Abstract
Objective
Bromelain, a clinically used pineapple extract and natural product, has reported anti-inflammatory and immunomodulatory activities. The purpose of this study was to determine the effect of bromelain treatment in an ovalbumin (OVA)-induced murine model of allergic airway disease (AAD).
Methods
To establish AAD, mice were sensitized with intraperitoneal (i.p.) OVA/alum and challenged with daily OVA aerosols. Mice were treated i.p. with either saline, 2 or 6 mg/kg bromelain, twice daily for four consecutive days. Bronchoalveolar lavage leukocytes and cytokines, lung histology, airway hyperresponsiveness, and lymphocyte populations via flow cytometry were compared between groups.
Results
Bromelain treatment of AAD mice resulted in reduced total BAL leukocytes, eosinophils, CD4+ and CD8+ T lymphocytes, CD4+/CD8+ T cell ratio, and IL-13.
Conclusion
Bromelain attenuated development of AAD while altering CD4+ to CD8+ T lymphocyte populations. The reduction in AAD outcomes suggests that bromelain may have similar effects in the treatment of human asthma and hypersensitivity disorders.
Keywords: Cysteine protease, Asthma, Airway inflammation, CD4+ T cells, IL-13, Immunomodulation
1. Introduction
Asthma is one of the most common diseases of children and adults and is a major burden to the national heath-care system. The pathophysiology of asthma is associated with: airway inflammation, hyperresponsiveness and bronchospasm, mucus hypersecretion, and remodeling. The inflammatory aspects of the disease are complex with mast cells, dendritic cells, T and B lymphocytes, and eosinophils playing important roles. Increases in eosinophils and T lymphocytes in the bronchial mucosa and bronchoalveolar lavage (BAL) fluid are distinctive features of the inflammatory response in patients with asthma and appear to correlate with the severity of the disease [1-3].
The ovalbumin (OVA)-induced model of allergic airway disease (AAD) in mice has proven to be useful in studying specific mechanisms underlying airway inflammation [4-8]. The OVA-AAD model produces measurable outcomes similar to those found in humans including increased BAL total white blood cells, eosinophils, CD4+ T lymphocytes, inflammatory cytokines (IL-4, IL-5, and IL-13) and airway hyperresponsiveness to methacholine [9-11]. In addition, it has been shown that depletion of CD4+ T cells in the model prevents pulmonary eosinophilia and airway hyperresponsiveness [12].
Despite new therapeutic advances, the effective treatment and management of asthma still remains a high clinical priority. Botanicals such as Ananas comosus (pineapple) and their extracts (bromelain) have been used clinically as anti-inflammatory agents in rheumatoid arthritis, soft tissue injuries, colonic inflammation, chronic pain and asthma[13-19]. The major mechanism of action of bromelain appears to be proteolytic in nature, although evidence also suggests an immunomodulatory and hormone-like activity acting via intracellular signaling pathways. In vitro studies have shown that bromelain can inhibit PMA-induced T cell production of the Th2 cytokine IL-4, and to a lesser degree the Th1 cytokines IL-2 and IFN-γ via modulation of the extracellular regulated kinase-2 intracellular signaling pathway [20]. Bromelain has also been shown to reduce cell surface receptors such as the hyaluronan receptor CD44, which is associated with leukocyte migration and induction of proinflammatory mediators [21-23]. Also bromelain has been shown to significantly reduce CD4+ T lymphocytes, which are primary effectors in animal models of inflammation [24]. Despite increased uses of natural anti-inflammatory products such as bromelain, the in vivo efficacy and mechanisms of action have not been rigorously studied in asthma models of inflammation. The purpose of the present study was to determine whether bromelain treatment has anti-inflammatory/immunoregulatory effects in an OVA-induced murine model of AAD.
2. Materials and methods
2.1. Animals
Female C57BL/6J mice, 3-6 months of age and weighing 18-25 g, were purchased from the Jackson Laboratory (Bar Harbor, ME), and housed conventionally in plastic cages with corncob bedding. The animal room was maintained at 22-24°C with a daily light/dark cycle (light from 06:00 to 18:00 h). Chow and water were supplied ad libitum. The protocols for animal use were approved by the Animal Care Committee at the University of Connecticut Health Center.
2.2. Ovalbumin sensitization and aerosol exposure protocol
Mice were immunized with three weekly intraperitoneal(i.p.) injections of a suspension containing 25 μg of OVA (grade V, Sigma Chemical, St. Louis, MO) and 2 mg of aluminum hydroxide (alum) in 0.5 ml of saline. One week after the last injection the mice were exposed to 1% aerosolized OVA in physiologic saline, 1 h/day, for 3 days (acute AAD model) [9]. The mice were placed in plastic restraint tubes (Research and Consulting, Basel, Switzerland) for nose-only aerosol exposure. The aerosols were generated by a BANG nebulizer (CH Technologies, Westwood, NJ) into a 7.6-L inhalation exposure chamber to which restraint tubes were attached. Chamber airflow was 6 L/min, and aerosol particle size of OVA was monitored by gravimetric analysis with a Mercer cascade impactor (In-Tox Products, Moriarty, NM). The mass median aerodynamic diameter and geometric standard deviations were 1.4 and 1.6 μm, respectively. The estimated daily inhaled OVA dose approximated 30-40 μg/mouse. Twenty-four hours after the final aerosol exposure, the mice were killed by ketamine/xylazine overdose and exsanguination.
2.3. BAL fluid analysis
At sacrifice the lungs were lavaged in situ with five 1-ml aliquots of physiologic saline. The BAL fluid was centrifuged, the cellular pellet was washed, and the total nucleated cells were counted with a hemocytometer using trypan blue dye exclusion as a measure of viability. Leukocyte differentials were determined in BAL fluid using cytocentrifuged preparations stained with May-Grünwald/Giemsa. Stained BAL slide differentials were counted in a blind manner by three individuals. The remaining cells were analyzed phenotypically for T cell subpopulations using specific antibodies and fluorescence flow cytometry. BAL protein concentrations were measured in the supernatants by bicinchoninic acid (BCA) protein assay using bovine serum albumin as a standard (Pierce Biotechnology, Rockford, IL).
2.4. BAL-flow cytometry and immunofluorescence
BAL samples were analyzed via flow cytometry using the following fluorescence labeled monoclonal antibodies: CD4-PerCP (RM4-5), CD8a-FITC (53-6.7), CD25-PE (PC61), and CD44-AvCy5 (IM7) (Pharmingen, San Jose, CA). Samples were washed in PBS containing 0.2% bovine serum albumin and 0.1% NaN3. Aliquots containing 104-105 cells were incubated with 100 μl of appropriately diluted antibodies for 30 min at 4 °C. After staining, the cells were washed twice with the above PBS solution, and relative fluorescence intensities were determined on a 4-decade log scale by flow cytometric analysis using a FACSCalibur (Becton-Dickinson, San Jose, CA).
2.5. BAL-cytokine analysis
After centrifugation to remove cells the BAL fluid component was concentrated 10-fold using an Amicon Centriplus YM-10 filtration device (Millipore, Bedford, MA). Samples were analyzed for the Th2 cytokines IL-4, IL-5 and IL-13 using enzyme-linked immunosorbent assay (ELISA) kits (Pierce Biotechnology Rockford, IL; R&D Systems Minneapolis, MN) according to the manufacturer’s directions. The limits of detection for IL-4, IL-5 and IL-13 were 6, 5, and 1.5 pg/ml, respectively.
2.6. Histology
After sacrifice, unmanipulated (not subject to BAL) and non-inflated lungs from separate animals were removed, fixed with 10% buffered formalin, and processed in a standard manner. Tissue sections were stained with hematoxylin and eosin, Mallory’s trichrome for collagen and periodic acid-schiff for mucus detection [12]. Sections from all five lobes were examined in a blind manner by three individuals and scored 0-4, four reflecting the greatest pathological change which include: perivascular and peribronchial inflammation and thickening of the bronchial smooth muscle layer.
2.7. Airway hyperresponsiveness
Airway responses to methacholine were assessed by whole-body barometric plethysmography, using the Buxco system (Buxco Electronics, Troy, NY), as previously described [12]. Mice were evaluated for maximal enhanced pause (Penh), 12 h after the third OVA aerosol was administered. Mice were placed in individual chambers and exposed for 2 min to aerosolized saline or increasing concentrations of methacholine from 3 to 300 mg/ml. Respiratory system variables including tidal volume, respiratory frequency, inspiratory and expiratory times, and changes in box pressure were recorded before and during aerosolization and for 4 min after each exposure. The Penh value response to methacholine was recorded at each dose. The interpolated concentration of methacholine needed to increase the Penh value to 2 U or the “Penh-2” was calculated. The Penh-2 value was selected as the portion of the dose-response curve in which the greatest changes in sensitivity would be manifested.
2.8. Peripheral white blood cell count
Peripheral blood was collected via intravenous puncture and total white blood cells (WBCs) were isolated using the Unopette system (Becton-Dickinson Vacutainer Systems, Franklin Lakes, NJ) according to the manufacturer’s directions. Total WBC’s were counted on a hemocytometer and adjusted for the dilution factor.
2.9. Natural product bromelain
A stock solution of stem bromelain (EC 3.4.22.32) 2400 GDU/GM, Lot# 1458 (Vital Nutrients Middletown, CT) was made using 60 mg of bromelain dissolved in 250 ml physiologic saline. Two doses of bromelain (2 and 6 mg/kg) in 0.5 ml of physiological saline were prepared from the stock solution. Each animal received two, i.p. injections (0.5 ml) per day, 6-8 h apart, beginning 1 day prior to aerosolization. The dosages used were based on previous animal and human studies [13-18,20-24] and in vivo dose response studies performed in our laboratory. Bromelain was independently tested for authenticity, potency, and contamination (Vital Nutrients, Middletown, CT; Chroma-Dex, Santa Ana, CA).
2.10. Statistical analysis
Statistical comparisons between groups were made with analysis of variance using StatView 4.5 (Abacus Concepts, Berkeley, CA). Dose-response data and cytokine levels were compared by repeated measures analysis of variance. Changes in Penh-2 values before and after aerosol exposure were made by paired t tests and were compared between groups by repeated measures analysis of variance. All data were expressed as means standard error of the mean, and differences were considered significant at p<0.05.
3. Results
3.1. Bromelain treatment demonstrated no in vivo toxicity
Relative to naïve-saline treated mice, naïve mice treated i.p. with 6 mg/kg of bromelain, two times per day, for 4 consecutive days showed no observed differences in body weight, BAL protein concentration, peripheral WBC count or lung histology (Table 1). Similar results were obtained in comparisons made between the AAD mice and AAD-bromelain treated mice (data not shown).
Table 1.
Bromelain treatment demonstrates no in vivo toxicity
| Animal groups | Weight (gm) | BAL Total WBC (× 104) | BAL EOS (× 104) | BAL protein (μg/ml) | Whole blood WBC (mm3) | Histology PS score (grade 0-4) |
|---|---|---|---|---|---|---|
| Naïve-saline | 18.3 ± 0.4 | 0.95 ± 0.2 | 0.8 ± 0.3 | 90 ± 12 | 2228 ± 257 | <1.0 |
| Naïve-bromelain (6mg/kg) | 17.9 ± 0.3 | 0.87 ± 0.2 | 1.5 ± 0.2 | 123 ± 30 | 1775 ± 366 | <1.0 |
All outcome measures were collected at the time of animal sacrifice. There were no significant differences when comparing the naïve-saline and naïve-bromelain treated groups (unpaired t test). Data represent means ± SEM with 6-8 animalscompared per group.
3.2. Bromelain treatment reduced BAL leukocytes in AAD
As previously shown [9], AAD mice displayed significant increases in BAL total leukocytes (1.0 × 104 vs 346 × 104; p<0.001), eosinophils (0.8 × 104; vs 268 × 104; p<0.001), and lymphocytes (0.2 × 104 vs 14 × 104; p<0.001), as compared to naïve mice (Fig. 1). As compared to the AAD mice the AAD-bromelain treated mice had significant reductions in BAL total leukocytes (p<0.001, Fig. 1A) and eosinophils (p<0.002, Fig. 1B) at both the 2 and the 6 mg/kg bromelain doses. Total BAL lymphocytes in the AAD-bromelain group were also significantly reduced at the 2 mg/kg dose (p<0.01, Fig. 1C).
Fig. 1.
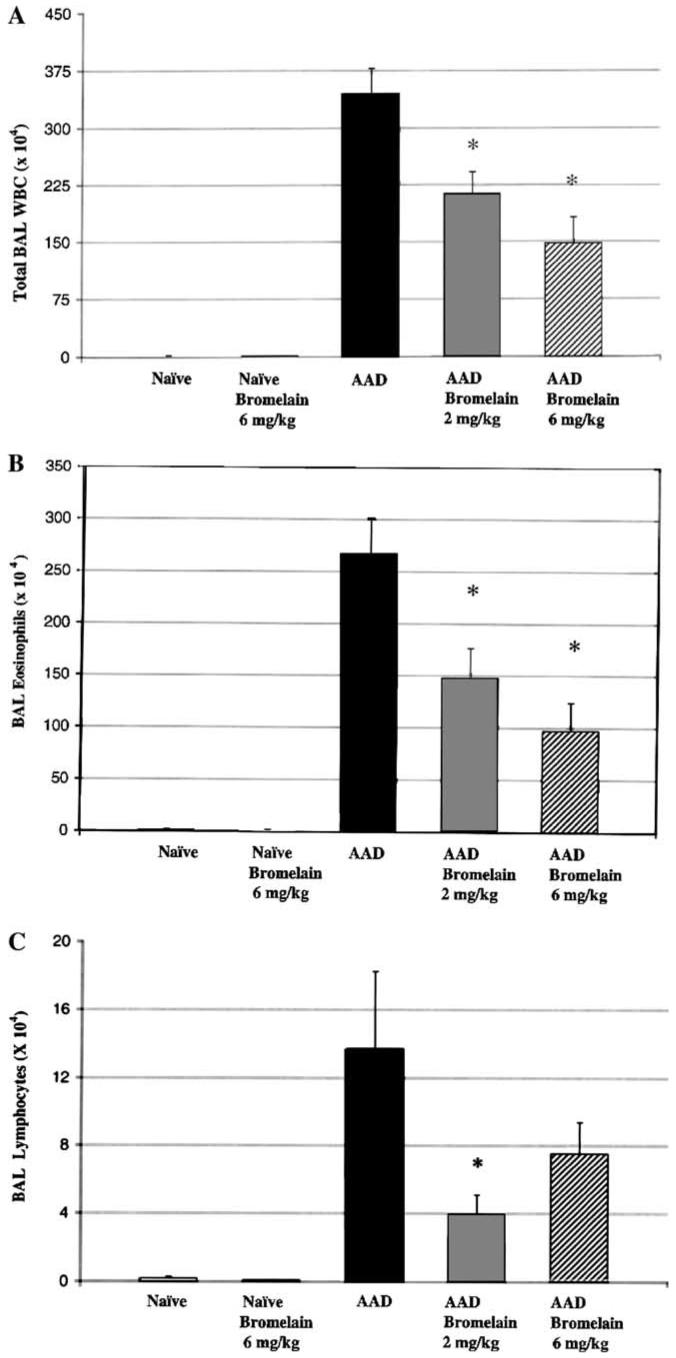
The effect of bromelain treatment on BAL leukocytes. Total leukocyte counts were significantly elevated in AAD mice as compared to naïve or naïve-bromelain treated mice. Bromelain treatment of AAD mice significantly reduced BAL total leukocytes (A), eosinophils (B), and lymphocytes (C). Naïve animals treated with 6 mg/kg Bromelain were similar to naïve animals in all comparisons. Data represent means ± SEM (n = 6-8 animals per group); *p ≤ 0.01 by ANOVA when comparing AAD-bromelain treated groups to the AAD group.
3.3. Bromelain treatment reduced CD4+, CD8+, and CD4+CD25+ T cells in AAD
AAD mice showed a significant increase in total CD4+ (p<0.01) and CD8+ (p<0.01) T cells when compared to naïve mice (Figs. 2A and B). As compared to AAD mice, CD4+ T cells were significantly reduced in both the 2 mg/kg (p<0.001) and 6 mg/kg (p<0.01) AAD-bromelain mice (Fig. 2A), whereas CD8+ T cells were significantly reduced only in the 2 mg/kg animals (p<0.05, Fig. 2B). The CD4:CD8 ratio was also altered from a ratio of 0.8 in the AAD mice to approximately 0.3 in both AAD-bromelain treated groups. When compared to the AAD mice, total CD4+CD25+ T cells were significantly reduced in both the 2mg/kg (p<0.01) and 6 mg/kg (p<0.02) AAD-bromelain treated groups (Fig. 2C).
Fig. 2.
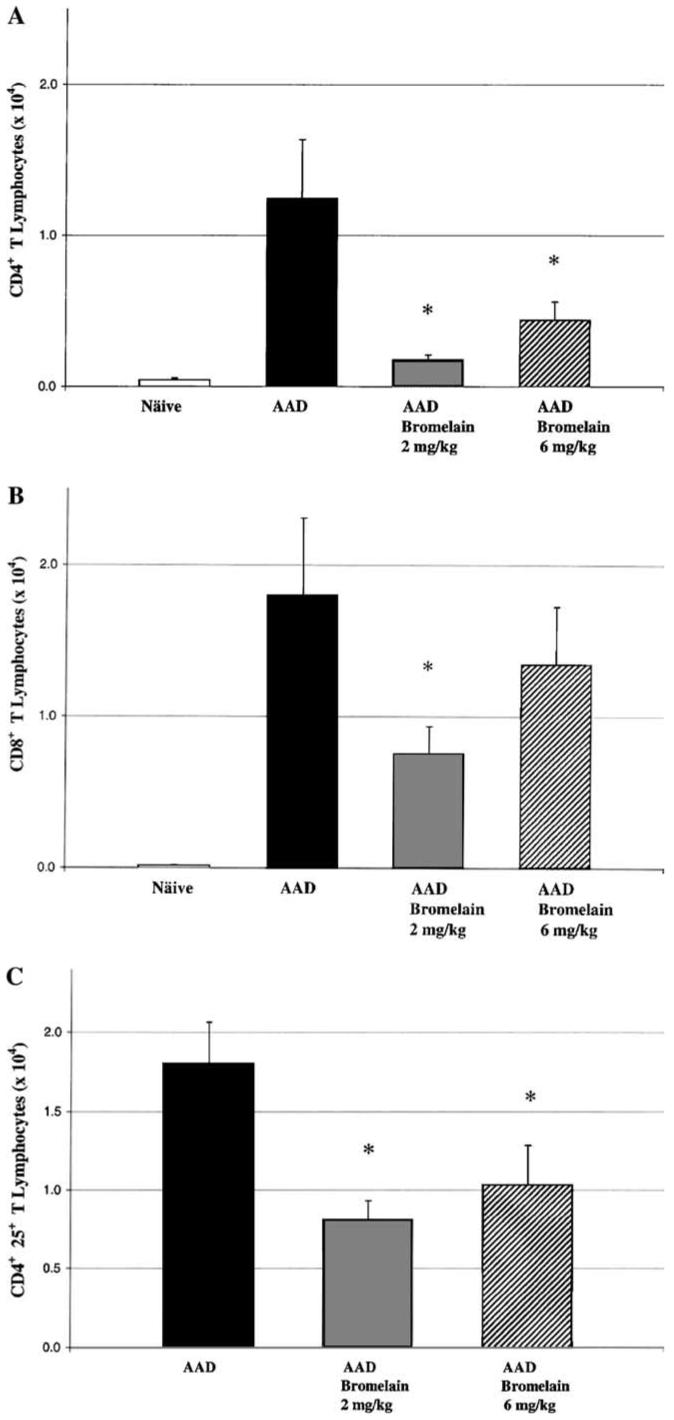
The effect of bromelain treatment on BAL CD4+ and CD8+ T lymphocytes. CD4+ T lymphocytes were significantly elevated in AAD mice as compared to naïve mice and declined significantly in both the 2 and the 6 mg/kg AAD-bromelain treatment groups as compared to the AAD group (A). There was a significant reduction in the CD8+ T lymphocytes only at the 2 mg/kg bromelain dose (B). Bromelain treatment significantly reduced BAL CD4+CD25+ T cells at both doses when compared to AAD (C). Data represent means ± SEM n = 6-8 animals per group); *p ≤ 0.05 by ANOVA when comparing naïve and AAD-bromelain treated groups to the AAD group.
3.4. Bromelain treatment had no effect on the expression of CD44
CD44 expression was measured by mean fluorescence intensity (MFI) of CD44 on BAL eosinophils and lymphocytes in the AAD and AAD-bromelain treatment groups. CD44 expression on BAL eosinophils in AAD mice (MFI: 667 U ± 112) was not significantly different from either the 2 mg/kg (MFI: 548 U ± 75) or the 6 mg/kg (MFI: 771 U ± 101) AAD-bromelain treated ± mice (Fig. 3). Similarly, the CD44 expression on BAL lymphocytes was unchanged when the AAD mice (MFI: 333 ± 56) were compared to either the 2 mg/kg (MFI: 276 ± 32) or 6 mg/kg (MFI: 394 U ± 59) AAD-bromelain treated mice.
Fig. 3.
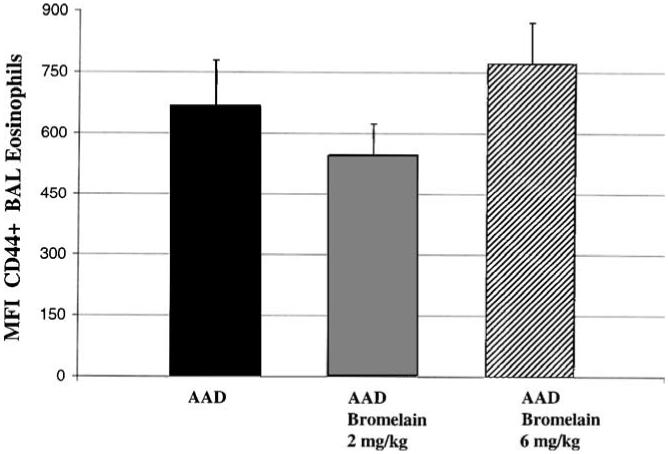
The effect of bromelain treatment on BAL eosinophil CD44 expression. There was no significant difference in the MFI of CD44+ on BAL eosinophils when comparing the AAD group to the AAD-bromelain treated groups. Data represent means ± SEM (n = 6-8 animals per group); statistical analyses by ANOVA.
CD44 expression was also measured on peripheral blood lymphocytes and eosinophils in naïve and naïve-bromelain treated mice (6 mg/kg). There were no differences when MFI of CD44 was compared between these mice (data not shown).
3.5. BAL IL-5 and IL-13 levels were altered in bromelain treated AAD mice
Inflammatory cytokines, IL-5 and IL-13, were detected at low limits in BAL from naïve mice. In comparison, the concentrations of both cytokines were significantly elevated in AAD mice IL-5 (p<0.0001; Fig. 4A) and IL-13 (p<0.0001; Fig. 4B). The 2 mg/kg AAD-bromelain mice had a significantly increased BAL IL-5 concentration as compared to AAD mice (p<0.02), whereas at the 6 mg/kg dose there was no significant change from AAD (Fig. 4A). IL-13 levels significantly decreased in the 6 mg/kg AAD-bromelain mice (p<0.005), as compared to the AAD mice (Fig. 4B). The BAL IL-4 concentration in both the AAD and Bromelain-AAD mice were below the detectable limits of the ELISA (data not shown).
Fig. 4.
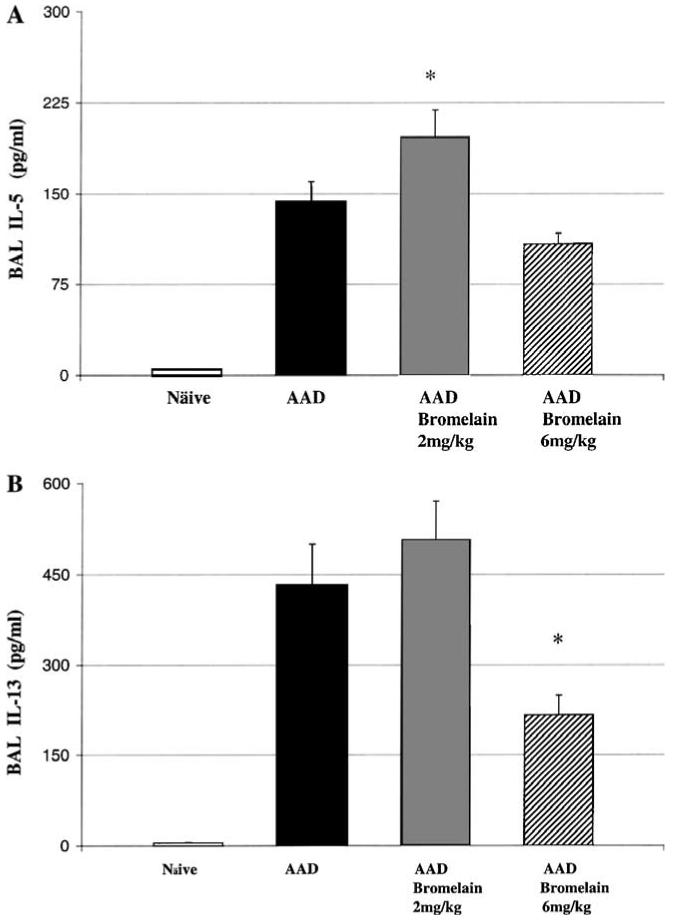
The effect of bromelain treatment on BAL IL-5 and IL-13 concentrations. A significant increase in IL-5 was observed in the 2 mg/kg bromelain treated AAD mice as compared to the AAD mice (A) and a significant decrease in IL-13 was observed when comparing the 6 mg/kg bromelain treated AAD mice to the AAD mice (B). Data represent means ± SEM (n = 6-8 animals per group); *p ≤ 0.05 by ANOVA.
3.6. Bromelain treatment did not affect lung pathology
Qualitative histological evaluations were made on unmanipulated, uninflated formalin-fixed lungs from separate groups of AAD and AAD-bromelain treated mice stained with hematoxylin and eosin, Mallory’s trichrome and periodic acid-schiff. As previously reported [9], AAD mice develop pathologic changes, which include perivascular and peribronchial inflammation characterized by infiltrates of lymphocytes, plasma cells and eosinophils. Pathologic changes were mild at this early 3 day time point and not significantly different when comparing the Pathology score in AAD (PS 1.5), AAD-bromelain 2 mg/kg (PS 1.4), and AAD-bromelain 6 mg/kg (PS 1.6) comparisons. Naïve (PS<1.0) and naïve-bromelain 6 mg/kg (PS<1.0) treated groups did not display differences in pathology and both presented less qualitative pathology when compared to AAD groups.
3.7. Bromelain treatment did not affect the airway hyperresponsiveness to methacholine challenge
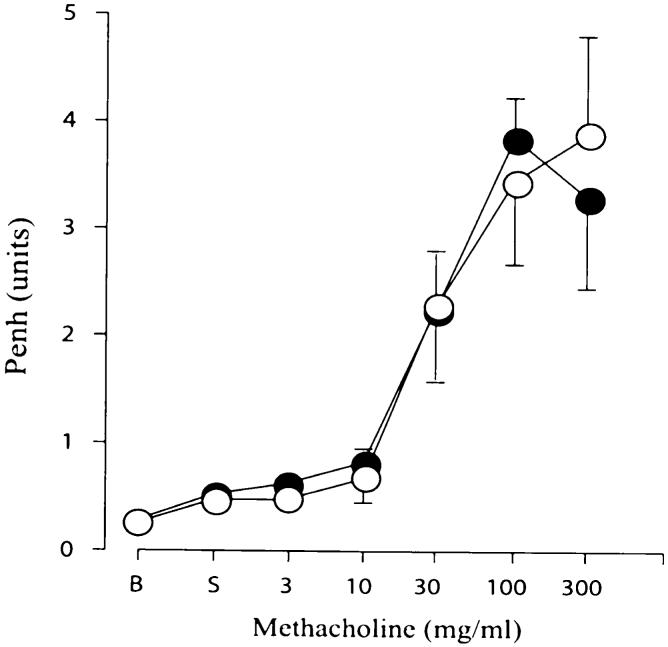
Airway responses to methacholine were assessed by whole-body barometric plethysmography using the Buxco system. Sensitivity to aerosolized methacholine was evaluated using increasing concentrations of methacholine from 3 to 300 mg/ml. The enhanced pause (Penh) response did not differ between the 6 mg/kg AAD-bromelain treated mice (Fig. 5). In addition, sensitivity to methacholine (Penh-2 value) was also similar between the two AAD groups (bromelain 28.1±9 mg/ml versus control 40.5±29.7 mg/ml).
Fig. 5.
The effect of bromelain treatment on airway hyperresponsiveness to methacholine challenge. Penh responses to aerosolized methacholine did not differ between AAD-bromelain treated mice (filled circles) and AAD mice (empty circles) over increasing concentrations of methacholine from 3 to 300 mg/ml.
4. Discussion
Bromelain a cysteine protease, has been shown to have anti-inflammatory effects in other animal disease models such as EAE and inflammatory bowel disease [18,32]. This study is the first to demonstrate the anti-inflammatory and immunomodulating effects of the natural product, bromelain, in a murine model of AAD.
Bromelain treatment was found to be well tolerated and non-toxic in both naive and AAD mice. There were no significant changes in body weight, BAL protein concentration, BAL total WBC, BAL eosinophils, peripheral blood WBC count or lung histology when comparing animals from naïve to naïve-bromelain treated groups (Table 1). Bromelain treatment significantly reduced the primary outcomes of murine AAD: total BAL leukocytes (eosinophils and lymphocytes), IL-13, CD4+ T cells, CD8+ T cells and CD4+CD25+ T cells, while also altering the CD4+/CD8+ ratio. These findings indicate that systemic bromelain treatment reduced an allergen induced localized airway inflammatory process. These results add to the continued characterization of bromelain and its use in the management of T cell mediated inflammatory conditions, such as asthma and autoimmunity [32].
Antigen-activated CD4+ T cells, such as those investigated during this study, have been shown to induce many of the characteristic features of asthma, including the secretion of cytokines such as IL-4, IL-5, and IL-13, which regulate mucus production, inflammation and adhesion molecules [25-27]. CD4+ T cells have also been shown to express high levels of CD44, an adhesion molecule involved with trafficking of antigen activated lymphocytes and granulocytes through the extracellular matrix [28,29]. CD4+ T cells expressing high levels of CD44 accumulate in the lung in murine models of asthma [30] and stimulation through CD44 aids in activating eosinophils [31]. Bromelain treatment has previously been shown to reduce the surface expression of CD44 without cellular toxicity [23,24,33-35]. Thus, the reduction in BAL CD4+, CD8+, and CD4+CD25+ T cells and eosinophils noted in the AAD-bromelain treated mice may have been due to the cleavage and reduction of CD44. However, there was no significant difference observed in the CD44 expression on isolated BAL eosinophils (Fig. 3) or T lymphocytes (data not shown) evaluated via flow cytometry. In addition, the CD44 expression on peripheral blood T lymphocytes cells did not differ when naïve mice were compared to naïve-bromelain treated mice (data not shown). Numerous investigators have demonstrated the effectiveness of CD44 cleavage by bromelain or bromelain containing compounds in in vitro systems [18,21-24,33,34]. The fact that CD44 expression of peripheral cells or those recovered from BAL did not change suggests that cells may have either: (1) re-established the CD44 receptor, (2) the in vivo response may be limited to the tissue matrix and the dynamic alteration in CD44 expression may be difficult to detect in in vivo samples [23,32], or (3) bromelain may not cleave CD44 well in vivo. Bromelain has been previously shown to be inactivated by β2-macroglobulin thus inhibiting its ability to cleave CD44 in vivo [23,36].
To further investigate mechanisms by which bromelain treatment could alter AAD, the Th2 cytokines IL-5 and IL-13 were measured in BAL. IL-13 levels were significantly reduced in the AAD-bromelain (6 mg/kg) mice as compared to the ADD mice, whereas a significant increase in IL-5 was observed in AAD-bromelain (2 mg/kg) mice. Bromelain has not previously been shown to effect IL-13, but Phlogenzyme, a bromelain containing preparation, has been shown to increase the production of IL-5 from splenic T cells in an experimental model of encephalomyelitis [32]. Since IL-5 has been shown to mediate the recruitment of eosinophils [25], our data would suggest that IL-5 was not the primary mechanism by which BAL eosinophils were reduced. The reduction in BAL IL-13 levels would suggest a beneficial therapeutic effect via decreased mucus production. However, histological lung tissue evaluation for mucus (PAS stain) was not able to differentiate any differences in PAS positive cells. The variation in cytokine responses with the two doses of bromelain, compared with the consistent reduction in airway inflammation, suggests that the inhibitory effect of bromelain on murine AAD may not be directly mediated by the modulation of cytokine levels.
In summary, bromelain treatment has been shown to inhibit and modulate critical components of the allergic airway disease response in this murine model, which includes influx of lymphoctyes and eosinophils into the lung, reduction of CD4+, CD8+, and CD4+CD25+ T lymphocytes, and BAL IL-13 levels. Botanicals such as Ananas comosus and their extracts (bromelain) are in current use as anti-inflammatory agents [37-39] and require more rigorous evaluation in both basic science models and in properly designed clinical trials [40-42]. The present study utilized a well established OVA-induced model of AAD [9-11]. The exact mechanism by which bromelain reduced BAL CD4+ and CD8+ T cells and eosinophils in this model remains elusive but does not appear to be related to the cleavage of CD44, or BAL IL-5 levels.
Acknowledgments
We thank the members of the Pulmonary Research Consortium, Mellisa Pensa, Dr. Anurag Singh and Sharale Walker for their technical assistance and Dr. Enrico P. Liva of Vital Nutrients for providing the stem bromelain extract and botanical medicine expertise. This work was supported by sponsored research grants: NIH/NCCAM FG32-AT001569; NIH/AI R01 HL-43573.
Footnotes
Grant support: This research was supported by NIH/NCCAM FG32-AT001569 and NIH/AI R01 HL-43573.
References
- [1].Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J. Allergy Clin. Immunol. 1991;88:935–942. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- [2].Caramori G, Pandit A, Papi A. Is there a difference between chronic airway inflammation in chronic severe asthma and chronic obstructive pulmonary disease? Curr. Opin. Allergy Clin. Immunol. 2005;5:77–83. doi: 10.1097/00130832-200502000-00014. [DOI] [PubMed] [Google Scholar]
- [3].Tillie-Leblond I, Gosset P, Tonnel AB. Inflammatory events in severe acute asthma. Allergy. 2005;1:23–29. doi: 10.1111/j.1398-9995.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- [4].Kennedy JD, Hatfield CA, Fidler SF, Winterrowd GE, Haas JV, Chin JE, Richards IM. Phenotypic characterization of T lymphocytes migrating into the lung tissue and airway lumen after antigen inhalation in sensitized mice. Am. J. Respir. Cell Mol. Biol. 1995;12:613–623. doi: 10.1165/ajrcmb.12.6.7766426. [DOI] [PubMed] [Google Scholar]
- [5].Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez C, Siegelman MH, Cybulsky M, Gutierrez-Ramos JC. Eosinophil recruitment to the lung in a mouse model of allergic inflammation. The role of T cells, chemokines and adhesion molecules. J. Clin. Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Renz H, Smith R, Henson JE, Ray BS, Irvin CG, Gelfand EW. Aerosolized antigen exposure without adjuvant causes increased IgE production and increased airway responsiveness in the mouse. J. Allergy Clin. Immunol. 1992;89:1127–1138. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- [7].Holt PG, Rose AH, Batty JE, Turner KJ. Induction of adjuvant-independent IgE responses in bred mice: primary, secondary and persistent IgE responses to ovalbumin and ovomucoid. Int. Arch. Allergy Appl. Immun. 1981;65:42–50. doi: 10.1159/000232736. [DOI] [PubMed] [Google Scholar]
- [8].Kung TT, Jones H, Adams GK, Umland SP, Kreutner W, Egan RW, Chapman RW, Watnick AS. Characterization of a murine model of allergic pulmonary inflammation. Int. Arch. Allergy Immunol. 1994;105:83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- [9].Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, Whiteley HE, Thrall RS. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am. J. Pathol. 1999;154:1191–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR) gamma delta and TCR alpha beta lymphocytes in a murine model of asthma. Am. J. Respir. Cell. Mol. Biol. 2000;2:218–225. doi: 10.1165/ajrcmb.22.2.3620. [DOI] [PubMed] [Google Scholar]
- [11].Schramm CM, Puddington L, Wu C, Guernsey L, Gharaee-Kermani M, Phan SH, Thrall RS. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am. J. Pathol. 2004;1:295–304. doi: 10.1016/S0002-9440(10)63119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gavett SH, Chen F, Finkelman X, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyper-reactivity and pulmonary eosinophilia. Am. J. Respir. Cell. Mol. Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- [13].Kelly GS. Bromelain: a literature review and discussion of its therapeutic applications. Altern. Med. Rev. 1996;1:243–257. [Google Scholar]
- [14].Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001;58:1231–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Taussig SJ, Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J. Ethnopharmacol. 1988;22:191–203. doi: 10.1016/0378-8741(88)90127-4. [DOI] [PubMed] [Google Scholar]
- [16].Cooreman WM, Scharpe S, Demeester J, Lauwers A. Bromelain, biochemical and pharmacological properties. Pharm. Acta Helv. 1976;4:73–97. [PubMed] [Google Scholar]
- [17].Izaka KI, Yamada M, Kawano T, Suyama T. Gastrointestinal absorption and anti-inflammatory effect of bromelain. Jpn. J. Pharmacol. 1972;4:519–534. doi: 10.1254/jjp.22.519. [DOI] [PubMed] [Google Scholar]
- [18].Hale LP, Greer PK, Trinh CT, Gottfried MR. Treatment with oral bromelain decreases colonic inflammation in the IL-10 deficient murine model of inflammatory bowel disease. Clin. Immunol. 2005;5:783–793. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- [19].Jaber R. Respiratory and allergic diseases: from upper respiratory tract infections to asthma. Prim. Care. 2002;2:231–261. doi: 10.1016/s0095-4543(01)00008-2. [DOI] [PubMed] [Google Scholar]
- [20].Mynott TL, Ladhams A, Scarmato P, Engwerda CR. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J. Immunol. 1999;163:2568–2575. [PubMed] [Google Scholar]
- [21].Engweda CR, Andrew D, Ladhams A, Mynott TL. Bromelain modulates T cell and B cell immune responses in vitro and in vivo. Cell. Immunol. 2001;210:66–75. doi: 10.1006/cimm.2001.1807. [DOI] [PubMed] [Google Scholar]
- [22].Eckert K, Grabowska E, Stange R, Schneider U, Eschmann K, Maurer HR. Effects of oral bromelain administration on the impaired immunocytotoxicity of mononuclear cells from mammary tumor patients. Oncol. Rep. 1999;6:1191–1199. doi: 10.3892/or.6.6.1191. [DOI] [PubMed] [Google Scholar]
- [23].Hale LP, Greer PK, Sempowski GD. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin. Immunol. 2002;104:183–190. doi: 10.1006/clim.2002.5254. [DOI] [PubMed] [Google Scholar]
- [24].Manhart N, Akomeah R, Bergmeister H, Spittler A, Ploner M, Roth E. Administration of proteolytic enzymes bromelain and trypsin diminish the number of CD4+ cells and the interferon-gamma response in Peyer’s patches and spleen in endotoxemic balb/c mice. Cell. Immunol. 2002;2:113–119. doi: 10.1016/s0008-8749(02)00019-9. [DOI] [PubMed] [Google Scholar]
- [25].Li J, Saito H, Crawford L, Inman MD, Cyr MM, Denburg JA. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005;114:386–396. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hogan MB, Weissman DN, Hubbs AF, Gibson LF, Piktel D, Landreth KS. Regulation of eosinophilopoiesis in a murine model of asthma. J. Immunol. 2003;171:2644–2651. doi: 10.4049/jimmunol.171.5.2644. [DOI] [PubMed] [Google Scholar]
- [27].Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Arch. Allergy Immunol. 2004;135:173–186. doi: 10.1159/000080899. [DOI] [PubMed] [Google Scholar]
- [28].DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J. Exp. Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- [30].Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: co-conspirators or independent triggers of allergic respiratory pathology? J. Allergy Clin. Immunol. 2001;107:945–957. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- [31].Ohkawara Y, Tamura G, Iwasaki T, Tanaka A, Shirato K. Activation and transforming growth factor-β production in eosinophils by hyaluronan. Am. J. Respir. Cell. Mol. Biol. 2000;23:444–451. doi: 10.1165/ajrcmb.23.4.3875. [DOI] [PubMed] [Google Scholar]
- [32].Targoni OS, Tary-Lehmann M, Lehmann PV. Prevention of murine EAE by oral hydrolytic enzyme treatment. J. Autoimmune. 1999;12:191–198. doi: 10.1006/jaut.1999.0271. [DOI] [PubMed] [Google Scholar]
- [33].Hale LP. Proteolytic activity and immunogenicity of oral bromelain within the gastrointestinal tract of mice. Int. Immunopharmacol. 2004;2:255–264. doi: 10.1016/j.intimp.2003.12.010. [DOI] [PubMed] [Google Scholar]
- [34].Tysnes BB, Maurer HR, Porwol T, Probst B, Bjerkvig R, Hoover F. Bromelain reversibly inhibits invasive properties of glioma cells. Neoplasia. 2001;6:2469–2479. doi: 10.1038/sj.neo.7900196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kleef R, Delohery TM, Bovbjerg DH. Selective modulation of cell adhesion molecules on lymphocytes by bromelain protease. Pathobiology. 1996;64:339–346. doi: 10.1159/000164070. [DOI] [PubMed] [Google Scholar]
- [36].Castell JV, Friedrich G, Kuhn CS, Poppe GE. Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. Am. J. Physiol. 1997;273:139–146. doi: 10.1152/ajpgi.1997.273.1.G139. [DOI] [PubMed] [Google Scholar]
- [37].Ammon HP. Boswellic acids (components of frankincense) as the active principle in treatment of chronic inflammatory diseases. Wien. Med. Wochenschr. 2002;152:373–378. doi: 10.1046/j.1563-258x.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- [38].Lemay M, Murray MA, Davies A, Roh-Schmidt H, Randolph RK. In vitro and ex vivo cyclooxygenase inhibition by a hops extract. Asia Pac. J. Clin. Nutr. 2004;13:S110. [Google Scholar]
- [39].Darshan S, Doreswamy R. Patented anti-inflammatory plant drug development from traditional medicine. Phytother. Res. 2004;18:343–357. doi: 10.1002/ptr.1475. [DOI] [PubMed] [Google Scholar]
- [40].Katiyar SK. Silymarin and skin cancer prevention: anti-inflammatory. antioxidant and immunomodulatory effects, Int. J. Oncol. 2005;26:169–176. [PubMed] [Google Scholar]
- [41].Bielory L. Complementary and alternative interventions in asthma, allergy, and immunology. Ann. Allergy Asthma Immunol. 2004;93:S45–S54. doi: 10.1016/s1081-1206(10)61486-x. [DOI] [PubMed] [Google Scholar]
- [42].Kralovec JA, Power MR, Liu F, Maydansk E, Ewart HS, Watson LV, Barrow CJ, Lin TJ. An aqueous Chlorella extract inhibits IL-5 production by mast cells in vitro and reduces ovalbumin-induced eosinophil infiltration in the airway in mice in vivo. Int. Immunopharmacol. 2005;4:689–698. doi: 10.1016/j.intimp.2004.11.016. [DOI] [PubMed] [Google Scholar]