Abstract
Mice sensitized to OVA and subjected to acute OVA aerosol exposures develop allergic airway disease (AAD). However, chronic continuous Ag exposure results in resolution of AAD and the development of local inhalational tolerance (LIT). Because we have previously observed the persistence of B cells in the bronchoalveolar lavage (BAL) and hilar lymph nodes (HLN) at the resolution stage of this model, we investigated the role of B cells in the modulation of AAD. Although B cell-deficient mice developed LIT, adoptive transfer of HLN B cells from LIT mice to OVA-sensitized recipients resulted in attenuated AAD following subsequent OVA aerosol exposure, as determined by reduced BAL leukocytosis and eosinophilia, decreased tissue inflammation, and absent methacholine hyper-responsiveness. In similar adoptive transfer studies, HLN B cells from AAD mice were without effect. The protection transferred by LIT HLN B cells was Ag specific and was associated with accumulation of Foxp3+ T regulatory cells regionally in BAL and HLN, but not systemically in the spleen. Fluorescent labeling of LIT HLN B cells before adoptive transfer demonstrated that these cells had the capacity to migrate to local inflammatory sites. In vitro assessment demonstrated that the LIT HLN B cells exerted this regulatory effect via TGF-β induced conversion of CD4+CD25− T effector cells into functionally suppressive CD4+CD25+Foxp3+ T regulatory cells. These findings illustrated a novel regulatory role for regional B cells in AAD and suggested a possible contributory role of B cells, along with other cell types, in the establishment of LIT.
The OVA-induced mouse model of allergic airway disease (AAD)3 is a Th2-mediated disease characteristic of human asthma (1). Mice sensitized to OVA Ag and then exposed to OVA aerosols for 3–10 days develop Ag-specific IgE production, airway and lung tissue eosinophilia, increased pulmonary B and T lymphocytes, airway mucus hypersecretion, and hyper-responsiveness to inhaled methacholine. However, the continued exposure of mice to OVA aerosols for 42 days results in resolution of the pulmonary AAD responses (2, 3). We have termed this resolution of disease as local inhalational tolerance (LIT), because in comparison to acutely exposed animals, the chronically challenged mice show minimal airway and tissue eosinophilia, diminished airway lymphocytosis, no airway hyper-reactivity, and altered cytokine profiles, while still maintaining high systemic levels of OVA-specific IgE and IgG1.
The ability to remain tolerant to inhaled Ags or to re-establish homeostasis once inflammation has occurred has led to an increased interest in immunoregulatory immune cells (4), particularly CD4+CD25+Foxp3+ regulatory T (Treg) cells (4, 5). CD4+CD25+Foxp3+ Treg cells consist of both thymus-derived natural occurring Treg cells and peripheral inducible Treg cells. It is thought that natural Tregs respond primarily to self Ags, whereas induced Tregs react to environmental Ags (6). Murine AAD responses to dust mite Ag are potentiated in mice subjected to anti-CD25-mediated Treg cell depletion before or during intra-tracheal exposure to house dust mite extract (7). Conversely, adoptive transfer of OVA-specific CD4+CD25+ Treg cells to OVA-sensitized mice attenuates the AAD response to allergen challenge (5). In sensitized rats chronically exposed to OVA aerosols, ongoing T cell activation and AAD are attenuated by CD4+CD25+Foxp3+ Treg cells, which appear in the airway mucosa and regional lymph nodes within 24 h of initiation of exposure (8). The mechanisms of peripheral Treg induction are incompletely understood, but include TGF-β (9, 10), IL-2 (11), and negative CTLA-4 costimulation (12). We have recently observed an accumulation of CD4+CD25+ Foxp3+ Treg cells in the draining lymph nodes of the lung that correlated with the appearance of LIT, suggesting an important role for these cells in the resolution of AAD (13).
Recent evidence indicates that in addition to Treg cells, regulatory types of B cells (Breg) capable of suppressing inflammation and enhancing tolerance are also specifically induced under inflammatory conditions (14–16). These Breg cells have functions independent to that of Ab production, such as secretion of the immunoregulatory cytokines IL-10 and TGF-β (17–19). Despite resolution of the airway eosinophilia, we have observed persistent expansion of T and B lymphocytes in bronchoalveolar lavage (BAL) fluid and regional hilar lymph nodes (HLN) from mice with LIT (2, 3). Other investigators have implicated putative Breg cells in the development of nasal tolerance to aeroallergens (20) and in the suppression of AAD (21). Therefore, the purpose of this study was to investigate the role of B cells in the resolution of AAD and induction of LIT in this OVA model. We identified a novel mechanism whereby B cells isolated from HLNs of LIT mice could contribute to the regulation of airways inflammation and the generation of LIT.
Materials and Methods
Mice
Studies were performed in C57BL/6J (Ly5.1) mice (The Jackson Laboratory), C57BL/6J (Ly5.2) mice (Charles River), wild-type BALB/c mice, and congenic BALB/c B cell-deficient JhD−/− mice (generously provided by Dr. T. V. Rajan, University of Connecticut Health Center, Farmington, CT). Mice were housed conventionally in the animal facility at the University of Connecticut Health Center in accordance with institutional and Office of Laboratory Animal Welfare guidelines. All experimental procedures were approved by the institution’s Center for Laboratory Animal Care.
Induction of AAD and LIT
Mice were immunized with 3 weekly i.p. injections of 0.5 ml saline containing 25 μg OVA (grade V; Sigma-Aldrich) and 2 mg of aluminum hydroxide. Then, 1 wk after the last injection, the mice were exposed to 1% aerosolized OVA in physiologic saline for 1 h/day for either 7 (AAD) or 42 days (LIT), as previously described (2, 3). The aerosols were generated by a BANG nebulizer (CH Technologies) into a 7.6 L nose-only inhalation exposure chamber to which individual restraint tubes were attached. The estimated daily inhaled OVA dose approximated 30 – 40 μg/mouse, with particle mass median aerodynamic diameter and geometric SDs of 1.4 and 1.6 μm, respectively. The protocol for immunization of mice to BSA (Sigma-Aldrich) was identical with OVA, with 3 weekly i.p. injections of 25 μg BSA in aluminum hydroxide. BSA-immunized mice were then exposed to 1% aerosolized BSA in physiologic saline for 7 days (AAD) using a different BANG nebulizer and aerosol chamber. A total of 24 h after the final aerosol exposure, the mice were killed by ketamine/xylazine overdose and subsequent exsanguination.
Reagents and cell isolation
B cells were positively selected using a mouse CD19+ isolation kit, and CD4+CD25− T effector cells (Teffs) were isolated with a CD4+CD25+ mouse Treg isolation kit (Miltenyi Biotech). Isolation yields were >95% for CD19+ cells and CD4+CD25− cells. For cell proliferation and stimulation assays, anti-CD3 (145-2C11) and anti-CD28 (37.51) were purchased from BD Pharmingen. Abs used for flow analysis were anti-CD19-PerCp (1D3), anti-CD4-PE-Cy7 (L3T4), anti-CD25-PE (PC61), anti-I-A/I-E (2G9) purchased from BD Pharmingen, anti-Foxp3 (FJK-16s), anti-Ly5.2 (104), anti-B220 (RA3– 6B2), anti-CD86 (GL1), neutralizing Ab to IL-10 (JES5–16E3) obtained from eBioscience, and anti-TGF-β latency-associated protein (LAP) (TGF-β1), pan-specific neutralizing Ab to TGF-β purchased from R&D Systems. CFSE dye was purchased from Molecular Probes.
Flow cytometry
Cell samples were washed in PBS containing 0.2% BSA and 0.1% NaN3. Aliquots containing 104–106 cells were incubated with 100 μl of appropriately diluted Abs for 30 min at 4°C. After staining, the cells were washed with the above PBS solution, and relative fluorescence intensities were determined on a 4-decade log scale by flow cytometric analysis, using an LSRII (BD Biosciences). For the identification of Foxp3+ Treg cells, cells stained with anti-CD3e, anti-CD4, and anti-CD25 were permeabilized using fixation/permeabilization buffer, following the manufacturer’s protocol, and stained using anti-Foxp3-allophycocyanin (FJK-16s) with corresponding isotype controls, IgG2a-allophycocyanin (eBioscience). Flow cytometry analysis was done using FACSDiva (BD Biosciences) and FLOWJO (Tree Star) software.
AAD assessment
Lung injury was assessed by BAL fluid analysis, histological evaluation and by responsiveness to methacholine. BAL was performed in situ with saline. Total nucleated cells were counted, and leukocyte differentials were determined from cytocentrifuged preparations stained with May-Grünwald/Giemsa. For lung histology, unmanipulated lungs from animals not subjected to BAL were removed, fixed with 10% buffered formalin, processed in a standard manner and tissue sections stained with H&E. Sections from all five lobes were examined in their entirety in a blinded manner. Functional assessment of AAD was performed by assessing changes in work of breathing in response to inhaled methacholine, as measured in unrestrained mice using whole body plethsymography (Buxco Electronics). Changes in sensitivity to methacholine were defined by shifts in the interpolated concentration of methacholine needed to increase the Penh value to 2 units (~5-fold increase greater than baseline) in individual animals before and after OVA aerosol challenge (3).
Adoptive transfer
CD19+ cells (1 × 106) isolated from HLNs from AAD or LIT mice were injected through a tail vein into two groups of sensitized mice. Control sensitized mice were injected in a similar manner with vehicle (physiological saline). A total of 2 days after the injection, the three groups of mice received OVA aerosols for 7 days. The mice were sacrificed 24 h after the last aerosol and the lungs were assessed for the development of AAD. For homing studies, CD19+ cells isolated from HLNs of LIT mice were labeled with CFSE (5 μM), and 1 × 106 viable cells were transferred into OVA-sensitized recipients.
Treg induction assay
Freshly isolated CD4+CD25− Teff cells were obtained from the spleen of naive mice. The purity of the Teff cells was >95%. The intracellular expression of Foxp3 was assessed preculture on a representative sample of Teff cells. Teff cells (1 × 106) were cocultured for 5 days with viable irradiated (2600 Rad) CD19+ B cells from spleens or HLNs of AAD or LIT mice (1 × 106) and soluble anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml). The cultures were in RPMI 1640 supplemented with 10% FCS and 50 μM 2-ME in 24-well plates. After 5 days in culture, the cells were taken out from the wells and the intracellular expression of Foxp3 was assessed on gated CD4+ T cells. Similar assays were performed in the presence of in vitro cytokine blockade with neutralizing Abs to either IL-10 (1 μg/ml) or pan-specific TGF-β (20 μg/ml). All studies were performed in triplicate.
Treg functional assay
Ly5.1 CD4+CD25− T cells (1 × 106) were cultured with irradiated AAD or LIT B cells (1 × 106) and soluble anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) in 24-well plates as described above to induce Treg cells. After 5 days of culture, viable T cells (2 × 105) from the B cell cocultures (B cell induced T cells) were taken and cultured with responder CFSE-labeled (2.5 μM) Ly5.2 CD4+CD25− T cells (5 × 104) from spleens of naive Ly5.2 animals in 96-well round-bottom plates. CFSE labeling was performed by incubating Ly5.2 CD4+CD25− T cells (1 × 107) with 2.5 μM CFSE at 37°C for 15 min. CFSE-labeled cells were washed three times with 10% FCS. Soluble anti-CD3 (1 μg/ml) and irradiated splenocytes (1 × 105) from Ly5.2 mice were used to stimulate the responder cells. After 60 h of coculture, the cells were harvested from the plate, stained with CD4 and Ly5.2 Abs, and observed for proliferation via CFSE dilution on flow cytometry.
Immunofluorescence and confocal imaging
CD19+ cells were isolated from the HLNs of AAD and LIT mice, placed in suspension, and stained with biotinylated anti-LAP followed by streptavidin Alexa Fluor 647. Another sample of LIT HLN CD19+ cells were stained with isotype control Ab for LAP (biotinylated goat IgG) followed by streptavidin Alexa Fluor 647. Cells were washed twice in PBS containing 0.2% BSA and 0.1% NaN3. Labeled cells were fixed in 3% paraformaldehyde, cytospun onto glass slides, and sealed with a cover slip. Confocal images were collected for the presence of LAP expression on CD19+ B cells using a Zeiss LSM 510 confocal laser scanning microscope with a 63 × 1.4 numerical aperture oil immersion objective (Zeiss) by recording in the 647-nm channel.
Statistical analysis
Statistical comparisons between group means were made with ANOVA followed by Fisher’s protected least significant difference test between possible pair-wise combinations of means (StatView 4.5; Abacus Concepts or JMP7; SAS Institute). Methacholine dose-response relationships were compared with single-factor and multifactor, repeated measures ANOVA, and sensitivities were compared pre- and post-OVA aerosol exposures by paired t tests. In all comparisons, p < 0.05 was used to determine statistical significance.
Results
Adoptive transfer of LIT HLN B cells inhibited BAL leukocytosis and eosinophilia at AAD
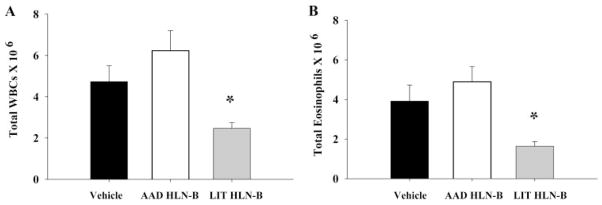
To examine the role of B cells in the resolution of AAD, adoptive transfer studies were done to determine whether CD19+ B cells isolated from the HLN of LIT mice (LIT HLN B cells) could inhibit the development of AAD in this OVA-induced model. Recipient mice, which received LIT HLN B cells, developed an attenuated AAD as compared with mice that received vehicle control or AAD HLN B cells. As previously reported, BAL leukocytosis and eosinophilia are prominent features of this OVA-induced model and AAD in general (2, 3). Numbers of BAL white blood cells (WBCs) (Fig. 1A) and eosinophils (Fig. 1B) were dramatically increased in the BAL of mice that received either vehicle alone (473 ± 77 × 104 WBCs; 391 ± 81 × 104 eosinophils) or AAD HLN B cells (623 ± 97 × 104 WBCs; 490 ± 76 × 104 eosinophils). In contrast, BAL WBCs (247 ± 27 × 104) and eosinophils (164 ± 23 × 104) were significantly reduced in mice that received LIT HLN B cells as compared with mice that received either vehicle alone or AAD HLN B cells (p < 0.05 for all comparisons).
FIGURE 1.
Adoptive transfer of LIT HLN B cells (1 × 106) into OVA-sensitized mice before OVA aerosol challenge attenuated BAL leukocytosis (A) and eosinophilia (B) after 7 days of OVA aerosol exposures. Total BAL leukocytes and BAL eosinophils were elevated in vehicle (black bars) and AAD HLN B cell transfer groups (white bars) but were decreased in the LIT HLN B cell transfer group (shaded bars). The data is expressed as mean ± SEM from eight animals per group; *, p < 0.05 compared with vehicle and AAD HLN B cell groups.
Adoptive transfer of LIT HLN B cells reduced lung inflammation and methacholine responsiveness
The reduction in airway leukocytosis and eosinophilia seen in mice given LIT HLN B cells was paralleled by reduced lung inflammation and methacholine responsiveness in these animals. Qualitative histological evaluation of lung tissue from mice that received adoptive transfer of LIT HLN B cells appeared to have reduced peribronchial and perivascular cellular (lymphocytes and esosinophils) infiltration as compared with mice that received vehicle alone or AAD HLN B cells (Fig. 2A). We also compared changes in sensitivity to inhaled methacholine before and after OVA aerosol challenge in control mice, mice receiving AAD HLN B cells, and mice receiving LIT HLN B cells. These studies used Penh measurements from whole-body plethysmography of unrestrained animals to measure Penh. This approach measures changes in breathing pattern and expiratory timing, and therefore provides evidence of work of breathing, which in turn can be related to a number of specific parameters of respiratory mechanics, including tissue resistance, tissue compliance, and airway resistance (22). Although Penh does not always reflect changes in lung mechanics in all situations, we have shown that changes in Penh responses track changes in invasive pulmonary mechanics in mice during the transition from AAD to LIT (2, 3) and distinguish Th2-cytokine driven airway and lung inflammation from Th1-cytokine-mediated inflammation (23). Similar to what we have reported previously (3), sensitivity to methacholine increased 2.3-fold (95% confidence interval 1.7–3.2-fold; p = 0.0002 by paired t test) with development of AAD in control mice (Fig. 2B). Transfer of AAD HLN B cells did not affect this change, with a 2.8-fold increase (95% C.I. 1.1–7.3-fold; p = 0.039) seen with AAD in AAD-HLN B cell-recipient animals. In contrast, transfer of LIT HLN B cells not only attenuated cellular and histologic changes associated with AAD, but it also abrogated any increase in methacholine sensitivity (0.75-fold change, 95% C.I. 0.44–1.3-fold; p = 0.28).
FIGURE 2.

Adoptive transfer of LIT HLN B cells into OVA-sensitized mice before OVA aerosol challenge attenuated lung inflammation (A) and methacholine sensitivity (B) after 7 days of OVA aerosol exposures. A, Qualitative histological evaluation demonstrated that adoptive transfer of LIT HLN B cells reduced lung inflammation with subsequent OVA aerosol exposure. Representative histopathology sections demonstrated decreased peribronchial and perivascular inflammation in LIT HLN B cell transfer mice compared with vehicle and AAD HLN B cell transfer mice. Images are at ×20 magnification; the bar in each plate represents 100 microns. B, Adoptive transfer of LIT HLN B cells prevented the development of increased methacholine responsiveness following OVA aerosol exposures. Methacholine sensitivity was compared in sensitized animals before (open symbols) and after 3 days of OVA aerosol exposures (filled symbols). Baseline, preaerosol sensitivity to methacholine did not differ in control mice (left; n = 11), mice receiving AAD HLN B cells (middle; n = 8), and mice receiving LIT HLN B cells (right; n = 13). Both control and AAD HLN B cell-recipient animals developed increased sensitivity to methacholine after OVA aerosols, as demonstrated by leftward shifts in the Penh dose-response relationships. In contrast, transfer of LIT HLN B cells prevented the development of increased methacholine sensitivity.
LIT HLN B cell inhibition of AAD was Ag specific
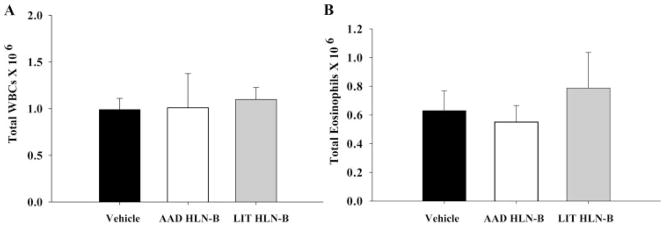
Because the generation of LIT in our model of AAD requires the constant presence of Ag (3), we next examined the role of Ag-specific B cells in the resolution of AAD. Adoptive transfer studies were done to determine whether OVA-specific LIT HLN CD19+ B cells could inhibit the development of AAD in mice sensitized and then aerosol-exposed to a different allergen (BSA). BSA-sensitized recipient mice, which received OVA-LIT HLN B cells, did not develop an attenuated AAD as compared with mice that received vehicle control or control B cells (OVA-AAD HLN B cells). Numbers of BAL WBCs (Fig. 3A) and eosinophils (Fig. 3B) were similar in the BAL of mice that received either vehicle alone (0.97 ± 0.2 × 106 WBCs; 0.61 ± 0.18 × 106 eosinophils), OVA-AAD HLN B cells (1.0 ± 0.4 × 106 WBCs; 0.57 ± 0.15 × 106 eosinophils), or OVA-LIT HLN B cells (1.1 ± 0.2 × 106 WBCs; 0.77 ± 0.25 × 106 eosinophils).
FIGURE 3.
Protection conferred by LIT HLN B cells was Ag-specific. Adoptive transfer of LIT HLN B cells (1 × 106) from OVA aerosolized animals into BSA-sensitized mice before BSA aerosol challenge did not attenuate BAL leukocytosis (A) or eosinophilia (B) after 7 days of BSA aerosol exposures. Data represent mean ± SEM responses; n = 5 in each group).
B cell-deficient mice develop AAD and LIT
The demonstration that B cells from regional draining lymph nodes of the lung (HLN) could modulate AAD led us to examine whether B cells were critical to the establishment of LIT. B cell-deficient JhD−/− mice and congenic, wild-type BALB/c mice were sensitized to OVA and then exposed to OVA aerosols for 7 or 42 days. Despite the absence of B cells and OVA-specific IgE, JhD−/− mice exposed acutely to OVA aerosols developed AAD characterized by increased BAL leukocytes and eosinophils, to a degree similar to that seen in the wild-type animals (Table I). Similarly, following chronic OVA exposure for 42 days, wild-type and JhD−/− mice developed LIT, with marked attenuation of BAL leukocytes and eosinophils. Although B cells did not appear to be essential for the establishment of AAD or LIT, it is likely that mechanisms of AAD and LIT differ in B cell-deficient vs wild-type animals, and these studies do not rule out the contribution of B cells along with other cell types to the regulation of airways inflammation.
Table I.
BAL leukocyte distributions in B cell-deficient and wild-type control micea
| Mice (n) | BAL WBCs (×106) | Eos (×106) | Neutrophils (×106) | Lymphs (×106) | Macrophages (×106) |
|---|---|---|---|---|---|
| Wild-type AAD | 6.44 ± 1.98 | 4.45 ± 0.99 | 0.71± 0.62 | 0.58 ± 0.33 | 0.68 ± 0.29 |
| JhD−/− AAD | 5.83 ± 1.92 | 3.22 ± 1.10 | 1.30 ± 0.50 | 0.58 ± 0.47 | 0.80 ± 0.38 |
| Wild-type LIT | 0.80 ± 0.14 | 0.15 ± 0.06 | 0.15 ± 0.03 | 0.13 ± 0.04 | 0.37 ± 0.14 |
| JhD−/− LIT | 1.21 ± 0.25 | 0.08 ± 0.03 | 0.17 ± 0.06 | 0.15 ± 0.06 | 0.83 ± 0.16 |
B cell-deficient mice developed AAD and LIT. JhD−/− mice and congenic, wild-type BALB/c mice were sensitized to OVA and then exposed to OVA aerosols for 7 or 42 days. JhD−/− mice exposed acutely to OVA aerosols developed AAD characterized by increased BAL leukocytes and eosinophils, to a degree similar to that seen in the wild-type animals. Similarly, following chronic OVA exposure for 42 days, wild-type and JhD−/− mice developed LIT, with marked attenuation of BAL leukocytes and eosinophils.
Adoptive transfer of LIT HLN B cells increased CD4+ Foxp3+ Treg cells locally but not systemically
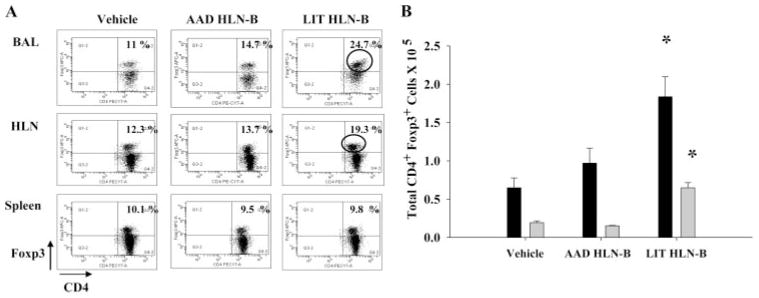
To further elucidate mechanisms whereby B cells could modulate AAD/LIT in normal, intact animals, we further characterized BAL lymphocyte profiles by flow cytometric analysis. BAL cells from mice with attenuated AAD following the adoptive transfer of LIT HLN B cells demonstrated significantly (p < 0.03) increased percentages of CD4+Foxp3+ Treg cells (mean 21.4%) as compared with AAD mice that received vehicle alone (mean 11.4%) or AAD HLN B cells (mean 14.6%; Fig. 4A). The increased percentage of CD4+Foxp3+ Treg cells in LIT HLN B cell-recipient animals was specific to regional lung compartments, namely the BAL and HLN (vehicle 12.3%; AAD HLN B cells 13.7%; and LIT HLN B cells 19.3%), but was not observed in systemic compartments such as the spleen (vehicle 10.1%; AAD HLN B cells 9.5%; and LIT HLN B cells 9.8%). Total Treg cell numbers in BAL and HLN also differed between the three adoptive transfer groups at AAD (Fig. 4B). Mice that received LIT HLN B cells had significantly increased (p < 0.05) total Treg cells in the BAL (6.2 ± 1.1 × 104) and HLN (1.8 ± 0.4 × 105) at AAD compared with mice that received either vehicle alone (BAL 2.1 ± 0.3 × 104 or HLN 0.64 ± 0.2 × 105) or AAD HLN B cells (BAL 1.61 ± 0.1 × 104 or HLN 0.88 ± 0.4 × 105; Fig. 3B). These findings suggested that the protective effect on AAD conferred by the adoptive transfer of HLN B cells from LIT mice was in part due to a corresponding increase in the number of Treg cells at local sites of inflammation.
FIGURE 4.
Adoptive transfer of LIT HLN B cells increased CD4+Foxp3+ Treg cells locally but not systemically. A, Representative FACS dot-plot demonstrating the percentage of CD4+Foxp3+ Treg cells in the BAL, HLN, and spleen after OVA aerosol exposures. Compared with vehicle and AAD HLN B cell transfer mice, the LIT HLN B cell transfer group demonstrated increased CD4+Foxp3+ cells in the BAL and HLN but not in the spleen after OVA aerosol exposures. B, The total number of Treg cells were significantly higher in the BAL (shaded bars) and HLN (black bars) of LIT HLN B cell transfer group compared with the other groups (*, p < 0.05; n = 4 in each group).
LIT HLN B cells were retained in draining lymph nodes of the lung following adoptive transfer
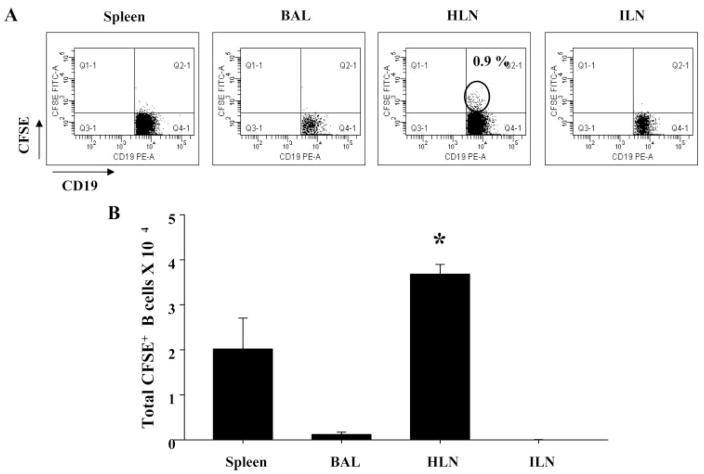
To assess whether the regional increase in Treg cells in the lung compartments was associated with homing of LIT HLN B cells to pulmonary sites of inflammation, CFSE-labeled LIT HLN B cells were adoptively transferred to OVA-sensitized recipients. These mice were then exposed to OVA aerosols for 7 days and later sacrificed. Flow cytometric analysis on gated CD19+ B cells in different local vs systemic sites demonstrated an increased percentage of CFSE+ B cells (0.9%) in the HLN of recipient mice (Fig. 5A) compared with other sites where the CFSE+ B cells were barely detectable. The total number of CFSE+ B cells was also significantly elevated in HLNs of recipient mice inhibited compared with other local vs systemic sites (p < 0.05; Fig. 5B). These findings suggested that the HLNs of LIT mice were enriched in a Breg cell population that had the ability to return to active sites of inflammation and regulate immune responses in vivo.
FIGURE 5.
Adoptively transferred LIT HLN B cells migrated to sites of inflammation. CFSE-labeled LIT HLN B cells (1 × 106) were injected i.v. into OVA-sensitized mice before OVA aerosol challenge. A, After 7 days of OVA aerosol exposure, CFSE+ B cells were found only at local sites (HLN) of recipient animals. B, Total numbers of CFSE+ B cells were significantly increased in regional draining lymph nodes (HLN) but were absent in BAL and inguinal lymph nodes (ILN) (*, p < 0.05 vs other groups).
LIT B cells were functionally mature and were a source of TGF-β
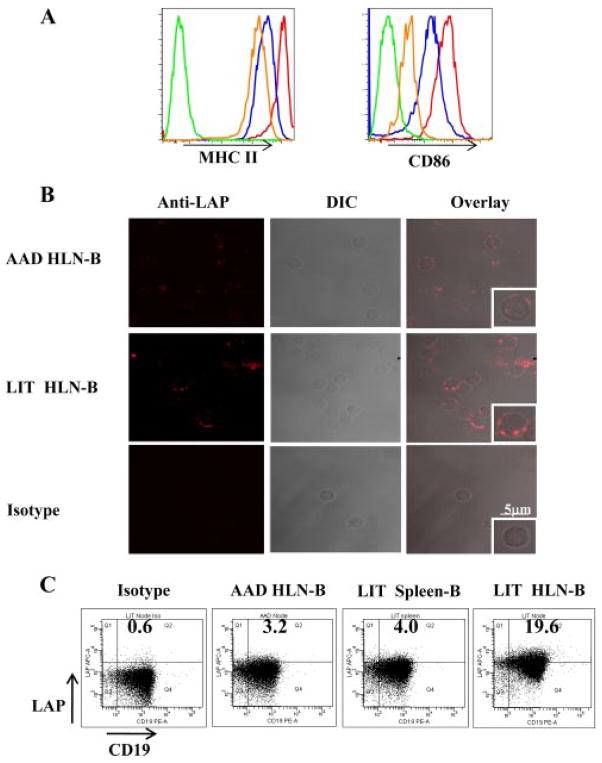
Because immature B cells can be tolerogenic and attenuate CD4+ T cell-mediated immune responses (24), we examined whether LIT HLN B cells differed from AAD HLN B cells in relation to the expression of MHC class II and costimulatory molecules. Compared with expression on HLN B cells in sensitized mice, the expressions of MHC II and CD86 were increased on AAD HLN B and LIT HLN B cells (Fig. 6A). Thus, HLN B cells were activated during AAD and remained activated with chronic Ag exposure at LIT. Although both AAD and LIT B cells were mature, the differences observed in our adoptive transfer experiments demonstrated that the AAD and LIT B cells were functionally different, with LIT HLN B cells possessing a suppressive phenotype. B cells have been shown to be capable of producing immunosuppressive cytokines, such as IL-10 (18, 19) and TGF-β (25, 26). Because we have previously shown that the development of LIT was not impaired in IL-10 deficient mice (27), we focused on the potential role of TGF-β in mediating the above in vivo and in vitro effects of LIT HLN B cells. Confocal imaging of isolated LIT HLN B cells labeled with biotinylated anti-LAP expressed increased amounts of staining as compared with AAD HLN B cells (Fig. 6A). Direct immunofluorescence evaluation of AAD HLN B cells and LIT HLN B cells demonstrated positive LAP staining in the LIT population only, with the isotype control Ab being negative (Panel 1). Direct phase contrast illustrated the location of the cells (Panel 2), and the overlay of both showed definitively that the staining was cell associated (Panel 3 low power and Panel 4 (inset) high power). Similarly, flow cytometric analysis revealed that a considerable population of LIT HLN B cells demonstrated enhanced LAP staining, as compared with AAD HLN B cells and LIT spleen B cells (Fig. 6B). Thus, the enhanced expression of TGF-β in B cells during LIT was regional, because it occurred in HLN B cells but not spleen B cells.
FIGURE 6.
LIT HLN B cells were mature and expressed TGF-β. A, Representative histograms of surface MHC II and CD86 expression on B cells isolated from HLNs from sensitized (orange), AAD (red), and LIT mice (blue), compared with isotype (green). LIT HLN B cells were mature, as they had comparable levels of MHC II expression and CD86 expression to AAD HLN B cells. B, CD19+ B cells isolated from AAD and LIT HLNs were stained with biotinylated anti-LAP followed by streptavidin Alexa Fluor 647 for confocal imaging and streptavidin allophycocyanin for flow cytometric analysis. Representative example of specific LAP staining, differential interference contrast (DIC) images, and overlay of staining and DIC images at multi- and single-cell levels. Top, AAD HLN B cells showed minimal staining for LAP. Middle, LIT HLN B cells demonstrate substantial LAP staining, which was localized to the surface (overlay). Lower, LIT HLN B cell isotype staining, DIC image, and overlay. C, Representative dot-plots of LAP expression on CD19+ cells demonstrated increased expression of anti-LAP (TGF-β1) on LIT HLN-B, whereas AAD HLN B cells and LIT spleen B cells had little anti-LAP expression, compared with isotype.
LIT HLN B cells induced formation of CD4+CD25+Foxp3+ Treg cells via a TGF-β-dependent mechanism
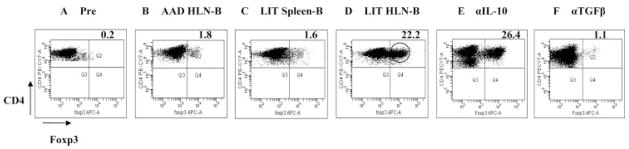
The demonstration that LIT HLN B cells expressed high levels of TGF-β, a cytokine known to induce Treg formation (9, 10, 28, 29), suggested that the in vivo increase in Treg cells following adoptive transfer of LIT HLN B cells was mediated by B cell-derived TGF-β. To assess this potential mechanism of action, we next investigated whether LIT HLN B cells could induce formation of CD4+CD25+Foxp3+ Treg cells in vitro from CD4+CD25− Teff cells taken from spleens of naive wild-type mice. Before coculture, only a small number (0.3%) of these Teff cells expressed Foxp3 (Fig. 7A). A total of 5 days of coculture with AAD HLN B cells (Fig. 7B) or LIT spleen B cells (Fig. 7C) failed to increase expression of Foxp3 in the Teff cells (1.9 ± 0.1 and 1.6 ± 0.1%, respectively; p > 0.2 vs naive). In contrast, coculture with LIT HLN B cells induced a high percentage (18.6 ± 1.8%) of the Teff cells to become Foxp3+ (p < 0.0005 vs naive or vs other groups; Fig. 7D). To determine whether the induction of Treg cells by LIT HLN B cells was dependent on the secretion of immunosuppressive cytokines like IL-10 or TGF-β by LIT HLN B cells, we added neutralizing Abs to these cytokines in the in vitro coculture system. Culture of the Teff cells with LIT HLN B cells in the presence of blocking Ab to IL-10 failed to block the induction of Foxp3 expression (26.4%; Fig. 7E). However, the abrogation of TGF-β via a pan-specific neutralizing Ab in culture led to failure of LIT HLN B cells to induce Foxp3 expression (1.1%; Fig. 7F). These results demonstrated that TGF-β was integral to the generation of Treg cells by LIT HLN B cells.
FIGURE 7.
LIT HLN B cells induced formation of CD4+CD25+Foxp3+ Treg cells from CD4+CD25− T effector cells. A, Precoculture, wild-type CD4+CD25− T cells did not express Foxp3. After in vitro coculture with B cells for 5 days in the presence of anti-CD3 and anti-CD28 Ab, CD4+CD25−Foxp3− T cells were evaluated for conversion to Foxp3+ T cells via flow cytometry (B and C). Coculture of wild-type Teff cells with AAD HLN B cells or LIT spleen B cells resulted in minimal intracellular Foxp3 expression on gated CD4+ cells. D, Coculture of wild-type Teff cells with LIT HLN B cells resulted in a greater than 10-fold increase in Foxp3 expression, as compared with the other B cell populations. E, Addition of IL-10 blocking Ab to the coculture did not abrogate the induction of Foxp3 expression. F, In vitro neutralization of TGF-β via a pan-specific TGF-β Ab inhibited Foxp3 induction by LIT HLN B cells. Results were representative of three independent experiments, each done in triplicate.
LIT HLN B cell-induced Treg cells were functional and suppressed the proliferation of Teff cells
To demonstrate that Treg cells generated by LIT HLN B cells were functionally suppressive in vitro, the converted T cells were cultured with CFSE-labeled responder CD4+CD25− Teff cells. Responder T cells alone did not proliferate in the absence of stimulation (0.7 ± 0.2% CFSE dilution; Fig. 8A) but proliferated well in response to anti-CD3 stimulation (23.9 ± 1.4%; Fig. 8B). Responder T cells also proliferated well in coculture with T cells previously cultured with AAD HLN B cells (35.0 ± 5.1%; p = 0.10 vs anti-CD3; Fig. 8C), suggesting that these B cell exposed T cells were primarily Teff cells. In contrast, responder T cells cocultured with T cells previously cultured with LIT HLN B cells proliferated poorly (10.4 ± 1.6%; p = 0.0016 vs anti-CD3; Fig. 8D), demonstrating suppressive abilities in this B cell exposed T cell population. The ability to induce suppressive Treg cells was regional, because T cells derived from LIT spleen B cell cocultures did not inhibit proliferation of responder T cells (34%, data not shown).
FIGURE 8.
Induced Foxp3+ Treg cells were functional and suppressed the proliferation of T effector cells. CD4+CD25− T cells from the spleen of Ly5.2 naive mice were labeled with CFSE (2.5 μM). A and B, CFSE-labeled responder Teff cells did not proliferate in unstimulated conditions but did in the presence of soluble anti-CD3 as demonstrated by the dilution of CFSE in the stimulated group. C, Ly5.1 CD4+CD25− T cells, cocultured with AAD HLN B cells as described, fail to suppress the proliferation of responder Teff cells. D, Ly5.1 CD4+CD25− T cells cocultured with LIT HLN B cells inhibited proliferation of Teff responder cells. Results were representative of two independent experiments, each done in duplicate.
Discussion
OVA-sensitized mice exposed to aerosolized OVA demonstrate a biphasic response, such that acute (3–14 day) OVA aerosol exposure elicits AAD while chronic (42 day) exposure results in resolution of AAD and apparent LIT. Although the airway eosinophilia and pulmonary inflammation resolve during LIT, regional expansion of T and B lymphocytes persists (2, 3). The recent description of Bregs in chronic models of inflammation (14–16) raised the consideration that subsets of such Breg cells could develop after the initiation of AAD and play a role in the resolution of disease.
The present study demonstrated a regulatory role for regional B cells in murine AAD. In contrast to transfer of AAD HLN B cells, the adoptive transfer of LIT HLN B cells inhibited the development of AAD in recipient mice. This phenomenon was Ag-specific, associated with regional increases in CD4+Foxp3+ Treg cells in the recipient animals, and correlated with migration of transferred B cells to regional lymph nodes of recipient mice. Interestingly, B cell-deficient JhD−/−mice were still capable of developing LIT. This observation is similar to what has been reported in B cell-deficient μMT mice (30) and could suggest that the inhibition of AAD by transfer of LIT HLN B cells was due instead to a different, LIT-specific unidentified cell type contaminating the transfer. This conclusion is unlikely, in that the transferred cells from both AAD and LIT HLNs were >95% B220+CD19+ cells and <0.3% CD3+ cells. B cell-knockout mice do exhibit defective nasal tolerance (20), but LIT differs from nasal tolerance in that LIT develops after a robust Th2-driven AAD response, whereas nasal tolerance prevents the generation of AAD when Ag is given intranasally to naive animals. It is likely that multiple regulatory mechanisms contribute to airway homeostasis. Different regulatory mechanisms may prevent development of AAD or promote its resolution to LIT. Similarly, different regulatory mechanisms may be activated in B cell-deficient vs wild-type animals (31, 32). The occurrence of LIT in B cell-deficient JhD−/−mice does not rule out a potential contributory role of suppressive B cells, along with other cell types, in the regulation of AAD and in the development or maintenance of LIT in intact animals.
The in vivo demonstration of a suppressive B cell phenotype found only in regional lymph nodes was supported by in vitro findings that LIT HLN B cells induced formation of functionally suppressive CD4+CD25+Foxp3+ Treg cells from CD4+CD25– Teff cells. The possibility of a contaminating Treg population expanding in the cultures was ruled out by the purity of the starting CD4+CD25− Teff cell population (>95% pure in all experiments) and the minimal expression of Foxp3 in the Teff cell population. This effect was specific to LIT HLN B cells, because it was not demonstrated by AAD HLN B cells, LIT spleen B cells, or LIT HLN non-B cell APCs. This regional effect, coupled with the specific homing of transferred HLN B cells back to pulmonary compartments, may account for the localized nature of LIT, such that lung inflammatory responses are inhibited while systemic IgE responses are maintained (2, 3). In contrast to AAD HLN B cells, LIT HLN B cells expressed high levels of TGF-β. Neutralization of TGF-β in vitro abrogated Treg generation by LIT HLN B cells, demonstrating the mechanistic role of TGF-β in this process. Collectively, these studies support a novel mechanism of regional immune regulation, mediated by B cell induction of Treg cells.
Tolerance to inhaled Ags has been shown to be mediated by IL-10 (33–35), as well as TGF-β (36–38). The present study focused on TGF-β-dependent mechanisms because of our previous demonstration that IL-10-deficient mice develop LIT (27). Nevertheless, we do not exclude the potential contribution of IL-10 in the development of tolerance to aeroallergens. IL-10 could originate from a Tr1 subset of Treg cells (39), dendritic cells (40), or potentially a different subset of Breg cells (18). Again, it is likely that a multitude of regulatory cell populations and compensatory regulatory mechanisms exist to counteract injurious inflammatory responses driven by constant Ag exposure (31, 32). The present study suggests that one of these regulatory mechanisms is the generation of a Breg population in regional lymph nodes. These local Breg cells are characterized by increased expression of TGF-β, and act to attenuate or abrogate the inflammatory response by TGF-β-dependent induction of functionally suppressive Treg populations from Teff cells. Interestingly, the local protective effect of Treg cells induced in the airway mucosa and regional lymph nodes is dependent on continuous Ag stimulation, because interruption of exposure leads to waning of Treg activity and recurrence of AAD in rats upon re-exposure to Ag (8). We have observed a similar process in mice, such that continuous OVA exposure induces LIT, but discontinuous exposure results in the failure to develop LIT and re-emergence of AAD (3).
B cells have also been shown to regulate the Ag presenting function and maturation of dendritic cells (16), and the possibility of B cells from LIT HLN influencing Ag presentation during the course of our model remains to be investigated. The concept of B cells in regional LIT HLNs contributing to the regulation of AAD is consistent with the recent description that such local regulatory networks exist in draining lymph nodes and regulate inflammatory responses at adjacent mucosal sites (41). Similarly, the development of tolerance to intranasal administration of Ag is dependent on B cells in regional lymphoid tissue (42). The localized nature of LIT, with preservation of systemic OVA-specific IgE responses, is consistent with this regional lymph node regulatory network and the role of local Breg cells in mediating the LIT response.
In conclusion, the present study identified a novel mechanism of regional immune regulation, whereby chronic Ag exposure results in the generation of a suppressive B cell subset in local lymph nodes. The suppressive activity of these Breg cells is Ag-specific and TFG-β-dependent. We have previously shown that LIT is dependent upon continuous Ag exposure and that disruption of Ag exposure results in the loss of LIT (2, 3). Whether this loss of LIT is associated with the disappearance of TGFβ+ B cells and/or CD4+CD25+Foxp3+ Treg cells remains to be determined. Further characterization of the lineage, function, and generation of these putative Breg cells may yield important insights into airway homeostatic responses and the establishment of tolerance to inhaled Ags.
Footnotes
This work was supported by National Institutes of Health/AI R01 HL-43573 and National Institutes of Health/National Center for Complementary and Alternative Medicine FG32-AT001569.
Abbreviations used in this paper: AAD, allergic airway disease; LIT, local inhalational tolerance; BAL, the bronchoalveolar lavage; HLN, hilar lymph nodes; WBC, white blood cell; Treg, T regulatory cell; Breg, B regulatory cell; DIC, differential interference contrast; Teff, T effector cell; LAP, TGF-βlatency-associated protein.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 2.Yiamouyiannis CA, Schramm CM, Puddington L, Stengel P, Baradaran-Hosseini E, Wolyniec WW, Whiteley HE, Thrall RS. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–1921. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schramm CM, Puddington L, Wu C, Guernsey L, Gharaee-Kermani M, Phan SH, Thrall RS. Chronic inhaled ovalbumin exposure induces antigen-dependent but not antigen-specific inhalational tolerance in a murine model of allergic airway disease. Am J Pathol. 2004;164:295–304. doi: 10.1016/S0002-9440(10)63119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umetsu DT, Dekruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212:238–255. doi: 10.1111/j.0105-2896.2006.00413.x. [DOI] [PubMed] [Google Scholar]
- 5.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408–414. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25 −naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 11.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25 −cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 12.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-β requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25− regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 13.Carson WF, IV, Guernsey LA, Singh A, Vella AT, Schramm CM, Thrall RS. Accumulation of regulatory T cells in local draining lymph nodes of the lung correlates with spontaneous resolution of chronic asthma in a murine model. Int Arch Allergy Immunol. 2008;145:231–243. doi: 10.1159/000109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 15.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 16.Serra P, Santamaria P. To “B” regulated: B cells as members of the regulatory workforce. Trends Immunol. 2006;27:7–10. doi: 10.1016/j.it.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J Exp Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 20.Tsitoura DC, V, Yeung P, DeKruyff RH, Umetsu DT. Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol. 2002;14:659–667. doi: 10.1093/intimm/dxf032. [DOI] [PubMed] [Google Scholar]
- 21.Lundy SK, Berlin AA, Martens TF, Lukacs NW. Deficiency of regulatory B cells increases allergic airway inflammation. Inflamm Res. 2005;54:514–521. doi: 10.1007/s00011-005-1387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanbing M, HayGlass KT, Becker AB, Fan Y, Yang X, Basu S, Srinivasan G, Simons FER, Halayko AJ, Peng Z. Novel recombinant interleukin-13 peptide-based vaccine reduces airway allergic inflammatory responses in mice. Am J Respir Crit Care Med. 2007;176:439–445. doi: 10.1164/rccm.200610-1405OC. [DOI] [PubMed] [Google Scholar]
- 23.Matson AP, Zhu L, Lingenheld EG, Schramm CM, Clark RB, Selander DM, Thrall RS, Breen E, Puddington L. Maternal transmission of resistance to development of allergic airway disease. J Immunol. 2007;179:1282–1291. doi: 10.4049/jimmunol.179.2.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croft M, Joseph SB, Miner KT. Partial activation of naïve CD4 T cells and tolerance induction in response to peptide presented by resting B cells. J Immunol. 1997;159:3257–3265. [PubMed] [Google Scholar]
- 25.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-β1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 26.Gonnella PA, Waldner HP, Weiner HL. B cell-deficient (μMT) mice have alterations in the cytokine microenvironment of the gut-associated lymphoid tissue (GALT) and a defect in the low dose mechanism of oral tolerance. J Immunol. 2001;166:4456–4464. doi: 10.4049/jimmunol.166.7.4456. [DOI] [PubMed] [Google Scholar]
- 27.Kabbur PM, Carson WF, IV, Guernsey L, Secor ER, Thrall RS, Schramm CM. Interleukin-10 does not mediate inhalational tolerance in a chronic model of ovalbumin-induced allergic airway disease. Cell Immunol. 2006;239:67–74. doi: 10.1016/j.cellimm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Park HB, Paik DJ, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-β-costimulated CD4+CD25− T cells. Int Immunol. 2004;16:1203–1213. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 29.Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P, Neurath MF. Transforming growth factor β induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680. doi: 10.1136/gut.2005.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jungsuwadeea P, Benkovszkya M, Dekanb G, Stingla G, Epsteina MM. Repeated aerosol allergen exposure suppresses inflammation in B-cell-deficient mice with established allergic asthma. Int Arch Allergy Immunol. 2004;133:40–48. doi: 10.1159/000075252. [DOI] [PubMed] [Google Scholar]
- 31.Niu N, Le Goff MK, Li F, Rahman M, Homer RJ, Cohn L. A novel pathway that regulates inflammatory disease in the respiratory tract. J Immunol. 2007;178:3846–3855. doi: 10.4049/jimmunol.178.6.3846. [DOI] [PubMed] [Google Scholar]
- 32.Van Hove CL, Maes T, Joos GF, Tournoy KG. Prolonged inhaled allergen exposure can induce persistent tolerance. Am J Respir Cell Mol Biol. 2007;36:573–584. doi: 10.1165/rcmb.2006-0385OC. [DOI] [PubMed] [Google Scholar]
- 33.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joetham A, Takada K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic responses depends on IL-10 induction of TGF-β. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 35.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane-bound TGF-β and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt-Weber CB, Blaser K. Regulation and role of transforming growth factor-β in immune tolerance induction and inflammation. Curr Opin Immunol. 2004;16:709–713. doi: 10.1016/j.coi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of IL-10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez D, Swirski FK, Yang TC, Fattouh R, Croitoru K, Bramson JL, Stampfli MR, Jordana M. Inhalation tolerance is induced selectively in thoracic lymph nodes but executed pervasively at distant mucosal and non-mucosal tissues. J Immunol. 2006;176:2568–2580. doi: 10.4049/jimmunol.176.4.2568. [DOI] [PubMed] [Google Scholar]
- 42.Kraal G, Samsom JN, Mebius RE. The importance of regional lymph nodes for mucosal tolerance. Immunol Rev. 2006;213:119–130. doi: 10.1111/j.1600-065X.2006.00429.x. [DOI] [PubMed] [Google Scholar]