Abstract
Acute exposure of sensitized mice to antigen elicits allergic airway disease (AAD) characterized by Th2 cytokine-dependent pulmonary eosinophilia, methacholine hyperresponsiveness and antigen-specific IgE elevation. However, chronic exposure induces a local inhalational tolerance (LIT), with resolution of the airway responses but persistent systemic IgE production. To further determine if systemic immunologic responses were maintained during LIT, we assessed subcutaneous late phase responses to ovalbumin in this model. Sensitized and AAD mice developed small subcutaneous responses to ovalbumin, with footpad thickness increasing to 113.7 and 113.6% of baseline, respectively. In comparison, LIT mice developed marked foot swelling (141.6%). Histologic examination confirmed increased inflammation in the chronic animals, with a significant contribution by eosinophils. Thus, the resolution of airway inflammatory responses with chronic antigen inhalation is a localized response, not associated with loss of systemic responses to antigen.
Keywords: asthma, mouse, ovalbumin, sensitization, tolerance
In antigen-sensitized humans and animals, inhalational exposure to the antigen can induce allergic airway disease (AAD), or asthma, characterized by eosinophilic airway inflammation, elaboration of Th2 cytokines and airway hyperresponsiveness. Such individuals also demonstrate systemic responses to antigen, including elevated levels of antigen-specific IgE. Numerous murine models exist in which inhalation of allergens in sensitized mice induces Th2-driven eosinophilic airway disease. We1,2 and others3-5 have shown that, in contrast to human subjects, mice exposed chronically to inhaled antigen can reverse their established airway inflammation and hyperresponsiveness, and develop what we have termed local inhalational tolerance (LIT). LIT is dependent upon continuous antigen exposure2 and is associated with the persistence of CD4+CD25+Foxp3+ regulatory T cells in the lung and regional lymph nodes6 and the establishment of bystander tolerance to unrelated antigens.2,5
We emphasized the term ‘local’ because despite resolution of the airway eosinophilia and Th2 cytokine production, systemic IgE responses to antigen are sustained.2 Although the persistence of the systemic IgE response has also been observed in other chronic inhalational models,5 it is not a uniform finding in sensitized mice chronically exposed to antigen.3,4 The maintenance of antigen-specific IgE elevation in the absence of airway inflammatory responses in LIT mice suggests that this form of inhalational tolerance is dependent upon local regulatory mechanisms rather than systemic lymphocyte clonal deletion or anergy. In addition, if chronic aerosolized antigen exposure induces clonal deletion or the development of a dominant, antigen-specific, inhibitory T-cell population, tolerance should be sustained in the absence of antigen or recalled with re-exposure to antigen. We found that such sustained tolerance did not occur in the absence of antigen, as interrupting ovalbumin (OVA) aerosol challenges for 4 weeks after establishment of the 6-week tolerant stage followed by re-exposure to OVA aerosols resulted in development of AAD.2 Although the above observations suggest that LIT is a regional response localized to the respiratory tract, they do not exclude the concomitant attenuation of systemic responses. Thus, the present study was designed to determine if systemic responses to antigen in sensitized mice were sustained in the presence of LIT.
RESULTS AND DISCUSSION
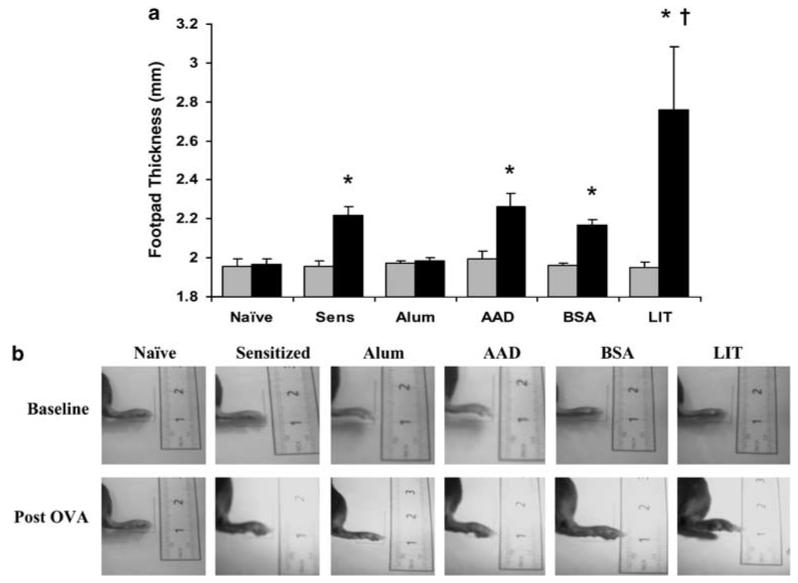
As shown in Figure 1, naïve and alum-only mice developed no foot swelling following local injection of OVA (post-OVA thickness 100.8 and 100.7% of baseline, respectively). OVA-sensitized/non-aerosol exposed mice, OVA-sensitized/OVA-exposed (AAD) mice, and OVA-sensitized/BSA-exposed (bovine serum albumin, BSA) mice showed modest but statistically significant delayed-type hypersensitivity responses, to 113.7, 113.6 and 110.5% of baseline, respectively (95% CI 111.5-115.8%, 111.2-115.9% and 108.3-112.8%; P<0.0001, P<0.0001 and P=0.0007 by paired t-tests). In comparison, LIT mice developed marked foot swelling, to 141.6% of baseline (95% CI 127.1-156.1%; P=0.0003) and significantly increased from responses in the sensitized, AAD and BSA animals (P<0.0001 by analysis of variance).
Figure 1.
Subcutaneous late phase responses in mice before and after footpad injection with OVA. (a) Footpad thicknesses were measured by a digital micrometer before (gray bars) and 24h after (black bars) local injection of OVA. Thickness did not change in naïve mice or mice given only intraperitoneal alum. Footpad thickness increased 10-14% in OVA-sensitized/non-aerosol challenged mice (Sens), OVA-sensitized/OVA-aerosol mice (AAD) and OVA-sensitized/BSA-aerosol mice (BSA). The swelling was significantly greater in LIT mice, increasing 42% above baseline thickness. Data represent mean±s.e. values; n=5-10 in each group. (b) Photographs of representative subcutaneous LPRs at baseline (upper photos) and 24h after OVA injection (lower photos) in a naïve, OVA-sensitized/non-aerosol (sensitized), alum-sensitized, OVA-sensitized/OVA-aerosol (AAD), OVA-sensitized/BSA-aerosol (BSA) and LIT mouse. *P<0.001 vs pre-OVA injection thickness; †P<0.0001 vs other post-OVA responses. AAD, allergic airway disease; BSA, bovine serum albumin; LIT, local inhalational tolerance; LPRs, late-phase responses; OVA, ovalbumin.
Histologic examination of the footpads confirmed that edema was representative of subcutaneous LPRs (late-phase responses). Tissues from sensitized and from AAD mice showed mild edema and perivascular inflammation, containing a mixed proportion of mononuclear cells and few eosinophils. These changes were qualitatively more pronounced in footpads from LIT mice, which consistently demonstrated increased edema, inflammation and eosinophilic infiltrates, along with areas of hemorrhage consistent with marked tissue inflammation (Figure 2).
Figure 2.
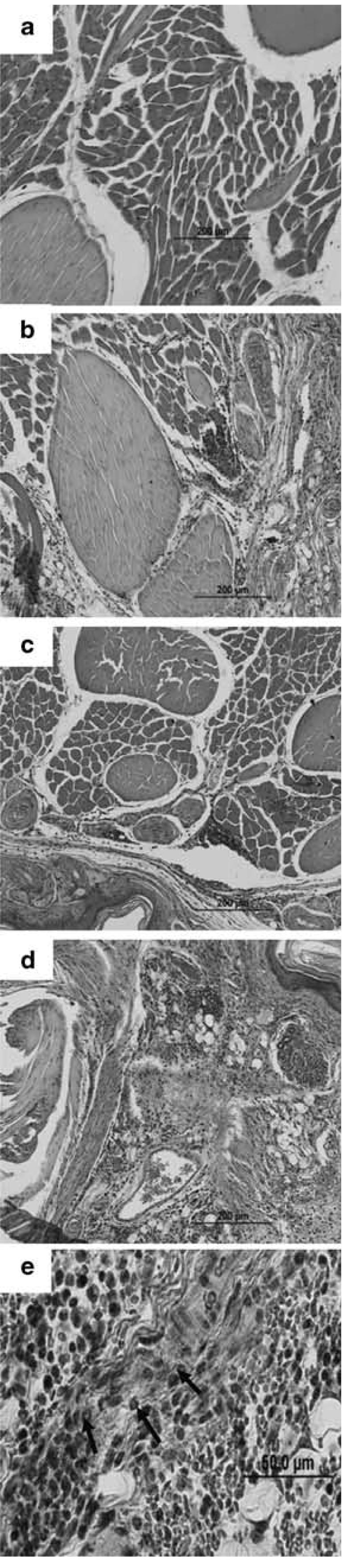
Histology of the footpad late phase responses in representative mice. Relative to naïve mice (a), sensitized and AAD mice (b and c) showed mild degrees of edema and perivascular inflammation. These responses were more pronounced in LIT mice, which demonstrated marked edema and areas of hemorrhage (d), and increased inflammation containing mononuclear cells and eosinophils (e; arrows identify eosinophils). Magnification ×20 (a-d) and ×60 (e). AAD, allergic airway disease; LIT, local inhalational tolerance.
Inflammatory responses occurring in subcutaneous LPRs are similar to those involved in allergic asthma and rhinitis.7 Subcutaneous LPRs are dependent upon antigen-specific IgE.8,9 In addition, T lymphocytes and activated eosinophils accumulate at subcutaneous LPR sites between 6 and 48 h after allergen injection.10 There is an association in allergic human subjects between CD4+ T-cell numbers and the persistence of activated eosinophils, suggesting that T cells are actively participating in the inflammatory process.10 Similarly, whereas classical delayed-type hypersensitivity is mediated by Th1 lymphocytes secreting predominantly IFN-γ and IL-2, subcutaneous LPRs to OVA given to mice without Freund's adjuvant are characterized by eosinophilic inflammation, mixed production of Th2 (IL-4 and IL-5) and Th1 (IFN-γ) cytokines and possible generation of Th2 (IgG1) and Th1 (IgG2) antibody isotype responses.11
There are several models of antigen-specific tolerance that are established in naïve animals before antigen sensitization12-17 or in sensitized animals prior to the development of AAD.18,19 The phenomenon of LIT differs significantly from the these tolerance models, in that it is established in the presence of AAD and serves to abrogate the allergic inflammatory responses and re-establish airway homeostasis. It is able to exert bystander protection against inflammatory responses to unrelated antigens. More importantly, it is a focal process, with tolerance localized to the lung but not to systemic responses. Although the above tolerance models inhibit antigen-specific IgE production, OVA-specific IgE and IgG1 remain elevated in our model.2 It is likely that the continued production of these antibodies contributes to the subcutaneous LPR in the present study. Moreover, the strongly positive LPR in LIT animals indicates that there remains a sizeable and perhaps increased pool of circulating CD4+ effector T cells that can respond to OVA during LIT. The attenuation of lung and airway responses during LIT may be attributed to the localization or focal expansion of CD4+CD25+Foxp3+ regulatory T cells in pulmonary compartments and regional lymph nodes.6 The absence of such regulatory T cells in the spleen or inguinal lymph nodes6 could allow circulating OVA-specific CD4+ effector T cells to generate a subcutaneous LPR to antigen.
In summary, the development of LIT with chronic aerosol exposure to OVA was not associated with loss of systemic effects to antigen. On the contrary, the subcutaneous LPR was enhanced, despite ablation of AAD. These findings support the regional nature of LIT and suggest that LIT represents a critical process for the control of allergic airways disease. The further elucidation of mechanisms underlying LIT may have major implications for the re-establishment of airway tolerance in allergic individuals with asthma.
METHODS
Animals
Adult (6-8 week) female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All work was approved by our institutional animal care committee, and animal welfare was in accordance with the ‘Guide for the Care and Use of Laboratory Animals’.20
OVA exposure protocol
Mice were sensitized and challenged to ovalbumin (OVA), as described previously.2 Briefly, animals first received 3 weekly intraperitoneal injections of 25 µg OVA (grade V; Sigma Chemical Co., St Louis, MO, USA) and 2 mg aluminum hydroxide (alum). One week after the last injection, the mice were exposed to 1% aerosolized OVA either for 1 h/day for 7 days (AAD group), or for 1 h per day for 7 days followed by 5 days per week for 6 weeks (LIT group). The OVA aerosols were generated by a BANG nebulizer (CH Technologies, Westwood, NJ, USA) into a 7.6-L, 48-port inhalation exposure chamber, to which mice placed in plastic restraint tubes were attached (CH Technologies). Control mice included animals that received intraperitoneal alum without OVA and animals that were sensitized to OVA/alum and subsequently exposed to 1% aerosolized BSA for 7 days using a different BANG nebulizer and aerosol chamber.
Subcutaneous LPR and histology
Naïve mice, sensitized/non-aerosol-exposed mice, AAD mice and LIT mice all received intradermal injections of 30 µl of PBS containing 100 µg OVA into the left hind footpad. Twenty-four hours later, the mice were anesthetized (ketamine 90 mg kg−1 and xylazine 10 mg kg−1), and the thickness of the injected and non-injected footpads was measured with a digital micrometer. After animal sacrifice, the injected feet were removed, fixed with 10% buffered formalin and processed in a standard manner. Tissue sections were stained with hematoxylin and eosin. Sections from all animals were examined in a blinded manner by one of the investigators (TVR), and representative areas were photographed.
Statistical analysis
Changes in footpad thickness before and after OVA injection were made by paired t-tests and were compared between groups by repeated measures analysis of variance (StatView 4.5, Abacus Concepts, Inc., Berkeley, CA, USA). Differences were considered significant at P<0.05.
ACKNOWLEDGEMENTS
Funding for this study came from the National Institutes of Health, Allergy-Immunology Section (R01 HL-43573).
References
- 1.Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, et al. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schramm CM, Puddington L, Wu C, Guernsey L, Gharaee-Kermani M, Phan SH, et al. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am J Pathol. 2004;164:295–304. doi: 10.1016/S0002-9440(10)63119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swirski FK, Sajic D, Robbins CS, Gajewska BU, Jordana M, Stampfli MR. Chronic exposure to innocuous antigen in sensitized mice leads to suppressed airway eosinophilia that is reversed by granulocyte macrophage colony-stimulating factor. J Immunol. 2002;169:3499–3506. doi: 10.4049/jimmunol.169.7.3499. [DOI] [PubMed] [Google Scholar]
- 4.Sakai K, Yokoyama A, Kohno N, Hamada H, Hiwada K. Prolonged antigen exposure ameliorates airway inflammation but not remodeling in a mouse model of bronchial asthma. Int Arch Allergy Immunol. 2001;126:126–134. doi: 10.1159/000049503. [DOI] [PubMed] [Google Scholar]
- 5.Van Hove CL, Maes T, Joos GF, Tournoy KG. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- 6.Carson WF, Guernsey LA, Singh A, Vella AT, Schramm CM, Thrall RS. Accumulation of regulatory T cells in the local draining lymph nodes of the lung correlates with the spontaneous resolution of a chronic model of murine asthma. Int Arch Allergy Immunol. 2008;145:231–243. doi: 10.1159/000109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleich GJ. The late phase of the immunoglobulin E-mediated reaction: a link between anaphylaxis and common allergic disease? J Allergy Clin Immunol. 1982;70:160–169. doi: 10.1016/0091-6749(82)90037-9. [DOI] [PubMed] [Google Scholar]
- 8.Dolovich J, Hargreave FE, Chalmers R, Shier KJ, Gauldie J, Bienenstock J. Late cutaneous allergic responses in isolated IgE-dependent reactions. J Allergy Clin Immunol. 1973;52:38–46. doi: 10.1016/0091-6749(73)90119-x. [DOI] [PubMed] [Google Scholar]
- 9.Solley GO, Gleich GJ, Jordon RE, Schroeter AL. The late phase of the immediate wheal and flare skin reaction; its dependence upon IgE antibodies. J Clin Invest. 1976;58:408–420. doi: 10.1172/JCI108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frew AJ, Kay AB. The relationship between infiltrating CD4+ lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J Immunol. 1988;141:4158–4164. [PubMed] [Google Scholar]
- 11.Akahira-Azuma M, Szczepanik M, Tsuji RF, Campos RA, Itakura A, Mobini N, et al. Early delayed-type hypersensitivity eosinophil infiltrated depend on T helper 2 cytokines and interferon-γ via CXCR3 chemokines. Immunology. 2004;111:306–317. doi: 10.1111/j.0019-2805.2004.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Halteren AG, van der Cammen MJ, Cooper D, Savelkoul HF, Kraal G, Holt PG. Regulation of antigen-specific IgE, IgG1, and mast cell responses to ingested allergen by mucosal tolerance induction. J Immunol. 1997;159:3009–3015. [PubMed] [Google Scholar]
- 13.Nakao A, Kasai M, Kumano K, Nakajima H, Kurasawa K, Iwamoto I. High-dose oral tolerance prevents antigen-induced eosinophil recruitment into the mouse airways. Int Immunol. 1998;10:387–394. doi: 10.1093/intimm/10.4.387. [DOI] [PubMed] [Google Scholar]
- 14.Haneda K, Sano K, Tamura G, Sato T, Habu S, Shirato K. TGF-beta induced by oral tolerance ameliorates experimental tracheal eosinophilia. J Immunol. 1997;159:4484–4490. [PubMed] [Google Scholar]
- 15.Yasue M, Yokota T, Kajiwara Y, Suko M, Okudaira H. Inhibition of airway inflammation in rDer f 2-sensitized mice by oral administration of recombinant der f 2. Cell Immunol. 1997;181:30–37. doi: 10.1006/cimm.1997.1184. [DOI] [PubMed] [Google Scholar]
- 16.Fox PC, Siraganian RP. IgE antibody suppression following aerosol exposure to antigens. Immunology. 1981;43:227–234. [PMC free article] [PubMed] [Google Scholar]
- 17.Tsitoura DC, Blumenthal RL, Berry G, DeKruyff RH, Umetsu DT. Mechanisms preventing allergen-induced airways hyperreactivity: role of tolerance and immune deviation. J Allergy Clin Immunol. 2000;106:239–246. doi: 10.1067/mai.2000.108429. [DOI] [PubMed] [Google Scholar]
- 18.Takabayashi K, Libet L, Chisholm D, Zubeldia J, Horner AA. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in Th2-sensitized mice. J Immunol. 2003;170:3898–3905. doi: 10.4049/jimmunol.170.7.3898. [DOI] [PubMed] [Google Scholar]
- 19.Vissers JL, van Esch BC, Hofman GA, Kapsenberg ML, Weller FR, Van Oosterhout AJ. Allergen immunotherapy induces a suppressive memory response mediated by IL-10 in a mouse asthma model. J Allergy Clin Immunol. 2004;113:1204–1210. doi: 10.1016/j.jaci.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Resources. National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC: 1996. [Google Scholar]