Abstract
We evaluated allelopathic interactions between strains of Cyanobacteria and green algae isolated from south and central Florida. Allelopathy, including inhibition or stimulation of growth, was assessed by cocultivation of each of the isolated strains, as well as by evaluation of extracts prepared from the isolates. All of the strains of Cyanobacteria, and four of the six isolates of green algae, showed some allelopathic activity (i.e. inhibition or stimulation of the growth of other strains). Of these, the most pronounced activity was observed for the cyanobacterial isolate Fischerella sp. strain 52−1. In the cocultivation experiments this cyanobacterium inhibited the growth of all tested green algae and Cyanobacteria. The crude lipophilic extracts from Fischerella sp. strain 52−1 isolated from both the biomass and the culture liquid inhibited photosynthesis of the green alga Chlamydomonas sp. in a concentration- and time-dependent manner and caused extensive loss of ultra- structural cell organization. Preliminary chemical characterization of compounds extracted from Fischerella sp. strain 52−1 indicated the presence of indole alkaloids, and further characterization has confirmed that these compounds belong to the hapalindoles previously isolated from other species of Fischerella and related genera. Further chemical characterization of these compounds, and further investigation of their apparent role in allelopathy is ongoing.
Keywords: allelopathy, Cyanobacteria, green algae, photosynthesis inhibition, Fischerella, indole alkaloid
Introduction
The composition and dynamics of algal communities is influenced not only by physical and chemical environmental factors, but also by interactions between members of these communities. In particular, the complex chemical signaling, referred to as allelopathy, includes an array of secondary metabolites that can act either as positive or negative regulators of the growth of sympatric species. As such, allelopathy can be considered as an adaptation to achieve a competitive advantage over other members within the same microbial community (Legrand et al., 2003).
Allelopathic activity of Cyanobacteria has attracted the attention of researchers for two primary reasons. First, understanding allelopathy can provide closer insight into successions in natural algal communities. In light of increased concern regarding harmful algal blooms (HAB), in particular, allelopathy is also seen as a possible factor in the dominance of toxin-producing species in a habitat (Rengefors & Legrand, 2001). Second, the active compounds are seen as environment-friendly herbicides or biocontrol agents that might have a potential commercial significance.
A number of authors have described antibiotic metabolites from microalgae, including compounds that inhibit bacteria (Falch et al., 1995; Chetsumon et al., 1998), fungi (Piccardi et al., 2000) and viruses (Loya et al., 1998), as well as Cyanobacteria and other microalgae (Flores & Wolk, 1986; Schlegel et al., 1999; Smith & Doan, 1999). Studies screening Cyanobacteria for their antialgal activity have shown that about 10% of strains exhibit this biological activity (Schlegel et al., 1999).
The primary aim in the present study was the assessment of the interactions between individual cultured strains of Cyanobacteria and green algae that were isolated from southern and central Florida. The mutual interactions of these potentially cohabitating organisms were studied in laboratory-designed experiments in which the strains were either cocultivated or grown in the presence of the extracts of other organisms. The most active species was identified and its compound isolated and partially characterized.
Materials and methods
Organisms and cultivation
The organisms investigated include eight strains of Cyanobacteria, and six strains of green algae (Chlorophyta) (Table 1). None of the cultures was axenic. Taxonomic identification of the strains to the genus level was based on morphological criteria given in Komarek & Anagnostidis (1986, 1989), Prescott (1962) and Whitford & Schumacher (1984). Due to the ambiguity in distinguishing Fischerella from Hapalosiphon, the taxonomic identification of strain 52−1 was supplemented by 16S rRNA gene sequencing followed by a blast search, which indeed showed that this strain belongs within the genus Fischerella.
Table 1.
Origin and morphological characteristics of cyanobacteria and chlorophytes
| Strain | Source | Characteristics |
|---|---|---|
| Cyanobacteria | ||
| Fischerella sp. 52−1 | Lake Tennessee, central Florida | Branched filaments, ellipsoid cells in main filament - 10 μm and cylindrical cells in the side filaments - 5 μm in diameter |
| Lyngbya sp. 15−2 | Floating periphyton mat, C111 canal, southern Florida | Filaments 20 μm in diameter |
| Nostoc sp. 23−2 | Lake Istokpoga, central Florida | Straight filaments, cylindrical cells 2.5 μm × 5.0 μm |
| Nostoc sp. Ev-1 | Shark Valley, Everglades, southern Florida | Straight filaments, spherical cells 5 μm in diameter |
| Nostoc sp. 37−7 | Crescent Lake, central Florida | Contorted filaments, cells spherical or cylindrical, diameter 3 μm |
| Nostoc sp. 58−2 | Lake Istokpoga central Florida | Straight filaments, cells oval 2.5 μm × 5.0 μm |
| Pseudanabaena sp. 21−9−3 | Storm Water Treatment Area (STA 1), southern Florida | Straight filaments, cells isodiametric, diameter 2.5 μm |
| Scytonema sp. 26−1 | Periphyton mat C-111 canal, southern Florida | Branched, sheathed filaments, cylindrical, diameter 12 μm, cells |
| Chlorophyta | ||
| Ankistrodesmus sp. 45−2 | Lake Howard, central Florida | Cells narrow contorted, 25−30 μm long |
| Chlamydomonas sp. Ev-29 | Shark Valley, Everglades southern Florida | Cells ovoid 7.5−10.0 μm in diameter |
| Excentrosphaera sp. 46−4 | Lake William Roe Park, central Florida | Spherical cells 10−60 μm and spores 3 μm in diameter |
| Chlorella sp. 2−4 | Everglades, Shark Valley, southern Florida | Cells spherical or ellipsoidal, 7−8 μm in diameter |
| Selenastrum sp. 34−4 | Periphyton mat, C-111 canal, southern Florida | Lunate cells, 5−7 μm long |
| Rhizoclonium sp. Ev-17 | Shark Valley, Everglades, southern Florida | Unbranched filaments, cylindrical cells 5 μm in diameter |
Except for Fischerella sp. strain 52−1 and Lyngbya sp. strain 15−2, which were known to be toxic from our previous work (Berry et al., 2004a, b) all other strains were randomly selected from our culture collection. The strains were isolated from various freshwater habitats in south and central Florida. To our knowledge none of the strains used in this work was part of HABs. The source of the isolates and their morphological characteristics are given in Table 1. The cultures were isolated by standard microbiological procedures, and maintained on BG11 medium (Rippka et al., 1979) in a light incubator at 24 °C under continuous fluorescent white light at an intensity of 30 μ Em−2 s−1.
Sequencing of the rRNA gene
For DNA isolation and sequencing, filaments of Fischerella sp. strain 52−1 from pure culture were first pelleted by centrifugation, washed and DNA isolated using the Bio101 Fast DNA isolation kit as per the manufacturer's instructions. A 675-bp 16S rRNA gene fragment was amplified by PCR using the primer set CYA106F/CYA781R (Nubel et al., 1997). The gene fragment was cloned using the Invitrogen TOPO TA cloning kit and sequenced through MWG biotech. A 663-bp fragment was sequenced from positions 85 to 747 in both directions. The result of the blast query showed 99.6% similarity to the sequence from Fischerella sp. strain 1711 (accession number AJ544076).
Cocultivation of Cyanobacteria and chlorophytes
The isolated strains were inoculated onto BG11 agar, and incubated in a light incubator under fluorescent white light (30 μ Em−2 s−1) for 10 days or until uniform growth appeared. Agar disks were excised using a sterile glass tube with a diameter of 7 mm and transferred to an empty Petri plate. The Petri plate containing three excised disks was overlayed with BG11 agar cooled to 45 °C. Plates were incubated in dim light for 24 h to allow diffusion of metabolites from the disk into the surrounding medium. Subsequently, this agar was overlayed with one of the test strains suspended in BG11 ‘soft agar’ (45 °C) to form a lawn. The same procedure was repeated for each combination of isolated strains. Plates were incubated at 24 °C and illumination of 30 μ Em−2 s−1. The inhibition or stimulation zone around the disks was recorded after 10 days of cocultivation. Each cocultivation combination was carried out in triplicate. Agar disks containing algal or cyanobacterial growth that were overlayed with the same strain were used as controls. In none of the cases was any effect produced.
Effect of extracts
Cellular extracts of Cyanobacteria and green algae were prepared from biomass that was obtained by growing the cultures in 4 L BG11 medium with aeration and constant illumination of 30 μ Em−2 s−1. The biomass was harvested after 4 weeks by centrifugation. Freeze-dried biomass (100 mg) was extracted first with 10 mL of chloroform to yield a lipophilic extract, and then with the same volume of 30% ethanol to obtain a hydrophilic extract. Both extracts were evaporated to dryness, and resuspended in absolute ethanol and 30% ethanol, respectively, to give a final concentration of 1 mg dry residue mL−1 of solvent.
In the case of the cyanobacterium Fischerella sp. 52−1, the culture liquid was additionally extracted. After removing the biomass by centrifugation, the culture liquid, which contained a visible whitish floating precipitate, was extracted with chloroform, evaporated and resuspended in ethyl alcohol to a final concentration of 1 mg mL−1.
To test for inhibition of the growth by the extracts, lawns of each strain on BG11 agar plates were incubated in dim light (10 μ Em−2 s−1) for 24 h. Subsequently, wells in the agar were made with a sterile glass tube (7 mm in diameter), and filled with 100 μL of extract. The plates were dried in a laminar flow cabinet. Each extract was tested in triplicate. The control plates contained the solvent only. In none of the cases did the solvent have any effect on the growth of the test organisms. The plates were incubated for 10 days after which the size of the inhibition zone was observed and measured. The data presented are the means of three replicate measurements.
Effect of Fischerella crude extract on the growth of Chlamydomonas
The effect of the cyanobacterial strain Fischerella sp. strain 52−1 on the growth of the green alga Chlamydomonas sp. strain Ev-29 was followed in a time-course experiment. The crude lipophilic extract from the culture liquid of Fischerella sp. strain 52−1 was added at different concentrations (5, 12, 25, 50, 500 μg mL−1) to the wells of 96-well plates. Each concentration was analyzed in three replicates. The control wells contained only the solvent. The extracts or the solvent in the case of controls were evaporated from the wells in vacuo. A suspension of 200 mL of Chlamydomonas cells in BG11 medium at a concentration of c. 104 mL−1 was added in each well. The plates were incubated at a light intensity of 30 μ Em−2 s−1 for 8 days. Plates were read on an HP plate reader at a wavelength of 600 nm. The first reading was taken immediately after inoculation, and readings were subsequently taken 2, 4, 6 and 8 days following inoculation. During the growth period, wells were mixed with a pipette tip once a day. After 4 days, an aliquot of fresh BG11 medium (100 μL) was added to each well to compensate for evaporation.
Effect of Fischerella extract on the photosynthetic activity of Chlamydomonas
The effect of the crude lipophilic extracts from the culture medium of Fischerella sp. strain 52−1 on the rate of photo-synthesis in Chlamydomonas sp. strain Ev-29 was assessed by the sensitive and selective fluorimetric measurement of the chlorophyll fluorescence of photosystem II (PSII) after pulse excitations at low (1 μmol quanta m−2 s−1, fluorescence F0,) and saturated actinic irradiance (3000−4000 μmol quanta m−2 s−1, fluorescence Fm) using the Phyto-Pam ED (Schreiber et al., 1986). Various volumes of the crude extract solution from 0 to 1.5 mL were mixed with Chlamydomonas culture (50 mL, final culture volume), which had a cell concentration of about 15 μg Chla L−1. In total, nine concentrations of crude extract were tested: 0 (= control), 0.1, 1, 3, 5, 7.5, 10, 20 and 30 μg mL−1. These test cultures were continuously stirred at low revolution with a magnetic stirrer under artificial light delivering 150−200 μmol m−2 s−1. Fluorimetric photosynthetic measurements were taken at 0, 10, 30 and 60 min after initial exposure to the crude extract on a 3-mL sample of the algal suspension, which was placed in a quartz cuvette. The photosynthetic capacity of the sample, or the maximum energy conversion efficiency (Y), was calculated as (Fm – F)/Fm. The percentage inhibition was determined over time by calculating: (Yt,0 – Yt,c)/Yt,0; where at time t, Yt,0 is Y in the control culture and Yt,c is Y in the culture exposed to the concentration of crude extract of concentration c. The data presented are representative results obtained from two separate experiments.
Electron microscopy
Chlamydomonas Ev-29 was grown in an aerated culture for 5 days under standard conditions. Extracts of Fischerella sp. strain 52−1 were added to test tubes, and the solvent evaporated. Subsequently, aliquots of Chlamydomonas cells were transferred to the test tubes, such that the final concentration of the crude extract was 1 mg mL−1. The tubes were incubated in the light incubator for 24 h. Control cells, without extract, were treated in the same fashion. Both control and extract-treated cells were harvested by centrifugation and embedded in 1.5% agar. The agar blocks were fixed in 2.5% (w/v) glutaraldehyde, 1% (w/v) OsO4 and 1% (w/v) KMnO4. The fixed cells were dehydrated in an increasing alcohol series, and infiltrated with Spurr's resin (Spurr, 1969). Sections were stained with uranyl acetate and lead citrate and observed under a Philips electron microscope.
Chemical characterization of Fischerella sp. strain 52−1
In the present study, preliminary chemical characterization of metabolites from Fischerella sp. strain 52−1 was conducted. Specifically, chloroform extracts were evaluated by thin-layer chromatography (TLC; silica gel, 1 : 1 hexane ethyl: acetate) visualized with the Ehrlich's reagent that is specific for indole alkaloids, including those that have been previously identified as biologically active metabolites from Fischerella and related genera. Indole alkaloids identified in this way have been subsequently isolated, and characterized by mass spectrometry and nuclear magnetic resonance (NMR; unpublished data).
Statistical analysis
The data presented are the means of three replicate measurements. SEs were calculated using stat-100 (Biosoft) software. Growth inhibition of Chlamydomonas sp. strain Ev-29 subsequent to six exposures to crude extract of Fischerella sp. strain 52−1 was compared over time using the multiple pairwise Tukey's honestly significant difference (HSD) test conducted at the 95% confidence level. All tests were performed using spss 14.0 after the homoscedasticity of the variance was checked.
Results
In the cocultivation experiments each strain was cultivated in the presence of each of the 13 other cyanobacterial and green algal strains. Of 182 total combinations, some effect, either inhibitory or stimulatory, was recorded in 37 cases (Table 2). Most of these cases (28) showed growth inhibition, while growth stimulation was recorded in nine cases. The only strain that was inhibitory to all others was the cyanobacterium Fischerella sp. strain 52−1 (Table 2). The strain that showed the greatest stimulatory effect towards the other strains was the green alga Chlamydomonas sp. strain Ev-29. The cyanobacterium Nostoc sp. strain 23−2 showed a stimulatory effect but only towards the green algae (Table 2). The strain that was most susceptible to an inhibitory effect was the cyanobacterium Nostoc sp. strain Ev-1, which was inhibited by two green algae and three cyanobacterial isolates.
Table 2.
Different types of interrelationships during cocultivation of cyanobacteria and green algae
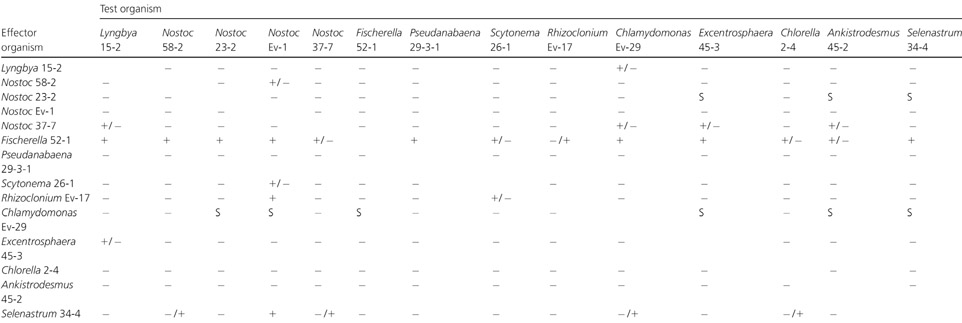 |
Agar disks containing growth of an effector organism were overlayered with the test organism and the zone of inhibition or stimulation was observed.[]
+, inhibition zone > 15 mm; +/−, weak inhibition zone 10−15 mm; −/+, very weak inhibition zone < 10 mm; −, no effect; S, stimulation.
In addition to cocultivation experiments, the growth of each organism was also tested in the presence of the extracts from the each of the other strains (Table 3). The extract that showed an inhibitory effect on the largest number of tested Cyanobacteria and green algae was a lipophilic extract obtained from the cyanobacterium Fischerella sp. strain 52−1 (Table 3). In particular, the most potent extract of this cyanobacterium was that obtained from the culture liquid. The strain that was most susceptible to inhibition by other green algae and Cyanobacteria was the green alga Chlamydomonas sp. strain Ev-29, and the organism that was most frequently stimulated was the cyanobacterium Nostoc sp. strain 23−2.
Table 3.
Effect of the aqueous (a) and chloroform (c) extracts from the biomass and the extract from the culture liquid (e) on the growth of test organisms
 |
The symbols represent a range of inhibition zones obtained as means from three replicates.
+, inhibition zone > 15 mm; +/−, weak inhibition zone 10−15 mm; −/+, very weak inhibition zone < 10 mm; −, no effect; S, stimulation.
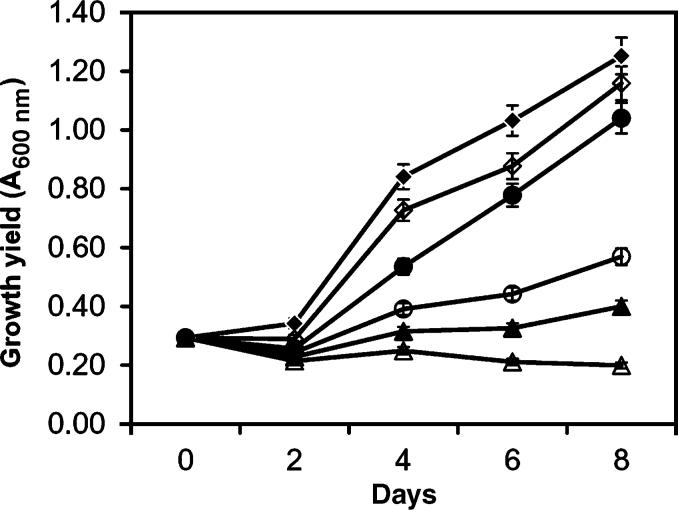
In order to determine the IC50 of the crude extract from Fischerella sp. strain 52−1, cells of Chlamydomonas sp. strain Ev-29 were grown in the presence of a range of concentrations of the extract that was obtained from the culture liquid. Growth of Chlamydomonas sp. strain Ev-29 was concentration-dependent (Fig. 1). Total lysis of cells was achieved at a concentration of 500 μg mL−1 of crude extract. Based on curve calculations, the IC50 for the crude extract was estimated to be 25 μg mL−1.
Fig. 1.
Concentration-dependent growth inhibition of Chlamydomonas sp. strain Ev-29 by the crude extract of Fischerella sp. strain 52−1 from the culture liquid. Chlamydomonas cells were grown in the presence of the following concentrations of Fischerella extract: 500 (△), 50 (▲), 25 (○), 12 (•) and 5 μg mL−1 (◆); control (◇). The controls contained the solvent only. Vertical bars are SEs generated from the mean of three replicates.
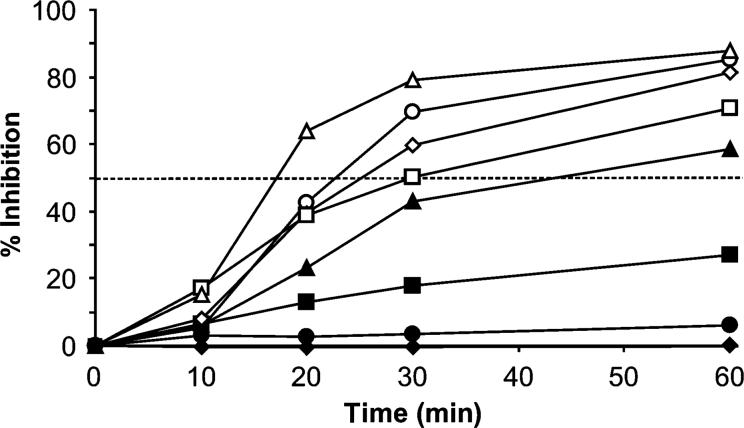
Fischerella crude extract showed inhibition of photosynthesis at a concentration as low as 1 μg mL−1. The inhibition kinetics described a sigmoidal curve over time that was concentration-dependent (Fig. 2). Inhibition of photosynthesis reached at least 50% after 1 h of incubation at concentrations of 5 μg mL−1 and above. Inhibition of photosynthesis of more than 80% was reached with concentrations above 10 μg mL−1.
Fig. 2.
Inhibition of photosynthesis in Chlamydomonas sp. strain Ev-29 by the lipophilic extract of Fischerella sp. strain 52−1. Inhibition was dependent on extract concentration and time. The crude extract was obtained from the culture liquid and dry residue dissolved in ethyl alcohol. The following concentrations of Fischerella crude extract were used: 30 (△), 20 (○), 10 (◇), 7.5 (□), 5 (▲), 3 (■), 1 (•) and 0.1 μg mL−1 (◆).
By using the multiple pairwise Tukey's HSD statistical test, it was shown that growth inhibition vs. the control was present at all crude extract concentrations as early as on day 2. On day 2, growth inhibition among the various treatments was similar for concentrations of 12 and 25 μg mL−1, 25 and 50 μg mL−1, and 50 and 500 μg mL−1. Thereafter, all pairwise comparisons conducted were significantly different.
After being exposed to the crude extract from the culture liquid of Fischerella for 24 h, cells of Chlamydomonas sp. strain Ev-29 showed distinctive morphological and structural changes. Light microscopy revealed bleaching and extensive vacuolization of the cells. Electron microscopy revealed degeneration of thylakoids into a system of irregularly arranged, sometimes swollen, membranes. In addition, disappearance of other cell structures including the nucleus was apparent (Fig. 3).
Fig. 3.
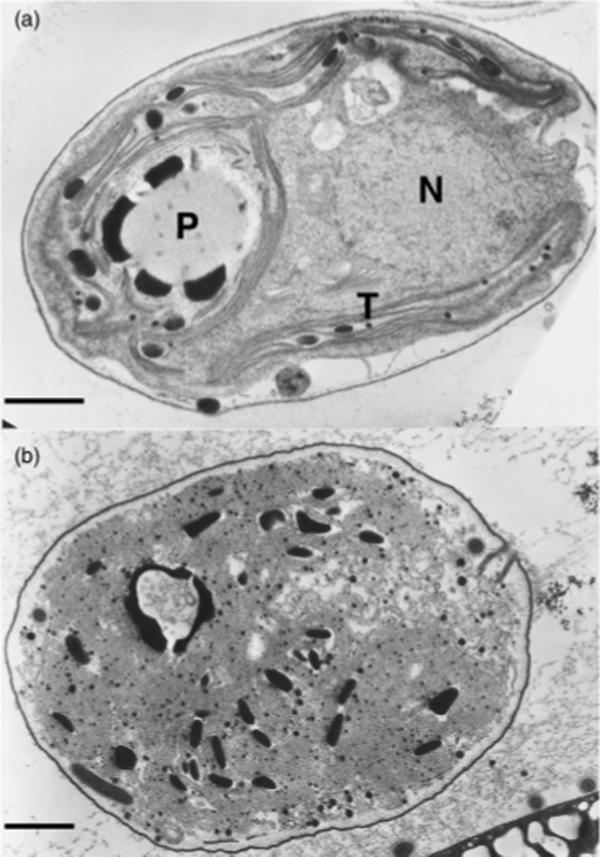
(a) Electron micrographs showing normal cells of Chlamydomonas sp. strain Ev-29 and (b) disintegration of cell structures after 24 h of exposure to the crude toxin extract from the culture liquid of Fischerella sp. strain 52−1. N, nucleus; T, tylakoids; P, pyrenoid. Scale bar, 1 μm.
Lipophilic (CHCl3) extracts prepared from both biomass and culture medium of Fischerella sp. strain 52−1 were evaluated by TLC. Visualization with Ehrlich's reagent indicated the presence of indole compounds, specifically by the appearance of diagnostic purple-colored ‘spots’ on TLC plates. Indole alkaloids identified in this way have been subsequently isolated and chemically characterized by mass spectrometry and NMR and shown to belong to the hapalindoles (unpublished data).
Discussion
Early studies of allelopathy among algae were limited to field observations by monitoring algal successions in natural environments (Keating, 1977). Later, diverse laboratory-based studies were directed to biochemical and ecological effects (Legrand et al., 2003). In the present study, we applied two methods to assess allelopathic interactions between Cyanobacteria and green algae – specifically, cocultivation of isolated strains and evaluation of stimulatory or inhibitory effects of cell extracts. These methods, as expected, did not produce identical results (Tables 2 and 3). Growth inhibition was recorded more frequently when extracts, rather than the cocultivation method, were used. This may be explained by the fact that some biologically active compounds are not released into the external environment until the cells are lysed, and therefore this kind of relationship cannot be considered to be truly allelopathic. In the case of Fischerella sp. strain 52−1, the cocultivation was inhibitory for all the tested Cyanobacteria and green algae (Table 2) while the crude extract was not effective against Pseudanabaena 29−3−1 and Scytonema 26−1 (Table 3). It is possible that the inhibitory effect is a result of a synergistic interaction of two or more compounds, some of which might have been lost in the process of extraction.
With the exception of Fischerella sp. strain 52−1, the inhibitory activity of individual strains was specific and it was observed only for one or a few test organisms. It should be also pointed out that, in some cases, only weak inhibition (inhibition zone < 10 mm) was observed (Table 3). Anti-algal activity is reported mostly for Cyanobacteria, although green algae can also produce allelopathic compounds such as the fatty acids described for Botryococcus braunii (Chiang et al., 2004). In our investigation, the green algal strains, with the exception of Rhizoclonium sp. strain Ev-17 and Selenastrum sp. strain 34−4, also showed some although low levels of inhibitory activity against other algae and Cyanobacteria.
The strain that was most susceptible to the inhibitory effect of the extracts was the green alga Chlamydomonas sp. strain Ev-29. At the same time, this alga had a pronounced stimulatory effect when cocultivated both with Cyanobacteria and with green algae (Table 2). The stimulatory effect of Chlamydomonas reinhardtii towards the cyanobacterium Anabaena flos-aquae was described and explained as a nutrifying effect of the extracelullar products of the green alga (Kearns & Hunter, 2000). We did not make any attempt to determine the nature of this stimulation, but it appears that the stimulatory compounds were excreted during active growth and were not present in the extracts.
The only organism that showed inhibition against all the tested Cyanobacteria and green algae was the cyanobacterium Fischerella sp. strain 52−1. This is in agreement with previous reports that allelopathic activity is found mostly among filamentous and nitrogen-fixing Cyanobacteria (Schlegel et al., 1999; Smith & Doan, 1999), including Fischerella (Doan et al., 2000) and the related genus Hapalosiphon (More, 1984). A larger zone of inhibition was observed in most of the cases when the crude extract was prepared from the culture liquid, rather than the cellular biomass (Table 3). As both extracts were prepared at 1 mg mL−1 concentration this indicates that the relative abundance of the active compound is higher in the extract from the culture liquid than from the cellular extracts.
Although there are previous reports on algicidal compounds from Fischerella (Smith & Doan, 1999) there is no information on possible ecological implications. Given that Fischerella is a benthic sessile organism, we can only speculate that production of allelopathic compounds by Fischerella provides a competitive advantage and plays an important role in deterring other organisms from colonizing its filaments.
The mode of action of allelopathic substances includes inhibition of growth (Keating, 1978; Schlegel et al., 1999), inhibition of PSII (Hagmann & Juttner, 1996) and inhibition of cellular motility (Kearns & Hunter, 2001). We have shown that the Fischerella crude extract from the culture medium inhibited photosynthesis in the green alga Chlamydomonas sp. strain Ev-29. Inhibition of photosynthesis by the Fischerella crude extract was relatively rapid but not instantaneous, becoming apparent only 10 min after exposure. The inhibition depended both on the concentration of the allelopathic compound and on the time of exposure (Fig. 3). A rate of inhibition of photosynthesis of 50% was achieved at a concentration of 5 μg mL−1 after 1 h of exposure. One-month-old cultures of Fischerella accumulated in the medium 4 μg mL−1 of crude extractable toxin, a concentration that would be sufficient to inhibit the photo-synthesis of other algae. It should be mentioned that we used the PHYTO-PAM ED fluorometer, which measures the electron transfer rate between PSII and PSI, and therefore at this point it is not known whether this inhibition is a direct result of a change in the electron transfer rate, or whether it occurred indirectly through the dark reaction pathway (i.e. Calvin cycle).
Cyanobacteria are known to harbor in their mucilaginous sheaths bacteria that are sometimes impossible to remove. In this work we used nonaxenic cultures, and therefore the potential effect of accompanying bacteria cannot be completely excluded. Not only might these bacteria degrade the biologically active compounds produced by Cyanobacteria (Jones et al., 1994), but there is also evidence that they may contribute to toxin production (Gallacher et al., 1997).
Ultrastructural study has revealed that 24 h after exposure to the crude extract, cells of Chlamydomonas sp. underwent dramatic changes primarily causing disintegration of the thylakoid system. Being soluble in organic solvents, and therefore presumably lipophilic, it is possible that the toxin primarily targets the lipid-rich thylakoid membranes and eventually leads to disruption of the electron transport system.
Algicidal compounds have been identified in different cyanobacterial genera (Smith & Doan, 1999) such as Hapalosiphon (Moore et al., 1984), Oscillatoria (Bagchi et al., 1990), Scytonema (Pignatello et al., 1983) and Nostoc (Todorova & Jüttner, 1995). It appears that species of Fischerella are the most typical producers of algicidal compounds among Cyanobacteria. The algicidal fischerellin was isolated from Fisherella muscicola (Gross et al., 1991; Hagmann & Jüttner, 1999) and was identified as an inhibitor of the oxygenic photosynthetic pathway. Etchegaray et al. (2004) identified two allelochemicals, an aminoacylpolyke-tide, fisherellin A, and an alkaloid, 12-epi-hapalindole F, from Fischerella sp. CENA 19. However, it appears that indole alkaloids are not characteristic of just the genus Fischerella. Norharmane [9H-pyrido(3,4-b)indol] was isolated from culture medium of Nodularia harveyana and had a strong algicidal activity against the cyanobacterium Arthrospira laxissima (Volk, 2005).
Based on our preliminary data, production of indole alkaloids by Fischerella species may indeed be a characteristic of the genus, as suggested by Gross et al. (1991). Compounds isolated from lipophilic extracts of Fischerella sp. strain 52−1 culture medium indicate the presence of such indole alkaloids. In addition to the preliminary characterization by TLC performed in the present study, two such apparent indole alkaloids have, in fact, been purified from Fischerella sp. strain 52−1 in separate studies conducted by us (unpublished data). Chemical characterization of these compounds by NMR and MS indicates that these compounds belong to the hapalin-doles previously isolated from other species of Fischerella and Hapalosiphon (Moore et al., 1984, 1987; Doan et al., 2000; Etchegaray et al., 2004). Further investigation of these compounds and their role in allelopathy is ongoing.
In conclusion, our study shows that allelopathy, in the form of either inhibition or stimulation, is broadly present among the organisms studied. Cocultivation proved to be an efficient tool in allelopathy research because it mimics the relationship between two actively growing community members. Using only a cell extract technique, the allelopathic effect may remain undetected due to the fact that the relationship between two organisms might be determined by a synergetic effect of two or more compounds. We have isolated two active compounds and identified them as indole alkaloids. Our finding that these compounds extracted from the culture liquid exhibited stronger activity than that from the cells might indicate that the toxin becomes activated only when excreted. This and other questions, together with identification of the toxin structure, will form the subject of our future research.
Acknowledgements
We would like to thank the National Institutes of Environmental Health Sciences (NIEHS) for financial support of this research through NIEHS ARCH grant S11 ES11181.
References
- Bagchi SN, Palod A, Chauhan VS. Algicidal properties of a bloom-forming blue-green alga Oscillatoria sp. J Basic Microbiol. 1990;30:21–29. [Google Scholar]
- Berry JP, Gantar M, Gawley RE, Wang M, Rein KS. Pharmacology and toxicology of pahayokolide A, a bioactive metabolite from a freshwater species of Lyngbya isolated from the Florida Everglades. Comp Biochem Physiol Part C. 2004a;139:231–238. doi: 10.1016/j.cca.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JP, Gantar M, Gawley RE, Rein KS. Isolation of bioactive metabolites from a Lyngbya species isolated from periphyton of the Florida Everglades. In: Steidinger KA, Landsberg JH, Tomas CR, Varg GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; St. Petersburg, FL: 2004b. pp. 192–194. [Google Scholar]
- Chetsumon A, Umeda F, Maeda I, Yagi K, Mizoguchi T, Miura Y. Broad spectrum and mode of action of an antibiotic produced by Scytonema sp. TISTR 8208 in a seaweed-type bioreactor. Appl Biochem Biotech. 1998;70:249–256. doi: 10.1007/BF02920141. [DOI] [PubMed] [Google Scholar]
- Chiang I-Z, Huang W-Y, Wu J-T. Allelochemicals of Botryococcus braunii (Chlorophyceae). J Phycol. 2004;40:474–480. [Google Scholar]
- Doan NT, Rickards RW, Rotschild JM, Smith GD. Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin A from Cyanobacteria of the genera Fischerella and Calothrix. J Appl Phycol. 2000;12:409–416. [Google Scholar]
- Etchegaray A, Rabello E, Dieckmann R, Moon DH, Fiore MF, von Dohren H, Tsai SM, Neilan BA. Algicide production by the filamentous cyanobacterium Fischerella sp. CENA 19. J Appl Phycol. 2004;16:237–243. [Google Scholar]
- Falch BS, Koening GM, Wright AD, Sticher O, Angerhofer CK, Pezzuto JM, Bachmann H. Biological activity of Cyanobacteria: evaluation of extracts and pure compounds. Planta Med. 1995;61:321–328. doi: 10.1055/s-2006-958092. [DOI] [PubMed] [Google Scholar]
- Flores E, Wolk CP. Production, by filamentous, nitrogen-fixing Cyanobacteria of a bacteriocin and of other antibiotics that kill related strains. Arch Microbiol. 1986;145:215–219. doi: 10.1007/BF00443648. [DOI] [PubMed] [Google Scholar]
- Gallacher S, Flynn KJ, Franco JM, Brueggemann EE, Hines HB. Evidence for production of paralytic shellfish toxins by bacteria associated with Alexandrium spp. (Dinophyta)in culture. Appl Environ Microbiol. 1997;63:239–245. doi: 10.1128/aem.63.1.239-245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross EM, Wolk CP, Jüttner F. Fisherellin, a new allelochemical from the freshwater cyanobacterium Fisherella muscicola. J Phycol. 1991;27:686–692. [Google Scholar]
- Hagmann L, Jüttner F. Fischerellin A, a novel photosystem-II-inhibiting allelochemical of the cyanobacterium Fischerella muscicola with antifungal and herbicidal activity. Tetrahedron Lett. 1999;36:6539–6542. [Google Scholar]
- Jones GJ, Bourne DG, Blakeley RL, Doelle H. Degradation of the cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat Toxins. 1994;2:228–235. doi: 10.1002/nt.2620020412. [DOI] [PubMed] [Google Scholar]
- Kearns KD, Hunter MD. Green algal extracellular products regulate antialgal toxin production in a cyanobacterium. Environ Microbiol. 2000;2:291–297. doi: 10.1046/j.1462-2920.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- Kearns KD, Hunter MD. Toxin-producing Anabaena flos-aquae induces settling of Chalmidomonas reinhardtii, a competing motile alga. Microbiol Ecol. 2001;42:80–86. doi: 10.1007/s002480000086. [DOI] [PubMed] [Google Scholar]
- Keating KI. Allelopathic influence on blue-green bloom sequence in a eutrophic lake. Science. 1977;196:885–886. doi: 10.1126/science.196.4292.885. [DOI] [PubMed] [Google Scholar]
- Keating KI. Blue-green algal inhibition of diatom growth: transition from mesotrophic to eutrophic community structure. Science. 1978;199:971–973. doi: 10.1126/science.199.4332.971. [DOI] [PubMed] [Google Scholar]
- Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes 2-Chroococcales. Arch Hydrobiol/Suppl 80 Algol Studies. 1986;43:157–226. [Google Scholar]
- Komarek J, Anagnostidis K. Modern approach to the classification system of cyanophytes 2-Nostocales. Arch Hydrobiol/Suppl 80 Algol Studies. 1989;56:247–345. [Google Scholar]
- Legrand C, Rengefors K, Fistarol GO, Graneli E. Allelopathy in phytoplankton – biochemical, ecological and evolutionary aspects. Phycologia. 2003;42:406–419. [Google Scholar]
- Loya S, Reshef V, Mizrachi E, Silberstein C, Rachamim Y, Carmeli S, Hizi A. The inhibition of the reverse transcriptase of HIV-1 by the natural sulfoglycolipids from Cyanobacteria: contribution of different moieties to their high potency. J Nat Prod. 1998;61:891–895. doi: 10.1021/np970585j. [DOI] [PubMed] [Google Scholar]
- Moore RE, Cheuk C, Patterson GML. Hapalindoles: new alkaloids from the blue-green alga Hapalosiphon fontinalis. J Am Chem Soc. 1984;106:6456–6457. [Google Scholar]
- Moore RE, Cheuk C, Yang X-QG, et al. Hapalindoles, antibacterial and antimycotic alkaloids from the Cyanophyte Hapalosiphon fontinalis. J Org Chem. 1987;52:1036–1043. [Google Scholar]
- Nubel U, Garcia-Pichel F, Muyzer M. PCR Primers to amplify 16S rRNA genes from Cyanobacteria. Appl Environ Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccardi R, Frosini A, Tredici MR, Margheri MC. Bioactivity in free-living and symbiotic Cyanobacteria of the genus Nostoc. J Appl Phycol. 2000;12:543–547. [Google Scholar]
- Pignatello JJ, Porwoll J, Carlson RE, Xavier A, Gleason FK, Wood JM. Structure of the antibiotic cyanobacterin, a chlorine-containing g-lactone from the freshwater cyanobacterium Scytonema hofmanii. J Org Chem. 1983;48:4035–4038. [Google Scholar]
- Prescott GW. Algae of the Western Great Lakes Area. WM. C. Brown Company Publishers; Dubuque, IA: 1962. [Google Scholar]
- Rengefors K, Legrand C. Toxicity in Peridinium aciculiferum – an adaptive strategy to outcompete other winter phytoplankton. Limnol Oceanogr. 2001;46:1990–1997. [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- Schlegel I, Doan NT, de Chazal N, Smith GD. Antibiotic activity of new cyanobacterial isolates from Australia and Asia against green algae and Cyanobacteria. J Appl Phycol. 1999;10:471–479. [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Smith GD, Doan NT. Cyanobacterial metabolites with bioactivity against photosynthesis in Cyanobacteria, algae and higher plants. J Appl Phycol. 1999;11:337–344. [Google Scholar]
- Spurr AR. A low viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–42. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Todorova AK, Jüttner F. Nostocyclamide: a new macrocyclic, thizole-containing allelochemical from Nostoc sp. 31 (Cyanobacteria). J Org Chem. 1995;60:7891–7895. [Google Scholar]
- Volk R-B. Screening of microalgal culture media for the presence of algicidal compounds and isolation and identification of two bioactive metabolites, excreted by the Cyanobacteria Nostoc insulare and Nodularia harveyana. J Appl Phycol. 2005;17:339–347. [Google Scholar]
- Whitford LA, Schumacher GJ. A Manual of Fresh-Water Algae. Sparks Press; Raleigh, NC: 1984. [Google Scholar]