Abstract
Hepatic gluconeogenesis is elevated in diabetes and a major contributor to hyperglycemia. Stromal cell-derived factor-1 (SDF-1) is a chemokine and an activator of Akt. In this study, we tested the hypothesis that SDF-1 suppresses hepatic gluconeogenesis through Akt. Our results from isolated primary hepatocytes show that SDF-1α and SDF-1β inhibited glucose production via gluconeogenesis and reduced transcript levels of key gluconeogenic genes glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK). Additionally, SDF-1α and SDF-1β both inhibited activation of the PEPCK promoter. In examining the mechanism by which SDF-1 inhibits gluconeogenesis, we found that SDF-1 promoted phosphorylation of Akt, FoxO1, and c-Src, but did not activate insulin receptor substrate-1-like insulin. Blockade of Akt activation by LY294002, FoxO1 translocation by constitutively nuclear FoxO1 mutant, or c-Src activation by the chemical inhibitor PP2, respectively, blunted SDF-1 suppression of gluconeogenesis. Finally, our results show that knocking down the level of SDF-1 receptor CXCR4 mRNA blocked SDF-1 suppression of gluconeogenesis. Together, our results demonstrate that SDF-1 is capable of inhibiting gluconeogenesis in primary hepatocytes through a signaling pathway distinct from the insulin signaling.
Hepatic gluconeogenesis is a major contributor to hyperglycemia in both types I and II diabetes (T1DM and T2DM) (1). Gluconeogenesis becomes unrestrained in diabetes due to either deficient insulin secretion in T1DM or deficient insulin action in T2DM (1). Therefore, the key to treat diabetes is to provide insulin to patients with T1DM and reverse or bypass the deficient insulin signaling pathway in patients with T2DM. Identification of agents that can inhibit hepatic gluconeogenesis through a signaling pathway distinct from insulin may provide new avenues to curtail the elevated gluconeogenesis caused by insulin resistance in T2DM.
Hepatic gluconeogenesis plays an essential role in maintaining the blood glucose level normal during the fasting or non-feeding phase, and is inhibited by insulin when nutrients are abundant in the blood such as shortly after food ingestion (1). It is well established that insulin suppresses hepatic gluconeogenesis through activation of Akt (2, 3). Akt subsequently phosphorylates FoxO1, a key transcription factor in regulation of gluconeogenic gene expression (4). The phosphorylated FoxO1 will be translocated to the cytoplasm from the nucleus (5). As a result, gluconeogenesis is inhibited. In T2DM, suppression of hepatic gluconeogenesis by the Akt-dependent insulin signaling is reduced or lost due to insulin resistance plus a relatively insufficient insulin production (1). Because stromal cell-derived factor-1 (SDF-1)3 is a known Akt activator and its receptor is expressed in liver (6), it is possible that SDF-1 can bypass the blunted insulin signaling and consequently inhibit hepatic gluconeogenesis.
SDF-1, also named CXC chemokine ligand 12 (CXCL12), belongs to the family of CXC chemokines (7, 8). There are at least two SDF-1 isoforms (α, β) due to alternative splicing. SDF-1 was initially discovered in bone marrow stromal cells, and now has been found in various immune and non-immune tissues and organs, including lymph node, thymus, thyroid gland, appendix, bone marrow, uterus, lung, salivary gland, heart, skeletal muscle, and liver (9–12). As the specific receptor for SDF-1, CXCR4 is also widely expressed in both immune cells (T cells, B cells, and NK cells) and non-immune cells (myocytes, epithelials, neurons, dendritic cells, and hepatocytes) (11, 13, 14). The wide spectrum distribution of the SDF-1/CXCR4 system is consistent with its diverse functions.
As a chemoattractant, SDF-1/CXCR4 attracts various kinds of cells such as hemopoietic progenitor cells, T lymphocytes, pre-B-cells, monocytes, and dendritic cells (8). SDF-1/CXCR4 is essential for normal hematopoietic progenitor cell movement and adherence within the bone marrow microenvironment (8). Roles for SDF-1/CXCR4 have been indicated in vascular remodeling by recruiting smooth muscle cells (15), bone remodeling by regulating osteoclastogenesis (16), regulation of pituitary function (8), and neuronal generation by promoting neuronal migration and axonal pathfinding (17). As a result, deletion of either the SDF-1 or CXCR4 genes leads to a similar embryonic lethal phenotype, which is characterized by deficient B-lympho- and myelopoiesis, deficient vasculogenesis, deficient myogenesis, and abnormal development of the heart and the central nervous system (13, 18).
In addition to its roles in the physiological events described above, SDF-1/CXCR4 has been implicated in inflammatory diseases (19), development and metastasis of cancers (20, 21), and atherosclerosis (22). Therefore, SDF-1/CXCR4 has become a potential therapeutic target for treatment of a variety of diseases such as human immunodeficiency virus infection, cancer metastasis, leukemia, rheumatoid arthritis, and stroke (23, 24).
Of our interest, differential roles for SDF-1/CXCR4 have been implicated in the development of diabetes. Elevated expression of SDF-1 in thymocytes may play a role in the development of autoimmune in non-obese diabetic (NOD) mouse, a T1DM model (25). Neutralization of SDF-1 function in NOD mice with antisera against SDF-1 reduces insulitis and significantly delays the onset of diabetes (26). These results suggest that SDF/CXCR4 is a mediator of the autoimmune in NOD mice.
In contrast, transgenic overexpression of SDF-1 in pancreas significantly increases the survival of β-cells and render normal mice more resistance to streptozotocin-induced β-cell inflammation and diabetes probably through promoting the survival and migration of progenitor cells in the pancreas (27, 28). Furthermore, blockade of SDF-1 function with an antagonist of CXCR4 (AMD3100) leads to programmed cell death of insulin producing MIN-6 cells (28). These reports support the notion that SDF-1/CXCR4 directly protects pancreatic β-cells in normal subjects. The protective role is associated with activation of Akt (28). In this study, we have tested the hypothesis that SDF-1/CXCR4 inhibits hepatic gluconeogenesis through Akt.
MATERIALS AND METHODS
Antibodies, Reagents, and Constructs—Recombinant SDF-1α, SDF-1β, and macrophage inflammatory protein-1α were from PeproTech Inc. (Rocky Hill, NJ). Antibodies against total/phospho-Akt (tyrosine 473 and 308), total and phospho-FoxO1 (serine 256), and total/phospho-Src (tyrosine 416) were from Cell Signaling Technology. Antibody against phospho-IRS-1 (tyrosine 612), human insulin, N6, 2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium (cAMP), and dexamethasome were from Sigma. Chemical inhibitors for Src tyrosine kinases PP2 and the negative control PP3 and the PI 3-kinase inhibitor LY294002 (catalog number 440202) were purchased from Calbiochem. The siRNA against CXCR4 (catalog number 161237) and related scrambled siRNA (catalog number 4635) were from Ambion (Austin, TX). Lipofectamine™ 2000 Transfection Reagents were from Invitrogen. The lactate dehydrogenase (LDH) assay kit was from Roche Applied Science. The phosphoenolpyruvate carboxykinase 1 (PEPCK) promoter construct, the constitutively nuclear form of FoxO1 encoded by adenovirus, and the adenoviral vector encoding GFP were kind gifts from Drs. Jianhua Shao, Domenico Accili, and Christopher Newgard, respectively (29–31).
Isolation and Culture of Hepatocytes—Primary hepatocytes were isolated from C57BL/6 male mice (8–10 weeks old) and cultured as previously described (32–37). In brief, under anesthesia with pentobarbital (intraperitoneal, 50 mg/kg body weight), isolated liver was perfused with Hanks' balanced solution containing 0.5 mm EGTA, 0.004 n NaOH, 10 mm HEPES (Invitrogen) at 5 ml/min for 8 min, followed by continuous perfusion with serum-free Williams' Medium E containing collagenase (Worthington, type II, 50 units/ml) (Invitrogen) supplemented with 10 mm HEPES for 12 min. Hepatocytes were harvested and purified with Percoll (Sigma) as previously described (32–37). The viability of hepatocytes was examined with trypan blue exclusion. Only cell isolates with viability over 95% were used. Hepatocytes were inoculated into collagen-coated plates (5 × 105 cells/well in 6-well plates and 1.25 × 105 cell per well in 24-well plates) in Williams' Medium E with 10% fetal bovine serum, and were incubated for 24 h before experimentation. All mice used in the present study for isolation of hepatocytes were fed a normal chow diet under using regular schedule and were not fasted. All studies were approved by The Hamner Institutes for Health Sciences Animal Care and Use Committee and complied with guidelines from the United States National Institutes of Health. Hepa1c1c7 mouse hepatoma cells (ATCC) were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum.
Measurement of Glucose Production—Glucose production was assayed as described before (36, 37). Briefly, hepatocytes were pre-treated with SDF-1α, SDF-1β, or insulin at concentrations as noted for 1 h, followed by stimulation with cAMP/dexamethasome (10 μm/50 nm) in glucose-free Dulbecco's modified Eagle's medium containing 2 mm sodium lactate. For inhibition assay, cells were pre-treated with chemical inhibitors at the indicated concentrations at 37 °C for 30 min. The culture media were subsequently collected for measuring glucose and LDH. Levels of LDH were measured with a LDH assay kit (Roche). Cells were lysed with the lysis buffer for protein determination using the Bio-Rad DC protein assay kit (Bio-Rad). Glucose concentrations were determined with an YSI glucose analyzer (YSI, Yellow Springs, OH) and normalized to protein concentrations. Glucose production via gluconeogenesis was calculated as previously described (24). Specifically, glucose production in the presence of the gluconeogenic substrate (sodium lactate) was considered as the total glucose production and glucose production in the absence of sodium lactate was defined as glycogenolysis. Glucose production via gluconeogenesis = total glucose production - glycogenolysis.
Immunoblotting Blotting—Cells were lysed in lysis buffer (20 mm Tris-HCl, pH 7.5, 137 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 2 μg/ml leupeptin, and 10 μg/ml aprotinin), supplemented with 1 mm phenylmethylsulfonyl fluoride before use (38). Cell lysates (15 μg/lane) were resolved in 4–20% Tris glycine gels (Invitrogen) and transferred to nitrocellulose membranes (Bio-Rad). The presence of proteins was detected by immunoblotting with primary antibodies as indicated and alkaline phosphatase-conjugated secondary antisera. The fluorescent bands were visualized with a Typhoon 9410 variable mode Imager from GE Healthcare, and then quantified by densitometry analysis using ImageQuant 5.2 software from GE Healthcare.
Gene Silencing, Transfection, and Viral Infection—The cognate siRNA against CXCR4 or scramble siRNA was introduced into primary hepatocytes by reverse transfection with Lipofectamine 2000 as previously described (36). Briefly, the siRNA transfection mixture was pre-applied to collagen-coated 6-well plates right before the plating of primary hepatocytes in culture media without antibiotics. The plasmid containing the PEPCK promoter and the luciferase reporter gene was introduced into Hepa1c1c7 hepatoma cells via standard transient transfection with Lipofectamine 2000. Recombinant adenoviruses encoding the nuclear form of FoxO1 (Ad-FoxO1-ADA) or GFP (Ad-GFP), amplified in HEK-293 cells, were applied to infect primary hepatocytes in 6-well plates as described before (32, 36, 37).
Real-time PCR—Total RNA was extracted from hepatocytes with an RNeasy Mini Kit (Qiagen), and reverse transcribed into cDNA at 42 °C for 30 min. The cDNAs were quantified by TaqMan® Real-time PCR with specific probes from Applied Biosciences, and normalized to levels of glyceraldehyde-3-phosphate dehydrogenase. Probes used in this study were glyceraldehyde-3-phosphate dehydrogenase (assay ID, Mm99999915_g1), glucose-6-phosphatase (G6Pase) (assay ID, Mm00839363_m1), and PEPCK (assay ID, Mm00440636_m1).
Statistical Analysis—Data are presented as mean ± S.E. Data were compared by Student's t test using GraphPad Prism version 4.0 for Windows (San Diego, CA). Differences at values of p < 0.05 were considered significant.
RESULTS
SDF-1 Suppresses Hepatic Gluconeogenesis in Primary Hepatocytes—It has previously been shown that SDF-1 can activate Akt in hepatocytes (12). Because Akt is a central mediator of insulin suppression of hepatic gluconeogenesis, we tested the hypothesis that SDF-1 plays an inhibitory role in hepatic gluconeogenesis. The glucagon/cAMP/PKA-dependent signaling is the primary stimulatory pathway of gluconeogenesis, and glucocorticoids play a prerequisite role in gluconeogenesis (1, 39). Thus, glucose production via gluconeogenesis in primary hepatocytes was stimulated with a membrane-permeable cAMP and dexamethasome in the presence or absence of SDF-1 or insulin, and quantified as detailed under “Materials and Methods.” As expected, glucose production via gluconeogenesis was promoted by cAMP/dexamethasome, but suppressed by insulin (Fig. 1A). The presence of either SDF-1α or SDF-1β inhibited cAMP/dexamethasome-induced glucose production via gluconeogenesis in a dose-dependent manner (Fig. 1A). To examine whether this inhibition by SDF-1 was due to cytotoxicity, levels of LDH in the cell culture media were quantified. Clearly, SDF-1 did not cause increased release of LDH, suggesting no detectable toxicity (Fig. 1B). In contrast, 1% Triton X-100 caused a significant release of LDH. These results support the hypothesis that SDF-1 inhibits gluconeogenesis in primary hepatocytes.
FIGURE 1.
Glucose production via gluconeogenesis is decreased by SDF-1α or -β in hepatocytes. Primary hepatocytes were isolated from mouse and cultured as described under “Materials and Methods.” A, measurement of glucose production. Cells were pretreated with either SDF-1 (α or β) or insulin (control) at indicated concentrations in serum-free Dulbecco's modified Eagle's medium for 1 h, and then treated for 2.5 h with cAMP (10 μm) and dexamethasome (Dex, 50 nm) in serum- and glucose-free Dulbecco's modified Eagle's medium supplemented with gluconeogenic substrate sodium lactate (2 mm) in the continuous presence or absence of SDF-11α or -1β. The glucose production via gluconeogenesis was determined, calculated, and normalized to protein concentrations as detailed under “Materials and Methods.” Results presented represent mean ± S.E. of three independent experiments, each in triplicate. B, measurement of LDH. Levels of LDH in the supernatants of the cell cultures described above were determined as detailed in “Materials and Methods” and compared with the positive control (1% Triton X-100). **, p < 0.01; and ***, p < 0.001 versus cells treated with cAMP/dexamethasome alone.
To further test this hypothesis, the effect of SDF-1 on expression of key gluconeogenic genes G6Pase and PEPCK was examined. Levels of G6Pase and PEPCK transcripts were elevated by treatment with cAMP/dexamethasome, but the elevations were significantly reduced by both SDF-1α and SDF-1β in a dose-dependent manner (Fig. 2A). Similarly, the activity of the PEPCK promoter measured by the luciferase reporter gene was stimulated by cAMP/dexamethasome, but blunted by insulin, or SDF-1 (α and β) (Fig. 2B). The effect of SDF-1 appears to be weaker than equal molar concentrations of insulin. Together, these results further support the hypothesis that SDF-1 inhibits gluconeogenesis in isolated hepatocytes.
FIGURE 2.
Inhibition of gluconeogenic gene expression by SDF-1. A, primary hepatocytes were pretreated with SDF-1α and -1β at the concentrations as noted for 1 h, followed by treatment with cAMP (10 μm) and dexamethasome (Dex, 50 nm) in the continuous presence or absence of SDF-1α or -1β for 2 h. Levels of G6Pase and PEPCK gene transcripts were evaluated with TaqMan Real-time reverse transcriptase-PCR. Results presented represent mean ± S.E. of two independent experiments, each in duplicate. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 compared with treatment with cAMP/dexamethasome alone. B, the plasmid containing the PEPCK promoter and a luciferase reporter gene was introduced into Hep1c1c7 cells via transient transfection with Lipofectamine 2000 for 24 h and then stimulated with cAMP (10 μm) and dexamethasome (Dex, 50 nm) for 4 h in the presence or absence of a pretreatment with SDF-1α,-1β, or insulin as noted. The promoter activity was measured by luciferase assays and normalized to protein concentrations. ***, p < 0.001 compared with treatment with cAMP/dexamethasome alone.
SDF-1 Stimulates Phosphorylation of Akt and FoxO1—To investigate the mechanism by which SDF-1 suppresses hepatic gluconeogenesis, we examined whether or not SDF-1 activates Akt, which is necessary for insulin suppression of hepatic gluconeogenesis (1). Activation of Akt is usually evaluated by measuring phosphorylation levels at either Ser473 or Thr308, or both sites (40). Thus, we measured phosphorylation levels at these two sites by immunoblottings with specific antisera. As shown in Fig. 3A, both SDF-1α and -β stimulated phosphorylation at Ser473 significantly in a dose-dependent manner. SDF-1α and -β also promoted phosphorylation at Thr308 although SDF-1α-induced phosphorylation at Thr308 appeared to be less than that induced by SDF-1β. As a positive control, insulin stimulated Akt phosphorylation at both sites as expected. To determine the possible role of PI 3-kinase, a key component of insulin signaling and a known activator of Akt (1), primary hepatocytes were pre-treated with the PI 3-kinase inhibitor LY294002 prior to treatment with SDF-1β. As shown in Fig. 3B, SDF-1β-induced Akt phosphorylation was prevented by LY294002 in a dose-dependent manner. We next evaluated the effect of LY294002 on SDF-1- or insulin-mediated suppression of gluconeogenesis. In the absence of LY294002, the cAMP/dexamethasome-induced glucose production via gluconeogenesis was blunted by either SDF-1β or the positive control insulin as expected (Fig. 3C). However, the presence of LY294002 prevented SDF-1β suppression of glucose production via gluconeogenesis. As anticipated, insulin-mediated suppression of glucose production via gluconeogenesis was also blocked by LY294002.
FIGURE 3.
SDF-1 inhibits glucose production through phosphorylation of Akt. A, primary hepatocytes were treated with SDF-1α, SDF-1β, or insulin at noted concentrations for 15 min. Subsequently, levels of phospho-Akt (at serine 473 or serine 308) and total Akt were evaluated by immunoblottings with specific antibodies. B, PI 3-kinase inhibitor LY294002 prevented SDF-1β phosphorylation of Akt. Primary hepatocytes were pretreated with LY294002 for 30 min followed by SDF-1β treatment for 15 min. Akt phosphorylation at serine 473 was detected by immunoblotting with specific antisera. C, SDF-1β suppression of glucose production was prevented by LY294002. Primary hepatocytes were treated with 1 μm LY294002 for 1 h prior to the treatment with cAMP/dexamethasome in the presence or absence of SDF-1β for 2.5 h. Glucose production was subsequently evaluated as detailed under “Materials and Methods.” D, SDF-1β reduction in levels of key gluconeogenic gene transcripts was prevented by LY294002. Primary hepatocytes were pre-treated with 1 μm LY294002 for 1 h, and then treated with cAMP/dexamethasome for 4 h. Levels of PEPCK and G6Pase transcripts were measured by real-time PCR. Results represent three independent experiments. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 in comparison to the first lane.
To further examine the role of Akt activation in SDF-1 suppression of gluconeogenesis, we studied the effect of LY294002 on SDF-1 inhibition of key gluconeogenic genes. As shown in Fig. 3D, application of SDF-1β significantly reduced the transcript levels of both G6Pase and PEPCK although the reduction was not as much as those induced by insulin (positive control). Importantly, application of LY294002 largely reversed the SDF-1β-induced decrease in levels of PEPCK and G6Pase transcripts (Fig. 3D). Similarly, the insulin-mediated reduction in levels of G6Pase and PEPCK transcripts was also largely prevented by LY294002. Together, these results indicate that SDF-1 inhibits gluconeogenesis through activation of Akt similarly to insulin.
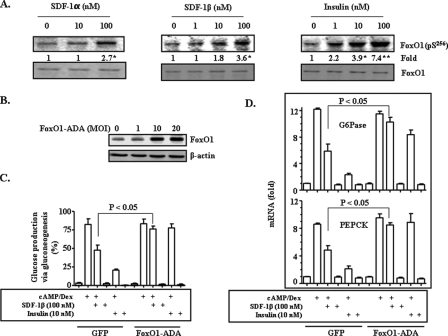
Next, the role of FoxO1, a downstream effector of Akt (1, 37), in SDF-1 inhibition of gluconeogenesis was investigated. Akt phosphorylation of FoxO1 at Ser256 will lead to FoxO1 translocation from the nucleus to cytoplasm, and consequently inhibit transcription of key gluconeogenic genes (G6Pase and PEPCK) (41, 42). Thus, we first studied the effect of SDF-1 on FoxO1 phosphorylation at Ser256. As shown in Fig. 4A, both SDF-1α and SDF-1β clearly stimulated phosphorylation of FoxO1 in a dose-dependent manner. It appeared that SDF-1 stimulation of FoxO1 phosphorylation at Ser256 was at a lower level than that induced by the positive control insulin. To determine the role FoxO1 in SDF inhibition of gluconeogenesis, we decided to overexpress a FoxO1 mutant, in which the Akt phosphorylation site (Ser256) was deleted, and could not be excluded from the nucleus (42, 43). As shown in Fig. 4B, FoxO1 protein levels in whole cell lysates were increased clearly after the mutant FoxO1 was introduced into primary hepatocytes via recombinant adenoviruses, indicating the presence of the mutant FoxO1 in the cells. We next examined the effect of the mutant FoxO1 on SDF-1 suppression of gluconeogenesis. Overexpression of the mutant (constitutively nuclear) FoxO1 prevented SDF-1β from inhibiting glucose production via gluconeogenesis (Fig. 4C). Insulin-mediated inhibition of gluconeogenesis was blocked by the mutant FoxO1 as expected. In contrast, the presence of overexpression of GFP encoded by recombinant adenoviruses (negative control) did not influence the effect of either SDF-1β or insulin.
FIGURE 4.
SDF-1 inhibition of glucose production is FoxO1-dependent. A, primary hepatocytes were treated with SDF-1α, SDF-1β, or insulin for 15 min. Levels of phospho-FoxO1 (at serine 256) and total FoxO1 were evaluated by immunoblottings with specific antibodies. The constitutively nuclear form of FoxO1 (FoxO1-ADA) or GFP encoded by adenoviruses (1–20 multiplicity of infection/well in 6-well plate) were introduced into isolated primary hepatocytes 36 h before the cells were treated with cAMP (10 μm)/dexamethasome (Dex) (50 nm) for 2.5 h in the presence of SDF-1β or insulin as noted. The expression level of the constitutively nuclear form of FoxO1 was determined by immunoblotting (B). Glucose production via gluconeogenesis was evaluated as detailed under “Materials and Methods” (C). Transcripts of G6Pase and PEPCK genes were quantified by TaqMan real-time PCR (D). Results represent three independent experiments. *, p < 0.05; and **, p < 0.01 in comparison to the first lane.
To further investigate the role of FoxO1 in SDF-1 inhibition of gluconeogenesis, we evaluated the effect of the mutant FoxO1 on transcript levels of G6Pase and PEPCK. As shown in Fig. 4D, overexpression of the mutant FoxO1 reversed SDF-1β reductions of both G6Pase and PEPCK mRNAs. The insulin-mediated decreases in G6Pase and PEPCK mRNA levels were also prevented by the mutant FoxO1 as predicted. However, overexpression of GFP (a control) had no effect. Together, these results indicate that FoxO1 is a target of SDF-1 in suppression of gluconeogenesis.
SDF-1 Suppresses Gluconeogenesis through a Signaling Pathway Distinct from Insulin—Tyrosine phosphorylation of insulin receptor substrate (IRS) proteins such as IRS-1 is necessary for activation of the insulin signaling pathway (1). To examine whether or not SDF-1 activates Akt in the same way as insulin, we measured levels of IRS-1 tyrosine phosphorylation. As shown in Fig. 5A, neither SDF-1α nor SDF-1β initiated any significant tyrosine phosphorylation of IRS-1, whereas insulin robustly stimulated IRS-1 tyrosine phosphorylation as expected. The negative control, macrophage inflammatory protein-1α, also did not promote any IRS-1 tyrosine phosphorylation.
FIGURE 5.
SDF-1 suppresses hepatic gluconeogenesis through c-Src. Primary hepatocytes were treated with SDF-1 (α or β), macrophage inflammatory protein-1α (MIP) (negative control), or insulin (positive control) for 15 min. Levels of phospho-IRS-1 (at tyrosine 612) and total IRS (A), and phospho-c-Src (at tyrosine 416, activating) and total c-Src (B) were determined by immunoblottings with specific antibodies. NS, nonspecific bands. C, primary hepatocytes were pretreated with PP2 or PP3 (negative control) for 30 min, and then treated with cAMP (10 μm)/dexamethasome (Dex) (50 nm) in the continuous presence or absence of SDF-1β for 3 h. Sodium lactate (2 mm) was added to some cells as a substrate of gluconeogenesis. Glucose production via gluconeogenesis was quantified, calculated, and normalized to protein levels as detailed under “Materials and Methods.” Results represent mean ± S.E. of two independent experiments performed in triplicate. D, levels of PEPCK gene transcripts from these same cells were determined by real-time reverse transcriptase-PCR using specific TaqMan PCR probes. Results represent mean ± S.E. from two independent experiments performed in duplicate. *, p < 0.05 in comparison to the first lane. NS, non specific.
It is known that the SDF-1 receptor CXCR4 is a Gi protein-coupled receptor, which is known to be able to activate c-Src (44, 45). It is also known that c-Src is an activator of Akt (46, 47). Therefore, we studied the effect of SDF-1 on activation of c-Src. Phosphorylations of c-Src at different sites can lead to either inhibition or activation of c-Src (48, 49). Activation of c-Src is usually evaluated by measuring phosphorylation levels at Tyr416. Therefore, we examined the effect of SDF-1 on c-Src phosphorylation at this site. Treatment of primary hepatocytes with either SDF-1α or -β stimulated c-Src phosphorylation at Tyr416 (Fig. 5B), suggesting that SDF-1 can activate c-Src.
Next, the role of c-Src in SDF-1 suppression of gluconeogenesis was evaluated. As shown in Fig. 5C, glucose production via gluconeogenesis was stimulated by cAMP/dexamethasome and inhibited by SDF-1β in a dose-dependent manner. Application of the c-Src inhibitor, PP2, significantly reversed the effect of SDF-1β in a dose-dependent manner. Application of PP3, a non-functional analogue of PP2, had no effect. Insulin also inhibited glucose production via gluconeogenesis. Importantly, blockade of c-Src with PP2 did not affect the insulin effect at all.
To further investigate the role of c-Src in SDF-1 suppression of gluconeogenesis, we examined the effect of PP2 on levels of PEPCK transcripts. The mRNA level of the PEPCK gene was increased by cAMP/dexamethasome and the increase was significantly reduced by SDF-1β (Fig. 5D). SDF-1β-mediated reduction in levels of PEPCK transcripts was largely prevented by PP2 in a dose-dependent manner. Again, PP3 showed no effect. It is noteworthy that the insulin-mediated decrease in the PEPCK mRNA level was not reversed by PP2 (Fig. 5D). These results indicate that SDF-1 inhibits gluconeogenesis through c-Src, whereas insulin suppression of gluconeogenesis is independent of c-Src.
Knocking Down the Level of SDF-1 Receptor CXCR4 Transcripts Prevents SDF-1 Suppression of Hepatic Gluconeogenesis— Because CXCR4 is the specific receptor of SDF-1 (44), we examined the role of CXCR4 in SDF-1 suppression of hepatic gluconeogenesis. The specific siRNA was introduced into primary hepatocytes to knock down the transcript level of the CXCR4 gene (Fig. 6A). Cells were then treated with cAMP/dexamethasome in the presence or absence of SDF-1, followed by measurements of glucose production via gluconeogenesis and levels of PEPCK gene transcripts. As shown in Fig. 6, B–D, the cAMP/dexamethasome-induced glucose production via gluconeogenesis and elevation of PEPCK transcript levels were significantly reduced by either SDF-1α or SDF-1β as predicted. In the presence of the siRNA against the CXCR4 gene, the inhibition of glucose production by either SDF-1α or SDF-1β was largely reversed (Fig. 6, B and C). Similarly, the SDF-1β-mediated reduction in PEPCK transcript levels was also significantly reversed by the specific siRNA (Fig. 6D). The control siRNA (scramble) had no effect. Together, these results show that SDF-1 inhibits hepatic gluconeogenesis through its receptor CXCR4.
FIGURE 6.
Reduction of SDF-1 receptor (CXCR4) mRNA levels prevents SDF-1-induced suppression of hepatic gluconeogenesis. A, the cognate siRNA against CXCR4 (siCXCR4) or a scramble siRNA was introduced into primary hepatocytes by reverse transfection for 36 h as detailed under “Materials and Methods.” Levels of CXCR4 transcripts were evaluated by Real-time reverse transcriptase-PCR using the specific CXCR4 TaqMan PCR probe, and normalized to levels of β-actin transcripts. Cells were then treated with either SDF-1α (B) or SDF-1β (C) for 1 h prior to stimulation with cAMP (10 μ)/dexamethasome (Dex) (50 nm) in the continuous presence or absence of SDF-1 (α or β). Results represent mean ± S.E. of three independent experiments, each in triplicate. D, levels of PEPCK transcripts in cells that were similarly treated were evaluated using TaqMan real-time reverse transcriptase-PCR. Results represent mean ± S.E. of two independent experiments, each in triplicate.
DISCUSSION
The predominant inhibitor of gluconeogenesis is insulin. That is why hepatic gluconeogenesis becomes a major contributor to hyperglycemia when the insulin function is faulted due to either absolute deficient insulin production in T1DM or deficient insulin action plus a relatively insufficient insulin production in T2DM (50). In this study, we have found that SDF-1 can inhibit hepatic gluconeogenesis through a signaling pathway that is distinct from the insulin signaling.
There are many similarities between SDF-1 and insulin in suppression of gluconeogenesis although SDF-1 appears less potent in comparison to equal molar concentrations of insulin. Both SDF-1 and insulin promote Akt phosphorylation, which is an integral component of insulin suppression of gluconeogenesis. Blockade of Akt activation by the PI 3-kinase inhibitor LY294002 prevents both insulin- and SDF-1-mediated inhibition of gluconeogenesis. Furthermore, introduction of the constitutively nuclear form of FoxO1, a downstream effector of Akt, reversed the inhibitory effect of both SDF-1 and insulin. Besides, under various conditions, both SDF-1 and insulin can activate other intracellular signaling pathways such as mitogen-activated protein kinases (ERK1/2 and p38), PKCs, NF-κB, and Akt (12, 51–53). Thus, it appears that the roles of SDF-1 and insulin may be interchangeable.
Importantly, the signaling pathways of SDF-1 and insulin are not exactly the same. The insulin activation of Akt and suppression of gluconeogenesis depend upon sequential activations of IRS1 and PI 3-kinase (50). Nevertheless, SDF-1 does not activate IRS1. Instead, SDF-1 activates c-Src, a known activator of Akt (46, 47). Blockade of c-Src activation blunts the effect of SDF-1 in gluconeogenesis, whereas having no effect on insulin suppression of gluconeogenesis. Therefore, SDF-1 may be used to bypass the blunted insulin signaling in subjects with insulin resistance.
Our results clearly show that SDF-1 suppression of gluconeogenesis is mediated by the specific receptor CXCR4. Because CXCR4 is obviously expressed in hepatocytes (14), it is anticipated that administration of CXCR4 agonist such as SDF-1 is able to inhibit gluconeogenesis. Additionally, it is established that CXCR4 is required for skeletal muscle development (18, 54). However, it is unclear whether or not a significant amount of CXCR4 is expressed in myotubes. Thus, it is hard to predict the effect of CXCR4 agonist on glucose metabolism in skeletal muscles, another major organ involved in metabolism.
Our results demonstrate that SDF-1 is able to inhibit hepatic gluconeogenesis in isolated hepatocytes at concentrations at or higher than 1 nm. It is currently unknown whether or not the focal concentrations of SDF-1 around hepatocytes in liver can reach 1 nm or higher. But it is unlikely that plasma levels of SDF-1 fluctuate with food intakes like insulin. Therefore, SDF-1/CXCR4 does not appear to play a significant role in regulation of hepatic gluconeogenesis under the physiological condition. Furthermore, it is unknown whether or not concentrations of SDF-1 in the liver or blood are altered in T2DM. Thus, it is unclear whether or not SDF-1 plays any role in the development of T2DM.
There is evidence that SDF-1/CXCR4 may be involved in the development of T1DM. It has been shown in a T1DM mouse model (NOD) that SDF-1 plays a role in the development of the autoimmune by promoting thymocyte migration (25, 26). Blockade of SDF-1 function with antisera can reduce insulitis and delays the onset of diabetes in the NOD mice (26). Conversely, overexpression of SDF-1 in pancreatic β-cells can prevent streptozotocin-induced inflammation and onset of diabetes in normal animals. Therefore, application of SDF-1 may be beneficial in preserving pancreatic β-cells in subjects with T2DM. Bedsides, it is also known that SDF-1/CXCR4 promotes vasculogenesis and neurogenesis (15, 17). It is possible that application of SDF-1 may prevent or slow vascular and neuronal degenerations that are frequently seen in diabetes. Here we show that SDF-1 can inhibit hepatic gluconeogenesis at concentrations that are not toxic to hepatocytes through a signaling pathway that is different from the insulin signaling. Therefore, SDF-1 may be potentially valuable in treatment of diabetes with insulin resistance by preserving β-cells, preventing or reversing degenerations of vasculatures and neurons, in addition to reduction of blood glucose levels through inhibition of hepatic gluconeogenesis.
In summary, we have identified a new inhibitor of hepatic gluconeogenesis in SDF-1/CXCR4. Importantly, SDF-1 suppresses gluconeogenesis through a signaling pathway distinct from the insulin signaling. Therefore, the SDF-1/CXCR4 system may be a potential target for modulating hepatic gluconeogenesis and blood glucose levels in diabetes.
Acknowledgments
We thank Drs. Christopher Newgard, Domenico Accili, and Jianhua Shao for kindly providing the necessary reagents for this study.
This work was supported, in whole or in part, by National Institutes of Health NIDDK Grant R01DK076039 (to W. C.). This work was also supported by the Investigator Development Fund from the Hamner Institutes for Health Sciences (to W. C.) and American Heart Association Grant SDG-0530244N (to W. C.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: SDF-1, stromal cell-derived factor-1; LDH, lactate dehydrogenase; IRS, insulin receptor substrate; NOD, non-obese diabetic; siRNA, small interfering RNA; PI, phosphatidylinositol; GFP, green fluorescent protein; PEPCK, phosphoenolpyruvate carboxykinase.
References
- 1.Accili, D. (2004) Diabetes 53 1633-1642 [DOI] [PubMed] [Google Scholar]
- 2.Whiteman, E. L., Cho, H., and Birnbaum, M. J. (2002) Trends Endocrinol. Metab. 13 444-451 [DOI] [PubMed] [Google Scholar]
- 3.Ono, H., Shimano, H., Katagiri, H., Yahagi, N., Sakoda, H., Onishi, Y., Anai, M., Ogihara, T., Fujishiro, M., Viana, A. Y., Fukushima, Y., Abe, M., Shojima, N., Kikuchi, M., Yamada, N., Oka, Y., and Asano, T. (2003) Diabetes 52 2905-2913 [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto, M., Pocai, A., Rossetti, L., Depinho, R. A., and Accili, D. (2007) Cell Metab. 6 208-216 [DOI] [PubMed] [Google Scholar]
- 5.Gross, D. N., van den Heuvel, A. P., and Birnbaum, M. J. (2008) Oncogene 27 2320-2336 [DOI] [PubMed] [Google Scholar]
- 6.Poggi, A., Catellani, S., Fenoglio, D., Borsellino, G., Battistini, L., and Zocchi, M. R. (2007) Curr. Med. Chem. 14 3166-3170 [DOI] [PubMed] [Google Scholar]
- 7.Broxmeyer, H. E. (2008) Curr. Opin. Hematol. 15 49-58 [DOI] [PubMed] [Google Scholar]
- 8.Barbieri, F., Bajetto, A., Porcile, C., Pattarozzi, A., Schettini, G., and Florio, T. (2007) J. Mol. Endocrinol. 38 383-389 [DOI] [PubMed] [Google Scholar]
- 9.Han, W., Yu, Y., and Liu, X. Y. (2006) Cell Res. 16 189-195 [DOI] [PubMed] [Google Scholar]
- 10.Shibuta, K., Mori, M., Shimoda, K., Inoue, H., Mitra, P., and Barnard, G. F. (2002) Jpn. J. Cancer Res. 93 789-797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tashiro, K., Tada, H., Heilker, R., Shirozu, M., Nakano, T., and Honjo, T. (1993) Science 261 600-603 [DOI] [PubMed] [Google Scholar]
- 12.Odemis, V., Boosmann, K., Dieterlen, M. T., and Engele, J. (2007) J. Cell Sci. 120 4050-4059 [DOI] [PubMed] [Google Scholar]
- 13.Nagasawa, T., Hirota, S., Tachibana, K., Takakura, N., Nishikawa, S., Kitamura, Y., Yoshida, N., Kikutani, H., and Kishimoto, T. (1996) Nature 382 635-638 [DOI] [PubMed] [Google Scholar]
- 14.Sutton, A., Friand, V., Brule-Donneger, S., Chaigneau, T., Ziol, M., Sainte-Catherine, O., Poire, A., Saffar, L., Kraemer, M., Vassy, J., Nahon, P., Salzmann, J. L., Gattegno, L., and Charnaux, N. (2007) Mol. Cancer Res. 5 21-33 [DOI] [PubMed] [Google Scholar]
- 15.Schober, A., and Zernecke, A. (2007) Thromb. Haemostasis 97 730-737 [PubMed] [Google Scholar]
- 16.Gronthos, S., and Zannettino, A. C. (2007) Trends Endocrinol. Metab. 18 108-113 [DOI] [PubMed] [Google Scholar]
- 17.Stumm, R., and Hollt, V. (2007) J. Mol. Endocrinol. 38 377-382 [DOI] [PubMed] [Google Scholar]
- 18.Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I., and Littman, D. R. (1998) Nature 393 595-599 [DOI] [PubMed] [Google Scholar]
- 19.De Paepe, B., Creus, K. K., and De Bleecker, J. L. (2008) Front. Biosci. 13 2548-2577 [DOI] [PubMed] [Google Scholar]
- 20.Koizumi, K., Hojo, S., Akashi, T., Yasumoto, K., and Saiki, I. (2007) Cancer Sci. 98 1652-1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger, J. A., and Burkle, A. (2007) Br. J. Haematol. 137 288-296 [DOI] [PubMed] [Google Scholar]
- 22.Braunersreuther, V., Mach, F., and Steffens, S. (2007) Thromb. Haemostasis 97 714-721 [PubMed] [Google Scholar]
- 23.Tsutsumi, H., Tanaka, T., Ohashi, N., Masuno, H., Tamamura, H., Hiramatsu, K., Araki, T., Ueda, S., Oishi, S., and Fujii, N. (2007) Biopolymers 88 279-289 [DOI] [PubMed] [Google Scholar]
- 24.Chang, Y. C., Shyu, W. C., Lin, S. Z., and Li, H. (2007) Cell Transplant. 16 171-181 [PubMed] [Google Scholar]
- 25.Mendes-da-Cruz, D. A., Smaniotto, S., Keller, A. C., Dardenne, M., and Savino, W. (2008) J. Immunol. 180 4639-4647 [DOI] [PubMed] [Google Scholar]
- 26.Matin, K., Salam, M. A., Akhter, J., Hanada, N., and Senpuku, H. (2002) Immunology 107 222-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayali, A. G., Van Gunst, K., Campbell, I. L., Stotland, A., Kritzik, M., Liu, G., Flodstrom-Tullberg, M., Zhang, Y. Q., and Sarvetnick, N. (2003) J. Cell Biol. 163 859-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano, T., Liu, Z., Donovan, J., Thomas, M. K., and Habener, J. F. (2007) Diabetes 56 2946-2957 [DOI] [PubMed] [Google Scholar]
- 29.Shao, J., Qiao, L., Janssen, R. C., Pagliassotti, M., and Friedman, J. E. (2005) Diabetes 54 976-984 [DOI] [PubMed] [Google Scholar]
- 30.Frescas, D., Valenti, L., and Accili, D. (2005) J. Biol. Chem. 280 20589-20595 [DOI] [PubMed] [Google Scholar]
- 31.An, J., Muoio, D. M., Shiota, M., Fujimoto, Y., Cline, G. W., Shulman, G. I., Koves, T. R., Stevens, R., Millington, D., and Newgard, C. B. (2004) Nat. Med. 10 268-274 [DOI] [PubMed] [Google Scholar]
- 32.Cao, W., Collins, Q. F., Becker, T. C., Robidoux, J., Lupo, J., Xiong, Y., Daniel, K., Floering, L., and Collins, S. (2005) J. Biol. Chem. 280 42731-42737 [DOI] [PubMed] [Google Scholar]
- 33.Xiong, Y., Collins, Q. F., Lupo, J., Liu, H. Y., Jie, A., Liu, D., and Cao, W. (2006) J. Biol. Chem. 282 4975-4982 [DOI] [PubMed] [Google Scholar]
- 34.Collins, Q. F., Xiong, Y., Lupo, J., Liu, H. Y., and Cao, W. (2006) J. Biol. Chem. 281 24336-24344 [DOI] [PubMed] [Google Scholar]
- 35.Collins, Q. F., Liu, H. Y., Pi, J., Liu, Z., Quon, M. J., and Cao, W. (2007) J. Biol. Chem. 282 30143-30149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, H. Y., Collins, Q. F., Xiong, Y., Moukdar, F., Lupo, J., Liu, Z., and Cao, W. (2007) J. Biol. Chem. 282 14205-14212 [DOI] [PubMed] [Google Scholar]
- 37.Liu, H. Y., Collins, Q. F., Fatiha, M., Zguo, D., Han, J., Hong, T., Collins, S., and Cao, W. (2008) J. Biol. Chem. 283 12056-12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, H. Y., MacDonald, J. I., Hryciw, T., Li, C., and Meakin, S. O. (2005) J. Biol. Chem. 280 19461-19471 [DOI] [PubMed] [Google Scholar]
- 39.Cassuto, H., Kochan, K., Chakravarty, K., Cohen, H., Blum, B., Olswang, Y., Hakimi, P., Xu, C., Massillon, D., Hanson, R. W., and Reshef, L. (2005) J. Biol. Chem. 280 33873-33884 [DOI] [PubMed] [Google Scholar]
- 40.Cameron, A. J., De Rycker, M., Calleja, V., Alcor, D., Kjaer, S., Kostelecky, B., Saurin, A., Faisal, A., Laguerre, M., Hemmings, B. A., McDonald, N., Larijani, B., and Parker, P. J. (2007) Biochem. Soc. Trans. 35 1013-1017 [DOI] [PubMed] [Google Scholar]
- 41.Huang, H., and Tindall, D. J. (2007) J. Cell Sci. 120 2479-2487 [DOI] [PubMed] [Google Scholar]
- 42.Nakae, J., Kitamura, T., Silver, D. L., and Accili, D. (2001) J. Clin. Investig. 108 1359-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitamura, T., Nakae, J., Kitamura, Y., Kido, Y., Biggs, W. H., 3rd, Wright, C. V., White, M. F., Arden, K. C., and Accili, D. (2002) J. Clin. Investig. 110 1839-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busillo, J. M., and Benovic, J. L. (2007) Biochim. Biophys. Acta 1768 952-963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGarrigle, D., and Huang, X. Y. (2007) Sci. STKE 2007, pe35. [DOI] [PubMed] [Google Scholar]
- 46.Warmuth, M., Damoiseaux, R., Liu, Y., Fabbro, D., and Gray, N. (2003) Curr. Pharm. Des. 9 2043-2059 [DOI] [PubMed] [Google Scholar]
- 47.Choudhury, G. G., Mahimainathan, L., Das, F., Venkatesan, B., and Ghosh-Choudhury, N. (2006) Cell Signal. 18 1854-1864 [DOI] [PubMed] [Google Scholar]
- 48.van Biesen, T., Luttrell, L. M., Hawes, B. E., and Lefkowitz, R. J. (1996) Endocr. Rev. 17 698-714 [DOI] [PubMed] [Google Scholar]
- 49.Levinson, N. M., Seeliger, M. A., Cole, P. A., and Kuriyan, J. (2008) Cell 134 124-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Accili, D., Drago, J., Lee, E. J., Johnson, M. D., Cool, M. H., Salvatore, P., Asico, L. D., Jose, P. A., Taylor, S. I., and Westphal, H. (1996) Nat. Genet. 12 106-109 [DOI] [PubMed] [Google Scholar]
- 51.Huang, Y. C., Hsiao, Y. C., Chen, Y. J., Wei, Y. Y., Lai, T. H., and Tang, C. H. (2007) Biochem. Pharmacol. 74 1702-1712 [DOI] [PubMed] [Google Scholar]
- 52.Wu, M., Chen, Q., Li, D., Li, X., Huang, C., Tang, Y., Zhou, Y., Wang, D., Tang, K., Cao, L., Shen, S., and Li, G. (2008) J. Cell. Biochem. 103 245-255 [DOI] [PubMed] [Google Scholar]
- 53.Zhu, W., Boachie-Adjei, O., Rawlins, B. A., Frenkel, B., Boskey, A. L., Ivashkiv, L. B., and Blobel, C. P. (2007) J. Biol. Chem. 282 18676-18685 [DOI] [PubMed] [Google Scholar]
- 54.Ma, Q., Jones, D., Borghesani, P. R., Segal, R. A., Nagasawa, T., Kishimoto, T., Bronson, R. T., and Springer, T. A. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 9448-9453 [DOI] [PMC free article] [PubMed] [Google Scholar]