Abstract
Hepatitis A virus (HAV) infection is rarely fatal except in patients with chronic liver disease. In the case reported here, an elderly women died of HAV infection 12 years after incomplete HAV vaccination. The possible role of a concordant Rift Valley fever virus infection acquired in Kenya is discussed.
CASE REPORT
A 71-year-old German woman reported to her physician at the beginning of January 2008 with a complaint of increasing fatigue, loss of appetite, and myalgias since 1 January. Her skin and sclera were jaundiced, and she had noticed a darkening of her urine in the absence of abdominal pain or fever. Four weeks before, the patient had returned from group travel to Kenya which comprised 5 days of a game-viewing safari and 9 days in a seaside hotel near Mombasa. She had not taken any antimalarial drugs. The patient occasionally took metamizole for arthralgias, but otherwise no serious illnesses or conditions were known. According to her vaccination record, the patient had received two injections of hepatitis A virus (HAV) vaccine (Havrix; SmithKline Beecham) within a 4-week interval in 1995 and a yellow fever vaccination in 1994.
After initial assessment in a German district hospital, she was referred to the Charité University Hospital on the same day (day 1) with a diagnosis of acute liver failure and painless jaundice. On examination the patient was icteric, afebrile, and hypotensive. Apart from discrete dyspnea, physical examination revealed no further findings of note and no external signs of bleeding in particular. Ultrasonographic examination of the liver and affiliated vessels did not display any abnormalities and especially no portal vein thrombosis or signs of malignant biliary obstruction. Routine laboratory examination (Fig. 1) showed excessive elevation of transaminase levels (aspartate aminotransferase, 7,875 U/liter; alanine aminotransferase [ALT], 9,077 U/liter), a bilirubin level of 13.33 mg/dl, and a prolonged prothrombin time (international normalized ratio [INR], 7.18). Serum creatinine and C-reactive protein levels were slightly elevated to 1.4 mg/dl and 2.26 mg/dl, respectively. Hematological investigation revealed a hemoglobin level of 15 g/dl, a white cell count of 20.31 × 109/liter, and a normal platelet count. Malaria was ruled out by conventional blood smears and immunochromatographic rapid testing. Drug screening tests likewise gave inconspicuous results.
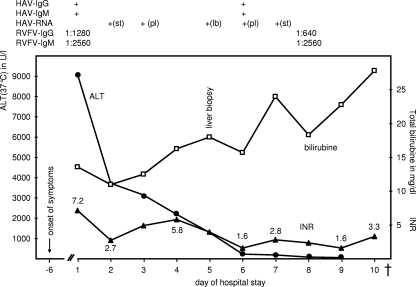
FIG. 1.
Development of INR (▴), serum ALT (37°C) (•), and total bilirubin (□) over time. The detection of HAV- and RVFV-specific IgG, IgM, and HAV RNA is depicted in the upper part. +(st), positive in stool; +(pl), positive in plasma; +(lb), positive in liver biopsy specimen. Shown are levels of ALT (in U/liter [U/l]; reference range, <34 U/liter) and total bilirubin (in mg/dl; reference range, <1 mg/dl) and INR of prothromb in time (0.9 to 1.25), influenced by fresh frozen plasma. The patient died (†) on day 11 (17 days after the onset of symptoms).
Serological examination of hepatitis parameters revealed unexpectedly high levels of positive HAV immunoglobulin M (IgM) antibodies (Abbott, Illinois). Since the patient had been immunized against HAV in the past, a second assay (BioMérieux, Marcy l'Etoile, France) was applied to prove the specificity of HAV IgM antibodies. Furthermore, detection of HAV RNA (Table 1) in serum and rectal swab samples confirmed an acute HAV infection (Fig. 1). Sequencing of the VP1/2A junction and phylogenetic analysis determined the presence of HAV genotype IB (5). HBV, HCV, and HEV as well as acute or reactivated cytomegalovirus and Epstein-Barr virus infections were excluded by serological or nucleic acid amplification assays.
TABLE 1.
Primer and probe sequences used to detect HAV and RVFV and primers for HAV genotyping
| Purpose | 5′→3′ primer or probe sequencea | Accession no. | nt positionb | Gene |
|---|---|---|---|---|
| HAV detection | TCACCGCCGTTTGCCTAG | EU131373.1 | 68-85 | 5′ UTRc |
| GGGAGAGCCCTGGAAGAAA | 242-224 | |||
| FAM-CCTGAACCTGCAGGAATTAA- MGB-NFQ | 169-150 | |||
| HAV genotyping | CTATTCAGATTGCAAATTAYAAT | EU131373.1 | 2396-2418 | VP1/2A |
| AAYTTCATYATTTCATGCTCCT | 3289-3268 | |||
| RVFV detection | AAAGGAACAATGGACTCTGGTCA | EF467178.1 | 1164-1186 | G2 |
| CACTTCTTACTACCATGTCCTCCAAT | 1258-1233 | |||
| FAM-AAAGCTTTGATATCTCTCAGTGCCCCAA-TAMRA | 1204-1231 |
FAM, 6-carboxyfluorescein; MGB-NFQ, molecular-groove binding nonfluorescence quencher; TAMRA, 6-carboxytetramethylrhodamine.
Nucleotide (nt) position of the binding site.
UTR, untranslated region.
Further serological investigations revealed an additional infection with Rift Valley fever virus (RVFV): the RVFV IgM titer (indirect immunofluorescence) was 1:2,560 and that for IgG was 1:1,280 (Fig. 1). The RVFV genome was undetectable in serum (Table 1), as could be expected due to the lag since the presumed time of infection. The patient denied any signs of febrile infection during or shortly after the journey. Antibodies to other bunyaviruses (sandfly fever virus, hantavirus) were not detectable. Apart from other viral etiologies of hepatitis, also leptospirosis, brucellosis, and rickettsial infections were ruled out serologically. Blood cultures remained without microbial growth.
The patient's condition deteriorated within 24 h after hospital admission. Despite supportive treatment including fresh frozen plasma, vitamin K, ornithine aspartate, and oral lactulose, she developed hepatic encephalopathy with somnolence on day 2. Incident renal failure was successfully averted by intravenous fluid substitution. An abdominal computed tomography scan showed no pathological findings apart from nonspecific thickening of the posthepatic bile ducts and duodenal mucosa.
Histology of a liver biopsy sample which had been obtained via a transjugular catheter (day 5) showed inflammatory infiltrations and hepatic cell degeneration with apoptosis and ballooning of cells. These findings are characteristic of a case of acute hepatitis but are not specific for hepatitis A. High amounts of HAV RNA were amplified from the tissue as expected, but the RVFV genome could not be traced.
On the basis of a hepatic coagulation failure, the patient developed hemorrhagic complications. Internal bleeding on day 8 ultimately led to hemodynamic instability with renal and ensuing multiorgan failure. Meanwhile, transaminase levels diminished progressively to levels below 100 U/ml, which, considering the clinical course, signified extensive liver necrosis rather than recovery. The bilirubin serum level peaked at 23 mg/dl (Fig. 1) and the ammoniac serum level at 150 μmol/liter. The patient died on the 11th day of her hospital stay, 17 days after the onset of clinical symptoms.
Hepatitis A is usually a self-limiting disease with a 0.3 to 1.5% case fatality rate (2). Age, coinfections with HBV or HCV, and female gender (3, 7, 10) are associated with a higher risk of liver failure and mortality. Early encephalopathy is indicative of a fulminant course (10). The incidence in Germany is about 1,400 to 2,300 cases per year, and about 50% of cases are imported (6). This report describes how a 71-year-old woman without any known hepatic disorder died of an HAV genotype IB infection 6 weeks after returning from a journey to Kenya and 17 days after the onset of symptoms. HAV genotype IB is known to be present in Africa as well as in Europe (5). However, an imported infection was likely, since the incubation time correlated with the stay in Kenya, and in Central Europe HAV infection with genotype IB is frequently travel associated (1, 6).
The rapid onset of encephalopathy as well as the persistence of high bilirubin levels signaled a poor prognosis, yet even in this situation recovery still could have been possible.
RVFV is endemic in Kenya, though no outbreaks were reported for November 2007. The infection has an incubation time of 3 to 6 days and, like in this case, often remains asymptomatic (4, 8). The virus has a tropism for the liver with a distinct midzonal pattern of lesions which is not fully understood (9). The route of transmission can be via aerosols, insect vectors, animal products, or secretions (8). Interviews with other participants of the journey did not clarify when and how the HAV and RVFV infections were presumably acquired. Irrespective of the time course, it is conceivable that RVFV infection caused subclinical liver damage and aggravated the patient's immune response to the HAV infection, as is known to be the case for HAV infection and underlying chronic liver diseases (7). Hepatic injury in HAV is mediated mainly by cytotoxic T cells (11), but little is known about the respective direct or cell-mediated effects of RVFV (9). The patient's age alone posed a risk factor for a severe course of HAV infection (7).
The fact that the patient had been vaccinated against HAV in 1995 initially raised doubts about the diagnosis. In 1995, Havrix was available with only 720 U/liter of HAV antigen per dose (today's dosage for children or for combined vaccination with HBV), and a schedule of vaccination at 0, 1, and 6 months was recommended (12). However, in this case the third dose was never documented and supposedly was not administered, so the immunization of the patient most probably remained incomplete.
HAV vaccination is considered very effective and long lasting, e.g., for at least 12 years and even for 25 years according to mathematical models (13). This may be misleading for descriptions of its efficacy in the elderly: Wolters et al. (14) found that even a complete HAV vaccination scheme was only 65% effective in vaccinees of over 40 years of age. Thus, complete vaccination at the age of 58 years might not have yielded protective immunity in the patient.
This case illustrates that HAV infection can be life threatening for elderly people traveling to areas of high HAV endemicity. A history of HAV vaccination does not necessarily rule out the infection. For people of over 40 years of age, HAV immunity should be verified before probable exposure. Additional boosts may be required to enhance the response rate in this age group (14).
Nucleotide sequence accession number.
The HAV RNA sequence has been assigned GenBank accession number EU930199.
Footnotes
Published ahead of print on 3 September 2008.
REFERENCES
- 1.Bruisten, S. M., G. M. Tjon, J. A. van den Hoek, C. J. Wijkmans, H. M. Gotz, and R. A. Coutinho. 2007. The molecular epidemiology of hepatitis A in The Netherlands: the usefulness of typing isolated viral strains. Ned. Tijdschr. Geneeskd. 1512779-2786. (In Dutch.) [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2008. Surveillance for acute viral hepatitis—United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 571-24. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2006. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 551-23. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2007. Rift Valley fever outbreak—Kenya, November 2006-January 2007. MMWR Morb. Mortal. Wkly. Rep. 5673-76. [PubMed] [Google Scholar]
- 5.de Paula, V. S., M. L. Baptista, E. Lampe, C. Niel, and A. M. Gaspar. 2002. Characterization of hepatitis A virus isolates from subgenotypes IA and IB in Rio de Janeiro, Brazil. J. Med. Virol. 6622-27. [DOI] [PubMed] [Google Scholar]
- 6.Frank, C., J. Walter, M. Muehlen, A. Jansen, U. van Treeck, A. M. Hauri, I. Zoellner, M. Rakha, M. Hoehne, O. Hamouda, E. Schreier, and K. Stark. 2007. Major outbreak of hepatitis A associated with orange juice among tourists, Egypt, 2004. Emerg. Infect. Dis. 13156-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyrlagkitsis, I., M. E. Cramp, H. Smith, B. Portmann, and J. O'Grady. 2002. Acute hepatitis A virus infection: a review of prognostic factors from 25 years experience in a tertiary referral center. Hepatogastroenterology 49:524-528. [PubMed] [Google Scholar]
- 8.LaBeaud, A. D., Y. Ochiai, C. J. Peters, E. M. Muchiri, and C. H. King. 2007. Spectrum of Rift Valley fever virus transmission in Kenya: insights from three distinct regions. Am. J. Trop. Med. Hyg. 76795-800. [PMC free article] [PubMed] [Google Scholar]
- 9.Quaresma, J. A., M. I. Duarte, and P. F. Vasconcelos. 2006. Midzonal lesions in yellow fever: a specific pattern of liver injury caused by direct virus action and in situ inflammatory response. Med. Hypotheses 67618-621. [DOI] [PubMed] [Google Scholar]
- 10.Rezende, G., A. M. Roque-Afonso, D. Samuel, M. Gigou, E. Nicand, V. Ferre, E. Dussaix, H. Bismuth, and C. Feray. 2003. Viral and clinical factors associated with the fulminant course of hepatitis A infection. Hepatology 38613-618. [DOI] [PubMed] [Google Scholar]
- 11.Vallbracht, A., K. Maier, Y. D. Stierhof, K. H. Wiedmann, B. Flehmig, and B. Fleischer. 1989. Liver-derived cytotoxic T cells in hepatitis A virus infection. J. Infect. Dis. 160209-217. [DOI] [PubMed] [Google Scholar]
- 12.Van Damme, P., S. Thoelen, M. Cramm, K. De Groote, A. Safary, and A. Meheus. 1994. Inactivated hepatitis A vaccine: reactogenicity, immunogenicity, and long-term antibody persistence. J. Med. Virol. 44446-451. [DOI] [PubMed] [Google Scholar]
- 13.Van Herck, K., P. Van Damme, M. Lievens, and M. Stoffel. 2004. Hepatitis A vaccine: indirect evidence of immune memory 12 years after the primary course. J. Med. Virol. 72194-196. [DOI] [PubMed] [Google Scholar]
- 14.Wolters, B., U. Junge, S. Dziuba, and M. Roggendorf. 2003. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 213623-3628. [DOI] [PubMed] [Google Scholar]