Abstract
Increasing recognition of the association of rhinovirus with severe lower respiratory tract illnesses has clarified the need to understand the relationship between specific serotypes of rhinovirus and their clinical consequences. To accomplish this, a specific and sensitive assay to detect and serotype rhinovirus directly from clinical specimens is needed. Traditional methods of serotyping using culture and serum neutralization are time-consuming, limited to certain reference laboratories, and complicated by the existence of over 100 serotypes of human rhinoviruses (HRVs). Accordingly, we have developed a sequence-based assay that targets a 390-bp fragment accounting for approximately two-thirds of the 5′ noncoding region (NCR). Our goal was to develop an assay permitting amplification of target sequences directly from clinical specimens and distinction among all 101 prototype strains of rhinoviruses. We determined the sequences of all 101 prototype strains of HRV in this region to enable differentiation of virus genotypes in both viral isolates and clinical specimens. We evaluated this assay in a total of 101 clinical viral isolates and 24 clinical specimens and compared our findings to genotyping results using a different region of the HRV genome (the VP4-VP2 region). Five specimens associated with severe respiratory disease in children did not correlate with any known serotype of rhinovirus and were found to belong to a novel genogroup of rhinovirus, genogroup C. Isolates were also found that corresponded to the genogroup A2 variant identified in New York and Australia and two other novel group A clusters (GAC1 and GAC2).
Human rhinoviruses (HRVs), members of the family Picornaviridae, are frequent etiological agents of acute upper respiratory tract infection. HRVs have been found to replicate effectively in lower airways and have been recovered from bronchoalveolar lavage fluids and bronchial biopsy samples (13, 19, 22, 27, 28). These viruses have been implicated as causes of asthma exacerbations (9, 25) and severe respiratory tract illnesses in children, the immunosuppressed, and the elderly (3, 5, 7, 21, 29). HRV-associated mortalities have also been recently reported (6, 11, 21, 35). Perhaps because HRV strains are often difficult to culture, few epidemiologic data exist on the relationship between the pattern and severity of clinical manifestations associated with individual serotypes (33), and no data are available regarding the biological impact of the serotype. A sensitive and specific assay that allows detection and genotyping of HRV strains in clinical specimens independently of viral isolation is needed to facilitate further investigation.
HRVs are nonenveloped positive-sense RNA viruses with a 7.2-kb genome (30). The 101 defined serotypes are currently grouped into two genogroups, A and B, based on molecular evidence from various regions of the HRV genome, including VP4, VP2, VP1, and polymerase coding regions (15, 18, 20, 31, 32). Serotyping of HRVs can be done only on HRVs grown in culture, depends on a limited supply of antibody reagents available in only a few reference laboratories, and is extremely laborious, due in part to the large number of serotypes. Molecular characterization, which is currently being used to type several viruses, including the closely related enteroviruses, is a suitable alternative (26). Assays developed for clinical detection of HRV based on reverse transcription (RT)-PCR exist but do not distinguish among HRV serotypes. Molecular analyses can distinguish all prototype HRVs but have not been fully evaluated for use with clinical isolates (14, 18, 31). The 5′ noncoding region (NCR) has been a target for some sequence-based methods, but these assays have not characterized all known prototype strains or serotypes of HRV (1, 4, 24). Here, we report our development of a method based on the 5′ NCR that allows rapid detection and typing of the all rhinovirus serotypes and compare it to genotyping by VP4-VP2 sequence analysis (31). While the manuscript was in preparation, Lee and coworkers (20) published an assay also based on the 5′ NCR that is capable of genotyping all prototype strains of rhinovirus. The differences between these two assays are highlighted in this report.
(Portions of the study were presented at the 23rd Annual Northern California American Society for Microbiology Meeting, Santa Clara, CA, 6 May 2006.)
MATERIALS AND METHODS
Virus strains and clinical isolates.
Eighty-nine prototype HRV strains were obtained from stocks maintained by the Viral and Rickettsial Disease Laboratory (VRDL) at the California Department of Public Health (Richmond, CA). HRV 90 to 97 and 100 were from stocks provided by the Centers for Disease Control (Atlanta, GA). HRV 98 and 99 were from the ATCC (Manassas, VA). As a state reference laboratory, the California Department of Public Health VRDL receives approximately 1,000 respiratory specimens annually for testing for a broad variety of viral respiratory pathogens. One hundred and one clinical isolates and 24 clinical specimens, collected from 2002 to 2007, were analyzed in this study. The clinical isolates were viruses isolated from specimen-inoculated cell culture. The clinical specimens were specimens that were culture negative and were identified as HRV positive by real-time PCR (10).
Virus isolation.
Respiratory specimens (i.e., nasopharyngeal swabs collected in viral transport media and endotracheal lavage fluids) were used to inoculate primary human fetal diploid lung and primary rhesus monkey kidney cells following standard procedures for virus isolation. In brief, viruses were passaged once onto a confluent monolayer of WI-38 cells and/or in-house human fetal diploid lung cells maintained in 90% Eagle minimal essential medium with Hanks balanced salt solution and 2% fetal bovine serum at 33°C. Cultures with full cytopathic effect were frozen and thawed three times and clarified by centrifugation at 1,100 × g for 10 min. The supernatants were collected and stored at −80°C.
Viral total-RNA extraction.
Total viral RNA was extracted from 150 μl of cell culture supernatant using a Qiaamp Viral RNA Mini Spin Kit (Qiagen, Valencia, CA) or by EasyMag (bioMérieux, Durham, NC) as recommended by the manufacturer.
RT-PCR.
First-strand cDNA was synthesized using 5 μl of extracted viral nucleic acid, random hexamer primers, and SuperScript II RTase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Primers for PCR amplification of a fragment within the 5′ NCR were designed based on an alignment of the complete 5′ NCR sequences from available full-length HRV sequences from the GenBank database (NCBI) and analysis of conserved regions within the 5′ NCR. The forward primer DK001 (11) and reverse primer DK004 (5′-CACGGACACCCAAAGTAGT-3′) were used to PCR amplify a region within the 5′ NCR as previously described (11). The PCR conditions were as follows: hot start at 95°C (5 min), followed by 40 cycles of denaturation at 95°C (15 s), annealing at 55°C (15 s), and elongation at 72°C (60 s), resulting in amplification of a fragment approximately 400 bp in length.
Purification and sequencing of PCR products.
The PCR products were purified using a Qiaquick PCR Purification Kit (Qiagen, Valencia, CA) and sequenced in both directions using the Sanger dideoxy cycle-sequencing method with the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit according to the manufacturer's instructions using the 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Sequence alignment and phylogenetic analyses.
Multiple sequences were aligned using Clustal X (v1.83). The multiple-sequence alignment was subjected to phylogenetic analyses using programs in the PHYLIP package (v3.6). Distance matrices were calculated using DNADIST. Bootstrap analysis was performed using SEQBOOT, in which 100 or 1,000 data sets were used, and phylogenetic relationships were assessed using neighbor-joining, maximum-parsimony, and maximum-likelihood methods. Consensus trees were computed using CONSENSE, and phylogenetic trees were visualized using TREEVIEW (v1.6.6). Twenty-seven published HRV sequences within the 5′ NCR were obtained from the GenBank database (NCBI). Distance matrices were calculated by the MegAlign program of the DNASTAR Lasergene 7.1 software (Madison, WI) using the Clustal W method.
Nucleotide sequence accession numbers.
Rhinovirus sequences have been submitted to the GenBank database (accession no. FJ231271 to FJ231290).
RESULTS
Sequence analysis of 101 prototype HRVs.
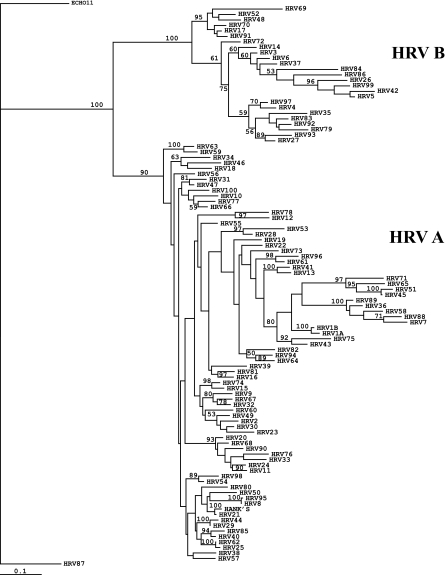
To demonstrate the broad reactivity of the 5′ NCR primers for PCR, a total of 74 HRV prototype strains (see Table S1 in the supplemental data) were amplified and sequenced for the approximately 390-bp region defined by primers DK001 and DK004. Corresponding sequences for HRV 1A, 1B, 2, 6, 7, 14, 16, 17, 21, 29, 37, 39, 49, 51, 52, 58, 59, 62, 69, 70, 72, 84, 85, 86, 87, 89, and 91 were obtained from the GenBank database. A total of 101 HRV prototype sequences were analyzed, along with a recent clinical isolate identified as HRV Hanks at the VRDL (T03-0053). For phylogenetic analysis, an approximately 310-nucleotide (nt) segment internal to the sequenced region that consistently provided clear sequence peaks was used. All HRV prototype strains had unique genomic sequences in the 5′ NCR and clustered into two groups, HRV-A and HRV-B (Fig. 1). HRV 87, which was determined to be very similar to enterovirus 68, a group D enterovirus (2), did not cluster with the remaining HRVs. The percent divergence for all 102 HRV sequences analyzed ranged from 0.3 to 63.3% at the nucleotide level (Fig. 2). Variation among the genogroup A strains ranged from 0.3 to 40.2%, and that among genogroup B strains was from 3.3 to 60.8%. Analysis of the frequencies of occurrence and pairwise divergences among HRVs in both genogroups is shown in Fig. 2. Heterologous HRV pairs that had a divergence of less than 7% are listed in Table 1. Among the group A HRVs, HRV 8/95, HRV 25/62, HRV 21/Hanks, HRV 29/44, and HRV 1A/1B had divergences of less than 3%. HRV 17/91 and HRV 17/70 were among the group B HRVs with divergence of less than 5%. Among all the prototype strains, the greatest divergence (63.3%) was between HRV 84, in group B, and HRV 36, in group A.
FIG. 1.
Phylogenetic tree of HRV prototype strains based on analysis of the 5′ NCR. HRVs cluster into genogroups A and B. HRV 87, which is more closely related to enteroviruses, demonstrates closer relationship to ECHO 11 (the outgroup) than other HRVs.
FIG. 2.
Pairwise nucleotide divergence betweeen HRV prototype strains. Percent divergence in increments of 10% was plotted versus the frequency of occurrence. The range was from 0.3 to 63.3%.
TABLE 1.
HRV pairs with less than 7% nucleotide divergence in the 5′ NCR
| No. | HRV serotype pair | % Nucleotide divergence |
|---|---|---|
| 1 | 8/95a | 0.3 |
| 2 | 25/62a | 1.0 |
| 3 | 21/Hanksa | 2.0 |
| 4 | 29/44a | 2.0 |
| 5 | 1a/1ba | 2.6 |
| 6 | 45/51 | 3.0 |
| 7 | 17/91 | 3.3 |
| 8 | 17/70 | 4.3 |
| 9 | 40/85 | 4.4 |
| 10 | 15/74 | 4.8 |
| 11 | 20/68 | 4.8 |
| 12 | 31/47 | 4.8 |
| 13 | 4/97 | 5.4 |
| 14 | 36/89 | 5.5 |
| 15 | 59/63 | 5.8 |
| 16 | 70/91 | 5.8 |
| 17 | 3/6 | 6.1 |
| 18 | 13/48 | 6.1 |
| 19 | 54/98 | 6.1 |
| 20 | 66/77 | 6.1 |
| 21 | 21/40 | 6.4 |
| 22 | 83/92 | 6.4 |
| 23 | 11/24 | 6.6 |
| 24 | 2/30 | 6.8 |
| 25 | 30/49 | 6.8 |
| 26 | 32/67 | 6.9 |
Pair with less than 3% divergence.
Phylogenetic analysis of clinical HRV isolates.
To evaluate the ability of the assay to genotype HRVs, a total of 101 unknown clinical respiratory isolates were tested in this study. Eighty-six isolates were successfully amplified, sequenced, and identified by comparison with our database of reference strain 5′ NCR sequences (Tables 2 and 3); 15 were HRV 5′ NCR PCR negative. Sequence identity comparisons and phylogenetic analysis of the prototype strains and clinically isolated strains led to the association of clinical isolates with a single prototype strain of HRV (Fig. 3 and 4). There were a total of 76 group A and 8 group B HRVs (Tables 2 and 3) representing 44 different types of HRVs and 1 type of enterovirus.
TABLE 2.
Serotyping results for clinical HRV isolates that yielded the same results in both 5′ NCR and VP4-VP2 sequence analysesa
| No. | RV isolate | Serotyping result |
|---|---|---|
| 1 | BMT306 | 31 |
| 2 | T02-0106 | 22 |
| 3 | T02-0786 | 31 |
| 4 | T02-0928 | Negb |
| 5 | T02-0968 | Neg |
| 6 | T02-1301 | Neg |
| 7 | T02-2397 | 42 |
| 8 | T02-2433 | 47 |
| 9 | T02-2476 | 47 |
| 10 | T02-2477 | Neg |
| 11 | T02-2616 | 34 |
| 12 | T02-2857 | 39 |
| 13 | T03-0037 | 49 |
| 14 | T03-0053 | Hanks |
| 15 | T03-0066 | Neg |
| 16 | T03-0078 | 87 |
| 17 | T03-0599 | 47 |
| 18 | T03-0600 | 47 |
| 19 | T03-0634 | 47 |
| 20 | T03-0655 | 95 |
| 21 | T03-1753 | 47 |
| 22 | T03-1808 | 39 |
| 23 | T03-2119 | 36 |
| 24 | T03-2151 | 55 |
| 25 | T03-2430 | 94 |
| 26 | T03-2431 | 94 |
| 27 | T03-2434 | 47 |
| 28 | T03-3194 | 44 |
| 29 | T03-3596 | 65 |
| 30 | T03-4111 | 47 |
| 31 | T03-4112 | 47 |
| 32 | T03-4196 | 1b |
| 33 | T03-4311 | 94 |
| 34 | T03-4474 | 47 |
| 35 | T03-4481 | 82 |
| 36 | T04-0424 | 1b |
| 37 | T04-0714 | 56 |
| 38 | T04-0946 | 16 |
| 39 | T04-0964 | 43 |
| 40 | T04-1004 | 28 |
| 41 | T04-1325 | 75 |
| 42 | T04-1411 | 59 |
| 43 | T04-2387 | 28 |
| 44 | T04-2896 | Neg |
| 45 | T04-3190 | 70 |
| 46 | T04-3247 | 7 |
| 47 | T04-3462 | Neg |
| 48 | T04-3552A | 33 |
| 49 | T04-3552B | 33 |
| 50 | T04-3607 | 72 |
| 51 | T04-3641 | 29 |
| 52 | T04-3642 | 29 |
| 53 | T04-3643 | 29 |
| 54 | T04-3644 | 29 |
| 55 | T04-3645 | 29 |
| 56 | T04-3738 | 61 |
| 57 | T04-3747 | 91 |
| 58 | T04-3900 | Neg |
| 59 | T04-3901 | Neg |
| 60 | T04-3903 | 48 |
| 61 | T04-3906 | 44 |
| 62 | T04-3933 | 7 |
| 63 | T04-3934 | 44 |
| 64 | T04-3935 | Neg |
| 65 | T04-3936 | Neg |
| 66 | T04-3937 | Neg |
| 67 | T04-3938 | Neg |
| 68 | T04-3939 | 44 |
| 69 | T04-4103 | 2 |
| 70 | T04-4113 | Neg |
| 71 | T04-4310 | 49 |
| 72 | T05-0000 | 46 |
| 73 | T05-1262 | 38 |
| 74 | T05-1430 | 22 |
| 75 | T05-1688 | 29 |
| 76 | T05-1738 | 76 |
| 77 | T05-1746 | Neg |
| 78 | T05-2142 | 19 |
| 79 | T05-2181 | 19 |
| 80 | T05-A001 | 13 |
| 81 | T06-1884 | 9 |
| 82 | T06-1895 | 52 |
| 83 | T06-2157 | 8 |
| 84 | T06-5376 | 49 |
| 85 | T06-5377 | 49 |
A total of 71 isolates were identified by both 5′ NCR and VP4-VP2 PCR. One isolate, T06-1482 (not listed), was identified as HRV 88 by 5′ NCR and HRV 63 by VP4-VP2 analysis. Fifteen isolates were negative by both PCRs. Group B HRVs are highlighted in boldface.
Neg, negative.
TABLE 3.
Serotyping results for HRV isolates that yielded positive results from 5′ NCR and negative results from VP4-VP2 PCR
| No. | RV isolate | 5′ NCR result | VP4-VP2 resultb |
|---|---|---|---|
| 1 | T03-3195 | 44 | Neg |
| 2 | T05-1034 | 58 | Neg |
| 3 | T05-1161 | 22 | Neg |
| 4 | T05-1169 | EV71a | Neg |
| 5 | T05-1711 | 58 | Neg |
| 6 | T05-2094 | 43 | Neg |
| 7 | T06-0477 | 88 | Neg |
| 8 | T06-3226 | 52 | Neg |
| 9 | T06-4424 | 44 | Neg |
| 10 | T06-4573 | 45 | Neg |
| 11 | T06-4862 | 49 | Neg |
| 12 | T06-5375 | EV71a | Neg |
| 13 | T05-5509 | 54 | Neg |
| 14 | T07-1647H | 1B | Neg |
| 15 | T06-5378 | 49 | Neg |
Isolate identified as enterovirus.
Neg, negative.
FIG. 3.
Phylogenetic tree of HRV group A prototype strains and clinical viral isolates based on 5′ NCR analysis. ECHO 11 was defined as the outgroup. T06-3575 and T05-1169 were more closely related to enterovirus 71 in the 5′ NCR and were identified as enteroviruses.
FIG. 4.
Phylogenetic tree of HRV group B prototype strains and clinical viral isolates based on 5′ NCR analysis. ECHO 11 was defined as an outgroup.
To determine the assay feasibility for performing genotyping, comparisons were done with a reference molecular assay with reliable genotyping results (31). Thus, in addition to 5′ NCR analysis, all 86 clinical isolates were subjected to genotyping by a previously described RT-PCR and sequencing assay using primers targeting the genes for structural proteins VP4 and VP2 (31). Using identical nucleic acid extracts, the VP4-VP2 region primers amplified 71 out of 86 (83%) 5′ NCR-positive clinical isolates (Tables 2 and 3). Comparison of the genotyping results based on the VP4-VP2 region with 5′ NCR results indicated that 70 out of 71 (99%) isolates resulted in the same genotype identification. One isolate, T06-1482, was identified as HRV 88 by 5′ NCR and HRV 63 by VP4-VP2. In two cases, the 5′ NCR RT-PCR assay could effectively detect and differentiate between HRV and human enterovirus.
Direct application to clinical specimens.
The applicability of this assay to genotyping clinical specimens was assessed. Total nucleic acid was extracted from original clinical specimens, including nasopharyngeal swabs, nasopharyngeal aspirates/washes, endotracheal aspirates, bronchoalveolar lavage fluids, and pleural fluid from patients with acute respiratory illnesses. A total of 24 direct clinical isolates that were cell culture negative were processed for genotyping. Among the 24 specimens, 10 were from children hospitalized in a pediatric intensive-care unit with severe respiratory illness, among which seven patients were coinfected with another agent (J. K. Louie, A. Roy-Burman, L. Guardia-Labar, E. Boston, D. Kiang, T. Padilla, S. Yagi, S. Messenger, C. A. Glaser, and A. Petru, unpublished data); 1 was from a 40-year-old bone marrow transplant patient with a coinfection by parainfluenza virus type 1; 1 was from an infant with encephalitis (California Encephalitis Project); and 8 were from a pediatric outbreak of enterovirus (Maniilaq Hospital, Alaska) with symptoms including fever and respiratory distress, myocarditis, and/or meningitis. All 24 samples amplified a specific product and were identified by amplicon sequencing and comparison with a 5′ NCR database of sequences. All amplicons yielded readable sequences. VP4-VP2 PCR failed to detect HRV in all 24 samples using cDNA templates identical to those used for 5′ NCR PCR.
A novel clade of HRVs, group C.
Phylogenetic analysis of the 24 direct clinical specimens identified five HRVs that belonged to the novel HRV group C (20). Other recent HRVs described as novel strains, and for which 5′ NCR sequences were available, were included in the analysis. Complete or nearly complete genome sequences were valuable in enabling comparisons between 5′ NCR strains and those analyzed by other regions of the HRV genome, such as VP4-VP2. Group C HRVs were identified in three hospitalized cases of severe respiratory illness requiring pediatric intensive care (Louie et al., unpublished), one bone marrow transplant patient on immunosuppressive agents, and one infant hospitalized with encephalitis.
Pairwise comparisons among the five group C HRVs indicated a range of 10.2% (BMT303/T07-2387) to 37.8% (T07-2385/T07-1639) divergence. Compared to E126788-W37 (a Wisconsin isolate), there was a range of 24.3 to 31.8% divergence. Among the 24 specimens, there were at least 10 genotypes represented (HRV 1B, 10, 12, 36, 45, 56, 65, 80, HRV A2, and HRV C, and two novel clusters within group A, GAC1 and GAC2).
DISCUSSION
Molecular typing of rhinovirus by 5′ NCR RT-PCR and sequence analysis represents a relatively simple and rapid method of identifying the HRV serotype. With human enteroviruses, the 5′ NCR is not the ideal region for genotyping because of the extensive recombination rate within this group of viruses (34, 36). However, HRVs do not appear to have the same level of recombination, as evidenced by the high level of correlation between the 5′ NCR genotyping results and those obtained by the analysis of HRV structural genes, encoding VP4 and VP2 (Table 2). Among the prototype strains, the HRV phylogenetic grouping (Fig. 1) was comparable to the A and B grouping from previous data obtained from VP4-VP2, VP1 and -2A, and VP1 and -3D analysis (14, 15, 18, 31, 32). Pairwise divergence distribution (Fig. 2) resulted in two peaks, demonstrating typical intraserotypic and interserotypic patterns among group A and B HRVs, with a maximum pairwise divergence of 63.3%. Strain pairs with divergence of less than 3% were Hanks/HRV 21, HRV 8/95, HRV 25/62, HRV 29/44, and HRV 1a/1b (Table 1), supporting neutralization data suggesting that the Hanks strain should be classified as HRV 21 and that HRV 8 and 95 are the same serotype (18). The close relationships among these five pairs are also reflected in studies analyzing VP4-VP2 and VP1 (18, 31). One difference from the study by Savolainen et al. (31) was noted for HRV 31 and 32 (less than 10% difference). In this study, HRV 31 and HRV 32 showed an 18.4% divergence and were closer to HRV 47 and HRV 67/HRV 9, respectively, an observation also noted by Ledford et al. (18) analyzing VP1. 5′ NCR RT-PCR demonstrated greater sensitivity than VP4-VP2 PCR, as reflected by the higher positivity rate in amplification of clinical isolates. The use of VP1 PCR analysis is complicated by the requirement for multiple primer pairs for PCR (18).
During this study, another independent study examining the 5′ NCR of all prototype strains for genotyping was under way (20). There are some key differences between the two studies. First, our method does not require cloning of the amplified PCR product, a process that adds expenditure of time and reagents. Our method was able to eliminate cloning, a step necessary in Lee et al.'s method because the region selected for evaluation in their phylogenetic analysis requires sequencing across the PCR primer annealing region, a region that typically yields unreliable sequences in the absence of cloning. We selected a 310-bp region internal to the 390-bp PCR product that consistently provided reliable sequence results directly from the PCR product. A second difference is our use of an extraction method that does not utilize phenol and so avoids the accumulation of toxic chemical wastes. Third, our use of a single primer set eliminates the need to perform an additional PCR and thus saves time. Fourth, our method eliminates the need for nested amplification (20), which is prone to contamination due to the handling of amplified products. Finally, our assay covers a larger fragment of the 5′ NCR, which may confer advantages in distinguishing among serotypes that are closely related. The maximum pairwise relationship between all prototype HRVs is 63.3% using the 310-nt region compared to 45% using the smaller 260- to 270-nt fragment used by Lee et al. (20).
A number of novel HRV genogroups have been identified recently, possibly due to the greater sensitivity of current molecular detection assays. Comparisons of these novel HRVs is difficult, since they utilize different regions of the HRV genome. Some of these novel HRVs have been notable for their association with severe respiratory illnesses among children and infants (17, 20, 23). Savolainen et al. noted the appearance of strains from a collection of more than 3,000 nasopharyngeal aspirates and middle ear fluid specimens in children with acute respiratory tract infections that were divergent from the 101 prototype strains based on the VP4-VP2 region of the HRV genome (region analyzed, VP4-VP2; study period, 1994 to 1996) (33). Lamson et al. in 2006 (16) reported a novel rhinovirus genotype associated with influenza-like illnesses in New York (VP4; 2004 and 2005). Strains were subsequently identified in Australia as the HRV A2 subtype among infants with bronchiolitis (23) (VP1 and VP4-VP2), in Hong Kong as HRV “C” (VP4, 5′ NCR; VP1, 3C and 3D) among children with acute respiratory illness (17), and in Germany as HRV “X” (VP4-VP2; 2003 to 2006) among children with severe respiratory infections (29). A study of adult volunteers performed at the University of California San Francisco (UCSF) (12) found some novel strains with homology to those identified by Lamson et al. (16), as well as some strains with less than 85% identity to these strains (VP4-VP2; 2001 to 2004). More recently, Lee et al. (20) identified a novel group, HRV C, in Wisconsin (5′ NCR; 1999 to 2001). Some of these novel strains for which the 5′ NCR sequences were available were included in our analysis for comparison.
Using our 5′ NCR-based assay, we identified an HRV group, group C, which is quite distinct from groups A and B (Fig. 5) and very similar to the HRV described in the Wisconsin study (20). In addition, other novel strains were identified, which fall into three distinct clusters. One of these clusters is identical to the QPM strain for which the complete genome sequence is available (EF186077). This strain was described by McErlean et al. (23) as an HRV A2 strain and has similarities to the strains described by Lamson et al. in New York (16). A recent report of HRV “C” by Lau et al. (17) showed closer similarities to HRV A2 than to HRV C. Phylogenetic analysis based on the 5′ NCR in the context of all 101 prototype strains indicated that QPM, HRV “C” strain 026 (17), and HRV X1 (12) should be grouped into the same A2 cluster (Fig. 5). T07-1643, identified in this study, also groups under this A2 cluster. Currently, the only reported HRV group C strains are identified in this and the Wisconsin study (20). In this study, five strains were identified as group C HRVs (T07-1639, T07-2385, T07-0049, T07-2387, and BMT303), which clustered with the HRV C strain W37 from Wisconsin (20). In addition, a different cluster (GAC1) was found that includes the HRV “C” strain 025 from Hong Kong (EF582386) (17), HRV X2 from UCSF (EF077280) (12), T07-4473, and T07-2103 (this study). A final cluster, GAC2, was found that includes strains W38 (E126789) (20) and T07-4480 (this study). Other strains, T06-0477 and the HRV “C” strain 024 (EF582385) (17), may also be emerging as additional novel strains of HRV.
FIG. 5.
Phylogenetic tree of all 101 HRV prototype strains clustering in group A (shown in blue), group B (green), and group C (purple) and clinical viral isolates (red) based on 5′ NCR analysis. Group C strains include T07-1639, T07-2385, T07-0049, and T07-2387 (this study) and strain W37 from Wisconsin (GenBank accession no. E126788). HRVA2 strains (highlighted by an orange arrow) include strain X1 from UCSF (EF077279), strain 026 from Hong Kong (EF582387), strain QPM from Australia (EF186077), and T07-1643 (this study). GAC1 strains (highlighted by a brown arrow) include strain 003 from Hong Kong (EF582386), strain X2 from UCSF (EF077280), and T07-4473 and T07-2103 (this study). GAC2 strains (highlighted by a pink arrow) include strain W38 from Wisconsin (E126789) and T07-4480 (this study). ECHO 11 was defined as an outgroup.
Typing of individual HRV isolates will allow better understanding of an association of genotypes with specific disease attributes or viral immunity. Recent data suggest that HRV infection can be associated with severe lower respiratory tract infection in children and the elderly (11, 21). Although asymptomatic infections have been reported (8), Andeweg et al. have shown that patients who had recovered from rhinovirus infections no longer had detectable levels of rhinovirus (1), suggesting that these patients were not carriers of HRV. The failure of the HRVs derived from the clinical specimens in this study to grow in culture suggests that these viruses have diverged from prototype strains. This is further supported by the tendency of some recent isolates to cluster some distance from prototypes and suggests that a database of recent isolates may prove useful to define currently circulating HRVs.
Supplementary Material
Acknowledgments
We are grateful to Shilpa Gavali, Cynthia Jean, and Somayeh Honarmand of the California Encephalitis Project and Erica Boston of the California Respiratory Project for their support in coordinating surveillance and the collection of the epidemiologic and clinical data, as well as specimens. We also thank Terry Schmidt and Elizabeth Funk from the State of Alaska Health and Social Services for providing specimens from the Maniilaq Hospital outbreak.
This study was supported by the California Department of Public Health and a grant from the National Institutes of Allergy and Infectious Diseases (Program Project grant AI-50496).
Footnotes
Published ahead of print on 27 August 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Andeweg, A. C., T. M. Bestebroer, M. Huybreghs, T. G. Kimman, and J. C. de Jong. 1999. Improved detection of rhinoviruses in clinical samples by using a newly developed nested reverse transcription-PCR assay. J. Clin. Microbiol. 37524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomqvist, S., C. Savolainen, L. Raman, M. Roivainen, and T. Hovi. 2002. Human rhinovirus 87 and enterovirus 68 represent a unique serotype with rhinovirus and enterovirus features. J. Clin. Microbiol. 404218-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheuk, D. K., I. W. Tang, K. H. Chan, P. C. Woo, M. J. Peiris, and S. S. Chiu. 2007. Rhinovirus infection in hospitalized children in Hong Kong: a prospective study. Pediatr. Infect. Dis. J. 26995-1000. [DOI] [PubMed] [Google Scholar]
- 4.Deffernez, C., W. Wunderli, Y. Thomas, S. Yerly, L. Perrin, and L. Kaiser. 2004. Amplicon sequencing and improved detection of human rhinovirus in respiratory samples. J. Clin. Microbiol. 423212-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutman, J. A., A. J. Peck, J. Kuypers, and M. Boeckh. 2007. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant. 40809-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hicks, L. A., C. W. Shepard, P. H. Britz, D. D. Erdman, M. Fischer, B. L. Flannery, A. J. Peck, X. Lu, W. L. Thacker, R. F. Benson, M. L. Tondella, M. E. Moll, C. G. Whitney, L. J. Anderson, and D. R. Feikin. 2006. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J. Am. Geriatr. Soc. 54284-289. [DOI] [PubMed] [Google Scholar]
- 7.Imakita, M., K. Shiraki, C. Yutani, and H. Ishibashi-Ueda. 2000. Pneumonia caused by rhinovirus. Clin. Infect. Dis. 30611-612. [DOI] [PubMed] [Google Scholar]
- 8.Ireland, D. C., J. Kent, and K. G. Nicholson. 1993. Improved detection of rhinoviruses in nasal and throat swabs by seminested RT-PCR. J. Med. Virol. 4096-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston, N. W., S. L. Johnston, J. M. Duncan, J. M. Greene, T. Kebadze, P. K. Keith, M. Roy, S. Waserman, and M. R. Sears. 2005. The September epidemic of asthma exacerbations in children: a search for etiology. J. Allergy Clin. Immunol. 115132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kares, S., M. Lonnrot, P. Vuorinen, S. Oikarinen, S. Taurianen, and H. Hyoty. 2004. Real-time PCR for rapid diagnosis of entero- and rhinovirus infections using LightCycler. J. Clin. Virol. 2999-104. [DOI] [PubMed] [Google Scholar]
- 11.Kiang, D., S. Yagi, K. A. Kantardjieff, E. J. Kim, J. K. Louie, and D. P. Schnurr. 2007. Molecular characterization of a variant rhinovirus from an outbreak associated with uncommonly high mortality. J. Clin. Virol. 38227-237. [DOI] [PubMed] [Google Scholar]
- 12.Kistler, A., P. C. Avila, S. Rouskin, D. Wang, T. Ward, S. Yagi, D. Schnurr, D. Ganem, J. L. DeRisi, and H. A. Boushey. 2007. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 196817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krilov, L., L. Pierik, E. Keller, K. Mahan, D. Watson, M. Hirsch, V. Hamparian, and K. McIntosh. 1986. The association of rhinoviruses with lower respiratory tract disease in hospitalized patients. J. Med. Virol. 19345-352. [DOI] [PubMed] [Google Scholar]
- 14.Laine, P., S. Blomqvist, C. Savolainen, K. Andries, and T. Hovi. 2006. Alignment of capsid protein VP1 sequences of all human rhinovirus prototype strains: conserved motifs and functional domains. J. Gen. Virol. 87129-138. [DOI] [PubMed] [Google Scholar]
- 15.Laine, P., C. Savolainen, S. Blomqvist, and T. Hovi. 2005. Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses. J. Gen. Virol. 86697-706. [DOI] [PubMed] [Google Scholar]
- 16.Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St George, T. Briese, and W. I. Lipkin. 2006. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J. Infect. Dis. 1941398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau, S. K., C. C. Yip, H. W. Tsoi, R. A. Lee, L. Y. So, Y. L. Lau, K. H. Chan, P. C. Woo, and K. Y. Yuen. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J. Clin. Microbiol. 453655-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz, M. S. Collett, and D. C. Pevear. 2004. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 783663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, B. E., J. L. Robinson, V. Khurana, X. L. Pang, J. K. Preiksaitis, and J. D. Fox. 2006. Enhanced identification of viral and atypical bacterial pathogens in lower respiratory tract samples with nucleic acid amplification tests. J. Med. Virol. 78702-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, W. M., C. Kiesner, T. Pappas, I. Lee, K. Grindle, T. Jartti, B. Jakiela, R. F. Lemanske, P. A. Shult, and J. E. Gern. 2007. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE 2e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie, J. K., S. Yagi, F. A. Nelson, D. Kiang, C. A. Glaser, J. Rosenberg, C. K. Cahill, and D. P. Schnurr. 2005. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin. Infect. Dis. 41262-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malcolm, E., E. Arruda, F. G. Hayden, and L. Kaiser. 2001. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J. Clin. Virol. 219-16. [DOI] [PubMed] [Google Scholar]
- 23.McErlean, P., L. A. Shackelton, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2007. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J. Clin. Virol. 3967-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori, J., and J. P. Clewley. 1994. Polymerase chain reaction and sequencing for typing rhinovirus RNA. J. Med. Virol. 44323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicholson, K. G., J. Kent, and D. C. Ireland. 1993. Respiratory viruses and exacerbations of asthma in adults. BMJ 307982-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nix, W. A., M. S. Oberste, and M. A. Pallansch. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 442698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopoulos, N. G., G. Sanderson, J. Hunter, and S. L. Johnston. 1999. Rhinoviruses replicate effectively at lower airway temperatures. J. Med. Virol. 58100-104. [DOI] [PubMed] [Google Scholar]
- 28.Puro, V., C. Minosse, G. Cappiello, F. N. Lauria, and M. R. Capobianchi. 2005. Rhinovirus and lower respiratory tract infection in adults. Clin. Infect. Dis. 401068-1069. [DOI] [PubMed] [Google Scholar]
- 29.Renwick, N., B. Schweiger, V. Kapoor, Z. Liu, J. Villari, R. Bullmann, R. Miething, T. Briese, and W. I. Lipkin. 2007. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J. Infect. Dis. 1961754-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rueckert, R. R. 1996. Picornaviridae: the viruses and their replication, p. 609-654. In B. N. Knipe, D. M. Howley, P. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, PA.
- 31.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83333-340. [DOI] [PubMed] [Google Scholar]
- 32.Savolainen, C., P. Laine, M. N. Mulders, and T. Hovi. 2004. Sequence analysis of human rhinoviruses in the RNA-dependent RNA polymerase coding region reveals large within-species variation. J. Gen. Virol. 852271-2277. [DOI] [PubMed] [Google Scholar]
- 33.Savolainen, C., M. N. Mulders, and T. Hovi. 2002. Phylogenetic analysis of rhinovirus isolates collected during successive epidemic seasons. Virus Res. 8541-46. [DOI] [PubMed] [Google Scholar]
- 34.Simmonds, P., and J. Welch. 2006. Frequency and dynamics of recombination within different species of human enteroviruses. J. Virol. 80483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald, T. G., P. Shult, P. Krause, B. A. Miller, P. Drinka, and S. Gravenstein. 1995. A rhinovirus outbreak among residents of a long-term care facility. Ann. Intern. Med. 123588-593. [DOI] [PubMed] [Google Scholar]
- 36.Yoke-Fun, C., and S. AbuBakar. 2006. Phylogenetic evidence for inter-typic recombination in the emergence of human enterovirus 71 subgenotypes. BMC Microbiol. 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.