Abstract
The hepatitis C virus (HCV) alternate reading frame protein or F protein of the HCV 1b genotype is a double-frameshift product of the HCV core protein. In order to assess the presence of antibodies specific for F protein and their clinical relevance in sera from HCV patients, we produced recombinant F protein and core protein of the HCV 1b genotype in Escherichia coli. An enzyme-linked immunosorbent assay was developed using purified recombinant HCV core, F protein, and a 99-residue synthetic F peptide (F99). The seroprevalences of anticore, anti-F protein, and anti-F99 synthetic peptide were 95%, 68%, and 36%, respectively, in 168 HCV patients. The prevalence of anti-F antibodies did not correlate with viral load, genotype, or alanine aminotransferase level. Interferon combination therapy induced a decline in the level of anti-F antibodies in 55 responders (P < 0.01). Thirteen responders (24%) lost their anti-F recombinant protein antibodies, and 17 (31%) lost their anti-F synthetic peptide antibodies, whereas no decrease was observed for the 17 nonresponders. These changes were significant between responders and nonresponders (P < 0.05). Meanwhile, no change was found in the anticore antibody titer of the 72 treated patients. The percentage of anti-F-protein-negative patients (15/15 [100%]) who achieved a sustained virological response (SVR) was higher than that of the anti-F-positive patients (70%) (P < 0.05). Based on these findings, HCV F protein elicits a specific antibody response other than the anticore protein response. Our data also suggest that the presence and level of anti-F antibody responses might be influenced by the treatment (interferon plus ribavirin) and associated with an SVR in Chinese hepatitis C patients.
An estimated 170 million people are infected with hepatitis C virus (HCV) worldwide. In developed countries, HCV infection accounts for 40% of end-stage cirrhosis and 60% of hepatocellular carcinomas and has become the leading cause of liver transplantations (21). The HCV genome is a positive-sense RNA approximately 9,600 bases long, and HCV is related to viruses of the Flaviviridae family. Genomic HCV RNA has a central, protein-coding domain that is flanked by nontranslated regions. The protein-coding domain has a large open reading frame (ORF) that encodes the classical HCV proteins: core, E1, E2, p7, NS2, NS3, NS4a, NS4b, NS5a, and NS5b (8, 20). Interestingly, recent reports indicate that the HCV genome contains an overlapping +1 reading frame encoding alternative core antigens (3, 6, 22, 24, 25), which has been called an alternate reading frame protein (ARFP) or F protein. The double-frameshift protein (DF) of HCV genotype 1b is composed of 42 amino acids of the core protein linked to 101 amino acids encoded in the ARF, followed by the C terminus of the core protein. For HCV genotype 1a-derived ARFP, the frameshifting appears to take place at or near codon 11 (24, 25), and the protein ends at codon 161. Although the shift junction and the length of the proteins are different, both genotype 1a and 1b ARFP contain a common central frameshifted domain of 101 residues starting at codon 43 and ending at codon 144.
Several studies using either synthetic peptides belonging to the F-protein ORF (F-ORF) (24), glutathione S-transferase recombinant F-ORF (22), His-tagged recombinant F protein (15), or in vitro-translated F protein (25) have reported the presence of anti-F protein antibodies in some HCV-positive sera. Bain et al. and Cohen et al. (1, 9) reported that no correlation was found between the presence of anti-F antibodies and viremia or therapy outcome. However, the potential role of ARFP in the development of chronicity and virus-associated pathogenesis remains an unanswered question.
MATERIALS AND METHODS
Patients.
All subjects were enrolled from the Department of Infectious Diseases of Ruijin Hospital affiliated with the Medical School of Shanghai Jiaotong University, Shanghai, China, from 2002 to 2007. Informed consent was obtained from each subject before inclusion in the study, and this study was approved by the hospital's ethics committee. Serum samples from 168 untreated HCV patients were collected and used to investigate the prevalence of specific anti-F protein antibodies in correlation with clinical parameters. The diagnosis of chronic hepatitis was based on internationally accepted criteria (2). The serum samples were centrifuged and stored at −70°C prior to use. The clinical characteristics of the patients are shown in Table 1. In order to investigate the influence of anti-F to antiviral treatment, 72 patients treated with combination therapy with pegylated alpha interferon (IFN) 2a (180 μg weekly) plus ribavirin (900 mg ribavirin daily) for 48 weeks were enrolled in this study. All sera were analyzed for anti-F and anticore antibodies before and 6 months after the end of antiviral therapy. Responders were defined as patients who had cleared viremia and sustained viremia for at least 6 months after the end of therapy, while nonresponders were defined as patients who had not cleared viremia at the end of therapy or relapse.
TABLE 1.
Clinical characteristics of 168 HCV-infected patients
| Characteristica | Value |
|---|---|
| Mean age (yr) (range) | 43 (6-76) |
| Sex | |
| No. of females | 68 |
| No. of males | 100 |
| No. of patients with the following HCV genotype: | |
| 1b | 104 |
| 1a | 1 |
| 2a | 16 |
| 3a | 11 |
| 3b | 5 |
| 6 | 1 |
| ND | 30 |
| Mean ALT (IU/ml) (range) | 111 (16-949) |
| No. of patients with ND ALT | 17 |
| Mean HCV load (copies/ml) | |
| (range) | 4.93 × 106 (2.3 × 104-2.20 × 108) |
| No. of patients with ND HCV | |
| RNA | 8 |
ND, not documented; ALT, alanine aminotransferase.
In addition, five consecutive blood samples from representative patients were investigated before and during the end of treatment and 6 months after the end of therapy. Serum samples from 40 blood donors and 40 HBV-infected patients were used as negative controls.
HCV RNA quantitation and HCV genotyping.
HCV RNA was quantified in serum samples by real-time PCR after reverse transcription of the 5′ noncoding region of the HCV genome (PJ Co. Ltd., China). The sensitivity of the assay was 1,000 RNA copies/ml.
The HCV genotypes were determined using a commercial kit (Realchip Biotechnology Co. Ltd., China) according to the manufacturer's recommendations. The assay allows the identification of the following HCV genotypes: 1a, 1b, 2a, 3a, 3b, and 6.
Construction of core protein and F-protein expression vectors.
Both recombinant HCV core (amino acids 1 to 169)- and F-protein-coding sequence were derived from the cDNA sequence encoding HCV core protein, which was obtained by PCR amplification using a serum sample of a genotype 1b HCV-infected patient. Briefly, for the core protein construct, a 507-bp fragment from nucleotide 342 to 848 corresponding to amino acids 1 to 169 of the HCV core protein was amplified by PCR. For the F-protein construct, the cDNA fragment from nucleotide 465 to nucleotide 768 of the HCV F-protein-coding sequence was achieved by the +1/−1 frameshifting artificially introduced at codon 43 and codon 144 of the core protein-coding sequence. DNA fragments covering the complete ARFP sequence corresponding to amino acids 1 to 191 were amplified and ligated by PCR. The resulting HCV F and HCV core cDNAs were cloned into the pET-28a (+) expression vector downstream of the six-His tag. The constructs were transformed into competent Escherichia coli DH5 bacteria (Invitrogen), and the purified plasmid DNA was verified by DNA sequencing.
Expression and identification of the recombinant proteins.
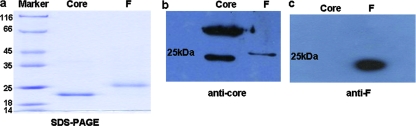
The recombinant proteins were expressed in E. coli with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Gibco/BRL) for F protein and 0.5 mM IPTG for core protein. Pelleted bacteria were suspended in a solution containing 10 mM β-mercaptoethanol, 0.1% dodecylmaltoside, and anti-protease phenylmethylsulfonyl fluoride (catalog no. P7627; Sigma), then homogenized by sonication, and centrifuged. Inclusion bodies were treated with either 6 M hydrochloride guanidine for F protein or with 6 M urea for core protein. Soluble fractions were loaded over a Ni-nitrilotriacetic acid-agarose column (Qiagen). After the column was washed, the six-His-tagged proteins were eluted from the column either by running 250 mM imidazole through the column for F protein or by decreasing the pH for the core protein. The concentration of the purified recombinant proteins was determined to be 92% following scanning of the Coomassie brilliant blue-stained gel (Fig. 1a) and quantitation by Quantity One software (Bio-Rad), with a protein concentration of the recombinant F protein of 0.92 mg/ml and a protein concentration of the core protein of 0.80 mg/ml, as determined by the Bradford method (5).
FIG. 1.
Expression and identification of the HCV F protein and core recombinant proteins. (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the purified F and core recombinant proteins expressed in E. coli. The positions of molecular mass markers (in kilodaltons) are indicated to the left of the gel. (b and c) Western blot analysis of the recombinant F and core proteins reacted with anticore antibody (b) and anti-F antibody (c).
The recombinant F and core proteins were subjected to 14% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting. The polyvinylidene difluoride membranes (Millipore) were blocked with and incubated with three distinct antibodies, anti-His monoclonal antibody (MAb) (Qiagen), anticore MAb, and anti-F MAb (CNRS, France). After several washes, the membranes were incubated with peroxidase-conjugated secondary goat anti-mouse immunoglobulin G (IgG) (Bio-Rad) and enhanced chemiluminescence solution (Amersham, Germany). As shown in Fig. 1b, the anticore antibody reacted with both the core and F proteins. It also revealed the typical dimerization of the core protein. In contrast, the anti-F MAb reacted only with the F protein (Fig. 1c), demonstrating the distinct antigenicity of the F protein compared to the core protein.
F synthetic peptide.
A synthetic 99-residue peptide encompassing amino acids 43 to 144 located at +1 core reading frame protein-coding sequence was synthesized by Gelson Chemical. The full sequence of the synthetic 99-residue peptide named F99 is as follows: GWVCARLGRLPSGRNLVEGDNLSPRLA VPRAGPGRSPGTLGPSMAMRAWGGQDGSCHPEAPGLVGAPQTPGVG RVIWVRSSIPSHAASPISWGTFRLSA.
ELISA for the detection of anti-F antibodies.
For the enzyme-linked immunosorbent assay (ELISA), the wells on microplates were coated overnight with either 100 μl of HCV core protein (0.2 μg/ml), HCV F protein (0.4 μg/ml), or the synthetic F peptide (4 μg/ml) in 50 mM sodium carbonate buffer, pH 9.6. After the wells were blocked and washed, 100 μl of diluted serum (1:200) was added in duplicate wells for the F-protein- or core protein-coated wells, and a 1:100 dilution was used for the F-peptide-coated wells. Following incubation at 37°C, the plates were washed and further incubated with 100 μl of peroxidase-conjugated affinity-purified goat anti-human IgG whole antibody (Merck KGaA, Germany) diluted 1:10,000. After the plates were washed, the substrate reaction was developed by adding tetramethyl benzidine buffer and stopped after 10 min by adding 1.8 M H2SO4. The absorbance was read at 450 nm in a microplate reader. For each experiment, the cutoff was determined as the mean plus 3 standard deviations of the results from three blood donor sera plus 0.1. A serum sample was considered positive when the absorbance was equal or superior to the cutoff (1, 15).
Statistical analysis.
Mean quantitative values were compared by Student's t test if the variances between two groups were equal or by the Cochran and Cox t test if the variances were unequal. Differences in proportion were tested by the chi-square test or Fisher's exact test if needed. Odds ratio and 95% confidence intervals were calculated along with Fisher's exact P values, where appropriate. All calculations were performed with SPSS software (SPSS Inc., Chicago, IL).
RESULTS
Prevalence of specific anti-F antibodies in HCV-infected patients.
To detect anti-F antibodies in sera from HCV patients, we developed an ELISA, using the core protein, the full-length F protein, and a synthetic F peptide corresponding to the frameshifted sequence of the core protein but having no sequence identity with the core protein. Interestingly, 95% of the patients were positive for anticore antibodies, while 68% were positive for anti-F recombinant protein antibodies and 36% were positive for anti-F99 synthetic peptide antibodies. In contrast, serum samples from 40 HBV-infected patients or 40 healthy controls were all negative. The seroprevalence of anti-F protein, anticore, and anti-F99 synthetic peptide antibodies in patients with different HCV genotypes was shown in Table 2.
TABLE 2.
Seroprevalence of anti-F protein, anti-F99 synthetic peptide, and anticore antibodies in168 HCV-infected patients by ELISA
| HCV-infected patients | Seroprevalence of antibodya to:
|
||
|---|---|---|---|
| F protein | Core protein | F99 synthetic peptide | |
| All patients | 114/168 (68) | 160/168 (95) | 61/168 (36) |
| Patients with the following HCV genotype: | |||
| 1b | 73/104 (70) | 97/104 (93) | 44/104 (42) |
| 1a | 0/1 (0) | 1/1 (100) | 0/1 (0) |
| 2a | 11/16 (69) | 16/16 (100) | 5/16 (31) |
| 3a | 6/11 (55) | 11/11 (100) | 4/11 (36) |
| 3b | 3/5 (60) | 5/5 (100) | 1/5 (20) |
| 6 | 0/1 (0) | 0/1 (0) | 0/1 (0) |
| NDb | 21/30 (70) | 30/30 (100) | 7/30 (23) |
Values are the numbers of positive serum or plasma samples/number of samples tested. The values in parentheses are percentages.
ND, not documented.
Correlation between the anti-F antibodies and HCV genotypes and other clinical parameters.
Analysis of the anti-F response (optical density [OD] value) revealed that the response did not correlate with HCV genotype 1b and non-1b genotype (P = 0.197 by the t test). With the correlation analysis, the presence and titer of anti-F recombinant protein antibodies did not correlate with the HCV RNA level in 168 untreated HCV patients (P = 0.955, r = 0.00003) or with the alanine aminotransferase level (P = 0.172, r = 0.136) (data not shown).
Changes in anti-F antibody titer before and after antiviral therapy.
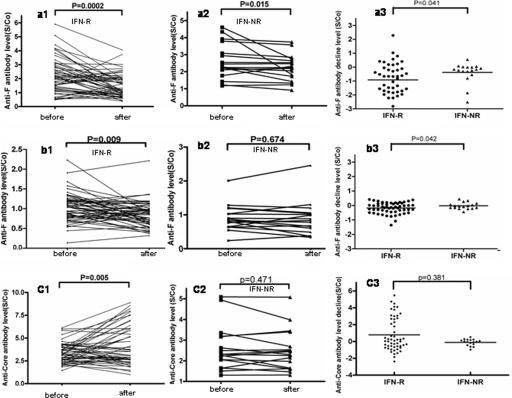
For 72 patients, we compared the titer of anti-F antibodies before and after IFN treatment. As shown in Fig. 2 a1 and a2, the titer of anti-F recombinant protein antibodies decreased during antiviral therapy in responders, which was statistically significant using the t test (P = 0.0002), whereas it was borderline significant (P = 0.049) in nonresponders. Thirteen (24%) out of 55 responders and none of 17 (0%) nonresponders lost their anti-F recombinant protein antibodies. In addition, the variation of the anti-F recombinant protein antibody titer between responders and nonresponders was statistically significant as shown by the unpaired t test (P = 0.041 [Fig. 2a3]).
FIG. 2.
Changes in the anti-F recombinant protein antibodies, anti-F99 synthetic peptide antibodies, and anticore antibodies before and after antiviral therapy in 72 HCV patients. (a) Comparison of the anti-F recombinant protein antibodies before and 6 months after treatment in responders (IFN-R) (a1) (n = 55, P = 0.0002) and nonresponders (IFN-NR) (a2) (n = 17, P = 0.015). (a3) Change in anti-F recombinant protein antibody levels between responders and nonresponders (P = 0.041). (b) Comparison of the anti-F99 synthetic peptide antibodies before and after interferon treatment in responders (b1) (n = 55, P = 0.009) and nonresponders (b2) (n = 17, P = 0.674). (b3) Change in anti-F99 synthetic peptide antibody levels between responders and nonresponders (P = 0.042). (c) Comparison of the anticore antibodies before and after interferon treatment in responders (c1) (n = 55, P = 0.005) and nonresponders (c2) (n = 17, P = 0.471). (c3) Change in anticore antibody levels between responders and nonresponders (P = 0.381). All comparisons were performed by using the t test. In panels a3, b3, and c3, each dot indicates the variation of antibody titers in 6 months after treatment (S/co [OD value]) minus the titer before treatment in each patient.
As shown in Fig. 2b1 and b2, the titer of anti-F99 synthetic peptide antibodies declined in patients who responded to IFN, which was statistically significant using the t test (P = 0.009), whereas it was not significant (P = 0.674) in nonresponders. Similarly, 17 (31%) out of 55 responders and none of 17 (0%) nonresponders lost their anti-F99 synthetic peptide antibodies. In addition, the variation of the anti-F99 synthetic peptide antibody titer between responders and nonresponders was also statistically significant as shown by the unpaired t test (P = 0.042 [Fig. 2b3]).
In contrast, no loss of anticore antibodies was observed. As shown in Fig. 2c1 and c2, the anticore antibody titer was stable or even increased in patients undergoing IFN treatment for both responders (P = 0.005) and nonresponders (P = 0.279). In addition, the variation in the anticore antibody titer between responders and nonresponders was not considered statistically significant by the unpaired t test (P = 0.381 [Fig. 2c3]).
Among 48 chronic hepatitis C patients of genotype 1b from a total number of 72, it is worth noting that 12 (34%) and 15 (43%) out of 35 responders lost their anti-F-protein antibodies and their anti-F synthetic peptide antibodies after treatment, respectively, whereas none of the 13 genotype 1b nonresponders became negative for anti-F antibodies and anti-F synthetic peptide antibodies (P < 0.01).
Overall, these results provide evidence that IFN treatment affects the titer of anti-F antibodies during antiviral therapy in HCV-infected patients.
Correlation between the presence of anti-F antibodies and SVR.
Seventy-two HCV-infected patients receiving antiviral therapy were evaluated for the presence of anti-F recombinant protein antibodies. Forty of the 57 anti-F-positive patients and all 15 anti-F-negative patients achieved a sustained virological response (SVR). The difference in the values for the anti-F positive (70%) and the anti-F negative patients (100%) was considered statistically significant by the χ2 test (P = 0.016).
Although the percentage of anti-F synthetic peptide antibody-negative patients (30/36 [83%]) who achieved an SVR was also higher than that of antibody-positive patients (25/36 [69%]), the difference was not statistically significant by the χ2 test (P = 0.268).
In contrast, 55 out of anticore antibody-positive 72 patients achieved an SVR.
Dynamics of the anti-F antibody titer during antiviral treatment.
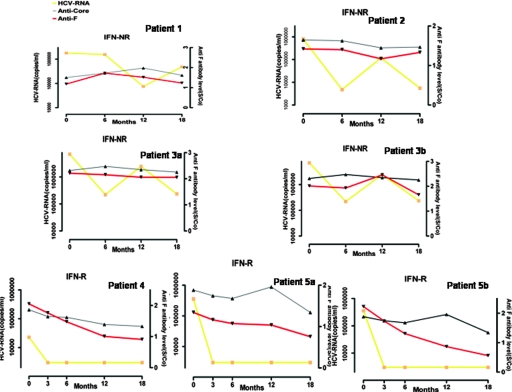
Among 72 patients, 5 representative patients were investigated at different time points during their treatment to observe the dynamic changes in anti-F antibody. As shown in Fig. 3, titers of anti-F recombinant protein or synthetic F peptide antibodies in three nonresponders remained stable, along with the HCV RNA level (patients 1, 2, and 3). In contrast, the anti-F recombinant protein or synthetic F peptide antibody titers of two responders decreased gradually and cleared the virus from their serum 3 months after the initiation of therapy (patients 4 and 5). The serum anti-F antibody titers show a well-defined trend along with HCV RNA level. However, the anticore antibody titers in these five patients did not change significantly during the treatment.
FIG. 3.
Evolution of antibody titers of five representative patients measured by ELISA (OD values) and HCV RNA level during treatment. Patients 1, 2, and 3 are nonresponders (IFN-NR), and their anti-F protein antibody titer (patients 1, 2, and 3a) or anti-F99 peptide antibody titer (patient 3b) remained stable, as did their HCV RNA level. Patients 4 and 5 are responders (IFN-R), and their anti-F protein antibody titer (patients 4 and 5a) or anti-F99 peptide antibody titer (patient 5b) decreased gradually, as did their HCV RNA level. The anticore antibody levels did not significantly change in the two responders and three nonresponders during antiviral therapy The red, black, and yellow lines represent anti-F antibody titer, anticore antibody titer, and HCV RNA level, respectively. Anti-F-protein antibody titers 6 months after treatment (S/co) are shown on the right-hand y axes.
DISCUSSION
In this study, we have investigated the presence of anti-F antibodies in sera from chronically HCV-infected patients using HCV recombinant core protein, F protein, and F99 synthetic peptide. The prevalences for anti-F protein, anti-F99 synthetic peptide, and anticore antibodies were 68%, 36% and 95%, respectively. The seroprevalence of anti-F antibodies (68%) in our study is compatible with the results reported by Komurian-Pradel et al. (15), who investigated specific anti-F antibodies in 62% of the hepatitis C patients they studied. The lower prevalence and the weaker anti-F response compared to the anticore protein response could be due to the unstable character of the F protein and its localization in the endoplasmic reticulum (20), a rare event in the translation of F-ORF (22).
The specific antigenicity of the HCV F protein compared to the HCV core protein is demonstrated in the Western blot. Only F protein, not the core protein, reacted with the anti-F MAb, despite the fact that the two proteins both possess the N-terminal and C-terminal domains of the core protein. The fact that 61 out of 114 (54%) anti-F-protein antibody-positive sera reacted with the F99 peptide further underlines the specific antigenicity of the frameshifted domain encompassing amino acids 43 to 144. However, the weaker reactivity of the sera with the full-length F protein compared to that of sera with the core protein could be caused by different antigenicities of the frameshifted domain and also different folding due to the presence of the first 42 amino acids in the F protein, modifying the overall antigenicity of the domain. In our study, the anti-F antibody titer decreased in HCV patients after treatment with IFN plus ribavirin, while the anticore antibody titer did not change significantly in these patients. These results also confirm that in chronically infected HCV patients, F protein elicits a specific antibody response distinct from the anticore protein response.
It has been shown that total HCV core antigen levels correlate with HCV RNA concentrations. The levels of total HCV core antigen in serum decreased and directly reflect the trends in HCV RNA levels in patients undergoing treatment, and total HCV core antigen levels were significantly higher in patients who did not achieve an SVR (4, 7, 11, 14, 17, 19, 23). In our study, 13 patients (24%) out of 55 responders lost their anti-F antibodies after interferon treatment, whereas none of the 17 (0%) nonresponders became anti-F negative. The results obtained in the present study indicate that the variation of anti-F antibodies before and after treatment gave similar results as HCV total core antigen testing did.
The recombinant F protein used in the ELISA comprises 42 N-terminal amino acids of the core protein linked to 101 amino acids of the +1 reading frame and 47 C-terminal amino acids of the core protein. In order to avoid cross-immunoreactivity of HCV recombinant core and F protein, measurements of anti-F99 synthetic peptide antibodies without unwanted portions of core protein was also performed for 72 patients before and after combined therapy. In our study, an even higher number of responders (17 [31%]) lost their anti-F99 peptide antibodies after IFN treatment. These changes occurred in 48 genotype 1b patients out of 72 patients given antiviral treatment and were significant. Antibodies reactive with recombinant F protein and synthetic F99 peptide were decreased or even undetectable in 55 responders, whereas no change occurred in 17 nonresponders. These changes between responders and nonresponders were statistically significant. Our findings indicated that the presence and the level of anti-F antibody responses may be influenced by treatment with IFN and ribavirin.
Although the in vivo function of the F protein is not yet known, experiments by Varaklioti et al. (22) have confirmed that the ribosomal frameshifting occurs at a high rate when HCV-1 transcripts are translated in cell extracts. Boulant et al. (3) have demonstrated that alternative HCV core proteins of the HCV 1b genotype are expressed in E. coli and that the frameshift signals can be reproduced both in an in vitro eukaryotic translation system and in cells in culture. The standard treatment of chronic HCV infection by pegylated interferon plus ribavirin is able to induce an SVR in only 42% of HCV genotype 1 patients compared to 80% of genotype 2 and 3 patients (10, 12, 16). Our present data showed that all the anti-F recombinant protein antibody-negative patients but only 40 out of 57 anti-F recombinant protein antibody-positive patients achieved an SVR. The percentage of anti-F99 synthetic peptide antibody-negative patients (83%) who achieved an SVR was higher than that of anti-F99 peptide antibody-positive patients (69%). These results suggest that patients with low anti-F activity or no activity are more likely to achieve an SVR than patients with anti-F antibodies in their sera. The presence or absence of specific antibodies to F protein may be an indicator that can predict the efficacy of antiviral treatment in these patients.
Until now, only two studies have reported a correlation between the immune response to F protein and the response to IFN combination therapy. In the study of Cohen et al. (9), 10 of the 27 patients showed biological and virological changes after treatment but no change in the anti-F antibody profile. Bain et al. (1) reported that anti-ARFP antibody responses have no link with viremia or therapy outcome in six patients during combined IFN and ribavirin therapy. The different geographical regions, races of the patients, different HCV genotypes, and sample sizes might be the reasons leading to the different outcomes of these studies. In our study, the data were obtained from 72 Chinese Han hepatitis C patients treated with interferon plus ribavarin with the majority having HCV 1b genotype (48 out of 72), which is different from the nature of the sample cohorts used by Bain et al. and Cohen et al. (1, 9). Previous studies have shown that the geographical distribution of HCV genotypes may have epidemiological relevance and clinical implications, such as response to therapy and disease progression (13). In addition, racial disparities might influence how HCV infection behaves, causing differences in the prevalence of HCV infection, clinical presentation, treatment response, and immunological recognition of HCV (18). It is deduced that these factors might have some impact on the characteristics of HCV anti-F antibody activity. Studies on different populations infected with different genotypes and a better understanding on how and why a patient generates an antibody response against HCV F protein might be required in vivo.
In summary, a specific antibody response to the alternative reading frame protein distinct from the HCV core protein might be associated with an sustained virological response in genotype 1b chronic hepatitis C patients. These data imply that the detection of anti-F antibodies might be an alternative assessment of the therapeutic response in HCV-infected patients. However, the prevalence of these antibodies is quite low in comparison to core antibodies. Therefore, the usage of this indicator for assessment of the therapeutic response in HCV-infected patients will be useful only in those cases that develop an antibody response against F protein. The mechanism of the relationship between antiviral therapy and expression of HCV F protein in vivo needs to be further investigated.
Acknowledgments
This work was supported by the Foundation of National Nature Science of China (grants 30471523 and 30671839).
We thank Geneviève Inchauspé (INSERM, France) for kindly providing anticore and anti-F monoclonal antibodies and helpful discussions. We also thank Nelly Kieffer (CNRS-UMR7151, Sino-French Research Center for Life Sciences and Genomics, Shanghai, China) for helpful discussions and revising the English of the manuscript.
Footnotes
Published ahead of print on 1 October 2008.
REFERENCES
- 1.Bain, C., P. Parroche, J. P. Lavergne, B. Duverger, C. Vieux, V. Dubois, F. Komurian-Pradel, C. Trepo, L. Gebuhrer, G. Paranhos-Baccala, F. Penin, and G. Inchauspe. 2004. Memory T-cell-mediated immune responses specific to an alternative core protein in hepatitis C virus infection. J. Virol. 7810460-10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedossa, P., T. Poynard, and The METAVIR Cooperative Study Group. 1996. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 24289-293. [DOI] [PubMed] [Google Scholar]
- 3.Boulant, S., M. Becchi, F. Penin, and J. P. Lavergne. 2003. Unusual multiple recoding events leading to alternative forms of hepatitis C virus core protein from genotype 1b. J. Biol. Chem. 27845785-45792. [DOI] [PubMed] [Google Scholar]
- 4.Bouvier-Alias, M., K. Patel, H. Dahari, S. Beaucourt, P. Larderie, L. Blatt, C. Hezode, G. Picchio, D. Dhumeaux, A. U. Neumann, J. G. McHutchison, and J. M. Pawlotsky. 2002. Clinical utility of total HCV core antigen quantification: a new indirect marker of HCV replication. Hepatology 36211-218. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 6.Branch, A. D., D. D. Stump, J. A. Gutierrez, F. Eng, and J. L. Walewski. 2005. The hepatitis C virus alternate reading frame (ARF) and its family of novel products: the alternate reading frame protein/F-protein, the double-frameshift protein, and others. Semin. Liver Dis. 25105-117. [DOI] [PubMed] [Google Scholar]
- 7.Buti, M., C. Mendez, M. Schaper, S. Sauleda, A. Valdes, F. Rodriguez-Frias, R. Jardi, and R. Esteban. 2004. Hepatitis C virus core antigen as a predictor of non-response in genotype 1 chronic hepatitis C patients treated with peginterferon alpha-2b plus ribavirin. J. Hepatol. 40527-532. [DOI] [PubMed] [Google Scholar]
- 8.Choo, Q. L., K. H. Richman, J. H. Han, K. Berger, C. Lee, C. Dong, C. Gallegos, D. Coit, R. Medina-Selby, and P. J. Barr. 1991. Genetic organization and diversity of the hepatitis C virus. Proc. Natl. Acad. Sci. USA 882451-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, M., L. Bachmatov, Z. Ben-Ari, Y. Rotman, R. Tur-Kaspa, and R. Zemel. 2007. Development of specific antibodies to an ARF protein in treated patients with chronic HCV infection. Dig. Dis. Sci. 522427-2432. [DOI] [PubMed] [Google Scholar]
- 10.Fried, M. W., M. L. Shiffman, K. R. Reddy, C. Smith, G. Marinos, F. L. Goncales, Jr., D. Haussinger, M. Diago, G. Carosi, D. Dhumeaux, A. Craxi, A. Lin, J. Hoffman, and J. Yu. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347975-982. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez, V., E. Padilla, M. Diago, M. D. Gimenez, R. Sola, L. Matas, S. Montoliu, R. M. Morillas, C. Perez, and R. Planas. 2005. Clinical usefulness of total hepatitis C virus core antigen quantification to monitor the response to treatment with peginterferon alpha-2a plus ribavirin*. J. Viral Hepat. 12481-487. [DOI] [PubMed] [Google Scholar]
- 12.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140346-355. [DOI] [PubMed] [Google Scholar]
- 13.Hnatyszyn, H. J. 2005. Chronic hepatitis C and genotyping: the clinical significance of determining HCV genotypes. Antivir. Ther. 101-11. [PubMed] [Google Scholar]
- 14.Komatsu, F., and K. Takasaki. 1999. Determination of serum hepatitis C virus (HCV) core protein using a novel approach for quantitative evaluation of HCV viraemia in anti-HCV-positive patients. Liver 19375-380. [DOI] [PubMed] [Google Scholar]
- 15.Komurian-Pradel, F., A. Rajoharison, J. L. Berland, V. Khouri, M. Perret, M. Van Roosmalen, S. Pol, F. Negro, and G. Paranhos-Baccala. 2004. Antigenic relevance of F protein in chronic hepatitis C virus infection. Hepatology 40900-909. [DOI] [PubMed] [Google Scholar]
- 16.Manns, M. P., J. G. McHutchison, S. C. Gordon, V. K. Rustgi, M. Shiffman, R. Reindollar, Z. D. Goodman, K. Koury, M. Ling, and J. K. Albrecht. 2001. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358958-965. [DOI] [PubMed] [Google Scholar]
- 17.Maynard, M., P. Pradat, P. Berthillon, G. Picchio, N. Voirin, M. Martinot, P. Marcellin, and C. Trepo. 2003. Clinical relevance of total HCV core antigen testing for hepatitis C monitoring and for predicting patients' response to therapy. J. Viral Hepat. 10318-323. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, G. C., and P. J. Thuluvath. 2008. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology 471058-1066. [DOI] [PubMed] [Google Scholar]
- 19.Rebucci, C., A. Cerino, A. Cividini, L. Timo, M. Furione, and M. U. Mondelli. 2003. Monitoring response to antiviral therapy for patients with chronic hepatitis C virus infection by a core-antigen assay. J. Clin. Microbiol. 413881-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed, K. E., and C. M. Rice. 2000. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr. Top. Microbiol. Immunol. 24255-84. [DOI] [PubMed] [Google Scholar]
- 21.Seeff, L. B. 2002. Natural history of chronic hepatitis C. Hepatology 36S35-S46. [DOI] [PubMed] [Google Scholar]
- 22.Varaklioti, A., N. Vassilaki, U. Georgopoulou, and P. Mavromara. 2002. Alternate translation occurs within the core coding region of the hepatitis C viral genome. J. Biol. Chem. 27717713-17721. [DOI] [PubMed] [Google Scholar]
- 23.Veillon, P., C. Payan, G. Picchio, M. Maniez-Montreuil, P. Guntz, and F. Lunel. 2003. Comparative evaluation of the total hepatitis C virus core antigen, branched-DNA, and Amplicor Monitor assays in determining viremia for patients with chronic hepatitis C during interferon plus ribavirin combination therapy. J. Clin. Microbiol. 413212-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walewski, J. L., T. R. Keller, D. D. Stump, and A. D. Branch. 2001. Evidence for a new hepatitis C virus antigen encoded in an overlapping reading frame. RNA 7710-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu, Z., J. Choi, T. S. Yen, W. Lu, A. Strohecker, S. Govindarajan, D. Chien, M. J. Selby, and J. Ou. 2001. Synthesis of a novel hepatitis C virus protein by ribosomal frameshift. EMBO J. 203840-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]