Abstract
Cadherins are a family of transmembrane glycoproteins that mediate cell-to-cell adhesion. Isoforms, including E- and N-cadherin, have been identified and shown to regulate morphogenesis through homophilic binding. In the ontogeny, the expressions of E- and N-cadherin change spatiotemporally, and the changes in cadherin isoforms, called cadherin switching, impact the mechanical adhesion of cells. Furthermore, cadherin functions as a receptor that transfers information from outside to inside cells, and in terms of switching, it affects cell phenotypes. To observe the expression patterns of E- and N-cadherins during embryogenesis and to identify cells that transiently coexpress both cadherins, we employed a recently developed immunohistochemical double staining technique in rat fetuses. At embryonic day 9, embryonic ectodermal cells more dominantly expressed E-cadherin, while mesodermal cells more dominantly expressed N-cadherin. At embryonic day 10, the expression pattern of E-cadherin in the surface ectoderm and endoderm and that of N-cadherin in the neuroectoderm were established. After embryonic day 10, unique co-expression of E- and N-cadherin was observed in primordia, such as the bulbus cordis, otic pit, notochord, and Rathke’s pouch. In the present study, it was possible to visualize the expression patterns of E- and N-cadherin during early fetal development, which enabled us to morphologically clarify cadherin switching.
Keywords: cadherin switching, organogenesis, immunohistochemistry, rat
I. Introduction
Cadherins are a family of transmembrane glycoproteins that mediate calcium-dependent cell-to-cell adhesion in solid tissues. Numerous isoforms of cadherins have been identified in a tissue-specific manner and shown to regulate morphogenesis through homophilic binding [13, 17]. E-cadherin and N-cadherin are important isoforms. In general, E-cadherin is a basic cell adhesion factor that is already functioning during the cleavage period, and N-cadherin is induced during the formation of certain organs. During the developmental period, the expression patterns of E-cadherin and N-cadherin change spatiotemporally to regulate organogenesis. Changes in cadherin isoforms, i.e., cadherin switching [20], markedly impact the mechanical adhesion of cells. In general, E-cadherin is responsible for stable cell-to-cell adhesion, while cells expressing N-cadherin are motile. For example, N-cadherin has been reported to play an essential role in the formation of the neural tube [17] and lens [19] and also in the process of epithelial-mesenchymal transition [5]. Furthermore, cadherin functions as a receptor that transfers information from outside to inside cells, and in terms of switching, it affects cell phenotypes via the changes in outside-in signal transduction [1, 14, 15].
Morphologically observing the expression patterns of E- and N-cadherins during embryogenesis is important for organogenesis. Immunohistochemical double staining can not only ascertain the difference between E- and N-cadherin distribution patterns but also identify cells that transiently coexpress E- and N-cadherins, and as a result, it appears to be the most effective technique for morphologically observing cadherin switching. However, most studies have morphologically observed fetuses by detecting N-cadherin, probably due to technical limitations; therefore, to the best of our knowledge, no study has detected both E- and N-cadherins by double staining. Therefore, in order to morphologically analyze cadherin switching, the recently developed immunohistochemical double staining technique of E- and N-cadherins in rat fetuses [7] was employed to ascertain the changes in the distribution of E- and N-cadherins in order to identify the primordium where E- and N-cadherins are uniquely coexpressed.
II. Materials and Methods
Animals
Pregnant Sprague-Dawley rats at various gestation periods were purchased from Japan SLC, Shizuoka, Japan. The rats were given ad libitum access to food and water and kept under a 12 hr-12 hr light-dark cycle. All animals were treated in accordance with The Guidelines for Animal Experimentation of Jichi Medical University based on the NIH Guidelines for the Care and Use of Laboratory Animals.
Immunohistochemistry
With the pregnant rats under deep Nembutal anesthesia, their embryos were collected at embryonic day 9 (E9), 9.5, 10, 10.5, 11, 12, 13, and 15 and fixed in Bouin’s fluid at 4°C. Six hours later, embryos were trimmed and returned to the same fixative for another 18 hr. Fixed tissues were routinely processed and embedded in Pathoprep embedding media (Wako Pure Chemical Industries, Osaka, Japan). Sagittal specimens were sectioned at 2 µm thickness for fluorescent microscopy or 50 µm thickness for confocal laser microscopy. Sections were incubated in 0.05% citraconic anhydride solution (Nissin EM, Tokyo, Japan) for 60 min at 95°C for antigenic retrieval. After immersion in phosphate-buffered saline (PBS) containing 2% normal goat serum for 20 min at room temperature, sections were incubated overnight at 30°C in PBS with mouse monoclonal antibody against E-cadherin (1.25 µg/ml, BD Transduction Laboratories, Franklin Lakes, NJ). After washing with PBS, sections were incubated in PBS with Alexa Fluor 488-labeled anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) for 30 min at 30°C. After all processes of immunostaining against E-cadherin were completed, another immunostaining against N-cadherin was performed using rabbit polyclonal primary antibody (dilution of 1:40, IBL, Gunma, Japan). Alexa Fluor 568-labeled anti-rabbit IgG (Invitrogen) was employed for N-cadherin. The absence of an observable nonspecific reaction was confirmed using normal animal serum. Two-µm thick specimens were observed through an AX80TR fluorescent microscope (Olympus, Tokyo, Japan) and imaged using a DP70 system (Olympus) with the aid of Photoshop software (Adobe Systems, San Jose, CA). Fifty-µm thick specimens were observed through an FV-1000 confocal laser microscope (Olympus).
III. Results and Discussion
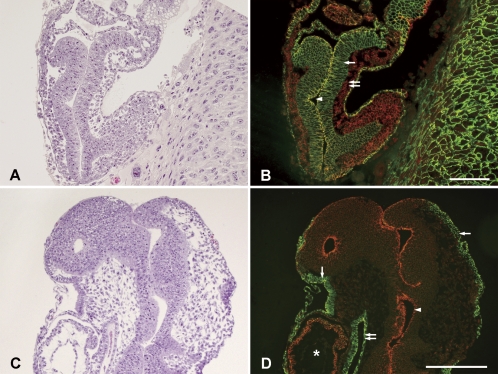
Figures 1A and 1B show hematoxylin and eosin staining and immunohistochemical double staining at E9. Embryonic ectodermal cells (single arrow) more dominantly expressed E-cadherin, while mesodermal cells (double arrow) more dominantly expressed N-cadherin. While ectodermal cells more dominantly expressed E-cadherin, all cells expressed N-cadherin, and cells facing the amniotic cavity (arrowhead) strongly expressed both cadherins. Figures 1C and 1D show hematoxylin and eosin staining and immunohistochemical double staining at E10. At E10 when embryonic turning begins, in both rostral and caudal regions, the expression pattern of E-cadherin in the surface ectoderm (epidermis, single arrow) and endoderm (foregut, double arrow) and that of N-cadherin in the neuroectoderm (arrowhead) were established. However, the separation of E- and N-cadherins was not complete at this stage, and N-cadherin was slightly expressed in the apical side of the foregut and the surface ectoderm. In addition, cells coexpressing E- and N-cadherins were seen in the heart primordial (asterisk).
Fig. 1.
Hematoxylin and eosin staining of embryos at E9 (A) and E10 (C) stages in sagittal sections and double fluorescent immunohistochemistry for E-cadherin and N-cadherin in adjacent sections (B and D). Immunoreactivity of E-cadherin and N-cadherin are shown in green and red, respectively. In panel B, the single arrow and double arrow indicate the embryonic ectoderm and mesoderm, respectively. The arrowhead indicates the amniotic cavity. In panel D, the single arrows, double arrow, and an arrowhead indicate the surface ectoderm (epidermis), endoderm, and neuroectoderm, respectively. The heart primordial is indicated by an asterisk. Bars=100 µm.
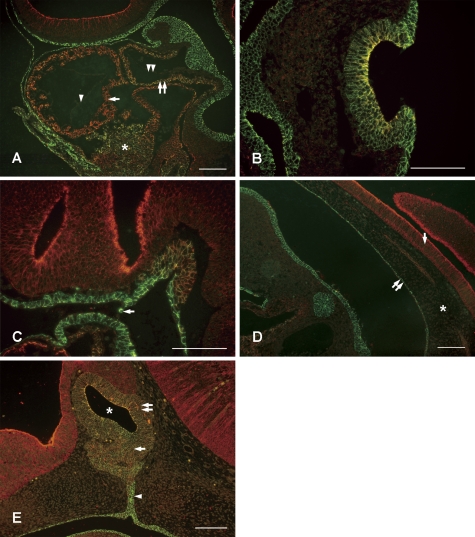
During organogenesis after E10, confocal laser microscopy showed the characteristic coexpression of E- and N-cadherins in several organ primordia on sagittal scans. Paraffin sections of the bulbus cordis (Fig. 2A), otic pit (Fig. 2B), notochord (Fig. 2C: near the prechordal plate and Fig. 2D: caudal) and Rathke’s pouch (Fig. 2E) were observed. In the bulbus cordis (Fig. 2A), the developing myocardial layer (single arrow), aortic sac smooth muscle layer (double arrow) and septum transversum tissue (asterisk) coexpressed E- and N-cadherins. Also, E- and N-cadherins were not expressed in the endocardium (single arrowhead) and aortic sac tunica intima (double arrowhead). While the expression of N-cadherin during organogenesis tends to be transient, N-cadherin continues to be expressed in the myocardium in adults [4]. Linask closely examined the expression patterns of N-cadherin in chicken embryos and documented that N-cadherin was involved with pericardial coelom formation and cardiomyocyte differentiation to from the myocardium, but not with endocardium formation [9], which supports our findings. Moreover, Nakagawa and Takeichi injected N-cadherin neutralizing antibody into whole embryo cultures and reported that N-cadherin was indispensable for heart formation [12].
Fig. 2.
Double fluorescent immunohistochemistry for E-cadherin and N-cadherin of embryonic organs. Immunoreactivity of E-cadherin and N-cadherin are shown in green and red, respectively. A: Bulbus cardialis at E11. The single arrow indicates the developing myocardial layer, the double arrow indicates the aortic sac smooth muscle layer, and the asterisk septum transversum tissue. Although both E- and N-cadherins were expressed in these tissues, the endocardium (single arrowhead) and aortic sac tunica intima (double arrowhead) expressed neither cadherin. B: Otic pit at E11. The surface ectoderm expressed only E-cadherin, and the otic pit expressed both E- and N-cadherins. C: Notochord (anterior) at E10. The arrow indicates the oral pharyngeal membrane. Cells expressing E- and N-cadherins were observed up to the prechordal plate. D: Notochord (posterior) at E10. The chorda dorsalis expressed E- and N-cadherins, and was independent of the neural tube (single arrow) and the midgut (double arrow). The asterisk indicates the interstitium. E: Rathke’s pouch at E14. Several layers of cells facing the lumen of Rathke’s pouch (asterisk) strongly expressed E- and N-cadherins. Note the variation in the expression ratio of E- and N-cadherins in the prospective pars distalis (single arrow) and pars intermedia (double arrow). An arrowhead indicates the pharyngohypophyseal stalk. Bars=100 µm.
In the otic pit (Fig. 2B), while the surface ectoderm only expressed E-cadherin, the otic pit expressed both E- and N-cadherins. In particular, N-cadherin was expressed strongly in the medial side of multilayered and invaginated cell layers. Simonneau et al. observed the development of the inner ear of fetuses after E16 and reported that E- and N-cadherins were expressed with mutually exclusive patterns [16], but in the primordium, both cadherins were expressed, and subsequent differentiation caused cells to express one or the other. However, according to Luo and colleagues, eight cadherin isoforms were involved in the formation of the cochlea in late chicken embryos [10], and in order to understand cell differentiation from the otic pit, it will be necessary to study the expression of other cadherins.
Cadherin expression of the cells in the notochord varied (Figs. 2C and 2D), and E- and N-cadherins were expressed at various ratios. In the rostral region, cells expressing E- and N-cadherins were observed up to the prechordal plate, and in this area, such cells were completely in contact with the foregut that only expresses E-cadherin. In the caudal region, the chorda dorsalis was inside the interstitium (asterisk) without E- and N-cadherin expressions and was independent of the neural tube (single arrow) expressing only N-cadherin and the midgut (double arrow) expressing only E-cadherin.
In the Rathke’s pouch (Fig. 2E), cells coexpressed E- and N-cadherins in all areas. In particular, several layers of cells facing the lumen of Rathke’s pouch (asterisk) strongly expressed E- and N-cadherins. In the proliferating pars distalis (single arrow) and pars intermedia (double arrow), the expression ratio of E- and N-cadherins varied. In the pharyngohypophyseal stalk (arrowhead), cells expressing only E-cadherin were seen continuous with the oral epithelia in the caudal side, and cells expressing E- and N-cadherins were seen in the rostral side, thus resulting in a two-layer structure. Our previous study clearly described the changes in cadherin expression due to the development of adenohypophyseal cells [7]. The study showed that as hormone producing cells differentiated, they began expressing only N-cadherin and stopped expressing E-cadherin. On the other hand, folliculo-stellate cells which do not produce hormones began expressing only E-cadherin and stopped expressing N-cadherin [7]. Fujita and Yasuda [2] reported that, in the matrix cell of the developing central nervous system, the dissociation and reassembly of N-cadherin-catenin complexes which occurs during mitosis causes elevator movement. It is likely that a similar mechanism plays a role during proliferation, differentiation and distribution of hormone-producing cells in the adenohypophysis. Future studies exploring the differences in how N-cadherin and E-cadherin interact with intracellular complexes will undoubtedly deepen our understanding of the roles of cadherin in histogenesis.
In the present study, it was possible to visualize the expression patterns of E-cadherin and N-cadherin during early fetal development. The distribution of E-cadherin and N-cadherin in fetuses was different from that in adults [18], thus suggesting that the expression of E-cadherin and N-cadherin is dynamic. Also, it was possible to clearly observe the specific coexpression of E-cadherin and N-cadherin during the formation of certain organs, and the present study enabled us to morphologically clarify cadherin switching. In general, E-cadherin is already expressed during the cleavage stage, while N-cadherin is induced later. Previous studies have shown that the switch from E-cadherin to N-cadherin is important for cell segregation, rearrangement and assembly [6, 11]. The switch appears to be involved with epithelial invagination and movement in the formation of the otic pit, Rathke’s pouch and notochord, which were examined in the present study, and the neural tube and eye, which are being closely examined [19]. Cadherin switching also appears to be involved with cell differentiation via intracellular signal transduction [3, 8, 20]. In the next step of our ongoing research, we are currently investigating temporal changes in cadherins in each of the organs in which co-expression of E- and N-cadherin was observed in the present study.
IV. Acknowledgements
We are grateful to Prof. Takashi Yashiro of the Department of Anatomy at Jichi Medical University School of Medicine for providing valuable insight and advice regarding this study.
V. References
- 1.Ferreira-Cornwell M. C., Veneziale R. W., Grunwald G. B., Menko A. S. N-cadherin function is required for differentiation-dependent cytoskeletal reorganization in lens cells in vitro. Exp. Cell Res. 2000;256:237–247. doi: 10.1006/excr.2000.4819. [DOI] [PubMed] [Google Scholar]
- 2.Fujita S., Yasuda Y. The molecular mechanism of elevator movement. Acta Histochem. Cytochem. 2003;36:393–398. [Google Scholar]
- 3.George-Weinstein M., Gerhart J., Blitz J., Simak E., Knudsen K. A. N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev. Biol. 1997;185:14–24. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- 4.Hatta K., Takagi S., Fujisawa H., Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev. Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 5.Hay E. D. An overview of epithelio-mesenchymal transformation. Acta Anat. (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 6.Kan N. G., Stemmler M. P., Junghans D., Kanzler B., de Vries W. N., Dominis M., Kemler R. Gene replacement reveals a specific role for E-cadherin in the formation of a functional trophectoderm. Development. 2007;134:31–41. doi: 10.1242/dev.02722. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi M., Yatabe M., Kouki T., Fujiwara K., Takigami S., Sakamoto A., Yashiro T. Changes in E- and N-cadherin expression in developing rat adenohypophysis. Anat. Rec. (Hoboken) 2007;290:486–490. doi: 10.1002/ar.20516. [DOI] [PubMed] [Google Scholar]
- 8.Larue L., Antos C., Butz S., Huber O., Delmas V., Dominis M., Kemler R. A role for cadherins in tissue formation. Development. 1996;122:3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- 9.Linask K. K. N-cadherin localization in early heart development and polar expression of Na+,K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Dev. Biol. 1992;151:213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- 10.Luo J., Wang H., Lin J., Redies C. Cadherin expression in the developing chicken cochlea. Dev. Dyn. 2007;236:2331–2337. doi: 10.1002/dvdy.21248. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y., Ferreira-Cornwell M., Baldwin H., Kostetskii I., Lenox J., Lieberman M., Radice G. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development. 2001;128:459–469. doi: 10.1242/dev.128.4.459. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa S., Takeichi M. N-cadherin is crucial for heart formation in the chick embryo. Dev. Growth Differ. 1997;39:451–455. doi: 10.1046/j.1440-169x.1997.t01-3-00006.x. [DOI] [PubMed] [Google Scholar]
- 13.Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 14.Pece S., Gutkind J. S. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J. Biol. Chem. 2000;275:41227–41233. doi: 10.1074/jbc.M006578200. [DOI] [PubMed] [Google Scholar]
- 15.Rubinek T., Yu R., Hadani M., Barkai G., Nass D., Melmed S., Shimon I. The cell adhesion molecules N-cadherin and neural cell adhesion molecule regulate human growth hormone: a novel mechanism for regulating pituitary hormone secretion. J. Clin. Endocrinol. Metab. 2003;88:3724–3730. doi: 10.1210/jc.2003-030090. [DOI] [PubMed] [Google Scholar]
- 16.Simonneau L., Gallego M., Pujol R. Comparative expression patterns of T-, N-, E-cadherins, beta-catenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development: implications for the nature of Kölliker’s organ. J. Comp. Neurol. 2003;459:113–126. doi: 10.1002/cne.10604. [DOI] [PubMed] [Google Scholar]
- 17.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya B., Sato Y., Kameya T., Okayasu I., Mukai K. Differential expression of N-cadherin and E-cadherin in normal human tissues. Arch. Histol. Cytol. 2006;69:135–145. doi: 10.1679/aohc.69.135. [DOI] [PubMed] [Google Scholar]
- 19.Xu L., Overbeek P. A., Reneker L. W. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- 20.Wheelock M. J., Shintani Y., Maeda M., Fukumoto Y., Johnson K. R. Cadherin switching. J. Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]