Abstract
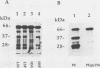
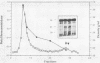
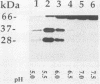
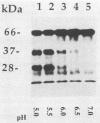
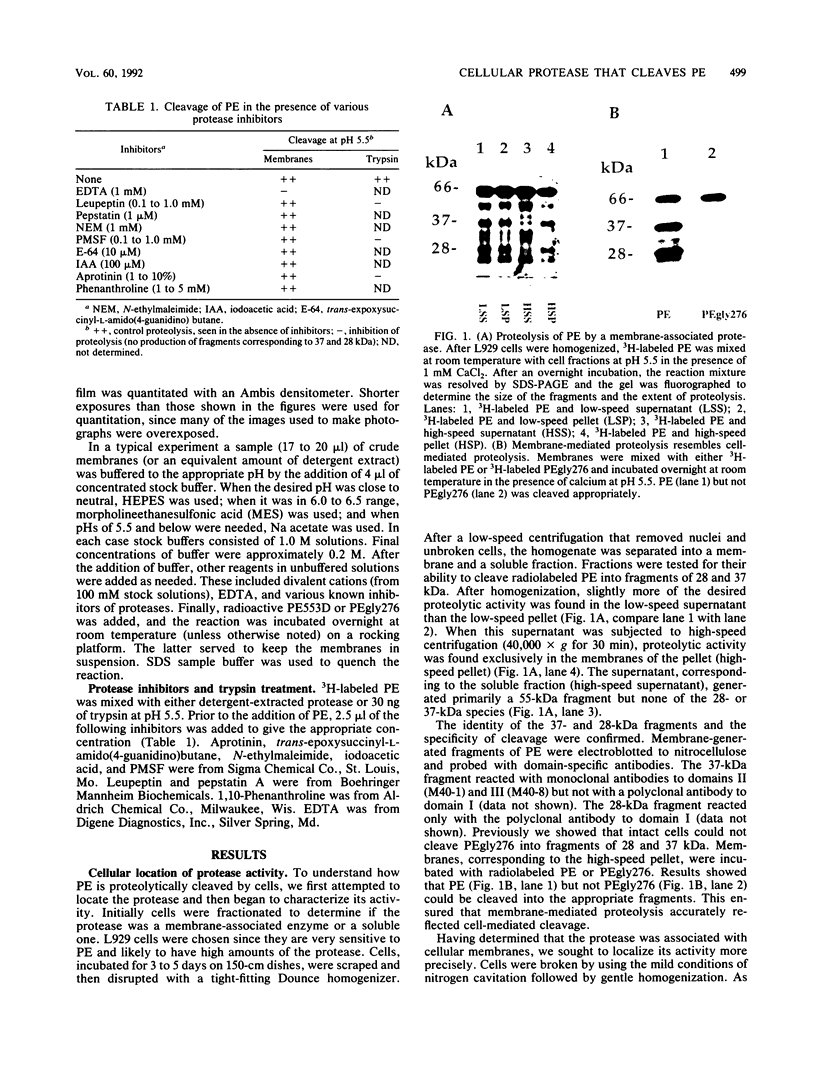
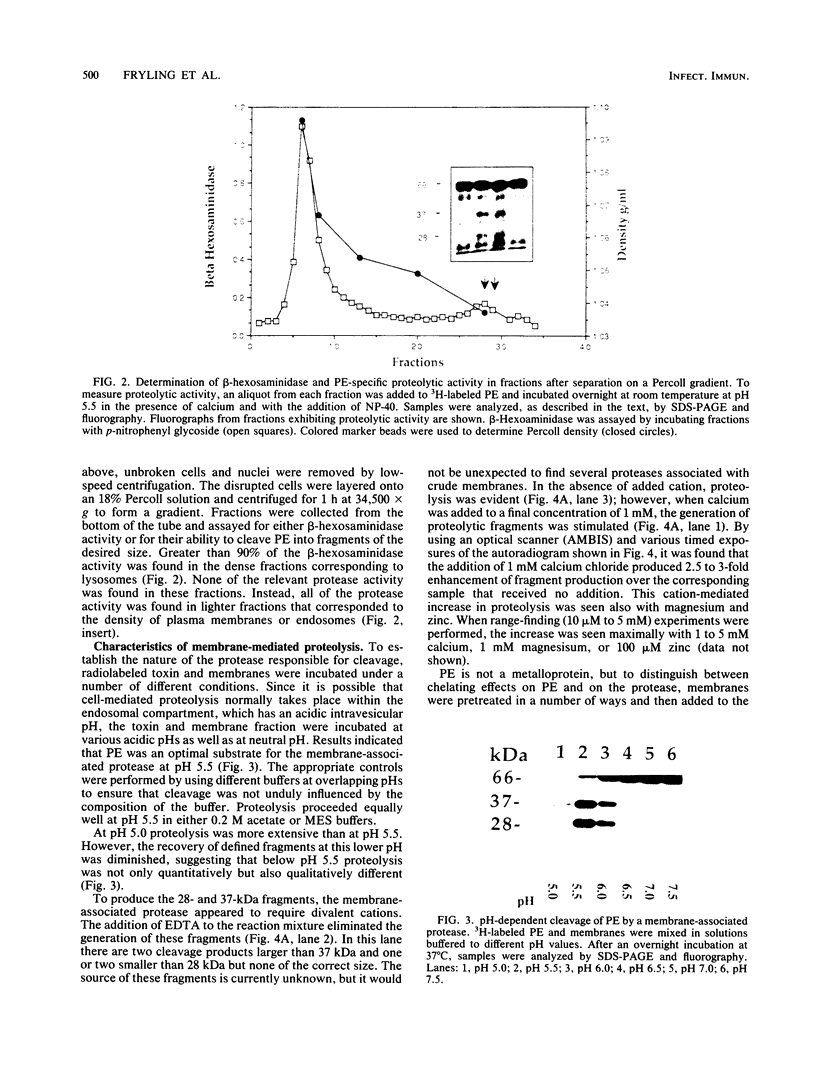
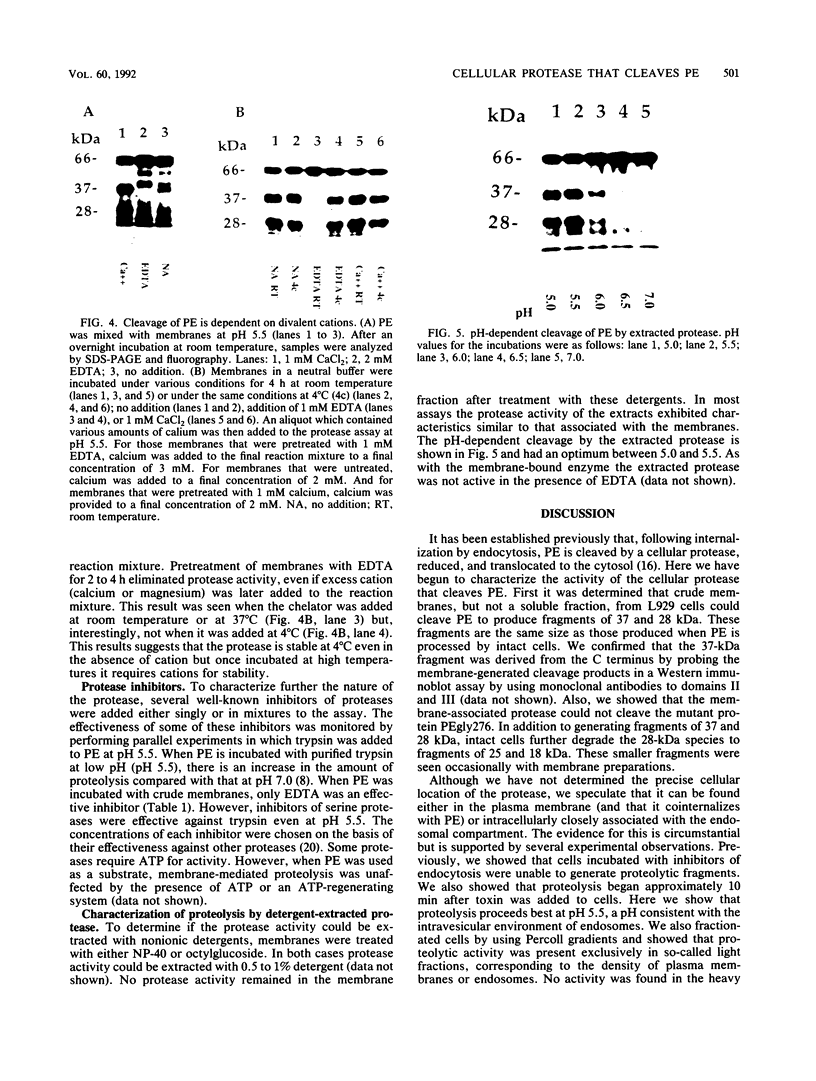
Pseudomonas exotoxin (PE) is a 66-kDa bacterial toxin that is proteolytically cleaved by cells to produce an N-terminal fragment of 28 kDa and a C-terminal 37-kDa fragment which translocates to the cytosol and inhibits protein synthesis (M. Ogata, V.K. Chaudhary, I. Pastan, and D.J. FitzGerald, J. Biol. Chem. 265:20678-20685, 1990). When cells were broken by homogenization, the appropriate proteolytic activity was found associated with cellular membranes and not in a soluble fraction. Proteolysis of PE by crude membranes was stimulated by divalent cations, was ATP independent, and had a pH optimum of 5.5. When cells were disrupted by nitrogen cavitation and fractionated on Percoll gradients, proteolytic activity was present in fractions corresponding to the density of plasma membranes or endosomes but not in fractions containing lysosomes. Proteolytic activity was recovered in detergent extracts after crude membranes were treated with Nonidet P-40 or octylglucoside. Proteolysis of PE by either crude membranes or detergent extracts generated fragments of 28 and 37 kDa. The sizes of these fragments resembled those produced by intact cells. However, when the nontoxic mutant, PEgly276, which cannot be cleaved appropriately by intact cells, was incubated with membranes or extracts there was no production of the 28- and 37-kDa fragments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Cassel D., Glaser L. Proteolytic cleavage of epidermal growth factor receptor. A Ca2+-dependent, sulfhydryl-sensitive proteolytic system in A431 cells. J Biol Chem. 1982 Aug 25;257(16):9845–9848. [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry G. J., Wilson R. B., Draper R. K., Clowes R. C. A dipeptide insertion in domain I of exotoxin A that impairs receptor binding. J Biol Chem. 1989 Sep 5;264(25):15151–15156. [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Structure-activity relationships in diphtheria toxin and Pseudomonas aeruginosa exotoxin A. Cancer Treat Res. 1988;37:25–35. doi: 10.1007/978-1-4613-1083-9_3. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Jiang J. X., London E. Involvement of denaturation-like changes in Pseudomonas exotoxin a hydrophobicity and membrane penetration determined by characterization of pH and thermal transitions. Roles of two distinct conformationally altered states. J Biol Chem. 1990 May 25;265(15):8636–8641. [PubMed] [Google Scholar]
- Jinno Y., Ogata M., Chaudhary V. K., Willingham M. C., Adhya S., FitzGerald D., Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep 25;264(27):15953–15959. [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac M., Pier G. B., Collier R. J. Toxoid of Pseudomonas aeruginosa exotoxin A generated by deletion of an active-site residue. Infect Immun. 1988 Dec;56(12):3095–3098. doi: 10.1128/iai.56.12.3095-3098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Manhart M. D., Saelinger C. B. Receptor-mediated entry of Pseudomonas toxin: methylamine blocks clustering step. Infect Immun. 1983 May;40(2):806–811. doi: 10.1128/iai.40.2.806-811.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Chaudhary V. K., Pastan I., FitzGerald D. J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990 Nov 25;265(33):20678–20685. [PubMed] [Google Scholar]
- Ogata M., Pastan I., FitzGerald D. Analysis of Pseudomonas exotoxin activation and conformational changes by using monoclonal antibodies as probes. Infect Immun. 1991 Jan;59(1):407–414. doi: 10.1128/iai.59.1.407-414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., FitzGerald D. Pseudomonas exotoxin: chimeric toxins. J Biol Chem. 1989 Sep 15;264(26):15157–15160. [PubMed] [Google Scholar]
- Roff C. F., Fuchs R., Mellman I., Robbins A. R. Chinese hamster ovary cell mutants with temperature-sensitive defects in endocytosis. I. Loss of function on shifting to the nonpermissive temperature. J Cell Biol. 1986 Dec;103(6 Pt 1):2283–2297. doi: 10.1083/jcb.103.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai Y., Morihara K., Tsuzuki H., Homma J. Y., Kato I. Proteolytic cleavage of exotoxin A from Pseudomonas aeruginosa: formation of an ADP-ribosyltransferase active fragment by the action of Pseudomonas elastase. FEBS Lett. 1980 Oct 20;120(1):131–134. doi: 10.1016/0014-5793(80)81063-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Thompson M. R., Forristal J., Kauffmann P., Madden T., Kozak K., Morris R. E., Saelinger C. B. Isolation and characterization of Pseudomonas aeruginosa exotoxin A binding glycoprotein from mouse LM cells. J Biol Chem. 1991 Feb 5;266(4):2390–2396. [PubMed] [Google Scholar]
- Watson D. G., Moehring J. M., Moehring T. J. A mutant CHO-K1 strain with resistance to Pseudomonas exotoxin A and alphaviruses fails to cleave Sindbis virus glycoprotein PE2. J Virol. 1991 May;65(5):2332–2339. doi: 10.1128/jvi.65.5.2332-2339.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]