Abstract
BK virus (BKV) is a polyomavirus that establishes a lifelong persistence in most humans and is a major impediment to success of kidney grafts. The function of the innate immune system in BKV infection and pathology has not been investigated. Here we examine the role of antimicrobial defensins in BKV infection of Vero cells. Our data show that α-defensin human neutrophil protein 1 (HNP1) and human α-defensin 5 (HD5) inhibit BKV infection by targeting an early event in the viral lifecycle. HD5 treatment of BKV reduced viral attachment to cells, whereas cellular treatment with HD5 did not. Colocalization studies indicated that HD5 interacts directly with BKV. Ultrastructural analysis revealed HD5-induced aggregation of virions. HD5 also inhibited infection of cells by other related polyomaviruses. This is the first study to demonstrate polyomavirus sensitivity to defensins. We also show a novel mechanism whereby HD5 binds to BKV leading to aggregation of virion particles preventing normal virus binding to the cell surface and uptake into cells.
BK virus is a member of the polyomaviridae family. Polyomaviruses are small (40-50 nm in diameter), non-enveloped, double-stranded DNA viruses that cause tumors in some species outside of their natural host range (1). BKV infects ∼80-90% of the human population and establishes a lifelong persistence within epithelial cells of the urinary tract (2, 3). Although BKV infection remains asymptomatic throughout the lifetime of most humans, immunocompromised individuals are susceptible to BKV-induced disease. Reactivation of BKV has been linked to hemorrhagic cystitis in bone marrow transplant recipients and is the etiological agent of polyomavirus-induced nephropathy (PVN),2 a complication found in 5% of kidney transplant recipients (4, 5). In PVN, BKV induces interstitial nephritis resulting in graft destruction and leads to transplant failure in ∼50% of diagnosed cases (6, 7). BKV reactivation causes a serious viral infection after renal transplantation and is a significant obstacle to the success of kidney grafts.
The factors that contribute to BKV-induced disease are largely unknown. As only a subset of kidney transplant recipients develop PVN, this raises the question of what unique factors are present in these situations for pathogenesis to ensue? The factors that contribute to PVN likely comprise multiple conditions that involve the virus, the host, and the graft. Some risk factors for the development of PVN include male gender, older age, and higher number of HLA mismatches (7, 8). Epidemiological studies suggest that the most significant cofactor leading to PVN is the degree of immunosuppression. The rise in PVN diagnoses is directly correlated to the increased use of the more potent anti-rejection drugs, tacromilus and mycophenolate mofetil (9, 10). Currently, a reduction of immunosuppressive therapy is effectively used to decrease viral replication in PVN patients suggesting that a functional immune system is critical in controlling BKV replication and pathology (11). The incidence of BKV reactivation also increases in many other groups of immunocompromised individuals (12-15). Some work has focused on dissecting which components of the immune system control BKV replication. A robust CD8+ T cell response in PVN patients correlates with decreased BKV replication, whereas a weak CD8+ T cell response correlates with increased viruria and viremia (16). CD4+ T cells also play a role in modulating BKV as elevated viral replication is associated with a decreased CD4+ count in HIV-positive patients (15). Together these studies indicate that cell-mediated immunity is important in controlling BKV replication and spread in the human host. Although the importance of T cell monitoring BKV infection is clear, virtually nothing is known about the role of the innate immune system in BKV infection.
Among the key mediators of the innate immune system are defensins, a family of antimicrobial peptides. The aim of this study was to examine if defensins block BKV infection of kidney cells. Defensins are small (18-45 amino acids), cationic peptides that form three intramolecular disulfide bonds. Human defensins are divided into two families, α- and β-defensins, based on the orientation of disulfide bonds. α-Defensins are composed of human neutrophil peptides 1-4 (HNP1-4), which are expressed in immune cells, and HD5-6, which are found in the small intestine and, described for HD5, in the urogenital tract (17-20). β-Defensins are produced by many different epithelial tissues and some immune cells (18). A third group, the cyclic θ-defensins, are found in rhesus monkeys and are not produced in humans (21). Overall, defensins have broad antimicrobial activity against fungi, bacteria, and viruses although the individual antimicrobial spectrum differs (22, 23). α-Defensins inhibits multiple enveloped viruses including herpes simplex virus, influenza virus, cytomegalovirus, rhabdovirus, and HIV-1 (23-26). β-Defensins exhibit antiviral activity against herpes simplex virus, respiratory syncytial virus, vaccinia virus, and HIV-1 (27-30). Interestingly, infection and replication of some viruses can induce the expression and secretion of defensins in host cells (27, 31). Recent studies have shown that α-defensins also inhibit non-enveloped viruses, such as adenovirus and papillomavirus, and have begun to unravel the mechanism of action (32-34). The aim of our study was to determine whether defensin antiviral activity extends to the polyomavirus family. In this study, we reveal that two α-defensins, HNP1 and HD5, significantly inhibit BKV infection and propose a novel mechanism by which these peptides inhibit viral infection.
EXPERIMENTAL PROCEDURES
Cells, Virus, Antibodies, and Defensins—Vero cells (American Type Culture Collection) and SVG-A cells (subclone of the original SVG human glial cell line established by SV40 transformation of human fetal glial cells) (35) were maintained in a humidified 37 °C CO2 chamber in minimal essential medium (MEM) (Mediatech) supplemented with 1% penicillin/streptomycin (HyClone) and 5 and 10% heat-inactivated fetal bovine serum (Mediatech), respectively. The Dunlop strain of BKV Gardner (ATCC) and wild-type 776 strain of SV40 (ATCC) were propagated in Vero cells and CV-1 cells, respectively, as described previously (36). The strain of JCV used contains JCV capsid proteins and SV40 regulatory regions, maintains JCV tropism, and was propagated in SVG-A cells (37). The PAB597 hybridoma produces a monoclonal antibody against the SV40 major capsid protein VP1 and was a generous gift from Ed Harlow. This antibody cross-reacts with JCV and BKV VP1 and was used for immunofluorescence staining of JCV-, BKV-, and SV40-infected cells. The preparation of rabbit BKV antisera used in dot blot fractionation experiments was generated as described (38). Rabbit HD5 antisera were previously described (39). HNP1, HD5, HBD1, and HBD2 (Peptide International) were suspended in water.
Virus Propagation, Purification, and Labeling—BKV was propagated and then purified as previously described (38). To label BKV, fluorochrome conjugation with 20 μg/ml Alexa Fluor 488 carboxylic acid-succinimidyl ester (AF488) was added to purified BKV and excess fluorescence was removed according to the manufacturer's labeling procedure (MP00143; Molecular Probes). The infectivity of CsCl purified BKV before and after labeling was equivalent (data not shown).
Infection of Host Cells—Subconfluent cells in 96-well culture plates were infected with an m.o.i. of 5 for BKV and JCV and an m.o.i. of 2 for SV40, except when indicated, at 37 °C for 1 h in the absence or presence of 5-50 μg/ml defensins. Infections took place in either serum-free MEM, PBS, 2 and 5% FBS containing MEM as indicated. After 1 h, cells were washed twice with MEM to remove unbound virus, and complete media containing defensins was added to cells for the duration of incubation. 72 h post-infection, cells were fixed using 2% paraformaldehyde (EMB Biosciences) and permeablized using 0.5% Triton X-100 (Shelton Scientific). The cells were incubated with 1:10 dilution of anti-V antigen (V-Ag) monoclonal antibody PAB597 and 4 μg/ml AF488-labeled goat anti-mouse antibody (Molecular Probes), and counterstained with 0.02% Evans Blue (red cytoplasmic dye). Cells were visualized using a Nikon epifluorescence microscope (Eclipse E800; Nikon, Inc.). Approximately 8,000 cells were screened for V-Ag expression.
Pretreatment of BKV with HD5—To test if incubation of HD5 and BKV inhibits infection of Vero cells, 50 μg/ml HD5 was incubated with the indicated m.o.i.s of purified BKV in a total volume of 100 μl (MEM, MEM containing 5% FBS, or PBS) at 4 °C for 1 h. Vero cells were infected, and stained for viral protein as described above.
Defensin Treatment Time Course—Cells were infected as described above. After infection and washing to remove unbound virus, complete cell culture media was added to the cells. At the indicated times post-infection, 50 μg/ml defensins were added to the media for the duration of the 72-h incubation. The 0-h time point had defensins present during infection and after cells were restored with cell culture media.
Flow Cytometric Analysis—To test if HD5 blocks BKV binding to host cells, 50 μg/ml HD5 or HBD1 was incubated with a 512 hemagglutination units (HAU) of purified AF488-BKV in a total volume of 100 μl (MEM, MEM containing 5% FBS, or PBS) at 4 °C for 1 h. Vero cells were detached from flasks with trypsin and washed with complete media to inactivate trypsin. Vero cells were incubated with the defensin/AF488-BKV mixture at 4 °C for 1 h. Washed cells were fixed with 2% paraformaldehyde and viral binding to Vero cells was measured using FACS caliber (BD Biosciences) and data analyzed using CellQuest software (BD Biosciences). To test if HD5 treatment of cells blocked BKV binding, cells were treated with 50 μg/ml HD5 as above. Washed cells were then incubated with either 512 HAU (m.o.i. 16) of purified AF488-BKV in PBS or the lectins: 4 μg/ml biotintylated Maackia amurensis lectin II (MALII) or biotintylated Sambucus nigra lectin (SNA) (Vector Laboratories) in lectin HEPES buffer (10 mm HEPES, 150 mm NaCl, 0.1 mm MgCl2, 0.1 mm CaCl2, pH 7.2) at 4 °C for 1 h. Lectin binding was followed by washing and incubation of 2 μg/ml streptavidin-labeled Alexa Fluor 488 (Molecular Probes) at 4 °C for 1 h. Cells were washed twice with PBS and fluorescence intensity determined as above.
Hemagglutination—50 μg/ml HD5 was incubated with a 1:100 dilution of purified BKV in MEM, MEM containing 5% FBS, or PBS at 4 °C for 1 h. 40 μl of PBS was aliquoted to all wells on 96-well round-bottom culture dishes and then 40 μl of the incubated BKV/HD5 mixture was serially diluted (2-fold) across the plate. Human type O erythrocytes were obtained from a healthy volunteer by venipuncture. The cells were washed several times in Alsevers buffer (0.1 m d-glucose, 0.027 m sodium citrate, 0.07 m NaCl, pH 6.5) and stored at 4 °C for 1 week. Before use, the cells were washed three times in PBS and adjusted to a concentration of 0.5% in PBS. Erythrocytes were added (40 μl) to serially diluted BKV in 96-well round-bottom culture dishes. Experiments were performed 4 times with unlabeled BKV and 2 times with AF488-BKV with identical results.
Confocal Colocalization—512 HAU (m.o.i. 16) of AF488-BKV was incubated with 50 μg/ml HD5 or H2O in either MEM containing 5% FBS or PBS at 4 °C for 1 h. Subconfluent Vero cells plated on glass coverslips were infected with BKV pretreated with or without HD5 at 37 °C for 1 h. Cells were washed twice with MEM, replaced with complete media, and cells were incubated at 37 °C for 1 h. Cells were fixed with 2% paraformaldehyde, incubated with a 1:1000 dilution of HD5 rabbit anti-serum at 37 °C for 1 h, washed 3 times with PBS, and incubated again with 4 μg/ml Alexa Fluor 568-labeled goat anti-rabbit (Molecular Probes) at 37 °C for 1 h. Cells were counterstained with 4′,6-diamidino-2-phenylindole (Vector Labs). Cells were visualized using Zeiss LSM 510 Meta laser-scanning confocal microscope.
Binding Assay Using Density Fractionation—The indicated combinations of BKV (6142 HAU) and HD5 (2.5 μg) in a total volume of 50 μl in PBS were incubated at 4 °C for 1 h. Using an 11 × 34-mm polycarbonate centrifuge tube (Beckman Coulter), this volume was overlaid on a nycodenz gradient (Accurate Chemicals & Scientific Corp.) containing 500 μl of 80% nycodenz and 450 μl of 30% nycodenz. Using a MLA-130 rotor and Optima MAX ultracentrifuge (Beckman Coulter), gradients were centrifuged at 209,000 × g (41,000 rpm) at 4 °C for 5 h. 75-μl fractions were collected. Immobilin-Psq polyvinylidene difluoride membranes (Millipore) were submerged in 100% methanol for 30 s, water for 1 min, then placed into a dot blot apparatus (Bio-Rad). Tandom membranes in a dot blot apparatus were loaded with 20 and 50 μl of collected 75-μl fractions and immobilized into membrane using a vacuum. The membranes were fixed with 0.5% gluteraldehyde in PBS for 20 min at room temperature, and blocked in 1× blocking buffer (Sigma) for 1 h at room temperature. The membranes were probed with 1:2000 HD5 rabbit antisera (50-μl sample) or 1:2500 BKV rabbit antisera (20-μl sample), 4 μg/ml Alexa Fluor 680-labled goat anti-rabbit antibody, and visualized using Odyssey 2.1 software (LiCor).
Electron Microscopy—768 HAU of purified BKV was treated with 50 μg/ml HD5, HBD1, or water at 4 °C for 1 h. Virus was immobilized on a carbon-coated formvar grid (Support Film Grid) for 20 s, removed through blotting with kimwipes, washed with water, and blotted again. The grid was stained with 3 μl of 2% uranyl acetate for 15 s and removed by blotting. Virus was visualized using Phillips 410 transmission electron microscope.
RESULTS
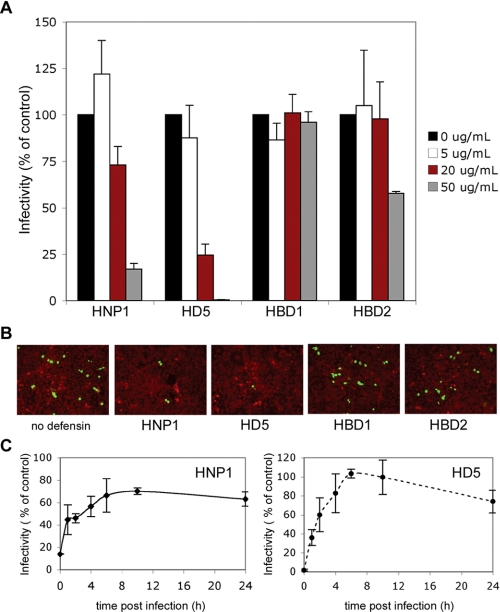
HNP1 and HD5 Inhibit BKV Infection of Vero Cells—To determine whether α- and β-defensins inhibit BKV infection, Vero cells were treated with defensins during and after BKV infection. Indirect immunofluorescence was used to quantify the number of cells expressing the viral protein, V-antigen (V-Ag), at 72 h post-infection. HNP1, HD5, and to a lesser extent human β-defensin 2 (HBD2) inhibited BKV infection (Fig. 1, A and B). To define which step in the BKV lifecycle α-defensins block, a time course experiment was performed (Fig. 1C). Cells were infected with BKV for 1 h, replaced with fresh complete media, and defensins were added to cell culture media at various times after viral infection and left in media for the duration of the experiment. These data suggest that HNP1 and HD5 inhibit an early event in the BKV life cycle.
FIGURE 1.
HNP1, HD5, and HBD2 block BKV infection of Vero cells. A, Vero cells plated at subconfluent density were infected with BKV (m.o.i. 4) in 2% FBS containing MEM at 37 °C for 1 h. Indicated concentrations of defensins were present during infection. Cells were washed to remove unbound virus and 5% FBS containing MEM with the same concentrations of defensins was added to the cells and remained present for the duration of the experiment. Cells were fixed at 72 h and infection was determined by scoring the number of cells expressing the viral protein V-antigen (V-Ag). The graph represents the average of three experiments each of which have been normalized to infected cells without defensin treatment. Error bars represent the standard deviation. B, representative images of BKV-infected Vero cells are shown (magnification ×100). V-Ag-expressing cells are in green and Evans blue cytoplasmic labeling in red. C, Vero cells were infected with purified BKV (m.o.i. 4) in 2% FBS containing MEM at 37 °C for 1 h. Unbound virus was removed by washing and complete media added to cells. At the indicated times, 50 μg/ml HNP1 or HD5 was added to culture media. At the 0-h time point, defensins were added during and also directly following infection. Cells were stained and scored for V-Ag expression at 72 h post-infection. The graph shows the average of three experiments, each of which have been normalized to infected cells without defensin treatment. Error bars represent the standard deviation.
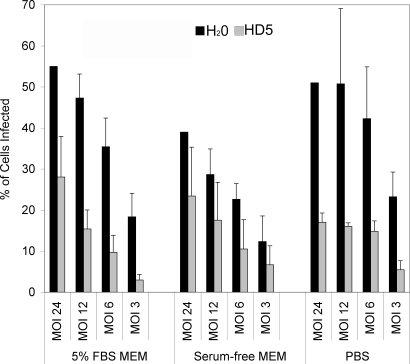
Pretreatment of BKV with HD5 Inhibits Viral Infection—To elucidate the mechanism that α-defensins inhibit BKV early after infection, we focused on HD5 as this defensin exhibited the greatest antiviral activity. Defensins inhibit viruses by either neutralizing the virus particle or by influencing the host cell (30, 40). To determine whether HD5 inhibits infection by acting on the virion or by acting on the host cell, BKV was first incubated with HD5 or H2O for 1 h at 4 °C in the indicated media. HD5 inhibited BKV infection at various multiplicity of infections, but was most active at lower multiplicity of infections (Fig. 2). We were interested in HD5 activity under different media conditions because previous studies had shown that HNP1 exhibits a direct effect on HIV-1 only at low multiplicity of infections and in the absence of serum (40). Serum is thought to prevent defensin activity because defensins can acts as lectins, binding anionic carbohydrates found in the serum, which would sequester the defensin away from the virus and limit antimicrobial activity (41, 42). To test the effect of serum on HD5 antiviral activity, virus was preincubated with HD5 in the presence of PBS, MEM, and MEM containing 5% FBS. The presence of serum did not block HD5 antiviral activity against BKV. In fact, inhibition was greatest under low multiplicity of infection conditions in the presence of PBS or MEM containing FBS.
FIGURE 2.
Pretreatment of BKV with HD5 inhibits infection. Purified BKV at the indicated m.o.i. was incubated with 50 μg/ml HD5 or the same volume of H2O for 1 h at 4 °C in MEM containing 5% FBS, serum-free MEM, or PBS. Vero cells were then infected with the HD5/BKV mixture at 37 °C for 1 h. Unbound virus was removed by washing and complete media was added to cells. Cells were fixed and the percentage of the total cells expressing V-Ag determined at 72 h post-infection. The graph shows the average of five experiments. Error bars represent the standard deviation.
HD5 Blocks BKV Binding to Vero Cells and BKV Hemagglutination of Red Blood Cells—Because HD5 inhibits an early event in the BKV lifecycle (Fig. 1C), we tested whether HD5 treatment blocks BKV binding to the surface of host cells. Alexa Fluor 488-labeled BKV (AF488-BKV) was incubated with HD5 or human β-defensin 1 (HBD1) for 1 h at 4 °C and then added to Vero cells in suspension for 1 h at 4 °C. Using flow cytometry, the amount of AF488-BKV bound to host cells was examined. HD5 treatment reduced BKV binding in both the presence and absence of serum suggesting that HD5 inhibits infection by preventing efficient virus attachment to the surface of host cells (Fig. 3A). HBD1 treatment had no effect of BKV attachment to host cells. The effect of HD5 on BKV HA was also tested (Fig. 3B). HD5 treatment reduced the capacity of BKV to hemagglutinate red blood cells. Under serum-free MEM and PBS conditions, HD5 reduced HA titer by a factor of 8, whereas HD5 in the presence of serum reduced HA titer by a factor of 2.
FIGURE 3.
Incubation of HD5 and BKV inhibits viral binding. A, AF488-BKV (m.o.i.: 16) was incubated with H2O(black line) or 50 μg/ml of the indicated defensins (dotted line) in MEM, MEM containing 5% FBS, or PBS at 4 °C for 1 h. Vero cells in suspension were then incubated with the BKV mixture at 4 °C for 1 h and viral binding was evaluated using flow cytometry. Filled histograms represent cells in the absence of AF488-BKV. B, a 1:100 dilution of purified BKV was incubated with H2O or 50 μg/ml HD5 in the indicated media at 4 °C for 1 h. This preparation was 2-fold serially diluted across a U-bottom plate containing PBS. The HA plate was incubated with 0.5% erythrocytes in PBS at 4 °C for 16 h. A representation image of a HA plate is shown. C, Vero cells in suspension were treated with 50 μg/ml HD5 (dotted line) or an equal volume of H2O(bold line) in the indicated solutions at 4 °C for 1 h. Cells were washed and incubated with AF488-BKV (m.o.i. 16), biotintylated-SNA (lectin specific for α(2,6)-linked sialic acid), or biotintylated-MALII (lectin specific for α(2,3)-linked sialic acid) at 4 °C for 1 h. For SNA and MALII samples, cells were washed and incubated with AF488-labeled streptavidin. Fluorescence intensity was measured using flow cytometry. Filled histograms represent unstained cells.
Anionic carbohydrates such as sialic acids and heparan sulfate molecules are found on the cell surface of eukaryotic cells. Because cationic defensins have high affinity for negatively charged carbohydrate groups and BKV uses α(2,3)-linked sialic acid as a host cell receptor, we tested whether treating Vero cells with HD5 prevents AF488-BKV attachment to host cells (Fig. 3C) (43-46). Incubation of Vero cells with HD5 at 4 °C for 1 h did not block AF488-BKV attachment to the cells. Additionally, incubating cells with HD5 did not reduce the cell surface binding of two lectins that interact with α(2,6)-sialic acid (SNA) and α(2,3)-linked sialic acid (MALII). These data suggest that the mechanism by which HD5 inhibits BKV infection does not involve HD5 binding to sialic acid moieties on the cell surface or obstructing virion engagement of the cell membrane.
HD5 Directly Associates with BKV—The antiviral activity of HD5 seems to act on BKV directly and not on host cells. To determine whether the HD5 peptide and BK virions are physically interacting, we examined the degree of colocalization using confocal immunofluorescence microscopy. Cells were infected with untreated AF488-BKV at 37 °C for 1 h and unbound virus was removed through repeated washings. Cells were given fresh media and viral internalization was allowed to proceed at 37 °C for 4 additional hours after which cells were fixed and stained with HD5 antisera. In the absence of HD5 treatment, AF488-BKV was internalized and localized to perinuclear areas when serum was present (Fig. 4A). In serum-free conditions, punctate viral particles were found along the cell surface and within the cytoplasm exhibiting a delayed internalization and intracellular trafficking. When AF488-BKV was incubated with HD5, BKV and HD5 showed some colocalization along the periphery of the host cell. In serum-free media, HD5 and BKV colocalized in round clusters that were distributed throughout the extracellular space and not associated with cells (Fig. 4A, bottom right panel). The extracellular HD5/BKV particles were found at a different focal plane from cells and may be associating with extracellular matrix (47). These HD5/BKV particles may be responsible for sequestering virions away from cell membranes and help to explain the reduced cell-surface binding (Fig. 2A). These extracellular particles were not seen when serum was present.
FIGURE 4.
HD5 colocalizes with BKV. A, subconfluent Vero cells plated on glass coverslips were challenged with AF488-BKV (m.o.i. 16) at 37 °C for 1 h in MEM with or without serum. Cells were washed with MEM, restored with complete media, and virus allowed to internalize by incubating cells at 37 °C for 4 h. Cells were fixed and stained for HD5 using HD5-specific antisera and AF568-labeled goat anti-rabbit antibodies. Representative images were obtained using immunofluorescence confocal microscopy. B, in a cell-free assay, PBS containing 2.5 μg of HD5, 6142 HAU of BKV, HD5, and BKV together, and HD5 and BKV in 750 mm NaCl supplemented PBS were incubated at 1 h at 4 °C. This was overlaid on a gradient containing 80 and 30% nycodenz and centrifuged at 209,000 × g for 5 h. Fractions of 75 μl were collected and immobilized onto two polyvinylidene difluoride membranes. Membranes were probed for either HD5 or BKV and binding was detected using AF680-labeled goat anti-rabbit antibody.
To determine whether HD5 and BKV bind to each other, the buoyant density of HD5 in the presence and absence of BKV was compared. HD5 with and without BKV was incubated at 4 °C for 1 h and then overlaid on a discontinuous nycodenz gradient. After centrifugation, fractions were immunobilized on two membranes and immunoblotted with either HD5-specific or BKV-specific antisera (Fig. 4B). HD5 alone localized to low-density fractions 1-3. When HD5 was pretreated with BKV, HD5 associated with higher density fractions that correspond to the density of BKV. Interestingly, BKV alone reproducibly showed a broad fractionation pattern. When HD5 was added to BKV, BKV sedimented at a lower density and was exclusively found at high dense fractions of 6 and 7. These findings suggest that HD5 physically interacts with BKV causing the HD5-BKV complex to migrate to a denser fraction. The anionic nature of NaCl has been shown to disrupt the interaction between HD5 and adenovirus (32). Under high salt concentrations more of the HD5 peptide was found at lower density fractions than it was at low salt concentrations. These data indicate that the high NaCl concentration can break the HD5/BKV interaction suggesting that electrostatic interactions are important for the attachment of HD5 to BKV.
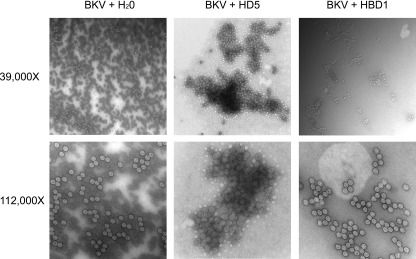
HD5-induced Aggregation of BKV Virions—The clustered pattern of HD5/BKV seen along the cell surface and in extracellular regions (Fig. 4A) led us to hypothesize that HD5 causes virion aggregation. To visualize the ultrastructural events that occur upon BKV incubation with HD5, we used electron microscopy. When BKV was mock-treated with H2O or incubated with HBD1, a fairly uniform distribution of virus that form loosely associated clusters of virions was observed (Fig. 5). In contrast, HD5 caused a striking aggregation of virions surrounded by adjacent regions that exhibited few or no virions. Examination of virions at ×112,000 magnification revealed a dense, lattice-like network of aggregated viral particles (Fig. 5). Dynamic light scattering analysis of purified BKV pentamers showed that the addition of HD5 reduced the percentage of particles that are pentamer size and increases the particle hydrodynamic radius by a factor of 1.8 (data not shown). Together, these data demonstrate that HD5 causes BKV clumping and the formation of large virion aggregates.
FIGURE 5.
Ultrastructure of BKV reveals HD5-induced aggregation of viral particles. 768 HAU of purified BKV was treated with 50 μg/ml HD5, HBD1, or an equal volume of H2O and incubated at 4 °C for 1 h. Virus was then immobilized on a carbon-coated formvar grid, washed with water, and stained with 2% uranyl acetate. Using transmission electron microscopy, BK virions were visualized (magnification ×39,000). The lower panels show that BK virions accumulate in dense aggregations when treated with HD5 (magnification ×112,000).
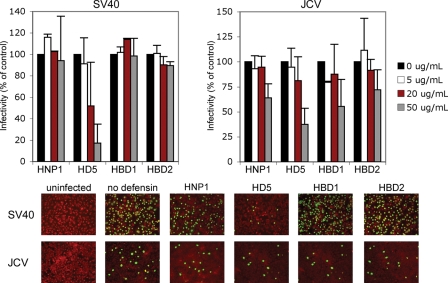
Other Polyomavirus Family Members Are Sensitive to HD5—To determine whether defensins block other members of the polyomavirus family, host cells were incubated with various defensins during and after simian virus 40 (SV40) and JC virus (JCV) infection. HD5 displayed a dose-dependent inhibition on both SV40 and JCV (Fig. 6). HNP1, HBD1, and HBD2 modestly inhibited JCV infection and had less of an effect on SV40. Together, these data show that the antiviral activity of HD5 extends to three polyomavirus members.
FIGURE 6.
HD5 inhibits SV40 and JCV infection. Subconfluent Vero cells or SVG-A were infected with SV40 (m.o.i. 2) or JCV (m.o.i. 5), respectively, in 2% FBS containing MEM at 37 °C for 1 h in the absence or presence of defensins at the indicated concentrations. After infection, cells were washed twice with MEM to remove unbound virus, and complete media containing defensins was added to cells for the duration of the incubation. Cells were fixed at 48 (Vero) and 72 h (SVG-A) post-infection and stained for V-Ag. Approximately 8,000 cells were screened for V-Ag expression. The graph shows the average of three experiments each of which has been normalized to infected cells without defensin treatment. Error bars represent the standard deviation. The bottom panel shows representative images of infected cells at the 50 μg/ml defensin concentration (magnification ×100). V-Ag-expressing cells are in green and Evans blue cytoplasmic labeling in red.
DISCUSSION
Defensins exhibit antimicrobial activity by either interacting with the microorganism or by effecting events in the target cells. Multiple mechanisms of inhibition have been described. In pathogens containing lipid membranes, the cationic and amphiphilic nature of defensins is thought to induce the formation of pores causing membrane depolarization and leakage (48). This characteristic contributes to membrane permeabilization and cell death of bacterial species and may also help to explain inhibition of some enveloped viruses (23, 49).
In vivo, defensins also inhibit infection by priming the adaptive immune response. Some defensins block viruses by up-regulating type I interferon response genes (26). Defensins, particular β-defensins, acts as chemoattractants for T-cells, monocytes, mast cells, and dendritic cells (50, 51). Once in contact with these immune cells, defensins can activate intracellular signaling to induce immune cell maturation, cytokine secretion, and antibody production (51-54).
In addition, the electrostatic charge and carbohydrate binding properties of defensins block infection by inhibiting the interactions between virus and host cells. Defensins have been shown to bind to both HIV glycoprotein gp120 and cell-surface receptor CD4, which is thought to prevent normal receptor interactions (43, 55). HNP1-3 block HSV-2 infection by binding to the viral glycoproteins that are required for receptor engagement at the cell surface (43). Retrocyclin, a synthetic θ-like defensin binds to glycoproteins of Sindbis virus, HIV-1, and influenza virus causing cross-linking and immobilization of viral glycoproteins resulting in a block in viral fusion to cells (44, 56).
Although the majority of studies have examined defensin inhibition of enveloped viruses, defensin antiviral activity extends to some non-enveloped viruses. HNP1 and HD5 inhibit both papillomavirus and adenovirus (32-34). HD5 treatment has no impact on papillomavirus binding to and entry into host cells, but HD5-treated viral particles become retained within the cellular endosome and cannot traffic to perinuclear regions. Accumulation of papillomavirus in endosomes may inactivate virions by shuttling them through the endosomal-lysosomal degradation system (34). HD5 inhibits adenovirus by physically binding and stabilizing the outer capsid proteins. This interaction prevents the partial disassembly of the virus, which normally induces lysis of the endosomal membrane. Endosomalysis releases the virus into the cytosolic space, an event necessary for productive infection (32).
Our findings extend the number of virus families that are sensitive to defensins and propose a novel mechanism for HD5-induced viral inhibition. Another polybasic molecule, HBD1, had no effect on BKV infectivity indicating that HD5 exhibits highly specific antiviral activity. Interestingly, we find inhibition is not serum-dependent. Previous studies demonstrated that defensin antiviral activity is reduced is the presence of serum (23, 40). HD5 pretreatment of the virus, but not cells, inhibited infection and cell-surface binding. These data suggest that HD5 blocks viral attachment to host cells by interacting with BKV directly. Colocalization studies substantiate these findings by showing that BKV and HD5 are located at the same regions on the periphery of the cell, possibly associating with the extracellular matrix, and fractionate at similar densities.
Ultrastructural examination revealed HD5-induced aggregation of viral particles into very large, dense masses and eliminated the even distribution of virions. We propose that this aggregation inhibits viral attachment to host cells by reducing the viral surface area and sequestering binding epitopes. The formation of HD5-induced viral aggregates also explains why BKV alone normally localizes to multiple higher density fractions on a nycodenz gradient, but when HD5 is present BKV is restricted to higher density fractions. Recent data shows that defensins may inhibit Clostridium difficile toxin B cytotoxicity by inducing high-molecular mass aggregates of toxin B (57).
Although BKV causes a lifelong persistent infection within the kidney, BKV is thought to initially infect humans by the fecal-oral route of transmission (58-60). As BKV travels through the gastrointestinal tract, the presence of HD5 in the small intestines may play an important role in inactivation of the virus. HD5 may also help clear BKV infection in urinary tract tissue during reactivation. Our study also demonstrates that HNP1 has anti-BKV activity. HNP1 is constitutively expressed in neutrophils, NK cells, B cells, subsets of T cells, and monocytes and detected in saliva, intestinal, and cervical mucosa (20, 61, 62). The antiviral activity of HNP1 could play a role in humans by limiting primary BKV infection in the blood-stream and also controlling and helping to clear virus during reactivation.
The importance of HNP1 and HD5 in BKV infection in vivo is currently unknown; however, the concentrations used in this study have biological relevance. Our findings show that the IC50 of HNP1 and HD5 on BKV infection was 23.2 and 9.1 μg/ml, respectively. HNP1 levels in the human plasma range from 400 ng/ml in healthy individuals to 13 μg/ml in individuals with bacteria infections and may be found in concentrations as high as 6 mg/ml within neutrophils (63-66). Less is known about the concentration of HD5 in the small intestines, however, analysis of urethral lavages from men with gonorrhea infections show HD5 concentrations in inflamed genital tracts at 0.6 ± 0.68 μg/ml (17). Lavages dilute urethra secretions so the authors of this study estimated the lumenal HD5 concentrations to be greater than 10-fold higher (17).
Although the expression of HD5 has not been compared in kidney transplant recipients, several medical conditions alter defensin production. HD5 expression is decreased in humans with Crohn disease, but increased in people who have experienced chlamydia or gonorrhea infections or undergone gastric bypass surgery. In general, defensin expression shows high variability between individuals and among different populations (67-71). Copy number polymorphisms are commonly found in defensin genes and contribute to variable defensin production among individuals (72). Differential expression of defensins among individuals could explain why 10-20% of humans never become infected with BKV or why only 5% of kidney transplant patients develop PVN (2, 8, 73). The impact of immunosuppression therapy on defensin expression has not been studied. It would be interesting to determine whether anti-transplant rejection drugs block defensin expression and whether this contributes to disease onset or progression.
This study demonstrates that HD5 has anti-polyomavirus activity and neutralize polyomaviruses in a serum-independent manner. Additionally, these findings have determined a role for the innate immune system in blocking BKV infection. Ongoing experiments are aimed at elucidating whether BKV infection leads to the up-regulation of defensin expression. The presence of nuclear factors of activated T cells-binding sites in the promoter regions of both the BKV genome and α-defensin genes, and an increase in nuclear factors of activated T cell-responsive genes after polyomavirus infection suggests that initial infection may stimulate defensin expression (74-76). Future studies will determine the relevance of these peptides in controlling BKV infection in human hosts.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA71878 from the NCI and R01 NS43097 from the NINDS (to W. J. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PVN, polyomavirus-induced nephropathy; HIV, human immunodeficiency virus; HBD2, human β-defensin 2; HNP1, human neutrophil protein 1; MEM, minimal essential medium; m.o.i., multiplicity of infection; PBS, phosphate-buffered saline; FBS, fetal bovine serum; HAU, hemagglutinin unit; MALII, Maackia amurensis lectin II; SNA, Sambucus nigra lectin.
References
- 1.Shah, K. V., Daniel, R. W., and Strandberg, J. D. (1975) J. Natl. Cancer Inst. 54 945-950 [PubMed] [Google Scholar]
- 2.Knowles, W. A., Pipkin, P., Andrews, N., Vyse, A., Minor, P., Brown, D. W., and Miller, E. (2003) J. Med. Virol. 71 115-123 [DOI] [PubMed] [Google Scholar]
- 3.Heritage, J., Chesters, P. M., and McCance, D. J. (1981) J. Med. Virol. 8 143-150 [DOI] [PubMed] [Google Scholar]
- 4.Arthur, R. R., Shah, K. V., Charache, P., and Saral, R. (1988) J. Infect. Dis. 158 563-569 [DOI] [PubMed] [Google Scholar]
- 5.Rosen, S., Harmon, W., Krensky, A. M., Edelson, P. J., Padgett, B. L., Grinnell, B. W., Rubino, M. J., and Walker, D. L. (1983) N. Engl. J. Med. 308 1192-1196 [DOI] [PubMed] [Google Scholar]
- 6.Randhawa, P. S., Finkelstein, S., Scantlebury, V., Shapiro, R., Vivas, C., Jordan, M., Picken, M. M., and Demetris, A. J. (1999) Transplantation 67 103-109 [DOI] [PubMed] [Google Scholar]
- 7.Ramos, E., Drachenberg, C. B., Papadimitriou, J. C., Hamze, O., Fink, J. C., Klassen, D. K., Drachenberg, R. C., Wiland, A., Wali, R., Cangro, C. B., Schweitzer, E., Bartlett, S. T., and Weir, M. R. (2002) J. Am. Soc. Nephrol. 13 2145-2151 [DOI] [PubMed] [Google Scholar]
- 8.Hirsch, H. H., Knowles, W., Dickenmann, M., Passweg, J., Klimkait, T., Mihatsch, M. J., and Steiger, J. (2002) N. Engl. J. Med. 347 488-496 [DOI] [PubMed] [Google Scholar]
- 9.Binet, I., Nickeleit, V., Hirsch, H. H., Prince, O., Dalquen, P., Gudat, F., Mihatsch, M. J., and Thiel, G. (1999) Transplantation 67 918-922 [DOI] [PubMed] [Google Scholar]
- 10.Mengel, M., Marwedel, M., Radermacher, J., Eden, G., Schwarz, A., Haller, H., and Kreipe, H. (2003) Nephrol. Dial. Transplant. 18 1190-1196 [DOI] [PubMed] [Google Scholar]
- 11.Drachenberg, C. B., Hirsch, H. H., Papadimitriou, J. C., Gosert, R., Wali, R. K., Munivenkatappa, R., Nogueira, J., Cangro, C. B., Haririan, A., Mendley, S., and Ramos, E. (2007) Transplantation 84 323-330 [DOI] [PubMed] [Google Scholar]
- 12.Sundsfjord, A., Osei, A., Rosenqvist, H., Van Ghelue, M., Silsand, Y., Haga, H. J., Rekvig, O. P., and Moens, U. (1999) J. Infect. Dis. 180 1-9 [DOI] [PubMed] [Google Scholar]
- 13.Sundsfjord, A., Spein, A. R., Lucht, E., Flaegstad, T., Seternes, O. M., and Traavik, T. (1994) J. Clin. Microbiol. 32 1390-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman, D. V., Wolfendale, M. R., Daniel, R. A., Dhanjal, N. K., Gardner, S. D., Gibson, P. E., and Field, A. M. (1980) J. Infect. Dis. 142 1-8 [DOI] [PubMed] [Google Scholar]
- 15.Jin, L., Pietropaolo, V., Booth, J. C., Ward, K. H., and Brown, D. W. (1995) Clin. Diagn. Virol. 3 285-295 [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., Trofe, J., Gordon, J., Du Pasquier, R. A., Roy-Chaudhury, P., Kuroda, M. J., Woodle, E. S., Khalili, K., and Koralnik, I. J. (2006) J. Virol. 80 3495-3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter, E., Yang, H., Yavagal, S., Preza, G. C., Murillo, O., Lima, H., Greene, S., Mahoozi, L., Klein-Patel, M., Diamond, G., Gulati, S., Ganz, T., Rice, P. A., and Quayle, A. J. (2005) Infect. Immun. 73 4823-4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klotman, M. E., and Chang, T. L. (2006) Nat. Rev. Immunol. 6 447-456 [DOI] [PubMed] [Google Scholar]
- 19.Svinarich, D. M., Wolf, N. A., Gomez, R., Gonik, B., and Romero, R. (1997) Am. J. Obstet. Gynecol. 176 470-475 [DOI] [PubMed] [Google Scholar]
- 20.Cunliffe, R. N., and Mahida, Y. R. (2004) J. Leukocyte Biol. 75 49-58 [DOI] [PubMed] [Google Scholar]
- 21.Tran, D., Tran, P. A., Tang, Y. Q., Yuan, J., Cole, T., and Selsted, M. E. (2002) J. Biol. Chem. 277 3079-3084 [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer, P. B., Harwig, S. S., Szklarek, D., Ganz, T., Selsted, M. E., and Lehrer, R. I. (1989) Infect. Immun. 57 2021-2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daher, K. A., Selsted, M. E., and Lehrer, R. I. (1986) J. Virol. 60 1068-1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasin, B., Pang, M., Turner, J. S., Cho, Y., Dinh, N. N., Waring, A. J., Lehrer, R. I., and Wagar, E. A. (2000) Eur. J. Clin. Microbiol. Infect. Dis. 19 187-194 [DOI] [PubMed] [Google Scholar]
- 25.Nakashima, H., Yamamoto, N., Masuda, M., and Fujii, N. (1993) AIDS 7 1129. [DOI] [PubMed] [Google Scholar]
- 26.Falco, A., Mas, V., Tafalla, C., Perez, L., Coll, J. M., and Estepa, A. (2007) Antiviral Res. 76 111-123 [DOI] [PubMed] [Google Scholar]
- 27.Kota, S., Sabbah, A., Chang, T. H., Harnack, R., Xiang, Y., Meng, X., and Bose, S. (2008) J. Biol. Chem. 283 22417-22429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howell, M. D., Streib, J. E., and Leung, D. Y. (2007) J. Allergy Clin. Immunol. 119 1022-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinones-Mateu, M. E., Lederman, M. M., Feng, Z., Chakraborty, B., Weber, J., Rangel, H. R., Marotta, M. L., Mirza, M., Jiang, B., Kiser, P., Medvik, K., Sieg, S. F., and Weinberg, A. (2003) AIDS 17 F39-F48 [DOI] [PubMed] [Google Scholar]
- 30.Hazrati, E., Galen, B., Lu, W., Wang, W., Ouyang, Y., Keller, M., Lehrer, R., and Herold, B. (2006) J. Immunol. 177 8658-8666 [DOI] [PubMed] [Google Scholar]
- 31.Duits, L. A., Nibbering, P. H., van Strijen, E., Vos, J. B., Mannesse-Lazeroms, S. P., van Sterkenburg, M. A., and Hiemstra, P. S. (2003) FEMS Immunol. Med. Microbiol. 38 59-64 [DOI] [PubMed] [Google Scholar]
- 32.Smith, J. G., and Nemerow, G. R. (2008) Cell Host Microbe 3 11-19 [DOI] [PubMed] [Google Scholar]
- 33.Gropp, R., Frye, M., Wagner, T. O., and Bargon, J. (1999) Hum. Gene Ther. 10 957-964 [DOI] [PubMed] [Google Scholar]
- 34.Buck, C. B., Day, P. M., Thompson, C. D., Lubkowski, J., Lu, W., Lowy, D. R., and Schiller, J. T. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 1516-1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Major, E. O., Miller, A. E., Mourrain, P., Traub, R. G., de Widt, E., and Sever, J. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 1257-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raptis, L. (2001) SV40 protocols, Humana Press, Totowa, NJ
- 37.Chen, B. J., and Atwood, W. J. (2002) Virology 300 282-290 [DOI] [PubMed] [Google Scholar]
- 38.Eash, S., Querbes, W., and Atwood, W. J. (2004) J. Virol. 78 11583-11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter, E. M., van Dam, E., Valore, E. V., and Ganz, T. (1997) Infect. Immun. 65 2396-2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang, T. L., Vargas, J., Jr., DelPortillo, A., and Klotman, M. E. (2005) J. Clin. Investig. 115 765-773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, Z., Cocchi, F., Gentles, D., Ericksen, B., Lubkowski, J., Devico, A., Lehrer, R. I., and Lu, W. (2005) FEBS Lett. 579 162-166 [DOI] [PubMed] [Google Scholar]
- 42.Svenson, J., Brandsdal, B. O., Stensen, W., and Svendsen, J. S. (2007) J. Med. Chem. 50 3334-3339 [DOI] [PubMed] [Google Scholar]
- 43.Wang, W., Owen, S. M., Rudolph, D. L., Cole, A. M., Hong, T., Waring, A. J., Lal, R. B., and Lehrer, R. I. (2004) J. Immunol. 173 515-520 [DOI] [PubMed] [Google Scholar]
- 44.Leikina, E., Delanoe-Ayari, H., Melikov, K., Cho, M. S., Chen, A., Waring, A. J., Wang, W., Xie, Y., Loo, J. A., Lehrer, R. I., and Chernomordik, L. V. (2005) Nat. Immunol. 6 995-1001 [DOI] [PubMed] [Google Scholar]
- 45.Dugan, A. S., Gasparovic, M. L., and Atwood, W. J. (2008) J. Virol. 82 2560-2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dugan, A. S., Eash, S., and Atwood, W. J. (2005) J. Virol. 79 14442-14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bdeir, K., Cane, W., Canziani, G., Chaiken, I., Weisel, J., Koschinsky, M. L., Lawn, R. M., Bannerman, P. G., Sachais, B. S., Kuo, A., Hancock, M. A., Tomaszewski, J., Raghunath, P. N., Ganz, T., Higazi, A. A., and Cines, D. B. (1999) Blood 94 2007-2019 [PubMed] [Google Scholar]
- 48.Wimley, W. C., Selsted, M. E., and White, S. H. (1994) Protein Sci. 3 1362-1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koo, S. P., Yeaman, M. R., Nast, C. C., and Bayer, A. S. (1997) Infect. Immun. 65 4795-4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, D., Chertov, O., Bykovskaia, S. N., Chen, Q., Buffo, M. J., Shogan, J., Anderson, M., Schroder, J. M., Wang, J. M., Howard, O. M., and Oppenheim, J. J. (1999) Science 286 525-528 [DOI] [PubMed] [Google Scholar]
- 51.Yang, D., Biragyn, A., Hoover, D. M., Lubkowski, J., and Oppenheim, J. J. (2004) Annu. Rev. Immunol. 22 181-215 [DOI] [PubMed] [Google Scholar]
- 52.Niyonsaba, F., Hirata, M., Ogawa, H., and Nagaoka, I. (2003) Curr. Drug Targets Inflamm. Allergy 2 224-231 [DOI] [PubMed] [Google Scholar]
- 53.Biragyn, A., Ruffini, P. A., Leifer, C. A., Klyushnenkova, E., Shakhov, A., Chertov, O., Shirakawa, A. K., Farber, J. M., Segal, D. M., Oppenheim, J. J., and Kwak, L. W. (2002) Science 298 1025-1029 [DOI] [PubMed] [Google Scholar]
- 54.Lillard, J. W., Jr., Boyaka, P. N., Chertov, O., Oppenheim, J. J., and McGhee, J. R. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 651-656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Furci, L., Sironi, F., Tolazzi, M., Vassena, L., and Lusso, P. (2007) Blood 109 2928-2935 [DOI] [PubMed] [Google Scholar]
- 56.Gallo, S. A., Wang, W., Rawat, S. S., Jung, G., Waring, A. J., Cole, A. M., Lu, H., Yan, X., Daly, N. L., Craik, D. J., Jiang, S., Lehrer, R. I., and Blumenthal, R. (2006) J. Biol. Chem. 281 18787-18792 [DOI] [PubMed] [Google Scholar]
- 57.Giesemann, T., Guttenberg, G., and Aktories, K. (2008) Gastroenterology 134 2049-2058 [DOI] [PubMed] [Google Scholar]
- 58.Bofill-Mas, S., Pina, S., and Girones, R. (2000) Appl. Environ. Microbiol. 66 238-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanchiere, J. A., Nicome, R. K., Greer, J. M., Demmler, G. J., and Butel, J. S. (2005) J. Infect. Dis. 192 658-664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanchiere, J. A., White, Z. S., and Butel, J. S. (2005) J. Med. Virol. 75 447-454 [DOI] [PubMed] [Google Scholar]
- 61.Agerberth, B., Charo, J., Werr, J., Olsson, B., Idali, F., Lindbom, L., Kiessling, R., Jornvall, H., Wigzell, H., and Gudmundsson, G. H. (2000) Blood 96 3086-3093 [PubMed] [Google Scholar]
- 62.Hein, M., Valore, E. V., Helmig, R. B., Uldbjerg, N., and Ganz, T. (2002) Am. J. Obstet. Gynecol. 187 137-144 [DOI] [PubMed] [Google Scholar]
- 63.Ihi, T., Nakazato, M., Mukae, H., and Matsukura, S. (1997) Clin. Infect. Dis. 25 1134-1140 [DOI] [PubMed] [Google Scholar]
- 64.Panyutich, A. V., Panyutich, E. A., Krapivin, V. A., Baturevich, E. A., and Ganz, T. (1993) J. Lab. Clin. Med. 122 202-207 [PubMed] [Google Scholar]
- 65.Shiomi, K., Nakazato, M., Ihi, T., Kangawa, K., Matsuo, H., and Matsukura, S. (1993) Biochem. Biophys. Res. Commun. 195 1336-1344 [DOI] [PubMed] [Google Scholar]
- 66.Lehrer, R. (1997) Clin. Infect. Dis. 25 1141-1142 [DOI] [PubMed] [Google Scholar]
- 67.Dhaliwal, W., Bajaj-Elliott, M., and Kelly, P. (2003) Mol. Immunol. 40 469-475 [DOI] [PubMed] [Google Scholar]
- 68.Wehkamp, J., Salzman, N. H., Porter, E., Nuding, S., Weichenthal, M., Petras, R. E., Shen, B., Schaeffeler, E., Schwab, M., Linzmeier, R., Feathers, R. W., Chu, H., Lima, H., Jr., Fellermann, K., Ganz, T., Stange, E. F., and Bevins, C. L. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 18129-18134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klotman, M. E., Rapista, A., Teleshova, N., Micsenyi, A., Jarvis, G. A., Lu, W., Porter, E., and Chang, T. L. (2008) J. Immunol. 180 6176-6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frye, M., Bargon, J., Lembcke, B., Wagner, T. O., and Gropp, R. (2000) Eur. J. Clin. Investig. 30 695-701 [DOI] [PubMed] [Google Scholar]
- 71.Sundbom, M., Elphick, D. A., Mahida, Y. R., Cunliffe, R. N., Midtvedt, T., Engstrand, L., Rubio, C., and Axelsson, L. G. (2007) J. Clin. Pathol. 60 1029-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aldred, P. M., Hollox, E. J., and Armour, J. A. (2005) Hum. Mol. Genet. 14 2045-2052 [DOI] [PubMed] [Google Scholar]
- 73.Lin, P. L., Vats, A. N., and Green, M. (2001) Pediatr. Transplant. 5 398-405 [PubMed] [Google Scholar]
- 74.Manley, K., O'Hara, B. A., and Atwood, W. J. (2008) Virology 372 48-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manley, K., O'Hara B, A., Gee, G. V., Simkevich, C. P., Sedivy, J. M., and Atwood, W. J. (2006) J. Virol. 80 12079-12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aceti, A., Mangoni, M. L., Pasquazzi, C., Fiocco, D., Marangi, M., Miele, R., Zechini, B., Borro, M., Versace, I., and Simmaco, M. (2006) J. Viral Hepat. 13 821-827 [DOI] [PubMed] [Google Scholar]