Abstract
Mammary gland homeostasis and the lactation-to-involution switch are regulated by serotonin (5-hydroxytryptamine (5-HT)). Mammary epithelial tight junctions are physiological targets of 5-HT, and their disruption marks an early stage of mammary gland involution. In these studies, we have identified signal transduction mechanism employed by 5-HT during regulation of mammary gland transepithelial resistance. Transepithelial electrical resistance and tight junction protein architecture were studied in cultures of MCF10A human mammary epithelial cells. Serotonin had biphasic effects on mammary epithelial resistance. At lower concentrations and earlier time points, 5-HT potentiated epithelial transmembrane resistance, whereas at higher concentrations and later time points, 5-HT decreased transepithelial electrical resistance and disrupted tight junctions. Both the early and delayed actions of 5-HT were mediated by the 5-HT7 receptor through activation of Gs/cAMP. 5-HT induced the activities of both protein kinase A and p38 mitogen-activated protein kinase. Inhibition of p38 mitogen-activated protein kinase abrogated 5-HT-induced disruption of mammary epithelial tight junctions (the delayed effect). In contrast, inhibition of protein kinase A prevented the increased epithelial resistance in response to 5-HT (the transient effect). These studies imply an integrated set of mechanisms whereby transient, modest activation of 5-HT7 promotes tight junction integrity, and sustained 5-HT7 activation drives involution by disrupting tight junctions.
The complex and specialized functions of mammary glands has evolved to meet the specific nutritional needs of the newborn. The rapid and extensive development of the mammary gland leading to establishment of the highly energy-consuming lactation stage is balanced by the massive tissue remodeling and disintegration of mammary epithelial tissue during the involution stage (1–3). Dysregulation of these events has severe consequences for maternal health and infant nutrition and health as well as from the practical perspective of dairy production.
Under the influence of endocrine hormones like estrogen, progesterone, and prolactin, the mammary gland reaches the fully developed alveolar stage by the end of pregnancy. However, lactation (milk synthesis and secretion) is not established until parturition, characterized by a drop in systemic progesterone levels along with a surge in the levels of prolactin and glucocorticoids (4–8). Isolation of the alveolar lumen from interstitial and vascular spaces by closure of tight junctions is a critical cellular event that regulates milk synthesis and secretion (9–11). The importance of tight junction closure is displayed by the complex interplay between the endocrine hormones in regulating them and thus, in turn, regulating lactation (11–13).
Each alveolar unit of a lactating mammary gland is independently regulated in an autocrine-paracrine manner through a feedback system of local factors (14–16). Absence of a suckling stimulus results in accumulation of milk within alveolar units (milk stasis), which serves as a signal for the mammary gland to undergo involution. Involution occurs in two stages. The first stage is accompanied by extreme distension of the alveoli due to continued milk secretion, resulting in leakage of milk constituents into the interstitium (2, 17). This phase is reversible, because reintroduction of suckling will bring the gland back into lactation. The second stage, which is irreversible, is characterized by massive tissue remodeling and adipocyte redifferentiation and requires a drop in systemic levels of lactogenic hormones. Older studies showed that neither the distension of alveoli nor the mechanical pressure due to milk accumulation is sufficient to initiate involution (18, 19). Moreover, forced weaning along with systemic administration of lactogenic hormones interrupted the second stage but did not affect the timing and occurrence of the first phase. These results implied that local chemical factors are involved in initiating involution (17). Although the components of systemic regulation have been extensively studied for years, the identities and the precise actions and mechanisms of local factors are less well known.
Using gene profiling studies, our laboratory previously reported serotonin (5-hydroxytryptamine (5-HT)2) to be one of the local factors synthesized and secreted by the mouse mammary epithelial cells (20). The gene encoding rate-limiting enzyme for serotonin synthesis, TPH1 (tryptophan hydroxylase 1), is tightly regulated by prolactin. However, by teat sealing experiments, Matsuda et al., (20) showed that increased TPH1 expression was driven by milk stasis, not prolactin, per se. Furthermore, increased serotonin was also shown to inhibit milk protein synthesis and induce epithelial apoptosis. Thus, serotonin, in the microenvironment of the mammary gland, functions as a feedback inhibitor of lactation in an autocrine-paracrine manner. As a first step toward understanding the complex physiology of serotonin in regulating mammary gland involution, our laboratory recently reported that mammary epithelial tight junctions are one of the targets of 5-HT (21).
In this study, we elucidate the dynamics and mechanisms of serotonin-mediated regulation of mammary epithelial tight junctions. Using our previously established in vitro Transwell® model of functionally differentiated human mammary MCF10A (MI Cancer foundation 10A) cells (21), we showed that 5-HT7 is resensitized in response to antagonist. Serotonin regulates mammary epithelial tight junctions in a biphasic manner, which is mediated by a switch in downstream signaling of 5-HT7 from protein kinase A (PKA) to p38 mitogen-activated protein kinase (MAPK).
EXPERIMENTAL PROCEDURES
Materials—Dulbecco's modified Eagle's medium/Ham's F-12 (50:50) and all medium supplements, including horse serum, were purchased form Invitrogen. 5-HT creatine sulfate salt, H89, forskolin, and horseradish peroxidase-conjugated mouse anti-glyceraldehyde-3-phosphate dehydrogenase antibodies were obtained from Sigma. SB269970 and SB203580 were obtained from Tocris Biosciences (Ellisville, MO). 2′,5′-Dideoxyadenosine and Y-27632 were obtained from Calbiochem. GF109302X was obtained from BioMol International (Plymouth, PA). Mouse anti-ZO1 (zona occludens 1), mouse anti-ZO2, and rabbit anti-occludin were purchased from Zymed Laboratories Inc., Inc. (South San Francisco, CA). The rabbit anti-phospho-PKA, rabbit anti-PKA, rabbit anti-phospho-p38 MAPK, and rabbit anti-p38 MAPK were obtained from Cell Signaling Technologies (Danvers, MA). Rabbit anti-5-HT7 receptor antibody was obtained from Imgenex (San Diego, CA).
Cell Culture and Measurement of Transepithelial Electrical Resistance—MCF10A (ATCC, Manassas, VA) cells were cultured in complete growth medium (Dulbecco's modified Eagle's medium/F-12 supplemented with 5% horse serum, 10 μg/ml insulin, 20 ng/ml epidermal growth factor, 0.5 μg/ml hydrocortisone, 1 IU/ml penicillin, 0.1 mg/ml streptomycin, 0.25 μg/ml amphotericin B, and 2 mm l-glutamine). For measurement of transepithelial electrical resistance (TEER), the MCF10A cells were seeded at a high plating density (1.25 × 105 cells/cm2) on clear polyester Transwell® permeable supports (Corning Glass) in growth medium. Medium (apical and basal) was changed every 24 h before and during treatments. Drugs were added to the culture medium when TEER reached a plateau (2–3 weeks after seeding, ∼3000 ohms·cm2). An EVOM epithelial volt/ohmmeter with STX (chopstick) electrodes (World Precision Instruments, Sarasota, FL) was used to measure TEER.
Paracellular Flux Measurements—MCF10A Transwell® cultures were incubated under experimental conditions in the presence of fluorescein isothiocyanate (FITC)-tagged inulin (3.5 kDa) (Sigma) (0.5 mg/ml) in the basal chamber. At regular 24-h intervals, 100 μl of apical and basal medium was removed for assay. FITC-inulin was measured using a fluorescence plate reader (Fluroskan II; Labsystems) (excitation, 495 nm; emission, 530 nm). Flux into the apical chamber was calculated as percentage of control (100% flux). The control is free flux of FITC-inulin across the Transwell® membrane in the absence of cells.
Immunofluorescence and Confocal Microscopy—Cells grown in monolayer or Transwell® configurations were treated as per the experiments. Portions were then washed with phosphate-buffered saline followed by fixing with 4% paraformaldehyde for 30 min. They were then permeabilized with 0.1% Triton X-100 and incubated with borate buffer overnight at 75 °C. They were then washed with phosphate-buffered saline and incubated in primary antibodies at 4 °C overnight after blocking with 10% appropriate secondary antibody serum in immunofluorescence buffer (NaCl, 130 mm; Na2HPO4, 7 mm; NaH2PO4, 3.5 mm; NaN3, 0.05%; Triton X-100, 0.2%; Tween 20, 0.05%; bovine serum albumin, 0.1%). Sections were then washed with immunofluorescence buffer followed by appropriate secondary antibody incubation for 2 h at room temperature. After a final wash with phosphate-buffered saline, sections were mounted on slides. Images were collected using a Zeiss LSM510 confocal microscope, and Z-stacks were reconstructed three-dimensionally using the Zeiss LSM Image Software version 3.5.
Western Blotting—Transwell® cultures were treated as indicated in the experiments, after which they were washed in ice-cold phosphate-buffered saline, followed by total cell protein extraction using cell lysis extraction buffer (Cell Signaling Technologies, Danvers, MA). Samples were normalized for protein content using the Lowry assay. Proteins were separated on gradient SDS-polyacrylamide gels (Fisher). Proteins were then transferred to nitrocellulose membranes (Whatman GmbH, Dassel, Germany) and blocked with 5% nonfat milk or bovine serum albumin in wash buffer (0.1 mm Tris HCl, pH 8.0, 150 mm NaCl, 0.05% Tween 20). The blots were then incubated in primary antibody overnight at 4 °C followed by secondary antibody for 2 h at room temperature. The blots were developed using an ECL chemiluminescent kit (GE Biosciences).
Statistical Analysis—All graphed results are presented as mean ± S.E. One-way ANOVA with Tukey's post-test or two-way ANOVA with Bonferroni's post-test were used to analyze the data. A probability level of 0.05 was considered as significant. Each experiment was done in triplicate and repeated at least three times.
RESULTS
Transwell® cultures of MCF10A cells undergo a sigmoidal increase in TEER concomitant with a differentiation process, with a plateau of ∼3000 ohms·cm2, indicative of a highly differentiated state (21). In the current studies, we used the Transwell® cultures of MCF10A in this highly differentiated state (∼3000 ohms·cm2) unless specified otherwise.
Transmembrane Conductance Is Regulated by the 5-HT7 Receptor—Transmembrane conductance is the sum of (a) paracellular flux, which is restrained by tight junctions, and (b) transcellular flux through pumps and ion channels located on the apical and basolateral membranes. In order to decipher the contribution of paracellular flux to 5-HT-mediated increases in transmembrane conductance (i.e. decreased TEER), paracellular inulin (3.5 kDa) flux and TEER changes were measured after 5-HT treatment (Fig. 1A). Inulin flux and TEER underwent coordinate, reciprocal changes in response to 5-HT, with the change observed for TEER slightly preceding the increase of inulin flux (Fig. 1A). The fact that the change of TEER preceded the change in inulin flux would be expected, because inulin flux requires pores that are large enough to allow passage of a relatively large (3.5-kDa) molecule. The size of the inulin molecule also means that it cannot be used to detect increases in paracellular resistance in the high resistance differentiated epithelial membranes used in these studies.
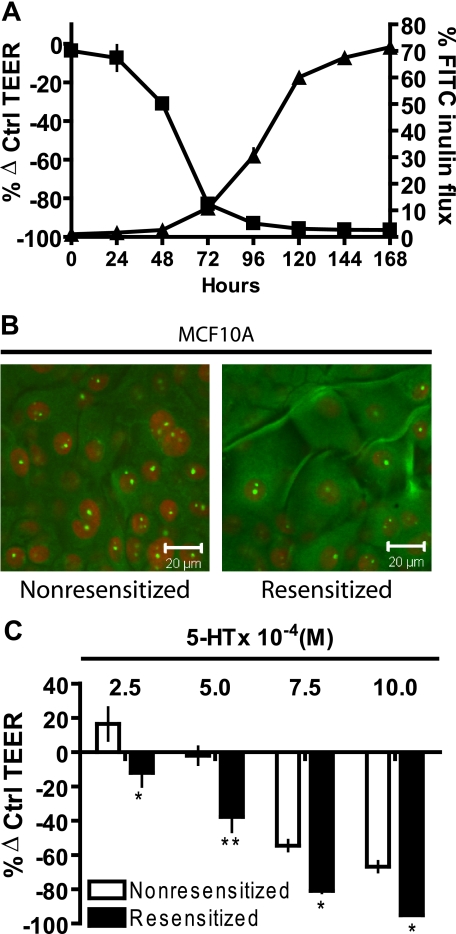
FIGURE 1.
5-HT7 receptor mediates 5-HT regulation of mammary epithelial tight junctions. A, TEER measurements (▪) and FITC-inulin (3.5 kDa) flux measurements (▴) on MCF10A Transwell® cultures at regular 24-h intervals after treatment with 5-HT (10 × 10-4 m). TEER is expressed as percentage change from control (untreated) values ± S.E. (n = 3). FITC-inulin flux is expressed as percentage compared with control ± S.E. (n = 3), the control (100%) being free flux of FITC-inulin across the Transwell® membrane in the absence of any cells. B, immunostaining for 5-HT7 receptor in MCF10A Transwell® cultures. Cells were either left untreated (nonresensitized) or treated with MG (10 μm) (resensitized) for 24 h prior to fixing and staining. C, TEER measurements of MCF10A Transwell® cultures expressed as percentage change from control (untreated) values ± S.E. (n = 3) at day 5 after the start of 5-HT treatment. Cultures were either left untreated (nonresensitized) or treated with MG (10 μm) (resensitized), 24 h prior to the start of 5-HT treatment. *, p < 0.05; **, p < 0.01 compared with respective controls (two-way ANOVA, Bonferroni's post-test).
Previously, we reported the presence of the 5-HT7 serotonin receptor in mammary epithelial cells and showed that 5-HT causes a disruption of tight junctions (21). 5-HT7 receptor is a G-protein-coupled receptor, coupled to Gs (22, 23). One of the characteristic features of 5-HT7 receptor is a high constitutive basal activity and results in homologous desensitization. Correspondingly, 5-HT7 receptors can be resensitized by a short exposure to antagonist, also known as reverse agonists, such as metergoline (MG) (24, 25). We used this characteristic behavior of 5-HT7 to confirm that responses of mammary epithelial cells to 5-HT were consistent with these known properties of 5-HT7. MCF10A Transwell® cultures were treated for 24 h with receptor antagonist MG (10 μm), and 5-HT7 staining was assayed by confocal imaging. We observed that MG caused redistribution of 5-HT7, resulting in predominantly peripheral staining, suggestive of a greater degree of membrane association. There was also an increase in overall receptor immunostaining (Fig. 1B). Further, to show an explicit relationship between 5-HT, 5-HT7, and disruption of tight junctions as measured by the decrease in TEER, one experimental group was left untreated (nonresensitized), and the other was treated with MG (resensitized) 24 h prior to 5-HT treatment for 120 h. Resensitized cells showed higher sensitivity to 5-HT, indicated by a significant leftward shift in the concentration response in comparison with the nonresensitized cells (Fig. 1C).
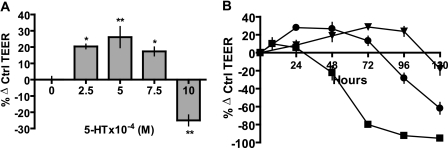
Biphasic Regulation of Mammary Epithelial Resistance by 5-HT—To gain a better understanding of the dynamics of 5-HT regulation of mammary epithelial resistance, we determined the concentration dependence of TEER on 5-HT. Resistance measurements at the 48 h time point were used to generate a concentration-response curve (Fig. 2A). Rather than showing a monotonic inhibition of TEER, the response to 5-HT was biphasic. At the lower end of the curve, 5-HT potentiated TEER, whereas at the high end, 5-HT decreased TEER. Moreover, when we measured TEER over time, there was a temporally biphasic effect of 5-HT at each concentration (Fig. 2B). At a given concentration, 5-HT had a transient effect of increasing the TEER and a delayed effect of decreased TEER. Increasing the [5-HT] shifted the time course to the left, so that the early transient effect was not significant at the highest [5-HT] (Fig. 2B). Thus, the relationship between 5-HT and membrane resistance was not linear, indicating a complex interplay of multiple signaling events.
FIGURE 2.
5-HT regulates mammary epithelial resistance in a biphasic manner. A, TEER measurements on MCF10A Transwell® cultures treated with indicated concentrations of 5-HT for 48 h and expressed as percentage change from control (untreated) values ± S.E. (n = 3). *, p < 0.05; **, p < 0.01 as compared with control (one-way ANOVA, Tukey's post-test). B, TEER measurements at 24-h intervals expressed as percentage change from control (untreated) values ± S.E. (n = 3) after 5-HT treatment (▴, 5 × 10-4; •, 7.5 × 10-4 m; ▪, 10 × 10-4 m).
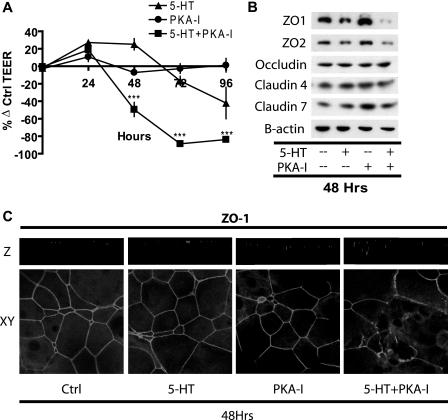
The Transient Response to 5-HT Is Mediated through PKA— The 5-HT7 receptor signals though Gs and, as we previously documented, stimulates cAMP accumulation (21, 22). Therefore, we decided to probe the involvement of the cAMP/PKA pathway in the 5-HT actions on TEER. MCF10A cultures were treated with 50 μm H89 (PKA inhibitor) for 1 h to inactivate PKA, followed by 5-HT treatment, in the presence of 5 μm H89 to inhibit further PKA activation. Untreated cultures served as controls. TEER was measured at regular intervals of 24 h (Fig. 3A). Parallel sets of experiments were Western blotted (Fig. 3B) and immunostained (Fig. 3C) at the 48 h time point to determine changes in tight junction protein levels and distribution.
FIGURE 3.
Inhibition of the PKA pathway blocks the potentiation of epithelial resistance by 5-HT and causes loss of tight junction protein integrity. A, TEER measurements on MCF10A Transwell® cultures at regular 24-h intervals, expressed as percentage change from control (untreated) values ± S.E. (n = 3) after the indicated treatments: 5-HT (7.5 × 10-4 m) and H89 (PKA-I) (5 μm). ***, p < 0.001 as compared with control (two-way ANOVA; Bonferroni's post-test). B, Western blotting for the indicated proteins on cell extracts from MCF10A Transwell® cultures after 48 h of the indicated treatments. C, representative xy and z sections of MCF10A Transwell® cultures immunostained for ZO1 protein after 48 h of the indicated treatments.
The early phase of increased TEER in response to 5-HT was eliminated by the PKA inhibitor H89 (Fig. 3A), which, by itself, did not cause a significant change in TEER. In combination with H89, the delayed phase of declining resistance was accelerated, so that TEER was reduced to 50% by 48 h (Fig. 3A).
Tight junction proteins were measured by Western blotting in protein extracts prepared by isotonic mild detergent (1% Triton) extraction. Fig. 3B shows images from one representative Western blot of the three replicate experiments. 5-HT treatment along with H89 caused a significant decrease in the Western blot levels of the tight junction proteins ZO1 and ZO2 at 48 h (Fig. 3B). These changes in ZO1 and ZO2 were seen consistently in all three experiments. Unlike the robust changes in ZO1 and ZO2, minor differences in the apparent levels of claudins and occludins were not consistent among the replicates. Confocal images of cells treated with 5-HT in the presence of H89 for 48 h showed a dispersed pattern of localization of ZO proteins (Fig. 3C) and occludin (data not shown) within the cells, as compared with the very regular and highly localized staining seen in each of the control groups. The marked reduction in Western blot levels of ZO proteins after treatment with 5-HT and H89 and the obvious cellular redistribution of ZO proteins in immunostained sections suggests a change in either extractability or detectability in the two assays (see “Discussion” for further consideration).
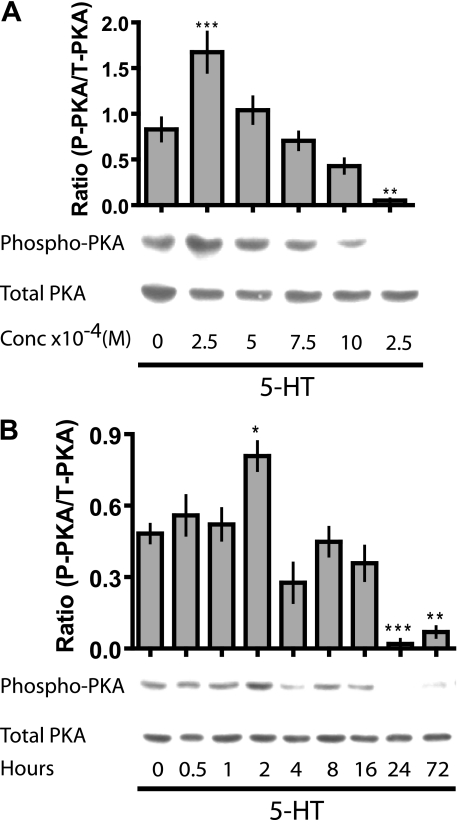
Since PKA inhibition abrogated the early potentiation of tight junctions in response to 5-HT, we decided to determine the concentration dependence of PKA activation at 24 h (Fig. 4A). The concentration-response curve for activated PKA was biphasic, with stimulation of phospho-PKA at the low end and inhibition of phospho-PKA at the high [5-HT]. To study the time course for changes in phospho-PKA during the first 24 h, we stimulated the cells with the highest concentration of 5-HT and sampled frequently. Under these conditions, we once again observed a biphasic response, with a transient stimulation of phospho-PKA at 2 h and a later inhibition by 24 h, which was maintained through 72 h (Fig. 4B).
FIGURE 4.
5-HT transiently activates PKA. A, Western blotting for detection of activated (phospho-PKA (P-PKA)) and total PKA (T-PKA) on total cell extracts from MCF10A Transwell® cultures treated with different concentrations of 5-HT for 24 h. Semiquantitative analysis of the experiment is represented in the bar graph (±S.E.; n = 4). B, Western blotting for phospho-PKA and total PKA on total cell extracts from Transwell® culture of MCF10A treated with 5-HT (10 × 10-4 m) for the indicated time periods. Semiquantitative analysis of the Western blots is represented in the bar graph (±S.E.; n > 5). *, p < 0.05; **, p < 0.01; ***, p < 0.001 as compared with control (untreated or 0 h) (one-way ANOVA, Tukey's post-test).
All of the above results (Figs. 3 and 4) indicated the involvement of a PKA signal in the early transient increase in TEER response to 5-HT. These results also hinted at the existence of a second, PKA-independent signal responsible for the decline in TEER that occurred after sustained stimulation with 5-HT. However, these results did not speak to the question of whether both phases were mediated by Gs/cAMP or an alternative upstream signaling branch.
Both Phases of the TEER Response to 5-HT Are Mediated through Gs/cAMP—There are three splice variants of the 5-HT7 receptor (5-HT7a, -b, and -d) in humans (25, 26), and previously we have reported the presence of all three splice variants in mammary epithelial cells (21). Although most accounts of 5-HT7 signaling link it to Gs, there are studies showing its association with G12 (27), whose downstream effectors are Rho family GTPases and/or PKC. Either of these effectors are known to influence cytoskeletal organization (27, 28) and thus could potentially affect TEER. On the other hand, cAMP can signal via a PKA-independent pathway (29), which could affect TEER.
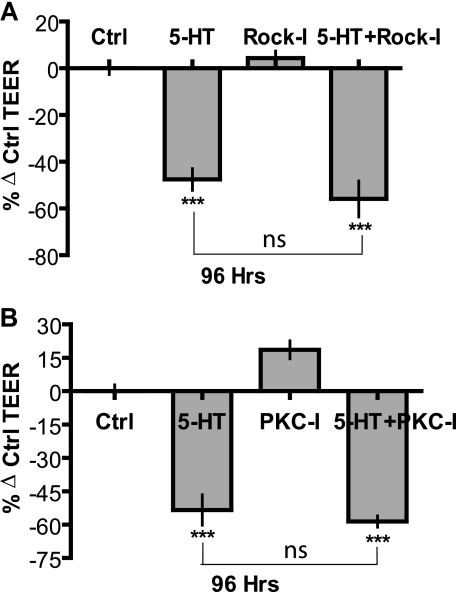
In order to test directly whether activation of Rho GTPases or PKC (G12-coupled) were involved in disruption of TEER by 5-HT, cultures were pretreated for 1 h with specific inhibitors of Rho kinase (ROCK), Y-27632 (Rock-I) (40 μm), and PKC GF109302X (PKC-I) (10 μm), followed by 0.5 μm treatment in presence of 5-HT. Untreated cultures served as respective controls. TEER measurements were taken at regular 24-h intervals, and results at 96 h are depicted. Inhibition of ROCK or PKC by themselves had little effect on TEER measurements. The decline in TEER induced by 5-HT alone (∼45–50%) was not statistically different form the decline in TEER induced by 5-HT in the presence of ROCK or PKC inhibitors (Fig. 5, A and B), thus indicating that neither Rho kinase nor PKC activity is necessary for the delayed disruption of tight junctions in response to 5-HT.
FIGURE 5.
Inhibition of ROCK or PKC does not affect 5-HT action on epithelial tight junction. TEER measurements on MCF10A Transwell® cultures expressed as percentage change from control (untreated) values ± S.E. (n = 3) after 96 h of the indicated treatment: 5-HT (7.5 × 10-4 m). A, ROCK inhibitor (ROCK-I; Y-27632 (0.5 μm)). B, PKC inhibitor (PKC-I; GF109302X (0.5 μm)). *, p < 0.05; **, p < 0.01 as compared with control (one-way ANOVA; Tukey's post-test).
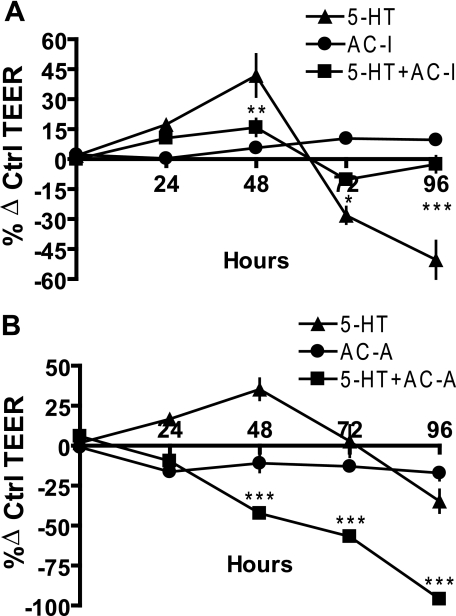
To test the hypothesis that a second cAMP-dependent pathway mediates the inhibition of TEER by 5-HT, we used an adenylyl cyclase inhibitor, 2′,5′-dideoxyadenosine (2′,5′-DDO). MCF10A cultures were pretreated with 20 μm 2′,5′-DDO for 1 h, followed by 5-HT treatment along with 10 μm 2′,5′-DDO to prevent reactivation of adenylyl cyclase. Untreated cultures served as controls. A parallel experiment using a potent adenylyl cyclase activator, forskolin, also was performed with a pretreatment of 2 μm for 1 h, followed by 5-HT in presence of 0.5 μm forskolin. Similarly, untreated cultures served as controls. In both experiments, TEER was measured at regular 24-h intervals, as depicted (Fig. 6, A and B). Neither 2′,5′-DDO nor forskolin alone had a significant effect on TEER. As expected, 5-HT caused a transient increase in TEER through 48 h, followed by a decrease in TEER by 96 h (∼50%). However, both effects were significantly abrogated by 2′,5′-DDO (inhibition of adenylyl cylase) (Fig. 6A). In the parallel experiment, forskolin, an adenylyl cylase activator, significantly shifted the 5-HT curve to the left (Fig. 6B), analogous to a concentration-dependent leftward curve shift observed in Fig. 2B. We infer from these results collectively that both the delayed, sustained suppression of TEER and the early, transient increase in TEER are mediated by cAMP.
FIGURE 6.
Both the potentiation and disruptive actions of 5-HT on epithelial tight junctions are mediated through cAMP. TEER measurements on MCF10A Transwell® cultures at regular 24-h intervals, expressed as percentage change from control (untreated) values ± S.E. (n = 3) after the indicated treatment: 5-HT (7.5 × 10-4 m). A, adenylyl cyclase inhibitor (AC-I;2′,5′-DDO (10 μm)). B, adenylyl cyclase activator (AC-A; forskolin (0.5 μm)). *, p < 0.05; **, p < 0.01; ***, p < 0.001 as compared with control (two-way ANOVA; Bonferroni's post-test).
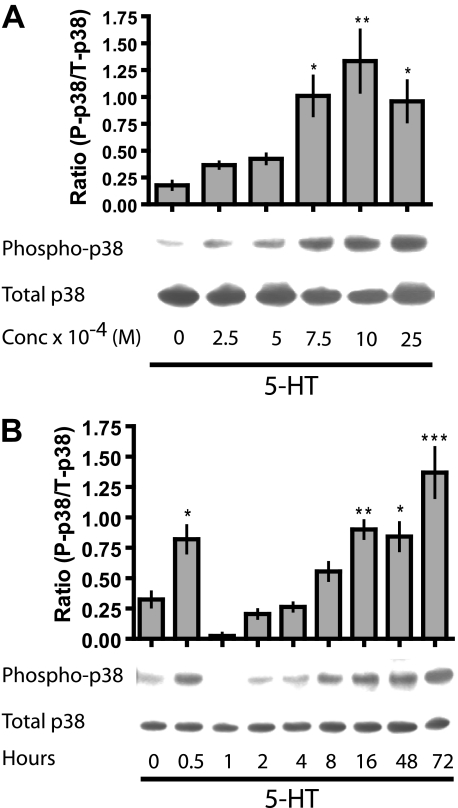
The Disruptive Action of 5-HT on Tight Junctions Is Mediated through a p38 MAPK Pathway—In an attempt to decipher the messenger downstream of cAMP that mediated the TEER inhibiting action of 5-HT, we screened for activation of various signaling molecules by 5-HT. Transwell® cultures of MCF10A cells were treated with 5-HT at several concentrations, and total cell extracts were collected. These extracts were then used for screening of activated downstream messengers using specific antibodies. A concentration-dependent activation of p38 MAPK was observed in response to 5-HT treatment (Fig. 7A), whereas other kinases (c-Jun N-terminal kinase and extracellular signal-regulated kinase) did not show any 5-HT-mediated activation (data not shown). The stimulation of p38 MAPK was confirmed by determining a detailed time course of p38 MAPK activation in response to 5-HT (10 × 10-4 m), which showed both transient and sustained stimulation of phospho-p38, with an intervening phase of dephosphorylation (Fig. 7B).
FIGURE 7.
5-HT induces sustained activation of p38 MAPK. A, Western blotting for detection of activated (phospho-p38 (P-p38)) and total p38 MAPK (T-p38) on total cell extracts from MCF10A Transwell® cultures treated with different concentrations of 5-HT for 24 h. Semiquantitative analysis of the experiment is represented in the bar graph (±S.E., n = 3). B, Western blotting for phospho-p38 and total p38 MAPK on total cell extracts from Transwell® cultures of MCF10A cells treated with 5-HT (10 × 10-4 m) for the indicated time periods. Semiquantitative analysis of the experiment is represented in the bar graph (±S.E., n = 4). *, p < 0.05; **, p < 0.01; ***, p < 0.001 as compared with control (untreated or 0 h) (one-way ANOVA; Tukey's post-test).
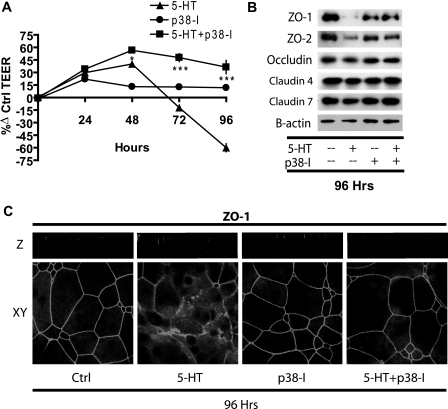
Since 5-HT induced sustained activation of p38 MAPK, we tested whether activation of p38 MAPK is the mode of action for tight junction disruption by 5-HT using a specific inhibitor of p38 MAPK, SB203580. MCF10A cells were pretreated with SB203580 (20 μm) for 1 h, followed by treatment with 5-HT along with 3 μm SB203580 to prevent reactivation of p38 MAPK. Untreated cultures served as controls. TEER was measured regularly at 24-h intervals as depicted (Fig. 8A). Changes in tight junction proteins, in response to the above treatments, were analyzed by Western blot analysis (Fig. 8B) and confocal microscopy (Fig. 8C).
FIGURE 8.
Inhibition of p38 MAPK prevents the disruptive action of 5-HT on epithelial tight junctions. A, TEER measurements on MCF10A Transwell® cultures at regular 24-h intervals, expressed as percentage change from control (untreated) values (±S.E., n = 3) after the indicated treatments: 5-HT (7.5 × 10-4 m), SB203580 (p38-I; 3 μm). *, p < 0.05; ***, p < 0.001 as compared with control (two-way ANOVA; Bonferroni's post-test). B, Western blotting for the indicated proteins, on cell extracts from the MCF10A Transwell® cultures after 96 h of the indicated treatments. C, representative xy and z sections of MCF10A Transwell® cultures immunostained for ZO1 protein after 96 h of the indicated treatment.
Inhibition of p38 MAPK by itself had no significant effect on TEER, whereas 5-HT alone, as predicted, caused a transient increase, followed by a decrease in TEER (∼60%) by 96 h (Fig. 8A). The opening of tight junctions by 96 h was reflected in the significant decrease in the detectable tight junction proteins ZO1 and ZO2 (Fig. 8B) and dispersion of ZO1 immunoreactivity (Fig. 8C). Levels of other tight junction proteins (occludin and claudin) remained largely unchanged (Fig. 8B).
Treatment with 5-HT in the presence of p38 MAPK inhibitor significantly increased the TEER at 48 h, and the elevated TEER persisted throughout the experiment with an obvious absence of the later declining phase of TEER (Fig. 8A). Correspondingly, the decrease in detectable ZO1 and -2 in response to 5-HT was blocked by SB203580 (Fig. 8B). Tight junction protein localization was more similar to controls in the 5-HT treatment in the presence of SB203580 with an absence of the dispersed signal for ZO1 (Fig. 7C) and occludin (data not shown). These results imply that p38 MAPK activation is required for the disruption of tight junctions in response to 5-HT.
DISCUSSION
Important aspects of the physiology of the mammary 5-HT system have been elucidated since our original observations of prolactin-induced up-regulation of TPH1, the rate-limiting enzyme for 5-HT synthesis, and presence of 5-HT in the mouse mammary epithelium and milk (20). Functionally, 5-HT was found to inhibit milk protein synthesis and initiate involution, thus acting as a homeostatic regulator of lactation. In our attempt to study the serotonergic function in the involuting mammary epithelium, we recently reported evidence for serotonergic regulation of mammary epithelial tight junctions along with the role of the 5-HT7 receptor in this process (21). A recent paper showed that 5-HT signaling regulates lactation in the mammary gland of the dairy cow (30). Thus, serotonergic signaling within the mammary epithelium is a phylogenetically conserved system with important physiological and practical implications. In the present study, we show that 5-HT mediates its effect on mammary epithelial resistance through two 5-HT7 receptor signaling systems.
Transwell® cultures of MCF10A cells were used in this study, because although they are immortalized, MCF10A cells are untransformed human mammary epithelial cells that undergo differentiation in Matrigel and collagen cultures (31–33). When cultured on permeable Transwell® supports, MCF10A undergoes extensive differentiation, forming a multilayered epithelium highly reminiscent of the organization of the mammary epithelium in vivo. The superficial cell layer constitutes a polarized barrier-forming epithelium that synthesizes human milk proteins and lipids.3
5-HT7 Receptor and Mammary Epithelial Resistance—There are seven classes of 5-HT receptors, totaling more than 20 different receptors (34). Previously, we reported the presence of only the 5-HT7 class of receptors in human and mouse mammary epithelium, correlatively implicating 5-HT7 as the mediator of 5-HT actions on tight junctions (21). 5-HT7 is a Gs-coupled receptor, with a high constitutive activity, which results in homologous desensitization (23–25). Blocking this constitutive activity by inverse agonists, such as metergoline or SB 269970, has been shown to resensitize the receptor (25, 36). In MCF10A cells, we observed this phenomenon in terms of increased intensity of immunoreactive 5-HT7, along with higher membrane localization and increased sensitivity to 5-HT-mediated tight junction regulation, thus explicitly indicating that 5-HT regulates epithelial resistance through the 5-HT7 receptor.
Biphasic Action of Serotonin on Mammary Epithelial Resistance—The premier function of mammary gland is tightly linked to the barrier forming ability of epithelial tight junctions, which compartmentalize milk within the alveolar lumen and maintain essential solute gradients across the epithelium (9–11, 37). During the two phases of involution (reversible and irreversible), multiple systemic and local factors, including 5-HT, act within the mammary gland to directly or indirectly affect tight junctions (11–13, 21). Initiation of involution is characterized by distension of alveoli because of weaning-induced milk accumulation, which results in a highly increased intramammary pressure (17, 38). This early phase of milk accumulation represents a challenge for the cellular junctions to maintain the integrity of the luminal space while maintaining an opportunity for reintroduction of the suckling stimulus to bring the gland back into lactation. Continued absence of milk removal brings about the ultimate disruption of tight junctions in response the accumulation of local factors (18, 19, 37, 39, 40). Here, we observed that 5-HT, in a dose- and a time-dependent manner, causes a transient increase of resistance, followed by a delayed disruption of tight junctions. It is formally possible that some of the actions of 5-HT are on transcellular, rather than paracellular, resistance. The early effect, although transient relative to the whole experiment, occurs over many h (up to 48 h), and because these membranes were in a high resistance state, the large (>600 ohms·cm2) absolute increase of resistance that we observed could be caused by small changes in either paracellular permeability or transcellular conductance, which ultimately traces to basolateral pump activity. However, sustaining such a change of resistance for many hours by altering active (transcellular) as opposed to passive (paracellular) mechanisms seems less likely.
Because of the biphasic actions on epithelial resistance, 5-HT is poised to act as an important integrator during the stages of lactation, milk stasis, and involution. It is tempting to speculate that by transiently potentiating cellular junctions, 5-HT may participate in the maintenance of the potential for milk production prior to the initiation of active involution. Subsequently, when 5-HT signaling is sustained at a high level, the disruptive action on tight junctions would transition the mammary gland into involution.
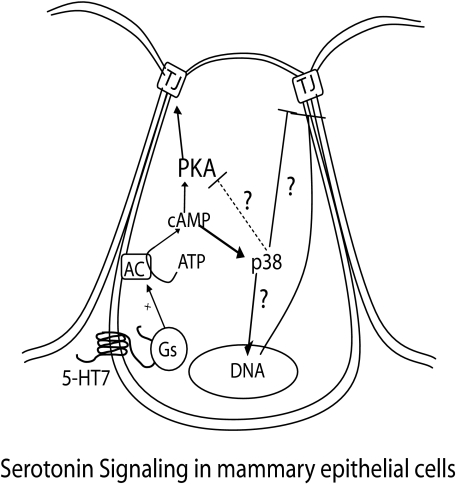
Signaling Pathways Induced by 5-HT in the Regulation of Epithelial Resistance—The 5-HT7 receptor, through which 5-HT acts in human and mouse glands, has been shown to be Gs-coupled, leading to adenylyl cylase activation (22, 23). In our previous study, we reported 5-HT-mediated cAMP accumulation (21). However, we did not establish a direct relationship between cAMP and the 5-HT action on tight junctions in those experiments. In this study, we report the interesting finding that both PKA and p38 MAPK are independently activated through cAMP (Fig. 9), and these two signals regulate the biphasic action of 5-HT on TEER. This is based on the finding that PKA inhibition abrogates the initial transient tight junction potentiation effect of 5-HT, leading to accelerated disruption of tight junctions (i.e. a leftward shift in TEER curves). On the other hand, p38 MAPK inhibition abrogates the later disruptive effect of 5-HT on tight junctions while leaving the early phase of tight junction potentiation unaffected. Analogous to its effect on TEER, 5-HT caused only a transient activation of PKA and activation at low concentrations. In the case of p38 MAPK, 5-HT caused a sustained concentration- and time-dependent activation, with only a brief interval of dephosphorylation. The sustained p38 MAPK activation by 5-HT is in accordance with ability of 5-HT to cause sustained elevation of intracellular cAMP in MCF10A cells (21). the possible involvement of a secondary or indirect effect of 5-HT in the later sustained p38 MAPK activation cannot be ruled out based only on our current data. Nonetheless, we can conclude that 5-HT specifically activates p38 MAPK.
FIGURE 9.
Proposed model for 5-HT signaling that regulates mammary epithelial tight junctions. We propose that in mammary epithelium, 5-HT regulates tight junction function in a biphasic manner by induction of multiple pathways. Early tight junction potentiation and later tight junction disruption are proposed to be mediated through cAMP activation by the Gs-coupled 5-HT7 receptor. PKA activation mediates the early potentiating action of 5-HT, whereas the p38 MAPK activation mediates the later disrupting action of 5-HT on tight junctions with possible cross-talk between the two pathways. The proposed model does not eliminate the possibility that transcellular pathways also contribute to the overall changes in membrane resistance.
Inhibition of adenylyl cylase inhibits both of the effects of 5-HT on epithelial resistance (transient potentiation and delayed disruption), indicating that they both are mediated through cAMP accumulation. PKA-independent activation of p38 MAPK may be mediated through the recently discovered exchange protein activated by cAMP (EPAC) (29). Involvement of EPAC in p38 MAPK activation by 5-HT remains to be determined, and a role for the βγ subunit of Gs protein cannot be ruled out (42–44). Interestingly, the transient activation of PKA by 5-HT is followed by a gradual inactivation during the same time that p38 MAPK is being activated. This correlation seriously hints at an autoregulatory mechanism wherein p38 MAPK directly and/or indirectly brings about inhibition of PKA activation by 5-HT, thus promoting its own activation and action in a positive feed-forward manner.
Effect of 5-HT on Tight Junction Proteins—Practically every signaling path that a cell can induce has a potential to affect tight junctions due to the massive multiprotein complex that forms the tight junctions (45–48). Extensive evidence gathered in recent years indicates that tight junctions can act as signaling platforms or relays that not only are targets of external factors but also are themselves regulators of multiple cellular processes (46, 49). Thus, factors such as 5-HT, which affect tight junctions, can simultaneously affect a variety of other cellular functions. 5-HT-mediated transient PKA activation and tight junction potentiation did not correspond with significant changes in the levels or apparent distribution of tight junction proteins. These changes are consistent with PKA-induced post-translational modifications (phosphorylation and dephosphorylation) that alter tight junction permeability without overall changes in cellular distribution of the proteins (50–53).
Interestingly, 5-HT-mediated p38 MAPK activation and tight junction disruption is associated with a scattered distribution of crucial tight junction scaffolding proteins. Although no obvious difference in total staining intensity of ZO proteins was observed among the test groups, the levels of Western blot-detectable (extractable) ZO proteins were decreased in the presence of 5-HT. This indicates the possible formation of insoluble protein complexes for these scaffolding proteins in the presence of 5-HT. These results suggest that 5-HT regulates ZO1 and -2 protein complex formation and their distribution across the membrane, thus regulating the barrier as well as membrane polarity functions of the mammary epithelial cells. Alternatively, ubiquitinylation and targeting of ZO proteins for degradation by 5-HT may also explain the decreased Western blot-detectable levels and scattered distribution of the proteins (41, 54).
In this study, we have elucidated the dynamics and mode of action of 5-HT in regulating mammary epithelial resistance that may occur during the homeorhetic process of involution. Understanding these mechanisms provides an opportunity to address practical issues of nursing mothers and dairy. Surely, 5-HT may have many other unexplored modes of action in regulating the complex process of involution. We do not yet know how 5-HT effects on the membrane “fencing” function of tight junctions might affect secretory and other cellular activities of the mammary epithelium; nor do we know about the roles of 5-HT in pathologies like breast cancers, which are known to be associated with dysregulated tight junctions, making them invasive and metastatic (35).
Acknowledgments
We thank Melissa Orr and Aaron Marshall for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant DK52134. This work was also supported by Department of the Army Grant BC052576 (to N. D. H.). This project was also supported by National Research Initiative Competitive Grant 2007-35206-17898 from the United States Department of Agriculture Cooperative State Research, Education, and Extension Service. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: 5-HT, serotonin (5-hydroxytryptamine); ZO, zona occludens; TEER, transepithelial electrical resistance; 5-HT7, type 7 serotonin receptor; PKA, protein kinase A; MAPK, mitogen-activated protein kinase; PKC, protein kinase C; FITC, fluorescein isothiocyanate; ANOVA, analysis of variance; MG, metergoline; 2′,5′-DDO, 2′,5′-dideoxyadenosine.
A. M. Marshall, V. Pai, M. A. Sartor, and N. D. Horseman, manuscript in preparation.
References
- 1.McManaman, J. L., and Neville, M. C. (2003) Adv. Drug Deliv. Rev. 55 629-641 [DOI] [PubMed] [Google Scholar]
- 2.Stein, T., Salomonis, N., and Gusterson, B. A. (2007) J. Mammary Gland Biol. Neoplasia 12 25-35 [DOI] [PubMed] [Google Scholar]
- 3.Watson, C. J. (2006) Breast Cancer Res. 8 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berle, P. (1972) Zentralbl. Gynakol. 94 1081-1086 [PubMed] [Google Scholar]
- 5.Davis, J. W., and Liu, T. M. (1969) Endocrinology 85 155-160 [DOI] [PubMed] [Google Scholar]
- 6.Grimm, S. L., Seagroves, T. N., Kabotyanski, E. B., Hovey, R. C., Vonderhaar, B. K., Lydon, J. P., Miyoshi, K., Hennighausen, L., Ormandy, C. J., Lee, A. V., Stull, M. A., Wood, T. L., and Rosen, J. M. (2002) Mol. Endocrinol. 16 2675-2691 [DOI] [PubMed] [Google Scholar]
- 7.Stucki, J. C., and Forbes, A. D. (1960) Acta Endocrinol. 33 73-80 [DOI] [PubMed] [Google Scholar]
- 8.Grosvenor, C. E., and Turner, C. W. (1959) Proc. Soc. Exp. Biol. Med. 101 699-703 [DOI] [PubMed] [Google Scholar]
- 9.Berga, S. E. (1984) Am. J. Physiol. 247 C20-C25 [DOI] [PubMed] [Google Scholar]
- 10.Morgan, G., and Wooding, F. B. (1982) J. Dairy Res. 49 1-11 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen, D. A., Parlow, A. F., and Neville, M. C. (2001) J. Endocrinol. 170 347-356 [DOI] [PubMed] [Google Scholar]
- 12.Stelwagen, K., McFadden, H. A., and Demmer, J. (1999) Mol. Cell. Endocrinol. 156 55-61 [DOI] [PubMed] [Google Scholar]
- 13.Flint, D. J., and Gardner, M. (1994) Endocrinology 135 1119-1124 [DOI] [PubMed] [Google Scholar]
- 14.Wilde, C. J., Calvert, D. T., Daly, A., and Peaker, M. (1987) Biochem. J. 242 285-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendry, K. A., Simpson, K. J., Nicholas, K. R., and Wilde, C. J. (1998) J. Mol. Endocrinol. 21 169-177 [DOI] [PubMed] [Google Scholar]
- 16.Knight, C. H., Peaker, M., and Wilde, C. J. (1998) Rev. Reprod. 3 104-112 [DOI] [PubMed] [Google Scholar]
- 17.Lund, L. R., Romer, J., Thomasset, N., Solberg, H., Pyke, C., Bissell, M. J., Dano, K., and Werb, Z. (1996) Development 122 181-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peaker, M. (1980) J. Physiol. 301 415-428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennison, M. E., Kerr, M., Addey, C. V., Handel, S. E., Turner, M. D., Wilde, C. J., and Burgoyne, R. D. (1993) J. Cell Sci. 106 641-648 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda, M., Imaoka, T., Vomachka, A. J., Gudelsky, G. A., Hou, Z., Mistry, M., Bailey, J. P., Nieport, K. M., Walther, D. J., Bader, M., and Horseman, N. D. (2004) Dev. Cell. 6 193-203 [DOI] [PubMed] [Google Scholar]
- 21.Stull, M. A., Pai, V., Vomachka, A. J., Marshall, A. M., Jacob, G. A., and Horseman, N. D. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 16708-16713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruat, M., Traiffort, E., Leurs, R., Tardivel-Lacombe, J., Diaz, J., Arrang, J. M., and Schwartz, J. C. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 8547-8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bard, J. A., Zgombick, J., Adham, N., Vaysse, P., Branchek, T. A., and Weinshank, R. L. (1993) J. Biol. Chem. 268 23422-23426 [PubMed] [Google Scholar]
- 24.Shimizu, M., Nishida, A., Zensho, H., Miyata, M., and Yamawaki, S. (1998) Brain Res. 784 57-62 [DOI] [PubMed] [Google Scholar]
- 25.Krobert, K. A., and Levy, F. O. (2002) Br. J. Pharmacol. 135 1563-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahe, C., Bernhard, M., Bobirnac, I., Keser, C., Loetscher, E., Feuerbach, D., Dev, K. K., and Schoeffter, P. (2004) Br. J. Pharmacol. 143 404-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvachnina, E., Liu, G., Dityatev, A., Renner, U., Dumuis, A., Richter, D. W., Dityateva, G., Schachner, M., Voyno-Yasenetskaya, T. A., and Ponimaskin, E. G. (2005) J. Neurosci. 25 7821-7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhanasekaran, N., and Dermott, J. M. (1996) Cell. Signal. 8 235-245 [DOI] [PubMed] [Google Scholar]
- 29.Lin, S. L., Johnson-Farley, N. N., Lubinsky, D. R., and Cowen, D. S. (2003) J. Neurochem. 87 1076-1085 [DOI] [PubMed] [Google Scholar]
- 30.Hernandez, L. L., Stiening, C. M., Wheelock, J. B., Baumgard, L. H., Parkhurst, A. M., and Collier, R. J. (2008) J. Dairy Sci. 91 1834-1844 [DOI] [PubMed] [Google Scholar]
- 31.Debnath, J., Muthuswamy, S. K., and Brugge, J. S. (2003) Methods 30 256-268 [DOI] [PubMed] [Google Scholar]
- 32.Debnath, J., Mills, K. R., Collins, N. L., Reginato, M. J., Muthuswamy, S. K., and Brugge, J. S. (2002) Cell 111 29-40 [DOI] [PubMed] [Google Scholar]
- 33.Soule, H. D., Maloney, T. M., Wolman, S. R., Peterson, W. D., Jr., Brenz, R., McGrath, C. M., Russo, J., Pauley, R. J., Jones, R. F., and Brooks, S. C. (1990) Cancer Res. 50 6075-6086 [PubMed] [Google Scholar]
- 34.Raymond, J. R., Mukhin, Y. V., Gelasco, A., Turner, J., Collinsworth, G., Gettys, T. W., Grewal, J. S., and Garnovskaya, M. N. (2001) Pharmacol. Ther. 92 179-212 [DOI] [PubMed] [Google Scholar]
- 35.Martin, T. A., Watkins, G., Mansel, R. E., and Jiang, W. G. (2004) Eur. J. Cancer 40 2717-2725 [DOI] [PubMed] [Google Scholar]
- 36.Guthrie, C. R., Murray, A. T., Franklin, A. A., and Hamblin, M. W. (2005) J. Pharmacol. Exp. Ther. 313 1003-1010 [DOI] [PubMed] [Google Scholar]
- 37.Stelwagen, K., Davis, S. R., Farr, V. C., Prosser, C. G., and Sherlock, R. A. (1994) J. Dairy Sci. 77 426-432 [DOI] [PubMed] [Google Scholar]
- 38.Nguyen, D. A., and Neville, M. C. (1998) J. Mammary Gland Biol. Neoplasia 3 233-246 [DOI] [PubMed] [Google Scholar]
- 39.Stelwagen, K., Farr, V. C., Davis, S. R., and Prosser, C. G. (1995) Am. J. Physiol. 269 R848-R855 [DOI] [PubMed] [Google Scholar]
- 40.Stelwagen, K., Farr, V. C., McFadden, H. A., Prosser, C. G., and Davis, S. R. (1997) Am. J. Physiol. 273 R379-R386 [DOI] [PubMed] [Google Scholar]
- 41.Hernandez, S., Chavez Munguia, B., and Gonzalez-Mariscal, L. (2007) Exp. Cell Res. 313 1533-1547 [DOI] [PubMed] [Google Scholar]
- 42.Seo, M., Lee, Y. I., Cho, C. H., Bae, C. D., Kim, I. H., and Juhnn, Y. S. (2002) J. Biol. Chem. 277 24197-24203 [DOI] [PubMed] [Google Scholar]
- 43.Seo, M., Cho, C. H., Lee, Y. I., Shin, E. Y., Park, D., Bae, C. D., Lee, J. W., Lee, E. S., and Juhnn, Y. S. (2004) J. Biol. Chem. 279 17366-17375 [DOI] [PubMed] [Google Scholar]
- 44.Faure, M., Voyno-Yasenetskaya, T. A., and Bourne, H. R. (1994) J. Biol. Chem. 269 7851-7854 [PubMed] [Google Scholar]
- 45.Zahraoui, A., Louvard, D., and Galli, T. (2000) J. Cell Biol. 151 F31-F36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneeberger, E. E., and Lynch, R. D. (2004) Am. J. Physiol. 286 C1213-C1228 [DOI] [PubMed] [Google Scholar]
- 47.Guillemot, L., Paschoud, S., Pulimeno, P., Foglia, A., and Citi, S. (2008) Biochim. Biophys. Acta 1778 601-613 [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Mariscal, L., Tapia, R., and Chamorro, D. (2008) Biochim. Biophys. Acta 1778 729-756 [DOI] [PubMed] [Google Scholar]
- 49.Shin, K., Fogg, V. C., and Margolis, B. (2006) Annu. Rev. Cell Dev. Biol. 22 207-235 [DOI] [PubMed] [Google Scholar]
- 50.Singer, K. L., Stevenson, B. R., Woo, P. L., and Firestone, G. L. (1994) J. Biol. Chem. 269 16108-16115 [PubMed] [Google Scholar]
- 51.Sakakibara, A., Furuse, M., Saitou, M., Ando-Akatsuka, Y., and Tsukita, S. (1997) J. Cell Biol. 137 1393-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreeva, A. Y., Krause, E., Muller, E. C., Blasig, I. E., and Utepbergenov, D. I. (2001) J. Biol. Chem. 276 38480-38486 [DOI] [PubMed] [Google Scholar]
- 53.D'Souza, T., Agarwal, R., and Morin, P. J. (2005) J. Biol. Chem. 280 26233-26240 [DOI] [PubMed] [Google Scholar]
- 54.Lui, W. Y., and Lee, W. M. (2005) J. Cell. Physiol. 203 564-572 [DOI] [PubMed] [Google Scholar]