Abstract
A disposable amperometric immunosensor was developed for the detection of Plasmodium falciparum histidine-rich protein 2 (PfHRP-2) in the sera of humans with P. falciparum malaria. For this purpose, disposable screen-printed electrodes (SPEs) were modified with multiwall carbon nanotubes (MWCNTs) and Au nanoparticles. The electrodes were characterized by cyclic voltammetry, scanning electron microscopy, and Raman spectroscopy. In order to study the immunosensing performances of modified electrodes, a rabbit anti-PfHRP-2 antibody (as the capturing antibody) was first immobilized on an electrode. Further, the electrode was exposed to a mouse anti-PfHRP-2 antibody from a serum sample (as the revealing antibody), followed by a rabbit anti-mouse immunoglobulin G-alkaline phosphatase conjugate. The immunosensing experiments were performed on bare SPEs, MWCNT-modified SPEs, and Au nanoparticle- and MWCNT-modified SPEs (Nano-Au/MWCNT/SPEs) for the amperometric detection of PfHRP-2 in a solution of 0.1 M diethanolamine buffer, pH 9.8, by applying a potential of 450 mV at the working electrode. Nano-Au/MWCNT/SPEs yielded the highest-level immunosensing performance among the electrodes, with a detection limit of 8 ng/ml. The analytical results of immunosensing experiments with human serum samples were compared with the results of a commercial Paracheck Pf test, as well as the results of microscopy. The specificities, sensitivities, and positive and negative predictive values of the Paracheck Pf and amperometric immunosensors were calculated by taking the microscopy results as the “gold standard.” The Paracheck Pf kit exhibited a sensitivity of 79% (detecting 34 of 43 positive samples; 95% confidence interval [CI], 75 to 86%) and a specificity of 81% (correctly identifying 57 of 70 negative samples; 95% CI, 76 to 92%), whereas the developed amperometric immunosensor showed a sensitivity of 96% (detecting 41 of 43 positive samples; 95% CI, 93 to 98%) and a specificity of 94% (correctly identifying 66 of 70 negative samples; 95% CI, 92 to 99%). The developed method is more sensitive and specific than the Paracheck Pf kit.
Each year, 300 to 500 million people develop malaria (20), and malaria is the fourth leading cause of children's deaths in developed countries (34). In humans, malaria is caused by four different protozoan species of the genus Plasmodium (35). The malarial parasite Plasmodium falciparum causes the most severe illness and is prevalent in sub-Saharan Africa, while Plasmodium vivax contributes significantly to malaria morbidity in Africa, Asia, and Latin America. The P. falciparum parasite synthesizes several proteins containing large amounts of the amino acid histidine, which are commonly referred to as P. falciparum histidine-rich proteins (PfHRPs) (7, 16). One of these, PfHRP-2, with 34% histidine and 37% alanine, shows a markedly high density compared to those of other proteins (32). PfHRP-2 is widely associated with the cytoskeletons of erythrocytes infected with P. falciparum, and it is produced and secreted by the parasite during its growth and development in infected red blood cells.
Due to the significance of PfHRP-2, an immunochromatographic dipstick test (14, 17, 29) and Western or dot blotting (24) to detect this protein are used in clinics to diagnose malaria. Several new diagnostic techniques have been developed in recent years, including photometric immunosensor detection (23), fluorescence microscopy and microarray analysis (6), PCR-based assays and colorimetric detection (33), laser desorption mass spectroscopy (8), and flow cytometry (with or without laser light depolarization monitoring) and chemodetection (28). However, these methods are either time-consuming, characterized by low levels of sensitivity and/or specificity, or expensive for mass screening. Most of the methods require highly qualified personnel (e.g., PCR) or sophisticated instrumentation (PCR, florescence microscopy, and flow cytometry). These features are potential drawbacks in the design of field-based portable devices based on these techniques for the diagnosis of malaria in large populations. By using electrochemical methods, cost-effective and field-deployable diagnostic devices can be made. The electrochemical techniques are also suitable for the analysis of samples that are turbid or colored, which may reduce the sensitivity and/or specificity of some of the diagnostic tests mentioned above (15).
Carbon nanotubes are a new form of carbon derivatives offering unique geometrical, mechanical, electronic, and chemical properties (12, 13). To utilize these unique properties for electrochemical detection, researchers have investigated carbon nanotube paste electrodes fabricated by mixing carbon nanotubes with mineral oil, as well as carbon nanotube-coated electrodes fabricated by dispersing carbon nanotubes into suitable solvents (21, 22, 30).
Gold nanoparticles are often cited in the literature due to their unique properties, such as a large surface area, excellent biocompatibility (4), and the capacity to provide a natural environment for biomolecules. In recent years, gold nanoparticles have attracted enormous interest in the application of analyses of hydrogen peroxide (9, 11, 19, 37), DNA, enzymes, and proteins (4, 5, 36), and antigens (31) and the functionalization of proteins (1). When such biomolecules are adsorbed onto gold nanoparticles, their bioactivity is retained (2).
In this study, efforts were directed toward the preparation of screen-printed electrodes (SPEs) modified with gold nanoparticles and carbon nanotubes (Nano-Au/multiwall carbon nanotube [MWCNT]/SPEs) and the evaluation of their immunosensing performance in the detection of PfHRP-2 in human serum by an amperometric method. The results obtained by this technique were compared with those obtained by microscopy and the use of a commercially available HRP-2 antigen detection kit (Paracheck Pf; Orchid Biomedical Systems, Goa, India). To the best of our knowledge, this is the first report proposing the use of an amperometric immunosensor for the detection of PfHRP-2.
MATERIALS AND METHODS
Apparatus.
Electrochemical experiments were performed with an autolab PGSTAT302 potentiostat/galvanostat (Eco Chemie, The Netherlands) with a conventional three-electrode system comprising a platinum rod as an auxiliary electrode, an Ag/AgCl/saturated-KCl (sat-KCl) reference electrode, and a bare or modified SPE (3 mm in diameter) as a working electrode. A Quanta 400 environmental scanning electron microscope equipped with an energy-dispersive X-ray (FEI, The Netherlands), Raman systems (Renishaw, United Kingdom), an ultrasonicator (UCB-30; Spectra Lab, India), and a pH meter (Eutech Instruments, Singapore) were utilized in this study.
Reagents and solutions.
1-Naphthyl phosphate, purified grade, was obtained from Lancaster, United Kingdom. 1-Naphthol was obtained from S.D.Fine-Chem (India). Tris buffer and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich (St. Louis, MO). Hydrochloroauric acid (HAuCl4) was purchased from Arora Matthey Ltd., Kolkata, India. Bovine serum albumin fraction V was obtained from John Baker Inc. Rabbit anti-mouse immunoglobulin G (IgG)-alkaline phosphatase (ALP) conjugate (code no. D0314) was received from Dako Cytomation, Denmark. Diethanolamine (DEA) was received from Acros Organics. All other chemicals were of extra-pure grade. MWCNTs (diameter, 110 to 170 nm; length, 5 to 9 μm) and 5% (wt/wt) Nafion solution were obtained from Aldrich. All solutions were prepared using deionized water purified with a Millipore system.
Preparation of PfHRP-2 antigen.
The PfHRP-2 gene sequence was amplified by PCR, and the PCR product was purified and cloned into the pQE-30 UA cloning vector. Escherichia coli strain M15 was transformed with the vector and used for the expression of histidine fusion protein. The expressed PfHRP-2 was purified using nickel-nitrilotriacetic acid affinity chromatography. The protein content of the purified sample was determined by the Lowry method and found to be 758 μg/ml.
Preparation of polyclonal antiserum.
BALB/c mice and New Zealand White rabbits were used for raising hyperimmune sera against recombinant PfHRP-2 antigen. The rabbits were initially immunized by the subcutaneous route with 100 μg of recombinant antigen and Freund's complete adjuvant at four different sites. They subsequently received booster doses of 100 μg of antigen along with Freund's incomplete adjuvant intramuscularly at 15-day intervals for 45 days. The rabbits were bled from the heart, and the sera were separated and stored at −20°C. The mice were immunized subcutaneously with 25 μg of recombinant antigen along with Freund's complete adjuvant, and after 1 week, a booster dose was given. The mice were bled after 4 weeks. Sera were separated and stored at −20°C.
IgG purification.
Five milliliters of saturated ammonium sulfate was slowly added dropwise to 5 ml of hyperimmune serum diluted 1:1, and the mixture was stirred for 30 min. The precipitated proteins (IgG) were removed by centrifugation (Sigma 4K15 centrifuge) at 5,000 rpm for 10 min at 4°C. The precipitated IgG was washed twice with 1:1 (vol/vol) saturated ammonium sulfate to remove remaining soluble proteins. The precipitate was dissolved in 1 ml of 0.1 M PBS, pH 7.2, and desalted by dialysis.
Collection of samples, immunochromatographic testing, and microscopic testing.
A total number of 113 samples were included in the study. Samples were collected from patients with fever (malaria-like symptoms) attending any of various hospitals in India (between August and September 2007). The samples were processed for serum separation and stored at 4°C. Microscopy and Paracheck Pf tests were conducted at the sample collection sites. For amperometric immunosensing, serum samples were brought to the laboratory Biosensor Development Division, DRDE, Gwalior, India) and, within 1 month, all the samples were analyzed. Serum samples determined by microscopy to be positive or negative for P. falciparum were collected from the National Institute of Malaria Research Field Station, Rourkela, India. Samples from patients with pyrexia of unknown origin (PUO) were collected from Hindu Rao Hospital, New Delhi, India, and were negative for P. falciparum by microscopy. Five samples positive for P. vivax were also collected from the Birla Institute of Medical Research, Gwalior, India. The samples were tested by the Paracheck Pf system for P. falciparum as a standard according to the protocol of the manufacturer (3).
Preparation of modified electrodes and measurement procedure.
SPEs were fabricated as reported previously in the literature (25). Prior to modification, the bare SPE was electrochemically pretreated in 0.1 M NaOH at a potential of 1.5 V for 5 min. Carboxylic functionalities were introduced onto MWCNTs (i.e., generating COOH-MWCNTs) with a 3:1 acid mixture of concentrated H2SO4 and HNO3. These COOH-MWCNTs were dispersed in 0.1% Nafion (1 mg/ml). The SPE was coated with 2 μl of this solution and dried in air. The resulting modified SPE was designated the MWCNT/SPE. The MWCNT/SPE was immersed in a 0.5 M H2SO4 solution containing 0.1 mM HAuCl4 (9, 10). The Au nanoparticles were electrodeposited via a potential step from +1.0 to 0.0 V (versus Ag/AgCl/sat-KCl) at a scan rate of 50 mV/s for 15 cycles. Then the electrode was rinsed with deionized water and stored in 0.1 M PBS (pH 7.0).
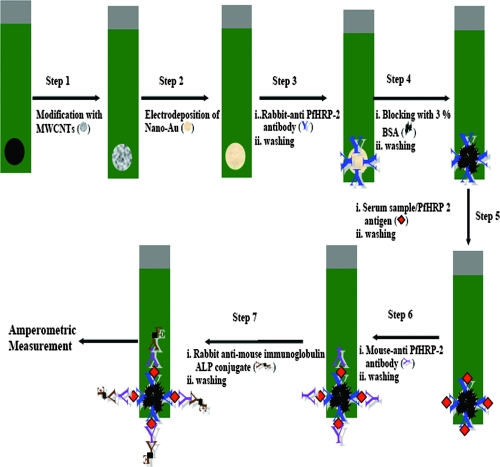
A sandwich immunoassay format was used to detect PfHRP-2 antigen (Fig. 1). Five microliters of rabbit anti-PfHRP-2 antibody (100 μg/ml) was pipetted onto the SPE (a modified and/or unmodified immunosensor), and the SPE was incubated for 1 h. Further, this immunosensor was incubated with 3% bovine serum albumin for 15 min to prevent nonspecific binding. In a further step, a 5-μl serum sample (a PfHRP-2-positive or -negative sample, a sample from a patient with PUO, or a sample from a healthy subject) was added to the SPE, and the SPE was incubated for 15 min each. In the next step, the SPE was incubated with 5 μl of mouse anti-PfHRP-2 antibody (optimized amount, 10 μg/ml) followed by 5 μl of rabbit anti-mouse IgG-ALP conjugate (1:100 dilution) for 15 min. All incubation steps were performed at 37°C, and thorough washing was performed with 0.1 M Tris buffer (pH 7.2) after each step.
FIG. 1.
Steps involved in the preparation of a modified SPE and steps involved in immunosensor detection. Red diamonds represent PfHRP-2 antigen. BSA, bovine serum albumin.
All amperometric experiments were performed at room temperature (27 ± 1°C) in an electrochemical cell containing 10 ml of DEA buffer, pH 9.8, stirred at a constant rate of 700 rpm. A potential of 450 mV versus the Ag/AgCl/sat-KCl reference electrode was applied. After the steady state of the background current was attained, the response was recorded by adding 1-naphthyl phosphate (the electrochemical substrate for ALP enzyme) to a final concentration of 5 mM (26).
RESULTS AND DISCUSSION
Cyclic voltammetry was used to characterize the electrochemical behavior of the electrodes (SPEs). Prior to the modification of SPEs with MWCNTs, we studied the effect of Nafion on the bare SPE surface. For this study, 1-μl samples of various concentrations (0.1 to 2%) of Nafion solution were pipetted onto the SPEs and the SPEs were dried at room temperature for 2 h. The electrodes were evaluated with cyclic voltammograms (CVs) recorded by using a 1 mM K3Fe(CN)6-K4Fe(CN)6 redox couple. Figure 2 depicts that the peak current was greatest in the case of the 0.1% Nafion solution, which indicated 0.1% to be the optimum concentration for making the MWCNT suspension. The higher concentrations of Nafion led to the blocking of the electrode surface.
FIG. 2.
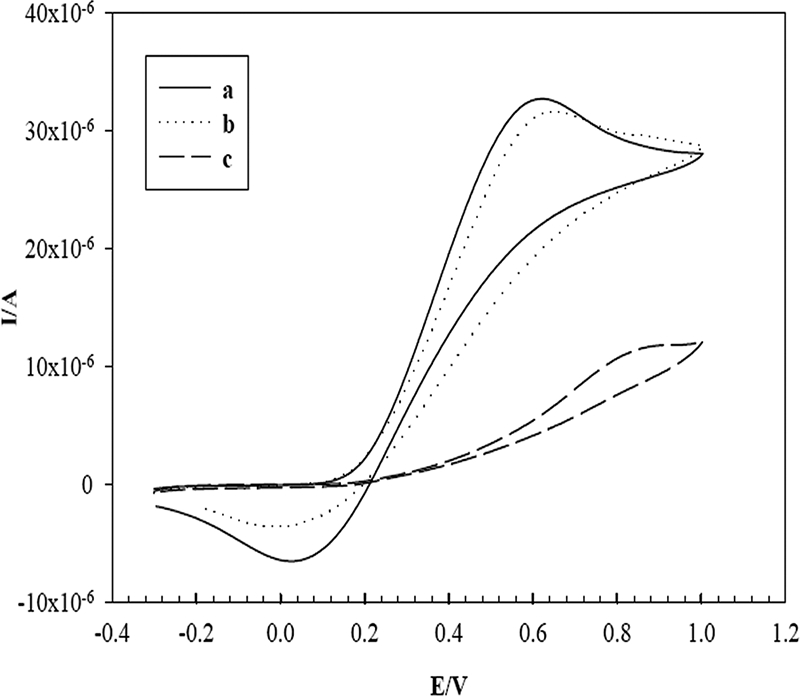
CVs measured with an SPE modified with 0.1% Nafion (a), 0.5% Nafion (b), and 1% Nafion (c) in an aqueous solution containing 1 mM K3Fe(CN)6-K4Fe(CN)6 (1:1) and 0.1 M KCl as the supporting electrolyte at a scan rate of 50 mV/s. I/A, current in amperes; E/V, electrode potential in volts.
The CVs for various modified SPEs were obtained with a 1 mM K3Fe(CN)6-K4Fe(CN)6 redox couple containing 0.1 M KCl (Fig. 3). The background currents for the MWCNT/SPE, the gold nanoparticle-modified SPE (Nano-Au/SPE), and the Nano-Au/MWCNT/SPE were higher than that for the bare SPE. The CV observed for the Nano-Au/MWCNT/SPE showed higher reduction and oxidation peak currents and also more cathodic shift in the oxidation peak potential than those observed for the MWCNT/SPE and bare SPE. This result clearly shows that the modification of SPEs with MWCNTs and Au nanoparticles significantly enhanced the effective electrode surface area (18).
FIG. 3.
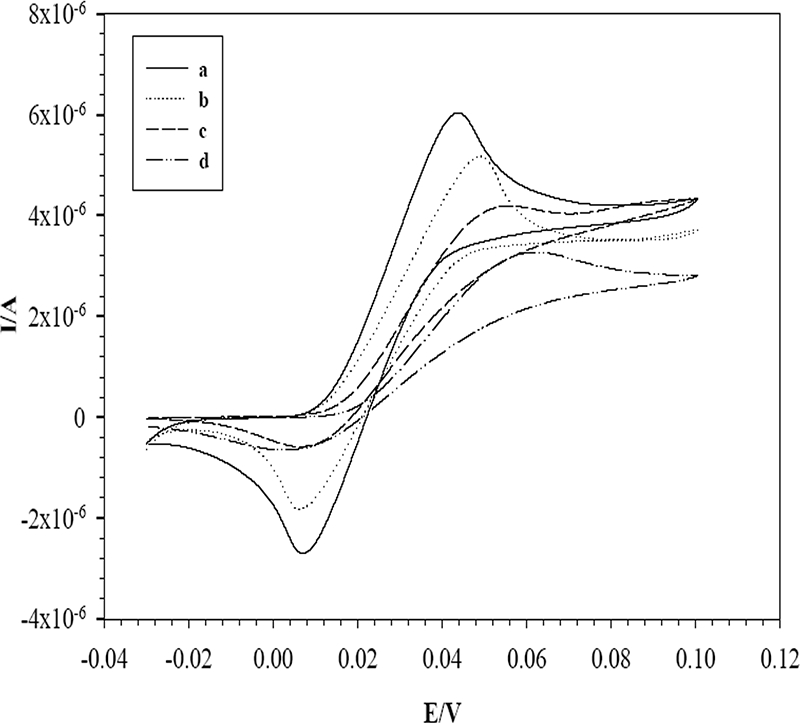
CVs measured with an SPE modified with Au nanoparticles and MWCNTs (a), Au nanoparticles (b), and MWCNTs (c) and with a bare SPE (d) in a solution containing 1 mM K3Fe(CN)6-K4Fe(CN)6 (1:1) and 0.1 M KCl as the supporting electrolyte at a scan rate of 50 mV/s. I/A, current in amperes; E/V, electrode potential in volts.
A sandwich enzyme-linked immunosorbent assay format was employed for amperometric immunosensor detection of PfHRP-2. This approach used ALP-conjugated antibodies which produce 1-naphthol as the hydrolyzed product of 1-naphthyl phosphate (the enzymatic substrate) during amperometric experiments. CVs for 0.2 mM 1-naphthol on bare and modified SPEs are displayed in Fig. 4. As can be seen, 1-naphthol exhibited a small sigmoid response on the bare SPE, while the Nano-Au/MWCNT/SPE exhibited the sharpest anodic peak. This remarkably enhanced signal from the Nano-Au/MWCNT/SPE was attributed to the synergistic effect of MWCNTs and Au nanoparticles. A similar experiment with a pure gold electrode was carried out. The oxidation peak potential (300 mV) of 1-naphthol on the pure gold electrode was found to be higher than the oxidation peak potential (270 mV) on the Nano-Au/MWCNT/SPE. This observation was supportive, as in the case of Fig. 3. The anodic peak current for 0.2 mM 1-naphthol on the Nano-Au/MWCNT/SPE was proportional to the square root of the scan rate over the sweep potential range from 50 to 250 mV/s, with a linear regression coefficient (r = 0.9989) showing a diffusion control process (data not shown). The oxidation peak current density value for the Nano-Au/MWCNT/SPE (1.289 μA/mm2) was higher than that for the pure gold electrode (0.185 μA/mm2). This significant enhancement in current density on the Nano-Au/MWCNT/SPE was due to Au nanoparticles.
FIG. 4.
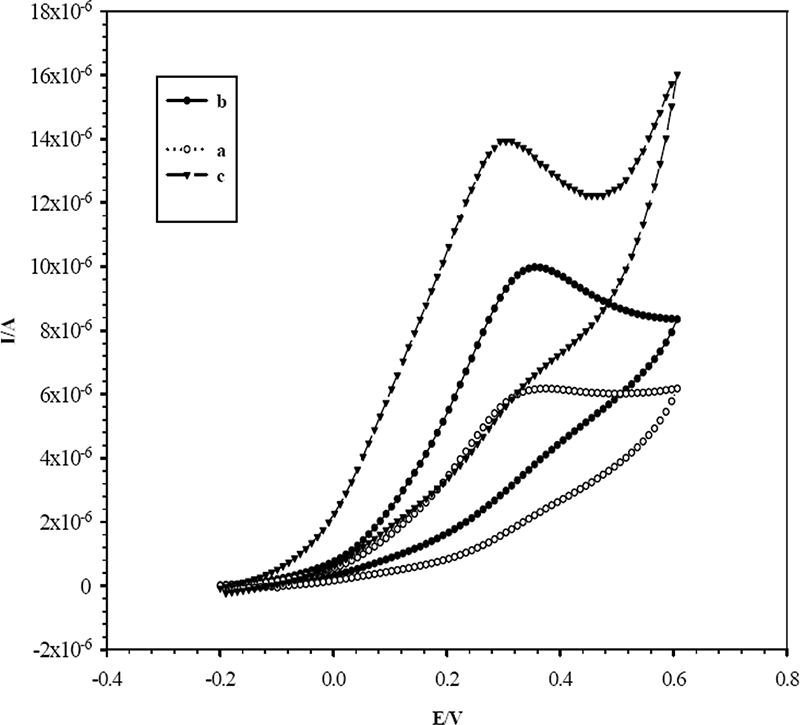
CVs measured with a bare SPE (a) and with an SPE modified with MWCNTs (b) and Au nanoparticles and MWCNTs (c) in 0.2 mM 1-naphthol in DEA buffer, pH 9.8, at a scan rate of 50 mV/s. I/A, current in amperes; E/V, electrode potential in volts.
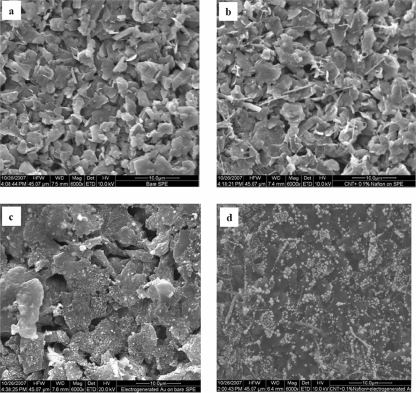
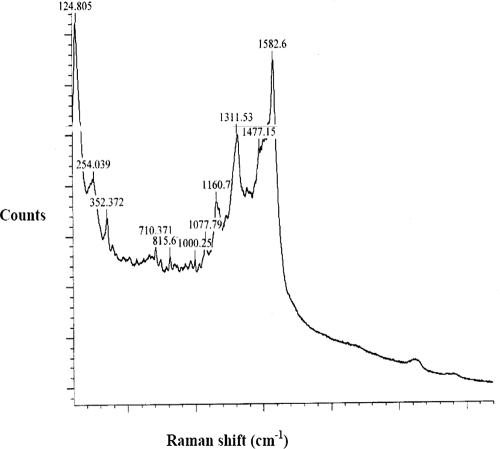
In order to investigate the surface morphologies of bare and modified SPEs, scanning electron microscopy (SEM) characterization was carried out. Figure 5a and b are SEM images of the bare SPE and the MWCNT/SPE, respectively. Electrogenerated Au nanoparticles on the bare SPE (Fig. 5c) and the MWCNT/SPE (Fig. 5d) suggest that Au nanoparticles can be easily adsorbed onto the MWCNT film to give a nanohybrid film. Figure 6 depicts the Raman spectra for the electrodeposited gold on the MWCNT/SPE surface; Raman shifts at 1,582.6 cm−1, 1,311.53 cm−1, and 1,160.7 cm−1 were the characteristic peaks for the Au on the modified surface of the SPE.
FIG. 5.
SEM images of the electrode surfaces of the bare SPE (a), the MWCNT/SPE (b), the Nano-Au/SPE (c), and the Nano-Au/MWCNT/SPE (d). Acceleration voltage, 10 kV.
FIG. 6.
Raman spectra of the Nano-Au/MWCNT/SPE. Laser source, 514 nm; exposure time, 10 s.
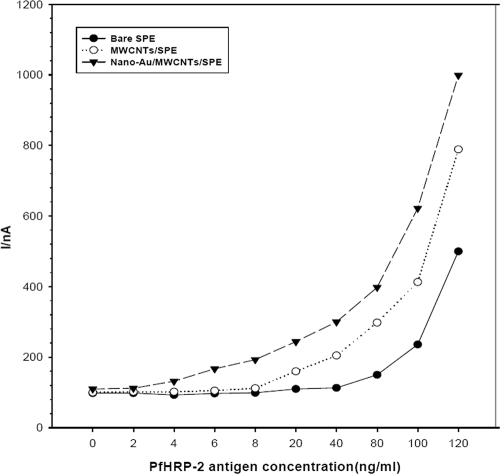
The electrodes were evaluated for their immunosensing performances in the detection of PfHRP-2. The calibration plot of the amperometric responses versus the PfHRP-2 concentrations on the bare SPE, MWCNT/SPE, and Nano-Au/MWCNT/SPE is shown in Fig. 7. The minimum detection limits of the bare SPE, MWCNT/SPE, and Nano-Au/MWCNT/SPE immunosensors for PfHRP-2 were found to be 80 ng/ml (standard deviation [SD], ±18; n = 6), 20 ng/ml (SD, ±16; n = 6), and 8 ng/ml (SD, ±12; n = 6), respectively; all of the limits of detection correspond to the baseline current plus three times the SD of the blank response (blank response + 3σ). This result clearly shows that after the modification of the bare SPE with MWCNTs and Au nanoparticles, the sensitivity of the immunosensor increased up to 10 times. This immunosensitivity effect for the Nano-Au/MWCNT/SPE was much greater than that for the MWCNT/SPE, even though the anodic peak current densities for the two electrodes were almost equal. Hence, we assume that gold nanoparticles help in the proper orientation of antibodies, which was essential for better immunosensing. Such effects with some proteins were reported previously in the literature (T. Lamar, http://www.prc.gatech.edu/academics/pre-college/hs-projects/fall2005/TyraLamarReport.pdf). Finally, based on these results, the Nano-Au/MWCNT/SPE immunosensor was selected for the detection of PfHRP-2 in human serum samples.
FIG. 7.
Calibration plot for the amperometric current with different concentrations of PfHRP-2 under optimized conditions for the bare SPE, the MWCNT/SPE, and the Nano-Au/MWCNT/SPE in DEA buffer, pH 9.8. Applied potential, 450 mV versus the Ag/AgCl/sat-KCl reference electrode, with a stirring speed of 700 rpm (n = 6). I/nA, current in nanoamperes.
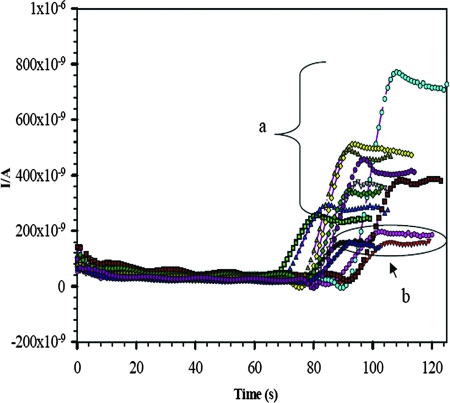
Figure 8 shows the amperometric responses for a representative set of 17 serum samples including PfHRP-2-positive samples and healthy human serum samples. There were considerably greater amperometric responses for the positive serum samples than for the healthy human serum samples. The magnitudes of the responses differed among individual samples and corresponded to the levels of infection. In addition, one can infer that an amperometric response of greater than 220 nA (blank response + 3σ: 160 + 3 × 20 = 220 nA) leads to the result of PfHRP-2 positive.
FIG. 8.
Amperometric responses on the Nano-Au/MWCNT/SPE immunosensor for P. falciparum-positive serum samples (a) and pooled healthy human serum samples (b) in a DEA buffer solution of pH 9.8. Applied potential, 450 mV versus the Ag/AgCl/sat-KCl reference electrode, with a stirring speed of 700 rpm. I/A, current in amperes.
A total of 113 patient samples were included in this study. Microscopy showed 43 serum samples to be P. falciparum positive and a total of 70 samples to be P. falciparum negative (5 positive for P. vivax but P. falciparum negative, 34 from healthy human subjects, and 31 from patients with PUO but P. falciparum negative). All these samples were tested by the developed amperometric immunosensor and the Paracheck Pf kit, which were assessed for sensitivity as well as specificity (Tables 1 and 2). There were five false-negative results with the Paracheck Pf kit, but only two false-negative results with the amperometric immunosensor. There was only one false-positive result with the amperometric immunosensor, whereas the Paracheck Pf kit gave eight false-positive results. There were three and nine doubtful results from the amperometric immunosensor and the Paracheck Pf kit, respectively.
TABLE 1.
Overall numbers of screening results comparing the Paracheck Pf and the present amperometric immunosensor method
| Microscopy resulta (no. of samples) | No. of samples with Paracheck Pf test result of:
|
No. of positive samples detected by Paracheck Pf at:
|
No. of samples with amperometric immunosensor result of:
|
No. of positive samples detected by amperometric immunosensor at:
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pf −ve | Pf +ve | Unclear | 1:10b | 1:50b | Pf −ve | Pf +ve | Unclear | 1:10b | 1:50b | |
| Pf +ve (43) | 5 | 34 | 4 | 12 | 0 | 2 | 41 | 0 | 41 | 36 |
| Pf −ve (70) | 57 | 8 | 5 | 66 | 1 | 3 | ||||
Pf +ve, P. falciparum positive; Pf −ve, P. falciparum negative.
Sample dilution.
TABLE 2.
Performances of Paracheck Pf and the developed amperometric immunosensor method in detecting PfHRP-2
| Test (no. of samples) | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | % of samples with unclear results (no. of unclear samples/no. of total samples) |
|---|---|---|---|
| Paracheck Pf (113) | 79 (75-86) | 81 (76-92) | 7.96 (9/113) |
| Amperometric immunosensor (113) | 96 (93-98) | 94 (92-99) | 2.7 (3/113) |
The amperometric immunosensor identified 96% (41 of 43; 95% confidence interval [CI], 93 to 98%) of the samples with P. falciparum infection, whereas the Paracheck Pf kit identified 79% (34 of 43; 95% CI = 75 to 86%). These patient serum samples were further diluted (1:10 and 1:50) in Tris buffer, pH 7.2, and tested. At 1:10 and 1:50 dilutions, the amperometric immunosensor detected 96% (41 of 43; 95% CI = 93 to 98%) and 84% (36 of 43; 95% CI = 80 to 89%) of the samples with P. falciparum infection, respectively. On the other hand, at a 1:10 dilution, the Paracheck Pf kit identified only 28% (12 of 43; 95% CI = 25 to 33%) of the samples with P. falciparum infection, and none of the samples were found to be P. falciparum positive at a 1:50 dilution. These results show that the present immunosensor is more sensitive than the Paracheck Pf kit.
In addition to the positive samples, 34 healthy human serum samples (negative for P. falciparum by microscopy) were tested to evaluate the specificities of the immunosensor and the Paracheck Pf kit. The immunosensor and Paracheck Pf kit showed specificities of 94% (correctly identifying 32 of 34 negative samples; 95% CI = 93 to 97%) and 88% (correctly identifying 30 of 34 negative samples; 95% CI = 82 to 92%), respectively. Five samples with P. vivax infection were also analyzed. The amperometric immunosensor showed 100% specificity (determining five of five to be negative for P. falciparum; 95% CI = 98 to 100%), whereas the Paracheck Pf kit showed 80% specificity (determining four of five to be negative; 95% CI = 77 to 84%). Based on these results, the developed immunosensor was more sensitive and specific than the commercial Paracheck Pf kit.
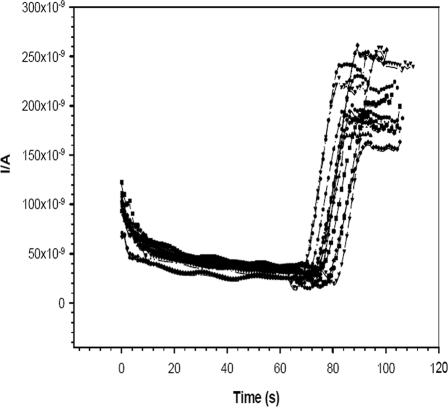
Cross-reactivity studies were also carried out to test the specificity of the developed detection method with serum samples from patients with PUO (31 samples) (Fig. 9) collected from Hindu Rao Hospital, New Delhi, India. The Paracheck Pf kit showed only 74% (23 of 31; 95% CI = 71 to 80%) to be negative for P. falciparum. The amperometric immunosensor showed 94% (29 of 31; 95% CI = 92 to 96%) to be negative. During the amperometric measurement, most of the samples showed responses in the range of 160 to 213 nA (the current subtracted from the steady-state background current), with a relative SD value of 5.3% (n = 6 repetitions of the amperometric experiment). These responses (currents of 160 to 213 nA) were very close to those shown for the healthy human serum samples (Fig. 6), indicating that the developed detection method is highly specific.
FIG. 9.
Amperometric responses for P. falciparum-negative PUO serum samples in DEA buffer, pH 9.8. Applied potential, 450 mV versus the Ag/AgCl/sat-KCl reference electrode, with a stirring speed of 700 rpm (n = 6). I/A, current in amperes.
Conclusion.
In essence, we observed that the Nano-Au/MWCNT/SPE immunosensor exhibited a higher level of sensitivity for the detection of PfHRP-2 than those of the bare SPE (by 10 times), the MWCNT/SPE, and the Nano-Au/SPE. In addition, the proposed detection method was more sensitive and specific than the commercial Paracheck Pf test. To the best of our knowledge, this is the first report proposing the use of a novel electrochemical immunosensor for the sensitive and quantitative detection of PfHRP-2. In conclusion, we have demonstrated the successful detection of P. falciparum in samples from infected patients by a newly developed electrochemical method. The current Nano-Au/MWCNT/SPE can be easily packaged into a handheld potentiostat device for the detection of PfHRP-2 under field conditions. The materials required to carry out the immunosensor method in the field are disposable SPEs (sensing part), a battery-operated potentiostat (we have developed a handheld, portable, battery-operated potentiostat [V. K. Rao and M. K. Sharma, 12 July 2007, patent application DEL/1669/07]), biomolecules (antibodies and ALP conjugate), and a magnetic stirrer with a magnetic bar. All these items are easily portable, and experiments can be performed in the field. In this setting, the potentiostat is not affected by any environmental condition. However, precautions should be taken with the modified SPEs (coated with capturing antibody) and biomolecules. These modified electrodes and biomolecules should be stored at 2 to 8°C.
Acknowledgments
We are grateful to P. Pandey and Anand Singh for helping in the SEM and Raman characterization of electrodes.
Footnotes
Published ahead of print on 17 September 2008.
REFERENCES
- 1.Abad, J. M., S. F. L. Mertens, M. Pita, V. M. Fernandez, and D. J. Schiffrin. 2005. Functionalization of thioctic acid-capped gold nanoparticles for specific immobilization of histidine-tagged proteins. J. Am. Chem. Soc. 1275689-5694. [DOI] [PubMed] [Google Scholar]
- 2.Bharathi, S., and M. Nogami. 2001. A glucose biosensor based on electrodeposited bio-composites of gold nanoparticles and glucose oxidase enzyme. Analyst 1261919-1922. [DOI] [PubMed] [Google Scholar]
- 3.Broek, I. V. D., O. Hill, F. Gordillo, B. Angarita, P. Hamade, H. Counihan, and J. Guthmann. 2006. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. Am. J. Trop. Med. Hyg. 751209-1215. [PubMed] [Google Scholar]
- 4.Brown, K. R., A. P. Fox, and M. J. Natan. 1996. Morphology-dependent electrochemistry of cytochrome c at Au colloid-modified SnO2 electrodes. J. Am. Chem. Soc. 1181154-1157. [Google Scholar]
- 5.Cai, H., C. Xu, P. G. He, and Y. Z. Fang. 2001. Colloid Au-enhanced DNA immobilization for the electrochemical detection of sequence-specific DNA. J. Electroanal. Chem. 51078-85. [Google Scholar]
- 6.Carret, C. K., H. Paul, K. Bernard, W. Elizabeth, Q. Matloob, N. Chris, and I. Alasdair. 2005. Microarray-based comparative genomic analyses of the human malaria parasite Plasmodium falciparum using Affymetrix arrays. Mol. Biochem. Parasitol. 144177-186. [DOI] [PubMed] [Google Scholar]
- 7.Chotia, C. 1984. Principles to determine the structure of proteins. Annu. Rev. Biochem. 53537-572. [DOI] [PubMed] [Google Scholar]
- 8.Demirev, P. A., A. B. Feldman, D. Kongkasuriyachi, P. Scholl, D. Sullivan, and N. Kumar. 2002. Detection of malaria parasites in blood by laser desorption mass spectroscopy. Anal. Chem. 743262-3266. [DOI] [PubMed] [Google Scholar]
- 9.El-Deab, M. S., T. Okajima, and T. Ohsaka. 2003. Electrochemical reduction of oxygen on gold nanoparticle-electrodeposited glassy carbon electrodes. J. Electrochem. Soc. 150A851-A857. [Google Scholar]
- 10.Finot, M. O., G. D. Braybrook, and M. T. Mcdermott. 1999. Characterization of electrochemically deposited gold nanocrystals on glassy carbon electrodes. J. Electroanal. Chem. 466234-241. [Google Scholar]
- 11.Gu, H., A. Yu, and H. Chen. 2001. Direct electron transfer and characterization of hemoglobin immobilized on a Au-colloid-cystamine-modified gold electrode. J. Electroanal. Chem. 516119-126. [Google Scholar]
- 12.Iijima, S. 1991. Helical microtubules of graphitic carbon. Nature 35456-58. [Google Scholar]
- 13.Iijima, S., and T. Ichihashi. 1993. Single shell carbon nanotubes of one nanometer diameter. Nature 363603-605. [Google Scholar]
- 14.Jelinek, T., M. P. Grobusch, and G. Harms. 2001. Evaluation of a dipstick test for the rapid diagnosis of imported malaria among patients presenting within the network TropNetEurop. Scand. J. Infect. Dis. 33752-754. [DOI] [PubMed] [Google Scholar]
- 15.Law, W. T., N. Akmal, and A. M. Usmani (ed.). 2002. Biomedical diagnostic science and technology. Informa Healthcare, New York, NY.
- 16.Lehninger, A. L. 1975. Biochemistry, p.101. Worth, New York, NY.
- 17.Leke, R. F. G., R. R. Djokam, R. Mbu, R. J. Leke, J. Fogako, R. Megnekou, S. Metenou, G. Sama, Y. Xhou, T. Cadigan, M. Parra, and D. W. Taylor. 1999. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J. Clin. Microbiol. 372992-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, J., and X. Q. Lin. 2007. Electrodeposition of gold nanoclusters on overoxidized polypyrrole film modified glassy carbon electrode and its application for the simultaneous determination of epinephrine and uric acid under coexistence of ascorbic acid. Anal. Chim. Acta 596222-230. [DOI] [PubMed] [Google Scholar]
- 19.Liu, S. Q., and H. X. Ju. 2002. Renewable reagentless hydrogen peroxide sensor based on direct electron transfer of horseradish peroxidase immobilized on colloidal gold-modified electrode. Anal. Biochem. 307110-116. [DOI] [PubMed] [Google Scholar]
- 20.Lo Re, V., III, and S. Gluckman. 2003. Prevention of malaria in travelers. Am. Fam. Physician 68509-514. [PubMed] [Google Scholar]
- 21.Luo, H., Z. Shi, N. Li, Z. Gu, and Q. Zhuang. 2001. Investigation of the electrochemical and electrocatalytic behavior of single-wall carbon nanotube film on a glassy carbon electrode. Anal. Chem. 73915-920. [DOI] [PubMed] [Google Scholar]
- 22.Musameha, M., J. Wang, A. Merkoci, and Y. Lin. 2002. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochem. Commun. 4743-746. [Google Scholar]
- 23.Mya, M. M., R. K. Saxena, A. Roy, and K. B. Roy. 2003. Design and development of an immunosensor for the detection of malaria in field conditions. Parasitol. Res. 89371-374. [DOI] [PubMed] [Google Scholar]
- 24.Parra, M. E., C. B. Evans, and D. W. Taylor. 1991. Identification of Plasmodium falciparum histidine-rich protein 2 in the plasma of humans with malaria. J. Clin. Microbiol. 291629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao, V. K., M. K. Sharma, A. K. Goel, L. Singh, and K. Sekhar. 2006. Amperometric immunosensor for detection of Vibrio cholerae O1 using disposable screen-printed electrodes. Anal. Sci. 221207-1211. [DOI] [PubMed] [Google Scholar]
- 26.Rao, V. K., G. P. Rai, G. S. Agarwal, and S. Suresh. 2005. Amperometric immunosensor for detection of antibodies of Salmonella typhi in patient serum. Anal. Chim. Acta 531173-177. [Google Scholar]
- 27.Reference deleted.
- 28.Sahal, D., R. Kannan, A. Sinha, P. Babbarwal, B. G. Prakash, G. Singh, and V. S. Chauhan. 2004. Specific and instantaneous one-step chemodetection of histidine-rich proteins by Pauly's stain. Anal. Biochem. 308405-408. [DOI] [PubMed] [Google Scholar]
- 29.Shiff, C. J., Z. Premji, and J. N. Minjas. 1993. The rapid manual ParaSight-F test, a new diagnostic tool for Plasmodium falciparum infection. Trans. R. Soc. Trop. Med. Hyg. 87646-648. [DOI] [PubMed] [Google Scholar]
- 30.Sun, Y., K. Wu, and S. Hu. 2003. Fabrication of a multi-wall carbon nanotubes modified glassy carbon electrode and its catalytic effect on the oxidation of estradiol, estrone and estriol. Microchim. Acta 14249-53. [Google Scholar]
- 31.Wang, M., L. Wang, G. Wang, X. Ji, Y. Bai, T. Li, S. Gong, and J. Li. 2004. Application of impedance spectroscopy for monitoring colloid Au-enhanced antibody immobilization and antibody-antigen reactions. Biosens. Bioelectron. 19575-582. [DOI] [PubMed] [Google Scholar]
- 32.Wellems, T. E., and R. J. Howard. 1986. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 836065-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiley, D. M., G. M. LeCornec, A. Baddeley, J. Savill, M. W. Syrmis, I. M. Mackay, D. J. Siebertb, D. Burnsb, M. Nissend, and T. P. Sloots. 2004. Detection and differentiation of Plasmodium species by polymerase chain reaction and colorimetric detection in blood samples of patients with suspected malaria. Diagn. Microbiol. Infect. Dis. 4925-29. [DOI] [PubMed] [Google Scholar]
- 34.World Bank. 2001. Malaria at a glance. World Bank, Washington, DC.
- 35.World Health Organization. 2003. The world health report 2003. World Health Organization, Geneva, Switzerland.
- 36.Xiao, Y., H. X. Ju, and H. Y. Chen. 1999. Hydrogen peroxide sensor based on horseradish peroxidase-labeled Au colloids immobilized on gold electrode surface by cysteamine monolayer. Anal. Chim. Acta 39173-82. [Google Scholar]
- 37.Zhang, J., and M. Oyama. 2004. A hydrogen peroxide sensor based on the peroxidase activity of hemoglobin immobilized on gold nanoparticles-modified ITO electrode. Electrochim. Acta 5085-90. [Google Scholar]