Abstract
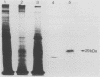
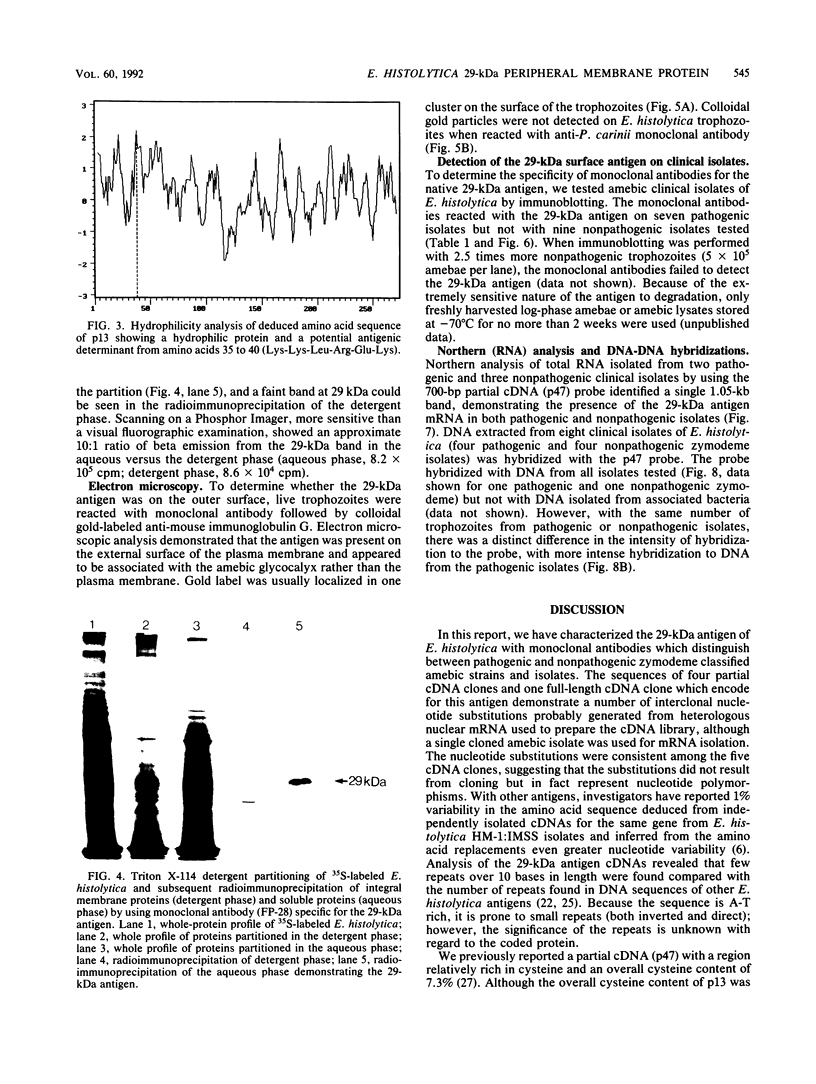
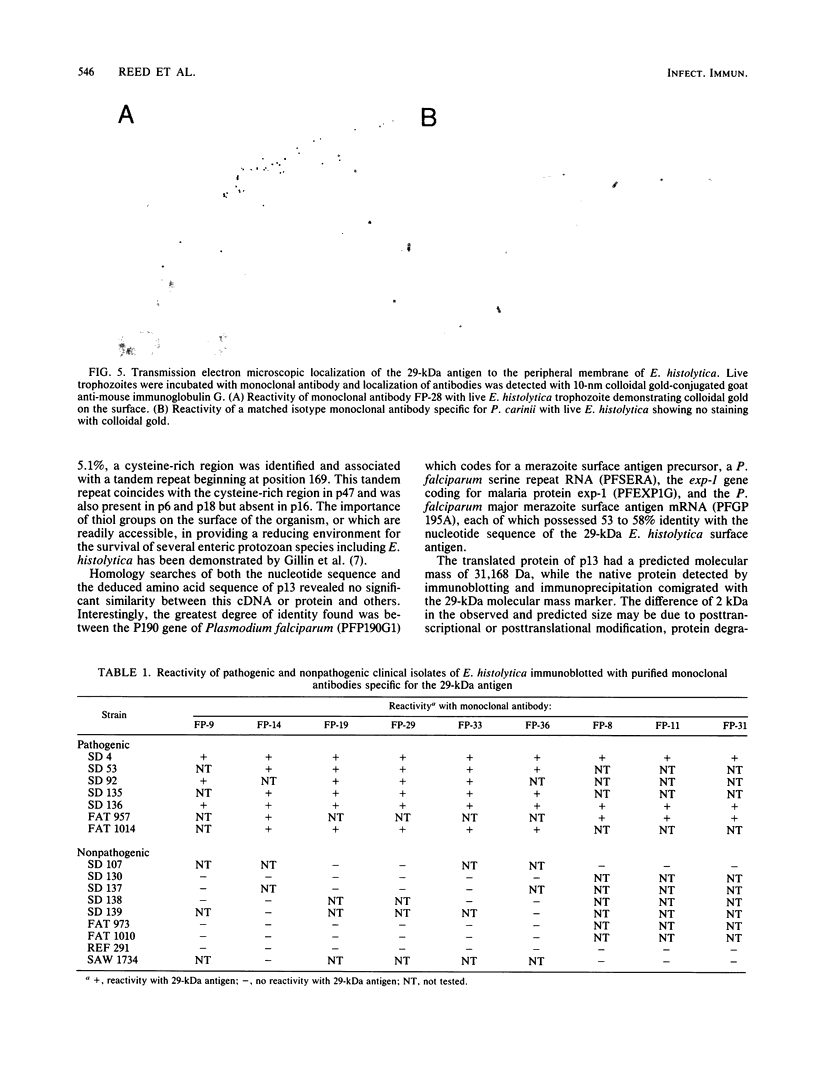
To further characterize the 29-kDa surface antigen of Entamoeba histolytica, we analyzed the complete nucleotide sequence and compared the immunoreactivity of this antigen in pathogenic and nonpathogenic strains. Five cDNA clones (one 1.0-kb full-length clone, designated p13, and four partial-length clones) encoding the antigen were analyzed for allelic variation. Comparison of the nucleotide sequences revealed several single-nucleotide substitutions in all five cDNAs, two of which resulted in amino acid differences. Localization of the antigen to the amebic surface in a previous report (B. E. Torian, B. M. Flores, V. L. Stroeher, F. S. Hagen, and W. E. Stamm, Proc. Natl. Acad. Sci. USA 87:6358-6362, 1990) was corroborated by transmission electron microscopy showing colloidal gold particles on the surface of the trophozoites. Computer analysis of the deduced amino acid sequence predicted that the protein encoded by p13 was a hydrophilic peripheral membrane protein, and these data were confirmed by a Triton X-114 membrane extraction showing the presence of the 29-kDa antigen primarily in the aqueous phase of the detergent partition. Monoclonal antibodies to a fusion peptide differentiated between pathogenic and nonpathogenic clinical strains of E. histolytica in immunoblots. Although no immunoreactive epitopes were detected on nonpathogenic strains, Northern (RNA) analysis and DNA-DNA hybridization with a 700-bp cDNA probe demonstrated that mRNA and the gene encoding the 29-kDa surface antigen were present in both pathogenic and nonpathogenic clinical isolates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharya A., Ghildyal R., Bhattacharya S., Diamond L. S. Characterization of a monoclonal antibody that selectively recognizes a subset of Entamoeba histolytica isolates. Infect Immun. 1990 Oct;58(10):3458–3461. doi: 10.1128/iai.58.10.3458-3461.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely P., Sargeaunt P. G., Reed S. L. An immunogenic 30-kDa surface antigen of pathogenic clinical isolates of Entamoeba histolytica. J Infect Dis. 1990 Oct;162(4):949–954. doi: 10.1093/infdis/162.4.949. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Edman U., Meraz M. A., Rausser S., Agabian N., Meza I. Characterization of an immuno-dominant variable surface antigen from pathogenic and nonpathogenic Entamoeba histolytica. J Exp Med. 1990 Sep 1;172(3):879–888. doi: 10.1084/jem.172.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin F. D., Reiner D. S., Levy R. B., Henkart P. A. Thiol groups on the surface of anaerobic parasitic protozoa. Mol Biochem Parasitol. 1984 Sep;13(1):1–12. doi: 10.1016/0166-6851(84)90096-3. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mann B. J., Torian B. E., Vedvick T. S., Petri W. A., Jr Sequence of a cysteine-rich galactose-specific lectin of Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3248–3252. doi: 10.1073/pnas.88.8.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Wexler A., Chayen A. Changes in isoenzyme patterns of a cloned culture of nonpathogenic Entamoeba histolytica during axenization. Infect Immun. 1986 Dec;54(3):827–832. doi: 10.1128/iai.54.3.827-832.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri W. A., Jr, Jackson T. F., Gathiram V., Kress K., Saffer L. D., Snodgrass T. L., Chapman M. D., Keren Z., Mirelman D. Pathogenic and nonpathogenic strains of Entamoeba histolytica can be differentiated by monoclonal antibodies to the galactose-specific adherence lectin. Infect Immun. 1990 Jun;58(6):1802–1806. doi: 10.1128/iai.58.6.1802-1806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. L., Curd J. G., Gigli I., Gillin F. D., Braude A. I. Activation of complement by pathogenic and nonpathogenic Entamoeba histolytica. J Immunol. 1986 Mar 15;136(6):2265–2270. [PubMed] [Google Scholar]
- Robinson G. L. The laboratory diagnosis of human parasitic amoebae. Trans R Soc Trop Med Hyg. 1968;62(2):285–294. doi: 10.1016/0035-9203(68)90170-3. [DOI] [PubMed] [Google Scholar]
- Sargeaunt P. G., Williams J. E. Electrophoretic isoenzyme patterns of Entamoeba histolytica and Entamoeba coli. Trans R Soc Trop Med Hyg. 1978;72(2):164–166. doi: 10.1016/0035-9203(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Sargeaunt P. G., Williams J. E., Grene J. D. The differentiation of invasive and non-invasive Entamoeba histolytica by isoenzyme electrophoresis. Trans R Soc Trop Med Hyg. 1978;72(5):519–521. doi: 10.1016/0035-9203(78)90174-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S. L., Jr, Becker A., Kunz-Jenkins C., Foster L., Li E. Cloning and expression of a membrane antigen of Entamoeba histolytica possessing multiple tandem repeats. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4976–4980. doi: 10.1073/pnas.87.13.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan W. D., Chiodini P. L., Spice W. M., Moody A. H., Ackers J. P. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet. 1988 Mar 12;1(8585):561–563. doi: 10.1016/s0140-6736(88)91355-4. [DOI] [PubMed] [Google Scholar]
- Tachibana H., Ihara S., Kobayashi S., Kaneda Y., Takeuchi T., Watanabe Y. Differences in genomic DNA sequences between pathogenic and nonpathogenic isolates of Entamoeba histolytica identified by polymerase chain reaction. J Clin Microbiol. 1991 Oct;29(10):2234–2239. doi: 10.1128/jcm.29.10.2234-2239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H., Kobayashi S., Kato Y., Nagakura K., Kaneda Y., Takeuchi T. Identification of a pathogenic isolate-specific 30,000-Mr antigen of Entamoeba histolytica by using a monoclonal antibody. Infect Immun. 1990 Apr;58(4):955–960. doi: 10.1128/iai.58.4.955-960.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannich E., Ebert F., Horstmann R. D. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannich E., Horstmann R. D., Knobloch J., Arnold H. H. Genomic DNA differences between pathogenic and nonpathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5118–5122. doi: 10.1073/pnas.86.13.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Flores B. M., Stroeher V. L., Hagen F. S., Stamm W. E. cDNA sequence analysis of a 29-kDa cysteine-rich surface antigen of pathogenic Entamoeba histolytica. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6358–6362. doi: 10.1073/pnas.87.16.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Lukehart S. A., Stamm W. E. Use of monoclonal antibodies to identify, characterize, and purify a 96,000-dalton surface antigen of pathogenic Entamoeba histolytica. J Infect Dis. 1987 Aug;156(2):334–343. doi: 10.1093/infdis/156.2.334. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Reed S. L., Flores B. M., Creely C. M., Coward J. E., Vial K., Stamm W. E. The 96-kilodalton antigen as an integral membrane protein in pathogenic Entamoeba histolytica: potential differences in pathogenic and nonpathogenic isolates. Infect Immun. 1990 Mar;58(3):753–760. doi: 10.1128/iai.58.3.753-760.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]