Abstract
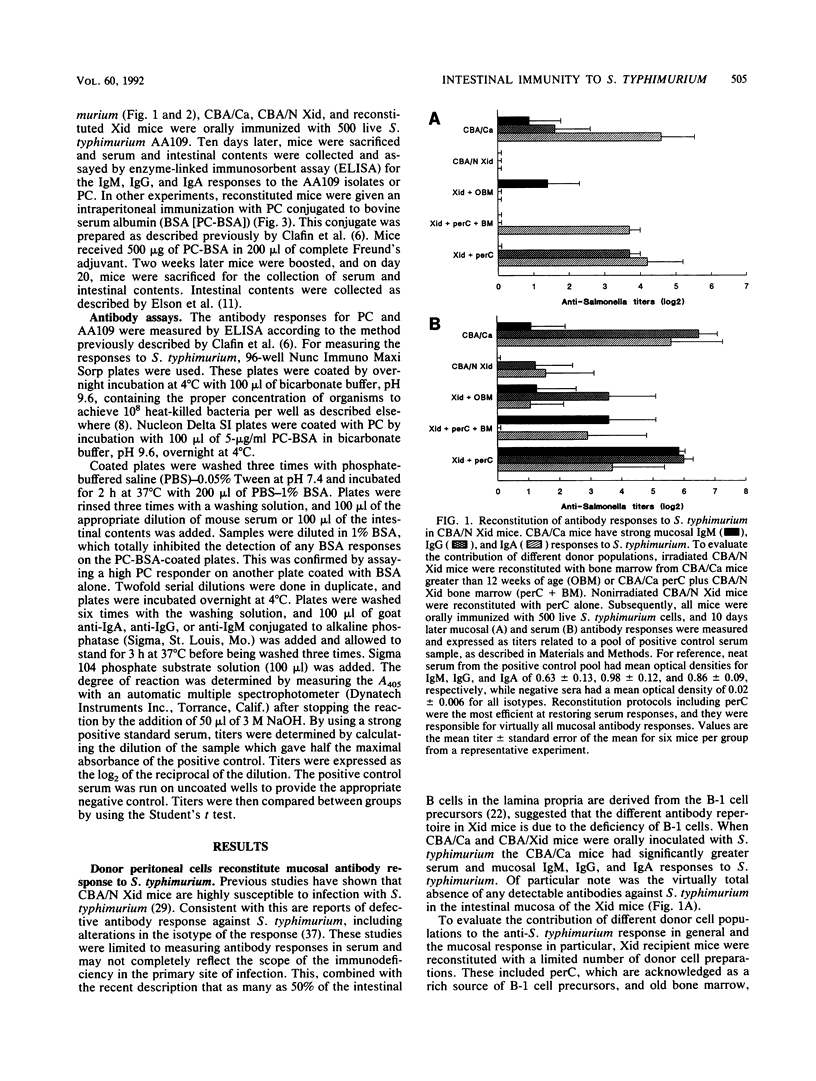
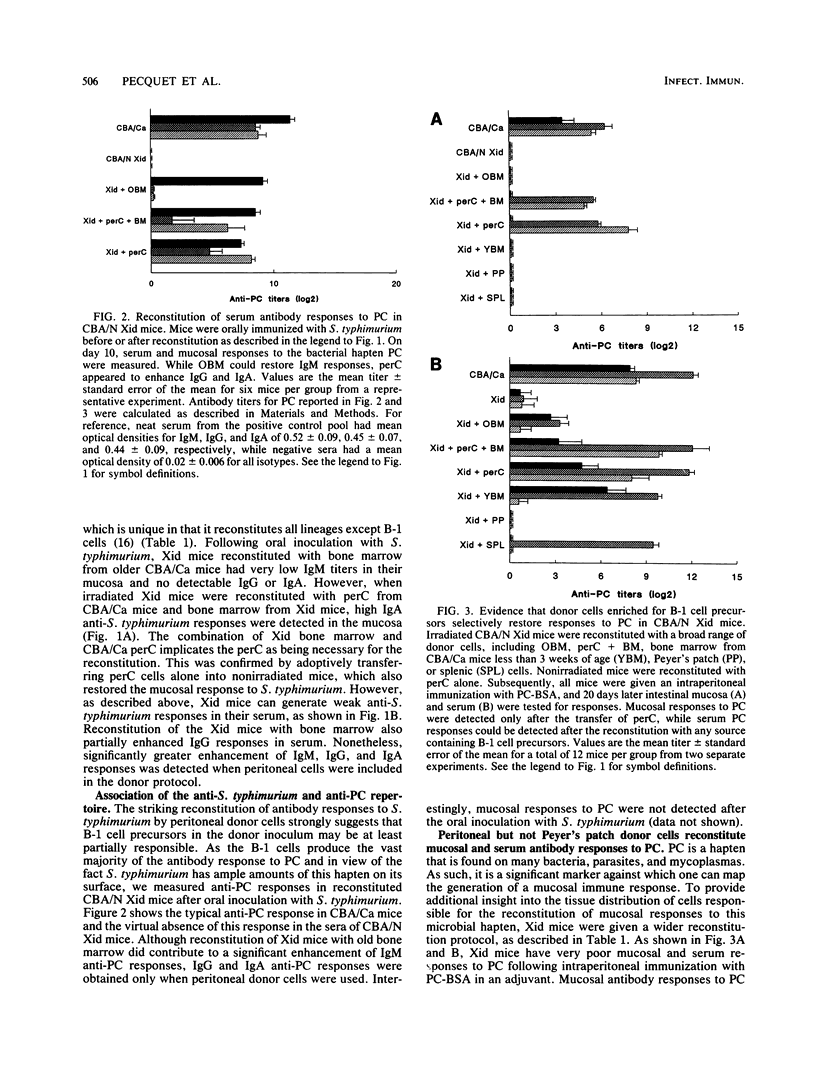
The observation that approximately half of the B cells in the murine intestinal lamina propria are derived from peritoneal CD5 B-cell precursors raises the question of their contribution to mucosal protection. Using mice with X-linked immunodeficiency which are deficient in CD5+ B cells, we showed that they mount little serum and virtually no intestinal immunoglobulin M (IgM), IgG, and IgA antibody responses following oral inoculation with live Salmonella typhimurium. Nonresponsive Xid mice were reconstituted with responsive CBA/Ca donor cell preparations which were constitutively enriched or depleted of CD5 B-cell precursors. Reconstitution of irradiated Xid mice with CD5 B-cell-deficient bone marrow from CBA/Ca donors marginally improved IgM responses in the intestinal mucosa but had no effect on IgG or IgA in response to oral immunization with live S. typhimurium. Whenever Xid mice were reconstituted with donor cells from the peritoneal cavity, which are enriched for CD5 B-cell precursors, strong IgA and in some cases IgG responses in the intestinal mucosa were stimulated in response to oral immunization. When mucosal and serum antibody responses were compared, the peritoneal donor cells again reinstated maximal serum antibody responses to S. typhimurium. Serum and mucosal responses to the bacterial hapten phosphorylcholine could be induced in Xid mice after immunization with S. typhimurium or hapten-carrier conjugates but only following reconstitution with donor cells containing CD5 B-cell precursors. These observations suggest that different lymphoid compartments are enriched for regulatory or effector cells which vary in their contributions to the mucosal antibody response against epitopes on S. typhimurium.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade L., Freitas A. A., Huetz F., Poncet P., Coutinho A. Immunoglobulin VH gene expression in Ly-1+ and conventional B lymphocytes. Eur J Immunol. 1989 Jun;19(6):1117–1122. doi: 10.1002/eji.1830190623. [DOI] [PubMed] [Google Scholar]
- Beckmann E., Levitt D. Phosphorylcholine on Streptococcus pneumoniae R36a is responsible for in vitro polyclonal antibody secretion by human peripheral blood lymphocytes. J Immunol. 1984 May;132(5):2174–2176. [PubMed] [Google Scholar]
- Braley-Mullen H. Requirement for B cells for activation of contrasuppressor T cells by type III pneumococcal polysaccharide. J Immunol. 1990 Apr 1;144(7):2465–2472. [PubMed] [Google Scholar]
- Brown A. R., Crandall C. A. A phosphorylcholine idiotype related to TEPC 15 in mice infected with Ascaris suum. J Immunol. 1976 Apr;116(4):1105–1109. [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J Exp Med. 1974 Jan 1;139(1):58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Rees A. S., Bergmeier L. A., Challacombe S. J. The detection and specificity of class specific antibodies to whole bacterial cells using a solid phase radioimmunoassay. Clin Exp Immunol. 1983 Jul;53(1):192–200. [PMC free article] [PubMed] [Google Scholar]
- Denkers E. Y., Wassom D. L., Hayes C. E. Characterization of Trichinella spiralis antigens sharing an immunodominant, carbohydrate-associated determinant distinct from phosphorylcholine. Mol Biochem Parasitol. 1990 Jun;41(2):241–249. doi: 10.1016/0166-6851(90)90187-q. [DOI] [PubMed] [Google Scholar]
- Duran L. W., Metcalf E. S. Antibody-defective, genetically susceptible CBA/N mice have an altered Salmonella typhimurium-specific B cell repertoire. J Exp Med. 1987 Jan 1;165(1):29–46. doi: 10.1084/jem.165.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984 Feb 24;67(1):101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Ernst P. B., Lee S. T., Maeba J., Bienenstock J., Stanisz A. M., Paraskevas F. A role for isotype-specific binding factors in the regulation of IgA- and IgG-specific responses by the anti/contrasuppressor T cell circuit. J Immunol. 1989 Sep 1;143(5):1426–1432. [PubMed] [Google Scholar]
- Ernst P. B., Maeba J., Lee S. I., Paraskevas F. A novel mechanism for the selection of isotype-specific antibody responses: the role of intestinal T cells in the regulation of IgA synthesis by the anti-suppressor circuit. Immunology. 1988 Sep;65(1):59–66. [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Cebra J. J. Differentiated B lymphocytes. Potential to express particular antibody variable and constant regions depends on site of lymphoid tissue and antigen load. J Exp Med. 1979 Jan 1;149(1):216–227. doi: 10.1084/jem.149.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A. Toward a layered immune system. Cell. 1989 Dec 22;59(6):953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kantor A. A new nomenclature for B cells. Immunol Today. 1991 Nov;12(11):388–388. doi: 10.1016/0167-5699(91)90135-G. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Holmes K. L. Differences in the repertoire expressed by peritoneal and splenic Ly-1 (CD5)+ B cells. J Immunol. 1990 Jun 15;144(12):4520–4525. [PubMed] [Google Scholar]
- Kroese F. G., Butcher E. C., Stall A. M., Lalor P. A., Adams S., Herzenberg L. A. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1(1):75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Morahan G. The peritoneal Ly-1 (CD5) B cell repertoire is unique among murine B cell repertoires. Eur J Immunol. 1990 Mar;20(3):485–492. doi: 10.1002/eji.1830200305. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Stall A. M., Adams S., Herzenberg L. A. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur J Immunol. 1989 Mar;19(3):501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- Masmoudi H., Mota-Santos T., Huetz F., Coutinho A., Cazenave P. A. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2(6):515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- Mattioli C. A., Tomasi T. B., Jr The life span of IgA plasma cells from the mouse intestine. J Exp Med. 1973 Aug 1;138(2):452–460. doi: 10.1084/jem.138.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Lieberman R., Inman J. K., Mosier D. E., Paul W. E. Inability of mice with a defect in B-lymphocyte maturation to respond to phosphorycholine on immunogenic carriers. J Exp Med. 1977 Oct 1;146(4):1138–1142. doi: 10.1084/jem.146.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- Pappo J., Steger H. J., Owen R. L. Differential adherence of epithelium overlying gut-associated lymphoid tissue. An ultrastructural study. Lab Invest. 1988 Jun;58(6):692–697. [PubMed] [Google Scholar]
- Perlmutter R. M., Nahm M., Stein K. E., Slack J., Zitron I., Paul W. E., Davie J. M. Immunoglobulin subclass-specific immunodeficiency in mice with an X-linked B-lymphocyte defect. J Exp Med. 1979 Apr 1;149(4):993–998. doi: 10.1084/jem.149.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Quintáns J., Kaplan R. B. Failure of CBA/N mice to respond to thymus-dependent and thymus-independent phosphorylcholine antigens. Cell Immunol. 1978 Jul;38(2):294–301. doi: 10.1016/0008-8749(78)90060-6. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Kitamura K., Kiyono H., Kurita T., Green D. R., McGhee J. R. Isotype-specific immunoregulation. Evidence for a distinct subset of T contrasuppressor cells for IgA responses in murine Peyer's patches. J Exp Med. 1986 Aug 1;164(2):501–516. doi: 10.1084/jem.164.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Forman C., Gray B. M., Briles D. E. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect Immun. 1982 Apr;36(1):184–188. doi: 10.1128/iai.36.1.184-188.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Hera A., Marcos M. A., Toribio M. L., Marquez C., Gaspar M. L., Martinez C. Development of Ly-1+ B cells in immunodeficient CBA/N mice. J Exp Med. 1987 Sep 1;166(3):804–809. doi: 10.1084/jem.166.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]



