR analysis. Products of β-actin is shown in upper panel. Total RNA extracts from the tissue of apical bud (Ab) showed the PCR product for podoplanin mRNA (lower panel) as well as the tissue of kidney (Ki), whereas no products were detected in the tissue of periodontal ligament (PDL). M, molecular weight marker (489 bp, arrows). (C) Real-time PCR analysis. Relative adhesion molecule production units are expressed as arbitrary units. The amount of podoplanin mRNA in the total RNA extracts from the tissue of apical bud (Ab) is 2-fold compared with the amount in the total RNA extracts from kidney (Ki), whereas little was detected in the tissue of periodontal ligament (PDL). *, Significantly different from Ki and PDL. **, Significantly different from PDL.
The podoplanin overexpression promotes formation of elongated cell extensions and significantly increases endothelial cell adhesion, migration, and tube formation [7]. The overexpression of podoplanin is also detected in almost all human intestinal tumors and podoplanin promotes tumor cell invasion in vitro, and in vivo [8, 21]. The expression of podoplanin in the proliferating epithelial cells of the cervical loop and apical bud may play a physical role that contributes to tooth development. Podoplanin is a mucin-type glycoprotein negatively charged by extensive O-glycosylation and a high content of sialic acid, which expresses the adhesive property. The podoplanin may contribute to form odontoblastic fiber or function as the anchorage to tooth development and in proliferating epithelial cells of cervical loop and apical bud [2, 16].
IV. References
- 1.About I., Laurent-Maquin D., Lendahl U., Mitsiadis T. A. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am. J. Pathol. 2000;157:287–295. doi: 10.1016/S0002-9440(10)64539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breiteneder-Geleff S., Matsui K., Soleiman A., Meraner P., Poczewski H., Kalt R., Schaffner G., Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 3.Dobbs L. G., Williams M. C., Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys. Acta. 1988;970:146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- 4.Harada H., Ohshima H. New perspectives on tooth development and the dental stem cell niche. Arch. Histol. Cytol. 2004;67:1–11. doi: 10.1679/aohc.67.1. [DOI] [PubMed] [Google Scholar]
- 5.Hata M., Ueki T., Sato A., Kojima H., Sawa Y. Expression of podoplanin in the mouse salivary glands. Arch. Oral Biol. 2008 doi: 10.1016/j.archoralbio.2008.02.006. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Hirakawa S., Hong Y. K., Harvey N., Schacht V., Matsuda K., Libermann T., Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneko M. K., Kato Y., Kameyama A., Ito H., Kuno A., Hirabayashi J., Kubota T., Amano K., Chiba Y., Hasegawa Y., Sasagawa I., Mishima K., Narimatsu H. Functional glycosylation of human podoplanin: glycan structure of platelet aggregation-inducing factor. FEBS Lett. 2007;581:331–336. doi: 10.1016/j.febslet.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y., Fujita N., Kunita A., Sato S., Kaneko M., Osawa M., Tsuruo T. Molecular identification of Aggrus/T1-α as a platelet aggregation-inducing factor expressed in colorectal tumors. J. Biol. Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 9.Kato Y., Kaneko M., Sata M., Fujita N., Tsuruo T., Osawa M. Enhanced expression of Aggrus (T1α/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol. 2005;26:195–200. doi: 10.1159/000086952. [DOI] [PubMed] [Google Scholar]
- 10.Kriehuber E., Breiteneder-Geleff S., Groeger M., Soleiman A., Schoppmann S. F., Stingl G., Kerjaschki D., Maurer D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001;194:797–808. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín-Villar E., Scholl F. G., Gamallo C., Yurrita M. M., Munoz-Guerra M., Cruces J., Quintanilla M. Characterization of human PA2.26 antigen (T1α-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int. J. Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 12.Mishima K., Kato Y., Kaneko M. K., Nishikawa R., Hirose T., Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa R., Saito C., Jung H. S., Ohshima H. Capacity of dental pulp differentiation after tooth transplantation. Cell Tissue Res. 2006;326:715–724. doi: 10.1007/s00441-006-0242-0. [DOI] [PubMed] [Google Scholar]
- 14.Ohshima H., Nakasone N., Hashimoto E., Sakai H., Nakakura-Ohshima K., Harada H. The eternal tooth germ is formed at the apical end of continuously growing teeth. Arch. Oral Biol. 2005;50:153–157. doi: 10.1016/j.archoralbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Rishi A. K., Joyce-Brady M., Fisher J., Dobbs L. G., Floros J., VanderSpek J., Brody J. S., Williams M. C. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev. Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- 16.Schacht V., Dadras S. S., Johnson L. A., Jackson D. G., Hong Y. K., Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schacht V., Ramirez M. I., Hong Y. K., Hirakawa S., Feng D., Harvey N., Williams M., Dvorak A. M., Dvorak H. F., Oliver G., Detmar M. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibahara J., Kashima T., Kikuchi Y., Kunita A., Fukayama M. Podoplanin is expressed in subsets of tumors of the central nervous system. Virchows Arch. 2006;448:493–499. doi: 10.1007/s00428-005-0133-x. [DOI] [PubMed] [Google Scholar]
- 19.Terling C., Rass A., Mitsiadis T. A., Fried K., Lendahl U., Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int. J. Dev. Biol. 1995;39:947–956. [PubMed] [Google Scholar]
- 20.Wetterwald A., Hoffstetter W., Cecchini M. G., Lanske B., Wagner C., Fleisch H., Atkinson M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 21.Wicki A., Lehembre F., Wick N., Hantusch B., Kerjaschki D., Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Williams M. C., Cao Y., Hinds A., Rishi A. K., Wetterwald A. T1α protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus and ciliary epithelia of adult rats. Am. J. Respir. Cell. Mol. Biol. 1996;14:577–585. doi: 10.1165/ajrcmb.14.6.8652186. [DOI] [PubMed] [Google Scholar]
- 23.Yuan P., Temam S., El-Naggar A., Zhou X., Liu D. D., Lee J. J., Mao L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–569. doi: 10.1002/cncr.22061. [DOI] [PubMed] [Google Scholar]