Abstract
Fonsecaea pedrosoi is the main agent of chromoblastomycosis, a skin disease presenting verrucous lesions, in which round, thick-walled sclerotic cells are found. In vitro induction of sclerotic cells is time-consuming (20 to 45 days) and temperature dependent. We present two new natural media that reduce the sclerotic-cell induction time to only 2 days.
Chromoblastomycosis is a subcutaneous mycosis with verrucous-nodular lesions, usually localized on the lower limbs of rural workers (12), appearing after accidental inoculation with thorns bearing dematiaceous fungi, such as Fonsecaea pedrosoi or Cladophialophora carrionii (11, 15). Laboratory diagnosis is performed by direct microscopic examination of skin scrapings after treatment with 10% KOH. Round, brownish fungal cells (called sclerotic or muriform cells) characterized by multiseptate division are observed in small aggregates or isolated in the lesion (9).
The vast majority of in vitro work done with F. pedrosoi, including tests with drugs, has used only hyphae (2) or the conidial stage of the life cycle of the fungus. In contrast to sclerotic cells, hyphae and conidia are never found in the lesions. Initial attempts to produce sclerotic cells in vitro were made in 1957 using a “synthetic lymph medium” made with alcoholic extracts of hair and nails in which spherical bodies, articulated in hyphal fragments similar to chlamydospores, were obtained (13). In 1985, F. pedrosoi strains cultured under constant shaking in Sabouraud medium (pH 2.5) after 30 days produced round, yeast-like cells with no septation (7). In 1993, a chemically defined medium (pH 2.5, 25°C) with 0.1 mmol liter−1 Ca2+ or the calcium chelant EGTA at 2 mmol liter−1 induced sclerotic cells after 21 days of culture under constant shaking (8).
The two media developed in this study were made from tree fruits. Theobroma grandiflorum (Willd. ex Spreng.) K. Schum. (cupuassu) is a native Amazonian tree that has a slightly fibrous, yellowish mesocarp, containing potassium (34.3 mg/100 g of mesocarp [fresh weight]), phosphorus (15.7 mg/100 g), magnesium (13.0 mg/100 g), and amino acids (10). Bactris gasipaes Kunth (peach palm) is an American palm tree, containing potassium (289.3 mg/100 g of mesocarp), calcium (24.7 mg/100 g), and magnesium (17.6 mg/100 g). It is rich in fatty acids, with oleic (46.3%), palmitic (38.2%), and palmitoleic (7.4%) acids (14).
Three different F. pedrosoi strains, obtained from skin scrapings, were used in this study. All samples presented the classic sclerotic cells (Fig. 1A and B) and were isolated in Mycosel (Becton Dickinson) and perpetuated in Sabouraud agar (Merck, Germany). These media promoted the formation of greenish-black colonies (Fig. 1C), which were analyzed by microculture (Fig. 1D); dematiaceous hyphae with cylindrical, intercalary or terminal, loosely branched conidiophores with small conidia compatible with F. pedrosoi (5) were observed.
FIG. 1.
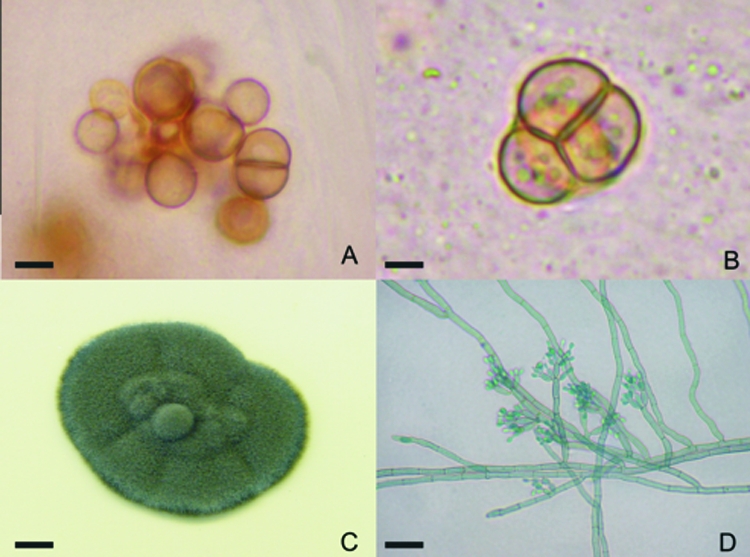
Fonsecaea pedrosoi sclerotic cells were obtained after scraping of chromoblastomycosis lesions. The cells gave rise to hyphae and conidia after in vitro culture. (A and B) Skin scrapings were collected and analyzed after clarification with 20% KOH, revealing well-defined, septated, sclerotic cells. (C) Greenish-black colonies grew from culture of this material on Mycosel. (D) Characteristic dematiaceous hyphae originating terminal cylindrical conidiophores with small subhyaline conidia were observed upon microculture. Bars, 4 μm (A), 3 μm (B), 0.5 cm (C), and 10 μm (D).
T. grandiflorum and B. gasipaes fruits were washed in the laboratory. The mesocarp was separated from the seeds, diluted 1:3 with distilled and deionized water, homogenized using a shaker, and centrifuged at 4,000 rpm for 5 min. The supernatant was collected and filtered through a 0.22-μm-pore-size filter (Advantec); the pH was adjusted to 2.7 with 1 M HCl; and the medium was autoclaved. The media and the processes for their production were registered with the Brazilian National Institute for Intellectual Property (11a).
Five fungal colonies, each with a diameter of around 2 to 3 cm (Fig. 1C), cultured for 15 to 20 days in potato agar (Merck, Germany), were harvested, suspended in 10 ml of distilled and deionized water, homogenized in a vortex mixer for 30 s, and filtered through a nylon membrane to separate hyphae from conidia. The conidia were collected and centrifuged at 4,000 rpm for 5 min, and the pellet was resuspended in deionized water to a final volume of 1 ml for counting using a Neubauer chamber. Sclerotic cells were induced from previously isolated conidia in 24-well cell culture plates (TPP, Switzerland) filled with one of the two new media at a concentration of 103 conidia/ml, with 20 μg/ml of gentamicin. For optical microscopy, scanning electron microscopy (SEM), and transmission electron microscopy (TEM), sclerotic cells were prepared as described previously (4).
More than 90% of conidia cultured for 48 h differentiated into sclerotic cells very similar to those found in lesioned tissue, with multiseptate division, a very thick wall, and a brownish color. After 15 days with no changes in the medium, there was differentiation from sclerotic cells toward hyphae. When the medium had been changed before 15 days, there was no hyphal formation, and it was possible to keep sclerotic cells viable for 15 days more, and so on. These cells became enlarged and septated, forming small aggregates, with no individualization of sclerotic cells. The longest culture time was 1 year, indicating the long-lasting viability of these cells.
The sclerotic cells induced in vitro in the two media displayed the same characteristics of size, color, and type of cellular division and multiseptation (Fig. 2A). An interesting characteristic of F. pedrosoi differentiation was observable for the first time: the conidial cellular wall breaks at one point, and a new sclerotic cell is formed by the expansion of the cytoplasm that was previously contained by the conidial wall (Fig. 2B and C). Multiseptation can be observed well by both SEM and TEM (Fig. 2C and D). Besides the well-defined septation, TEM also made it possible to observe vesicles and electron-dense granules near the thick cellular wall.
FIG. 2.
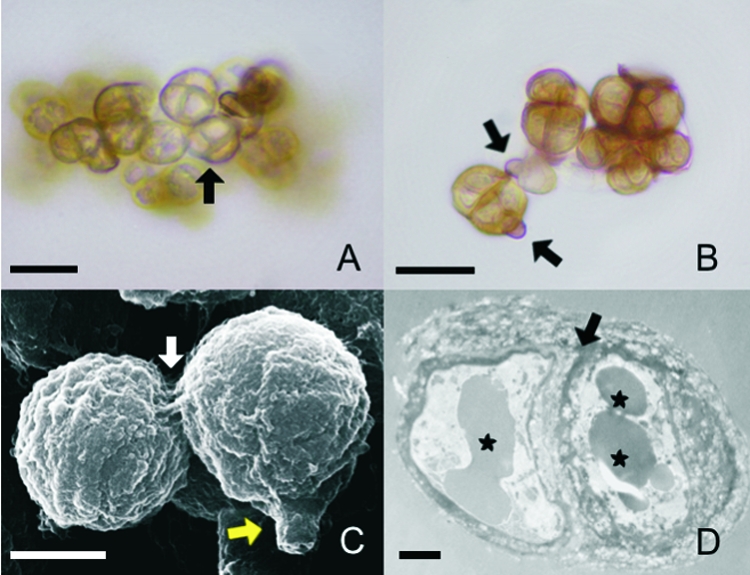
The morphological characteristics of sclerotic cells induced in either of the two natural media are similar. (A and B) Sclerotic cells obtained after culture of conidia in Theobroma grandiflorum or Bactris gasipaes natural media were analyzed by optical microscopy with no special staining. (A) Brownish, multiseptated cells (arrow) were evident. (B) Remnants of conidia adhering to the newly formed sclerotic cells (arrows) were observed. (C) SEM of aggregates of sclerotic cells demonstrates septated division (white arrow) and remnants of conidia (yellow arrow). (D) TEM of a sclerotic cell shows typical thick-walled septation (arrow) and vesicles containing electron-dense material (asterisks). Data are representative of one of three independent experiments, which were performed in triplicate. Bars, 10 μm (A and B), 5 μm (C), and 1 μm (D).
The techniques presently available for inducing sclerotic cells from F. pedrosoi hyphae or conidia are based on the chemically defined Butterfield medium, which, following addition of 800 μM dl-propranolol, can induce sclerotic cells after 45 days of culture at pH 2.5 with constant shaking (1).
Phytopathogenic fungi are common in the species used to develop the new media. Examples include Crinipellis perniciosa, the etiologic agent of witches' broom disease in T. grandiflorum, and a Mycospharella sp. that is responsible for brown leaf spot in B. gasipaes. Also, the pathogenic forms of chromoblastomycosis are observed in plant tissues, for example, in histological specimens of the cactus species Ritterocereus griseus and Ritterocereus deficiens (15), indicating that different plant species can be natural substrates for the growth of pathogenic fungi. Here we report that plant species can be employed in in vitro media to grow F. pedrosoi.
By using these natural media, it was possible to reduce the time required for the induction of sclerotic cells from about 45 days to only 48 h without the addition of other chemical components and without the use of specific temperature conditions or shaking. SEM and TEM enabled us to evaluate the morphological similarities between our in vitro-generated sclerotic cells and those of others (3, 6) and between in vitro-generated sclerotic cells and those from lesions. The main similarities are size, multiseptation, and the formation of a thick, pigmented cellular wall. Additionally, the vesicles observed inside the sclerotic cell cytoplasm by TEM are consistent with previous findings (3).
The new culture media presented here significantly reduce the time necessary for the induction of sclerotic cells, making labor-intensive conditions such as constant shaking or controlled temperature unnecessary, and open up new possibilities for studying F. pedrosoi pathogenic forms in vitro using a simple and economical technique.
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Pará (FAPESPA), the Programa de Apoio à Pesquisa da Universidade do Estado do Pará, the Secretaria Executiva de Saúde Pública (SESPA), the Secretaria de Vigilância Epidemiológica/MS-Instituto Evandro Chagas, the Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Ministério da Saúde do Brasil, and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Alviano, D. S., L. F. Kneipp, A. H. Lopes, L. R. Travassos, J. R. Meyer-Fernandes, M. L. Rodrigues, and C. S. Alviano. 2003. Differentiation of Fonsecaea pedrosoi mycelial forms into sclerotic cells is induced by platelet-activating factor. Res. Microbiol. 154689-695. [DOI] [PubMed] [Google Scholar]
- 2.Cermeño-Vivas, J. R., and J. M. Torres Rodriguez. 2001. In vitro susceptibility of dematiaceous fungi to ten antifungal drugs using an agar diffusion test. Rev. Iberoam. Micol. 18113-117. (In Spanish.) [PubMed] [Google Scholar]
- 3.da Silva, J. P., D. S. Alviano, C. S. Alviano, W. de Souza, L. R. Travassos, J. A. Diniz, and S. Rozental. 2002. Comparison of Fonsecaea pedrosoi sclerotic cells obtained in vivo and in vitro: ultrastructure and antigenicity. FEMS Immunol. Med. Microbiol. 3363-69. [DOI] [PubMed] [Google Scholar]
- 4.da Silva, J. P., M. B. da Silva, U. I. Salgado, J. A. Diniz, S. Rozental, and C. G. Salgado. 2007. Phagocytosis of Fonsecaea pedrosoi conidia, but not sclerotic cells caused by Langerhans cells, inhibits CD40 and B7-2 expression. FEMS Immunol. Med. Microbiol. 50104-111. [DOI] [PubMed] [Google Scholar]
- 5.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures and Universitat Rovira i Virgili, Utrecht, The Netherlands.
- 6.Harada, S., and T. Kusunoki. 1983. Scanning electron microscopic observation of the parasitic forms of Fonsecaea pedrosoi in a human skin lesion. Mycopathologia 8233-37. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim-Granet, O., C. de Bievre, F. Romain, and S. Letoffe. 1985. Comparative electrophoresis, isoelectric focusing and numerical taxonomy of some isolates of Fonsecaea pedrosoi and allied fungi. Sabouraudia 23253-264. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza, L., S. M. Karuppayil, and P. J. Szaniszlo. 1993. Calcium regulates in vitro dimorphism in chromoblastomycotic fungi. Mycoses 36157-164. [DOI] [PubMed] [Google Scholar]
- 9.Queiroz-Telles, F., M. R. McGinnis, I. Salkin, and J. R. Graybill. 2003. Subcutaneous mycoses. Infect. Dis. Clin. N. Am. 1759-85, viii. [DOI] [PubMed] [Google Scholar]
- 10.Rogez, H., R. Buxant, E. Mignolet, J. N. S. Souza, E. M. Silva, and Y. Larondelle. 2004. Chemical composition of the pulp of three typical Amazonian fruits: araca-boi (Eugenia stipitata), bacuri (Platonia insignis) and cupuacu (Theobroma grandiflorum). Eur. Food Res. Technol. 218380-384. [Google Scholar]
- 11.Salgado, C. G., J. P. da Silva, J. A. Diniz, M. B. da Silva, P. F. da Costa, C. Teixeira, and U. I. Salgado. 2004. Isolation of Fonsecaea pedrosoi from thorns of Mimosa pudica, a probable natural source of chromoblastomycosis. Rev. Inst. Med. Trop. Sao Paulo 4633-36. [DOI] [PubMed] [Google Scholar]
- 11a.Salgado, C. G., U. I. Salgado, M. B. da Silva, P. F. da Costa, and J. P. da Silva. June 2008. Processo para a preparação de meios de cultura naturais e fabricação de um meio quimicamente difinido para alterações morfológicas e crescimento de microorganismos. Brazilian Natioal Institute for Inetellectual Property (INPI) patent P10520730-4.
- 12.Silva, J. P., W. de Souza, and S. Rozental. 1998. Chromoblastomycosis: a retrospective study of 325 cases on Amazonic Region (Brazil). Mycopathologia 143171-175. [DOI] [PubMed] [Google Scholar]
- 13.Silva, M. 1957. The parasitic phase of the fungi of chromoblastomycosis: development of sclerotic cells in vitro and in vivo. Mycologia 49318-331. [Google Scholar]
- 14.Yuyama, L. K. O., J. P. L. Aguiar, K. Yuyama, C. R. Clement, S. H. M. Macedo, D. I. T. Favaro, C. Afonso, M. B. A. Vasconcellos, S. A. Pimentel, E. S. G. Badolato, and H. Vannucchi. 2003. Chemical composition of the fruit mesocarp of three peach palm (Bactris gasipaes) populations grown in Central Amazonia, Brazil. Int. J. Food Sci. Nutr. 5449-56. [DOI] [PubMed] [Google Scholar]
- 15.Zeppenfeldt, G., N. Richard-Yegres, and F. Yegres. 1994. Cladosporium carrionii: hongo dimórfico en cactáceas de la zona endémica para la cromomicosis en Venezuela. Rev. Iberoam. Micol. 1161-63. [Google Scholar]


