Abstract
We performed 24- and 48-h MIC determinations and disk diffusion testing of fluconazole against more than 11,000 clinical isolates of Candida species. By using the reference MIC breakpoints, the categorical agreement between the 24-h and reference 48-h broth microdilution results ranged from 93.8% (all Candida species) to 94.9% (all Candida species minus Candida krusei), with only 0.1% very major errors (VME). The essential agreement (within 2 log2 dilutions) between the 24-h and 48-h results was 99.6%. The categorical agreement between the 24-h disk diffusion results and the 24-h MIC results, using the previously established breakpoints, was 94.4%, with 0.1% VME. Both the MIC and the disk diffusion results obtained for fluconazole after only 24 h of incubation may be used to determine the susceptibilities of Candida spp. to this widely used antifungal agent.
Recent studies examining the clinical utility of “real-time” antifungal susceptibility testing in the treatment of candidemia have shown that when such testing is available on site, physicians find the results helpful and not infrequently alter therapy on the basis of results (2, 15, 17, 18). Collins et al. (7) found that susceptibility testing of Candida glabrata isolates results in lower overall treatment costs, based on de-escalation of therapy from an expensive echinocandin to fluconazole, for patients with documented fungemia. Thus, it would appear that routine antifungal susceptibility testing can serve as an adjunct in the treatment of candidemia in the same way that antibacterial testing aids in the treatment of bacterial infections (13, 19, 40).
In light of the need to provide clinicians with useful information sooner rather than later (14, 22) and to avoid the potentially confounding effects of trailing growth on 48-h fluconazole MICs (1, 37, 39), the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) Subcommittee for Antifungal Testing sought to determine if reading the broth microdilution (BMD) fluconazole MIC at 24 h would produce valid results when they were interpreted using the existing (48-h) breakpoints (25). Reanalysis of the MIC data set that was used to create the original CLSI M27 48-h fluconazole susceptibility breakpoints for Candida spp. (32, 38) showed good correlation between 24-h and 48-h MICs. Furthermore, when the 48-h fluconazole breakpoints were applied to MICs read at 24 h, the earlier reading predicted therapeutic outcomes as accurately as the 48-h MICs: 82% success for those episodes in which the 24-h MIC was ≤8 μg/ml (susceptible [S] isolates), 55% success for those episodes in which the MIC was 16 to 32 μg/ml (susceptible dose-dependent [SDD] isolates), and 39% success for those episodes in which the MIC was ≥64 μg/ml (resistant [R] isolates) (25). Based on these results, the CLSI Subcommittee has included the option to read fluconazole MICs for Candida species after a 24-h incubation, using the original interpretive breakpoints, in CLSI documents M27-A3 and M27-S3 (5, 6).
Although earlier studies of 24-h fluconazole readings in different data sets and with a variety of methods versus the 48-h reference BMD method have shown similar results and relevance (8, 11, 12, 28, 31, 35, 41), further evaluation of this concept in other data sets is warranted (25). The purpose of the present study was to provide further documentation of the correlation between 24-h and 48-h fluconazole BMD MICs by assessing the essential agreement (EA; calculated as the percent agreement within ±2 log2 dilutions of the reference MIC) as well as the absolute categorical agreement (CA) and error rates obtained with a large data set of 24- and 48-h MIC results compiled in the course of global surveillance studies (27, 29, 30, 33, 34, 36). We also provide a reassessment of the fluconazole disk diffusion zone diameters as they relate to the 24-h fluconazole MIC results.
MATERIALS AND METHODS
Study design.
A total of 11,654 clinical isolates of Candida spp. isolated from blood and other normally sterile body fluids via a global network of 105 sentinel hospital sites between January 2001 and December 2006 were included in the study. All isolates were saved on agar slants and were sent to the University of Iowa College of Medicine (Iowa City) for storage and further characterization by reference identification methods and susceptibility testing against fluconazole by BMD and disk diffusion methods (4-6, 16, 23).
Organism identification.
All Candida species isolates were identified at the participating institutions by the routine method used in each laboratory. Upon receipt at the University of Iowa, the isolates were subcultured onto potato dextrose agar (Remel, Lenexa, KS) and CHROMagar Candida medium (Hardy Laboratories, Santa Maria, CA) to ensure viability and purity. Confirmation of species identification was performed with Vitek and API products (bioMerieux, St. Louis, MO) as recommended by the manufacturer or by conventional methods as required (16). Isolates were stored as water suspensions until they were used.
Susceptibility testing.
Reference antifungal susceptibility testing of all 11,654 isolates was performed by BMD exactly as described in CLSI document M27-A3 (5). Fluconazole reference powder was obtained from Pfizer Pharmaceuticals (Groton, CT). Frozen BMD panels containing serial twofold dilutions of fluconazole (range, 0.12 to 128 μg/ml) in RPMI 1640 medium buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid buffer were thawed and inoculated with an organism suspension adjusted to attain a final inoculum concentration of 1.5 × 103 ± 1.0 × 103 cells/ml. The panels were incubated in air at 35°C and observed for the presence or absence of growth at 24 and 48 h. The fluconazole MIC was read as the lowest concentration that produced a prominent decrease in turbidity (a ca. 50% reduction in growth) relative to that of the drug-free control (5).
Disk diffusion testing of fluconazole was performed on 11,237 of the isolates as described in NCCLS document M44-A (23). Fluconazole disks (25 μg) were obtained from Becton Dickinson (Sparks, MD). For disk diffusion testing, 150-mm-diameter plates containing Mueller-Hinton agar (Difco Laboratories) supplemented with 2% glucose and methylene blue (0.5 μg/ml) at a depth of 4.0 mm were used. The agar surface was inoculated by using a swab dipped in a cell suspension adjusted to the turbidity of a 0.5 McFarland standard. The plates were incubated in air at 35°C and read at 24 h. Zone diameter end points were read at 80% growth inhibition by using the BIOMIC image analysis plate reader system (version 5.9; Giles Scientific, Santa Barbara, CA). MIC interpretive criteria for fluconazole were those published by Pfaller et al. (32) and the CLSI (5, 6) and were as follows: S, MIC of ≤8 μg/ml; SDD, MIC of 16 to 32 μg/ml; R, MIC of ≥64 μg/ml. The interpretive criteria for the fluconazole disk test were those published by Pfaller et al. (32) and the NCCLS/CLSI (4, 23): S, zone diameter of ≥19 mm; SDD, zone diameter of 15 to 18 mm; R, zone diameter of ≤14 mm.
QC.
Quality control (QC) was performed for BMD in accordance with CLSI documents M27-A3 and M27-S3 (6) by using Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019. QC determinations made on each day of testing were within the 24- and 48-h control limits described by the CLSI (6). QC for disk diffusion testing was performed by using Candida albicans ATCC 90028 and C. parapsilosis ATCC 22019 (4, 23).
Analysis of results.
The MIC results obtained for fluconazole after 24 h of incubation were compared with those obtained after 48 h of incubation by using regression statistics and a scattergram (Fig. 1). Both on-scale and off-scale results were included in the analysis. As with previous studies (31, 35), high off-scale MIC results were converted to the next highest concentration, and low off-scale MIC results were left unchanged. Discrepancies among MIC end points (24-h versus 48-h results) of more than 2 dilutions (two wells) were used to calculate the EA. The CLSI interpretive breakpoints for fluconazole were used to obtain CA percentages between the MICs determined after 24 h of incubation and the reference 48-h BMD results. Very major errors (VME) were identified when the reference MIC indicated R and the 24-h MIC indicated S. Major errors (ME) were identified when the isolate was classified as R at 24 h of incubation and as S at 48 h. Minor errors were identified when the result of one of the readings (at 24 or at 48 h) was either S or R and that of the other was SDD.
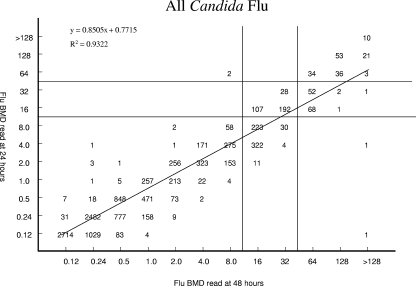
FIG. 1.
Comparison of fluconazole (Flu) BMD MICs at 24 and 48 h for 11,654 Candida species isolates. The horizontal and vertical lines indicate the interpretive MIC breakpoints.
In a similar fashion, the diameters of the zones of inhibition (in millimeters) surrounding the fluconazole disks at 24 h of incubation were plotted against their respective BMD MICs read at 24 h (Fig. 2). The method of least squares was used to calculate a regression line for each comparison. The interpretive breakpoints defined by the CLSI (4, 6) were used to determine the CA between the disk diffusion and 24-h BMD results for fluconazole. Error rates were calculated as described above using the BMD MIC as the reference test.
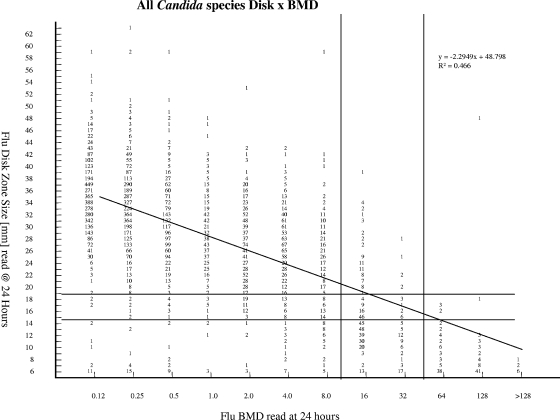
FIG. 2.
Comparison of fluconazole (Flu) disk diffusion zone diameters at 24 h and BMD MICs at 24 h for 11,237 Candida species isolates. The horizontal and vertical lines indicate the interpretive zone diameter and MIC breakpoints, respectively.
RESULTS AND DISCUSSION
Table 1 summarizes the in vitro susceptibilities of 11,654 isolates of Candida spp. (14 species) to fluconazole as determined by the CLSI BMD method and read at 24 and 48 h. The MIC results were typical of each species of Candida (28, 32), with the lowest MICs at both 24 and 48 h observed for C. albicans and the highest MICs observed for C. glabrata and C. krusei. In general, the MICs read at 24 h of incubation were twofold lower than those read at 48 h.
TABLE 1.
Susceptibilities of 11,654 isolates of Candida spp. to fluconazole as determined by CLSI BMD methods and read after 24 and 48 h of incubation
| Species | No. of isolates tested | Incubation time (h) | MIC (μg/ml)a
|
EA (%)b | ||
|---|---|---|---|---|---|---|
| Range | 50% | 90% | ||||
| C. albicans | 6,320 | 24 | 0.12->128 | 0.12 | 0.25 | 99.8 |
| 48 | 0.12->128 | 0.25 | 0.5 | |||
| C. parapsilosis | 1,664 | 24 | 0.12-64 | 0.5 | 2 | 99.8 |
| 48 | 0.12->128 | 0.5 | 2 | |||
| C. glabrata | 1,628 | 24 | 0.25->128 | 4 | 16 | 98.7 |
| 48 | 0.25->128 | 8 | 32 | |||
| C. tropicalis | 1,286 | 24 | 0.12-32 | 0.25 | 1 | 99.5 |
| 48 | 0.12-64 | 0.5 | 2 | |||
| C. krusei | 316 | 24 | 0.25-64 | 16 | 32 | 99.7 |
| 48 | 0.25->128 | 32 | 64 | |||
| C. guilliermondii | 142 | 24 | 0.5-32 | 2 | 4 | 100.0 |
| 48 | 0.5-32 | 4 | 8 | |||
| C. lusitaniae | 139 | 24 | 0.12-64 | 0.5 | 1 | 99.3 |
| 48 | 0.12-64 | 0.5 | 1 | |||
| C. kefyr | 58 | 24 | 0.12-1 | 0.25 | 0.5 | 100.0 |
| 48 | 0.12-2 | 0.25 | 1 | |||
| C. pelliculosa | 34 | 24 | 0.5-8 | 2 | 4 | 100.0 |
| 48 | 1-8 | 4 | 8 | |||
| Miscellaneous Candida spp.c | 67 | 24 | 0.12-16 | 2 | 8 | 98.5 |
| 48 | 0.12-64 | 2 | 8 | |||
| All Candida spp. | 11,654 | 24 | 0.12->128 | 0.25 | 4 | 99.6 |
| 48 | 0.12->128 | 0.25 | 16 | |||
| All Candida spp. minus C. krusei | 11,338 | 24 | 0.12->128 | 0.25 | 4 | 99.6 |
| 48 | 0.12->128 | 0.25 | 8 | |||
50% and 90%, MICs encompassing 50% and 90% of isolates tested, respectively.
Between 24- and 48-h BMD MICs.
Including C. famata (20 isolates), C. rugosa (14 isolates), C. dubliniensis (13 isolates), C. lipolytica (12 isolates), and C. zeylanoides (8 isolates).
The overall EA between the 24-h and 48-h MIC readings was 99.6% (91.7% were within ±1 dilution). Figure 1 illustrates the high degree of correlation between the two MIC readings (R2 = 0.9322). Of the 44 discrepancies noted between the two readings, the MICs read after 24 h of incubation were lower than those obtained at 48 h in 38 instances (86.4%). Among the various species, the greatest numbers of discrepancies were observed with C. albicans (11 discrepancies), C. glabrata (21 discrepancies), and Candida tropicalis (6 discrepancies), species noted by others to exhibit the trailing phenomenon following incubation for 48 h (1, 24).
Regarding the individual species of Candida, the EA between the 24-h and 48-h BMD MICs was >98% for each of the 14 species included in the survey (Table 1). Given the CLSI recommendation that C. krusei should be considered to be intrinsically resistant to fluconazole and thus should not be tested against this agent (5), we also determined the EA for all isolates minus C. krusei; 99.6% of these results were within ±2 dilution of one another.
The CA between the 24-h and 48-h fluconazole MICs is shown in Table 2. Excellent CA was observed for all comparisons with the exception of C. glabrata and C. krusei. The overall CA between the 24- and 48-h results was 93.8% when all isolates were included and 94.9% when the C. krusei results were omitted. Importantly, there were only two VME (false-susceptible results) and two ME (false-resistant results) in the entire 11,654-isolate comparison.
TABLE 2.
Categorical agreement between 24-h and 48-h CLSI BMD fluconazole MICs for 11,654 isolates of Candida spp.
| Species (no. of isolates tested) | Incubation time (h) | % of isolatesa that tested:
|
CA (%) | % of errors
|
||||
|---|---|---|---|---|---|---|---|---|
| S | SDD | R | VME | ME | Minor errors | |||
| C. albicans (6,320) | 24 | 99.5 | 0.4 | 0.1 | 99.9 | 0.0 | 0.0 | 0.1 |
| 48 | 99.4 | 0.5 | 0.1 | |||||
| C. parapsilosis (1,664) | 24 | 97.5 | 2.3 | 0.2 | 98.2 | 0.0 | 0.0 | 1.8 |
| 48 | 96.1 | 3.3 | 0.6 | |||||
| C. glabrata (1,628) | 24 | 84.8 | 6.9 | 8.3 | 67.5 | 0.1 | 0.1 | 32.3 |
| 48 | 53.7 | 36.7 | 9.6 | |||||
| C. tropicalis (1,286) | 24 | 99.6 | 0.4 | 0.0 | 99.5 | 0.0 | 0.0 | 0.5 |
| 48 | 99.1 | 0.8 | 0.1 | |||||
| C. krusei (316) | 24 | 14.9 | 81.3 | 3.8 | 56.6 | 0.0 | 0.3 | 43.1 |
| 48 | 1.6 | 65.5 | 32.9 | |||||
| C. guilliermondii (142) | 24 | 97.2 | 2.8 | 0.0 | 95.8 | 0.0 | 0.0 | 4.2 |
| 48 | 93.0 | 7.0 | 0.0 | |||||
| C. lusitaniae (139) | 24 | 97.1 | 2.2 | 0.7 | 99.3 | 0.0 | 0.0 | 0.7 |
| 48 | 97.1 | 1.4 | 1.5 | |||||
| C. kefyr (58) | 24 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| 48 | 100.0 | 0.0 | 0.0 | |||||
| C. pelliculosa (34) | 24 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 |
| 48 | 100.0 | 0.0 | 0.0 | |||||
| Miscellaneous Candida spp.b (67) | 24 | 91.0 | 9.0 | 0.0 | 97.0 | 0.0 | 0.0 | 3.0 |
| 48 | 89.6 | 9.0 | 1.4 | |||||
| All Candida spp. (11,654) | 24 | 94.8 | 3.9 | 1.3 | 93.8 | 0.02 | 0.02 | 6.16 |
| 48 | 89.7 | 7.9 | 2.4 | |||||
| All Candida spp. minus C. krusei (11,338) | 24 | 97.0 | 1.7 | 1.3 | 94.9 | 0.02 | 0.01 | 5.07 |
| 48 | 92.2 | 6.2 | 1.6 | |||||
Isolates were classified as S at a MIC of ≤8 μg/ml, as SDD at a MIC of 16 to 32 μg/ml, and as R at a MIC of ≥64 μg/ml.
Including C. famata (20 isolates), C. rugosa (14 isolates), C. dubliniensis (13 isolates), C. lipolytica (12 isolates), and C. zeylanoides (8 isolates).
Although the absolute CA for C. glabrata was only 67.5%, virtually all of the errors (99.4%) were minor; they were predominantly the result of isolates determined to be S at 24 h and SDD at 48 h (96.2% of all minor errors). This is not surprising given the tendency of fluconazole MICs for C. glabrata to fall close to the susceptible breakpoint: 59% of MICs determined at 24 h and 78% of MICs determined at 48 h fell between 4 and 16 μg/ml (data not shown). It should be noted, however, that 86% of the 156 C. glabrata isolates that were classified as R at the 48-h MIC determination were also R at the 24-h reading, and only 2 isolates were S at the 24-h reading and R at the 48-h reading (1.2% of all R C. glabrata isolates and 0.1% of all C. glabrata isolates tested). This accuracy in detecting fluconazole resistance among C. glabrata isolates is comparable or superior to that observed with the FDA-approved commercial products Sensititre YeastOne (TREK) and Vitek 2 yeast antifungal test (bioMerieux) (31, 35). In view of the shift of results from SDD (at 48 h) to S (at 24 h) for this species, the CLSI Subcommittee has cautioned physicians and laboratorians to be aware that when an isolate is identified as C. glabrata and the 24-h or 48-h fluconazole MIC is ≤32 μg/ml, patients should receive a maximum dosage of fluconazole (e.g., 12 mg/kg of body weight/day) (5, 6, 25, 26).
Disk diffusion testing of fluconazole has now been established as a simple and inexpensive qualitative method for determining the susceptibility of Candida to this agent, with results available within 24 h (30, 32, 34). Previously, the zone diameter breakpoints for fluconazole disk diffusion testing were derived by comparing the zone diameters read at 24 h with the BMD results at 48 h by using the error rate bounded method (21), whereby the number of discrepancies between the zone diameter and MIC categories was minimized (32). This process resulted in zone diameter breakpoints of ≥19 mm (S), 15 to 18 mm (SDD), and ≤14 mm (R), with an overall CA between the disk diffusion test results and the 48-h MIC test results of 92.8% (2,949 isolates) and very few VME (0.1%) or ME (0.4%) (32). Figure 2 shows the correlation between the fluconazole disk zone diameters read at 24 h and the BMD MIC results read at 24 h for 11,237 Candida isolates. By using the MIC and zone diameter breakpoints developed previously (32), the overall CA was 94.4%, with 0.1% VME and 1.1.% ME. Thus, the disk diffusion test for fluconazole performs comparably to the 24-h MIC test without necessitating a change in interpretive criteria.
The findings of the present study confirm and extend the results of previous studies regarding the feasibility, accuracy, and clinical utility of 24-h fluconazole MIC readings (8, 11, 12, 28, 31, 35, 41). Indeed, if the 24-h MIC reading were considered to be a “new test,” its performance relative to the 48-h reference BMD test would be considered superior to those reported for the fluconazole disk diffusion test, the Etest, the YeastOne colorimetric method, and the Vitek 2 yeast antifungal test (9, 10, 20, 35). An earlier multicenter study by Espinel-Ingroff et al. (11) not only documented excellent EA and CA for the comparison of 24- versus 48-h fluconazole MICs but also found a high degree (98%) of interlaboratory reproducibility among the six participating laboratories.
Clearly, the determination of fluconazole MICs after only 24 h of incubation would provide potentially important results in a more clinically useful time frame. Furthermore, previous investigations have shown that the 24-h fluconazole MIC end point correlated better than the 48-h end point with sterol quantification (1) and with treatment outcome both clinically (37) and in a murine model of invasive candidiasis (39). These findings suggest that fluconazole results for isolates of Candida spp. with significant trailing (e.g., C. albicans, C. glabrata, and C. tropicalis in the present study) should be interpreted on the basis of the lower MIC observed at the earlier (24-h) time point.
In addition to the data provided by Ostrosky-Zeichner et al. (25), the clinical validity of 24-h fluconazole MICs was also addressed in a recent study by Baddley et al. (3), in which the authors demonstrated the association between patient characteristics, MICs for Candida, fluconazole pharmacodynamics, and mortality among hospitalized patients with candidemia. These investigators confirmed our findings that fluconazole MICs read after 24 and 48 h of incubation were very similar (Spearman's rank correlation coefficient, 0.91). Furthermore, classification and regression tree (CART) analysis was used to identify breakpoints for survival of 11.5 for a fluconazole AUC (area under the concentration-time curve)-to-MIC ratio and of 64 μg/ml for MICs read after either 24 or 48 h of incubation. For 24-h MICs, 74% (57/77) of patients survived when the AUC/MIC ratio or MICs were above or below these thresholds, respectively (i.e., >11.5 or <64 μg/ml). Conversely, only 42.9% (3/7) of patients survived when either the AUC/MIC ratio was less than 11.5 or the MIC exceeded 64 μg/ml. Similar results were evident for 48-h MICs. Thus, regardless of the timing of MIC end point determination, infection with a fluconazole-resistant isolate was associated with increased mortality. Furthermore, these studies suggest that a clinician-controlled variable, fluconazole dose, may impact individual patient survival (3). In addition to host factors, the fluconazole dose and MICs may be helpful in managing and optimizing outcomes for patients with candidemia (3).
The simplicity and flexibility of disk diffusion testing makes it a very appealing method for use in the clinical laboratory. Although previous studies have already established the correlation between the 24-h fluconazole disk zone diameter and 48-h MICs (32), the data presented here demonstrate even better agreement between the zone diameters and 24-h fluconazole MICs and establish the continuing validity of the published zone interpretive criteria.
In summary, the MICs of fluconazole can be determined after 24 h of incubation for all species of Candida by using the CLSI BMD method. The high degree of accuracy of the 24-h reading compared to the 48-h reference method compared favorably to those reported previously for the FDA-approved methods Sensititre YeastOne and Vitek 2 yeast antifungal test. Both the 24-h MIC test and the 24-h disk diffusion test reliably identify fluconazole resistance among Candida spp. by using the previously established interpretive breakpoints. The availability of fluconazole susceptibility results within a 24-h time frame will be an important step in optimizing antifungal therapy for candidiasis.
Acknowledgments
Linda Elliott and Tara Schroder provided excellent support in the preparation of the manuscript.
This work was supported in part by Pfizer, Inc., Pfizer Global Pharmaceuticals, New York, NY.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock for the Candidemia Active Surveillance Group. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 462477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddley, J. W., M. Patel, M. Jones, G. Cloud, A. C. Smith, and S. A. Moser. 2004. Utility of real-time antifungal susceptibility testing for fluconazole in the treatment of candidemia. Diagn. Microbiol. Infect. Dis. 50119-124. [DOI] [PubMed] [Google Scholar]
- 3.Baddley, J. W., M. Patel, S. M. Bhavnani, S. A. Moser, and D. R. Andes. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 523022-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2007. Zone diameter interpretive standards, corresponding minimal inhibitory concentration (MIC) interpretive breakpoints, and quality control limits for antifungal disk diffusion susceptibility testing of yeasts: informational supplement, M44-S2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard, 3rd ed., M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts: informational supplement, M27-S3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Collins, C. D., G. A. Eschenauer, S. L. Salo, and D. W. Newton. 2007. To test or not to test: a cost minimization analysis of susceptibility testing for patients with documented Candida glabrata fungemias. J. Clin. Microbiol. 451884-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuenca-Estrella, M., W. Lee-Yang, M. A. Ciblak, B. A. Arthington-Skaggs, E. Mellado, D. W. Warnock, and J. L. Rodriguez-Tudela. 2002. Comparative evaluation of NCCLS M27-A and EUCAST broth microdilution procedures for antifungal susceptibility testing of Candida species. Antimicrob. Agents Chemother. 463644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff, A., M. A. Pfaller, S. A. Messer, C. C. Knapp, S. Killian, H. A. Norris, and M. A. Ghannoum. 1999. Multicenter comparison of the Sensititre YeastOne colorimetric antifungal panel with the National Committee for Clinical and Laboratory Standards M27-A reference method for testing clinical isolates of common and emerging Candida spp., Cryptococcus spp., and other yeast-like organisms. J. Clin. Microbiol. 37591-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff, A., M. Pfaller, S. A. Messer, C. C Knapp, N. Holliday, and S. Killian. 2004. Multicenter comparison of Sensititre YeastOne colorimetric antifungal panel with the NCCLS M27-A2 reference method for testing new antifungal agents against clinical isolates of Candida spp. J. Clin. Microbiol. 42718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinel-Ingroff, A., F. Barchiesi, M. Cuenca-Estrella, A. Fothergill, M. A. Pfaller, M. Rinaldi, J. L. Rodriguez-Tudela, and P. E. Verweij. 2005. Comparison of visual 24-hour and spectrophotometric 48-hour MICs to CLSI reference microdilution MICs of fluconazole, itraconazole, posaconazole, and voriconazole for Candida spp.: a collaborative study. J. Clin. Microbiol. 434535-4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A., F. Barchiesi, M. Cuenca-Estrella, M. A. Pfaller, M. Rinaldi, J. L. Rodriguez-Tudela, and P. E. Verweij. 2005. International and multicenter comparison of EUCAST and CLSI M27-A2 broth microdilution methods for testing susceptibilities of Candida spp. to fluconazole, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 433884-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrest, G. 2006. Role of antifungal susceptibility testing in patient management. Curr. Opin. Infect. Dis. 19538-543. [DOI] [PubMed] [Google Scholar]
- 14.Garey, K. W., M. Rege, M. P. Pai, D. E. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 4325-31. [DOI] [PubMed] [Google Scholar]
- 15.Hadley, S., J. A. Martinez, L. McDermott, B. Rapino, and D. R. Snydman. 2002. Real-time antifungal susceptibility screening aids management of invasive yeast infections in immunocompromised patients. J. Antimicrob. Chemother. 49415-419. [DOI] [PubMed] [Google Scholar]
- 16.Hazen, K. C., and S. A. Howell. 2007. Candida, Cryptococcus, and other yeasts of medical importance, p. 1762-1788. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 17.Hospenthal, D. R., C. K. Murray, and M. G. Rinaldi. 2004. The role of antifungal susceptibility in the therapy of candidiasis. Diagn. Microbiol. Infect. Dis. 48153-160. [DOI] [PubMed] [Google Scholar]
- 18.Magill, S. S., C. Shields, C. L. Sears, M. Choti, and W. G. Merz. 2006. Triazole cross-resistance among Candida spp.: case report, occurrence among bloodstream isolates, and implications for antifungal therapy. J. Clin. Microbiol. 44529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masterton, R., G. Drusano, D. L. Paterson, and G. Park. 2003. Appropriate antimicrobial treatment in nosocomial infections—the clinical challenges. J. Hosp. Infect. 55(Suppl. 1)1-12. [DOI] [PubMed] [Google Scholar]
- 20.Matar, M. J., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chan, and J. H. Rex. 2003. Correlation between E-test, disk diffusion, and microdilution methods for antifungal susceptibility testing of fluconazole and voriconazole. Antimicrob. Agents Chemother. 471647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzler, C. M., and R. M. DeHaan. 1974. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J. Infect. Dis. 130588-594. [DOI] [PubMed] [Google Scholar]
- 22.Morrell, M., V. J. Fraser, and M. J. Kollef. 2005. Delaying empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for mortality. Antimicrob. Agents Chemother. 493640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCCLS. 2004. Methods for antifungal disk diffusion susceptibility testing of yeasts: approved guideline, M44-A. NCCLS, Wayne, PA.
- 24.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 473149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrosky-Zeichner, L., J. H. Rex, M. A. Pfaller, D. J. Diekema, B. D. Alexander, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, D. J. Sheehan, and T. J. Walsh. 2008. Rationale for reading fluconazole MICs at 24 hours rather than 48 hours when testing Candida spp. by the CLSI M27-A2 standard method. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 26.Pappas, P. G., J. H. Rex, J. D. Sobel, et al. 2004. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 38161-189. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and D. J. Diekema. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 461723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 411440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaller, M. A., and D. J. Diekema. 2004. Twelve years of fluconazole in clinical practice: global trends in species distribution and fluconazole susceptibility of bloodstream isolates of Candida. Clin. Microbiol. Infect. 10(Suppl. 1)11-23. [DOI] [PubMed] [Google Scholar]
- 30.Pfaller, M. A., K. C. Hazen, S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2004. Comparison of results of fluconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS Global Antifungal Surveillance Program. J. Clin. Microbiol. 423607-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., A. Espinel-Ingroff, and R. N. Jones. 2004. Clinical evaluation of the Sensititre YeastOne colorimetric antifungal plate for antifungal susceptibility testing of the new triazoles voriconazole, posaconazole, and ravuconazole. J. Clin. Microbiol. 424577-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller, M. A., D. J. Diekema, and D. J. Sheehan. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19435-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, et al. 2007. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 451735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., D. J. Diekema, G. W. Procop, and M. G. Rinaldi. 2007. Multicenter comparison of the VITEK 2 yeast susceptibility test with the CLSI broth microdilution reference method for testing fluconazole against Candida spp. J. Clin. Microbiol. 45796-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaller, M. A., S. A. Messer, L. Boyken, S. Tendolkar, R. J. Hollis, and D. J. Diekema. 2008. Selection of a surrogate agent (fluconazole or voriconazole) for initial susceptibility testing of posaconazole against Candida spp.: results from a Global Antifungal Surveillance Program. J. Clin. Microbiol. 46551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24235-247. [DOI] [PubMed] [Google Scholar]
- 39.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, A. Espinel-Ingroff, and E. J. Anaissie. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing critical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rex, J. H., and M. A. Pfaller. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35982-989. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Tudela, J. L., J. P. Donnelly, M. A. Pfaller, E. Chryssantou, P. Warn, D. W. Denning, A. Espinel-Ingroff, F. Barchiesi, and M. Cuenca-Estrella. 2007. Statistical analyses of correlation between fluconazole MICs for Candida spp. assessed by standard methods set forth by the European Committee on Antimicrobial Susceptibility Testing (E.Dis. 7.1) and CLSI (M27-A2). J. Clin. Microbiol. 45109-111. [DOI] [PMC free article] [PubMed] [Google Scholar]