Abstract
Signals produced in leaves are transported to the shoot apex where they cause flowering. Protein of the gene FLOWERING LOCUS T (FT) is probably a long day (LD) signal in Arabidopsis. In the companion paper, rapid LD increases in FT expression associated with flowering driven photosynthetically in red light were documented. In a far red (FR)-rich LD, along with FT there was a potential role for gibberellin (GA). Here, with the GA biosynthesis dwarf mutant ga1-3, GA4-treated plants flowered after 26 d in short days (SD) but untreated plants were still vegetative after 6 months. Not only was FT expression low in SD but applied GA bypassed some of the block to flowering in ft-1. On transfer to LD, ga1-3 only flowered when treated simultaneously with GA, and FT expression increased rapidly (<19.5 h) and dramatically (15-fold). In contrast, in the wild type in LD there was little requirement for GA for FT increase and flowering so its endogenous GA content was near to saturating. Despite this permissive role for endogenous GA in Columbia, RNA interference (RNAi) silencing of the GA biosynthesis gene, GA 20-OXIDASE2, revealed an additional, direct role for GA in LD. Flowering took twice as long after silencing the LD-regulated gene, GA 20-OXIDASE2. Such independent LD input by FT and GA reflects their non-sympatric expression (FT in the leaf blade and GA 20-OXIDASE2 in the petiole). Overall, FT acts as the main LD floral signal in Columbia and GA acts on flowering both via and independently of FT.
Keywords: Arabidopsis, far-red light, flowering, FT, gibberellin, c long day.l
Introduction
Recent studies of floral signalling in Arabidopsis (Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007), Cucumis spp (Lin et al. 2007), and rice (Tamaki et al., 2007) have indicated that FLOWERING LOCUS T (FT) could be involved in long day (LD) floral signalling, its protein acting as a signal transported from the photoinduced leaves to the shoot apex where it evokes flowering (see reviews in Kobayashi and Weigel, 2007; Turck et al., 2008).
The nature of the LD photoresponse(s) can be critical for understanding FT regulation of flowering. As documented in the companion paper (King et al., 2008), in a high light intensity LD from red light (R)-rich lamps, photosynthesis up-regulates FT expression and causes flowering of Arabidopsis. In contrast, an LD from far-red-rich lamps (LD-FR) up-regulates FT, causes flowering, and, in addition, increases biosynthesis of the gibberellin (GA) class of plant growth regulator (Xu et al., 1997; Gocal et al., 2001; Hisamatsu et al., 2005). Comparable FR-regulated LD increases in GA content have been widely reported for other species (see reviews by García-Martínez and Gil, 2002; King and Evans, 2003) so GA could act as an additional LD signal.
For the LD grass, Lolium temulentum, both GA and FT may regulate its flowering (King et al., 2006), but genetic analysis has not been possible. For Arabidopsis, in contrast, genetic studies do not implicate GA in the LD response but show that it is needed for flowering in short days (SD) (see reviews in Boss et al., 2004; Searle and Coupland, 2004; Imaizumi and Kay, 2006). The evidence of large increases in shoot tip GA during the transition to flowering in SD (Eriksson et al., 2006) is consistent with the genetic evidence, but none of these studies rule out a role for GA in LD flowering.
Here the contribution of FT and GA to LD flowering of Arabidopsis has been examined. Using genetic and molecular approaches, the potential for FT and GA to act both independently and interactively in LD floral signalling is documented.
Materials and methods
Plant material, growing conditions, and LD treatment
Plants of Arabidopsis thaliana (L.) Heynh., ecotype Columbia, mutants and RNA interference (RNAi) silencing lines were grown vegetatively for 5 weeks in 8 h SD at 22 °C under an irradiance of 100 μmol m−2 s−1 from fluorescent lamps. In the case of the ga1-3 mutant, it was grown in these SD conditions for 3 months. When exposed to an LD for floral induction, the main 8 h light period was extended by 16 h to give a total of 24 h light. This single LD was at a low intensity (10 μmol m−2 s−1) from incandescent bulbs (FR-enriched light; LD-FR) or from R-rich fluorescent lamps (LD-lR). In a few instances the LD exposure was for two cycles and involved a low intensity FR-rich LD or an LD from R-rich fluorescent lamps at 100 μmol m−2 s−1 (LD-R). Treatments where LD was for more than one cycle enhanced the response somewhat. More details of such LD treatments and responses are given in the companion paper. Plants of Columbia retained in SD remained vegetative for at least another 6 weeks, whereas those of ga1-3 were still vegetative 3 months later.
Mutants and gene silencing lines were all in ecotype Columbia. The ga1-3 mutant from Landsberg erecta had been backcrossed six times into Columbia (Tyler et al., 2004). The GA 20-OXIDASE1 T-DNA insert null mutant, ga5-3, and RNAi silencing lines for GA 20-OXIDASE2 were described in Hisamatsu et al. (2005). Subsequently this ga5-3 mutant has been renamed by Rieu and co-workers (2007) as ga20ox1-3 but, for continuity, the original terminology has been retained here. The ft-1 mutant is described in the companion paper.
Chemical treatments
GA4 (1 mM) was applied either as a 10 μl drop to three leaves or as a spray to run off. Response was similar in these treatments, and this is in accordance with the known transport of GA4 in Arabidopsis (Ericksson et al., 2006). Control plants were treated with the same aqueous solvent containing 20% ethanol and 0.02% Tween-20. A commercially available GA biosynthesis inhibitor, paclobutrazol ([2S,3S; 2R,3R]-1-[4-chlorophenyl]-4,4-dimethyl-2-[1,2,4-triazol-l-yl] pentan-3-ol), was applied as a 6 ml pot drench at a dose of 0.05 mg ml−1 in water.
Errors are shown as means ±SE. In many instances the error was smaller than the symbol and is not evident in the figures. All experiments reported here were repeated at least once.
Quantitative real-time PCR analysis of gene expression
Conditions, primers, and materials for gene expression studies were as documented in the companion paper and previously by Hisamatsu et al. (2005).
Results
In the companion paper we detailed distinct LD light responses which trigger rapid and obligate flowering in Arabidopsis, ecotype Columbia. Briefly, in a high light intensity, R-rich LD, photosynthesis up-regulated FT expression and flowering while at a 10-fold lower intensity, an FR-rich LD acting independently of photosynthesis rapidly up-regulated FT and induced flowering. Plants in a low light intensity R-rich LD or in SD showed weak FT expression and remained vegetative for ≥6 weeks.
Because an FR-rich LD activates GA biosynthesis in the petioles of Columbia (Gocal et al., 2001; Hisamatsu et al., 2005), three approaches have been used to examine potential GA/FT regulation of flowering. First, to determine if FT and endogenous GA might act in concert, GA biosynthesis has been blocked in a mutant or with a GA biosynthesis inhibitor. Secondly, GA regulation of flowering has been examined in application studies with Columbia and the ft-1 mutant. Lastly, the role of GA biosynthesis in LD flowering has been examined by silencing a GA 20-OXIDASE genes along with analysis of tissue specificity of gene expression patterns.
Inhibition of GA synthesis, LD flowering, and a role for FT
The GA1 gene of Arabidopsis regulates an early step of GA biosynthesis (Zeevaart and Talon, 1992), and the ga1-3 mutant is dwarfed and flowers late in SD unless treated with GA over many weeks (Koorneef and van der Veen, 1980; Wilson et al., 1992; Putterill et al., 1995; Reeves and Coupland, 2001; Ericksson et al., 2006; Rieu et al., 2008). In LD, ga1-3 can flower reasonably rapidly although with some delay relative to GA-treated LD plants (Koorneef and van der Veen, 1980; Wilson et al., 1992; Putterill et al., 1995; Reeves and Coupland, 2001; Ericksson et al., 2006; Rieu et al., 2008).
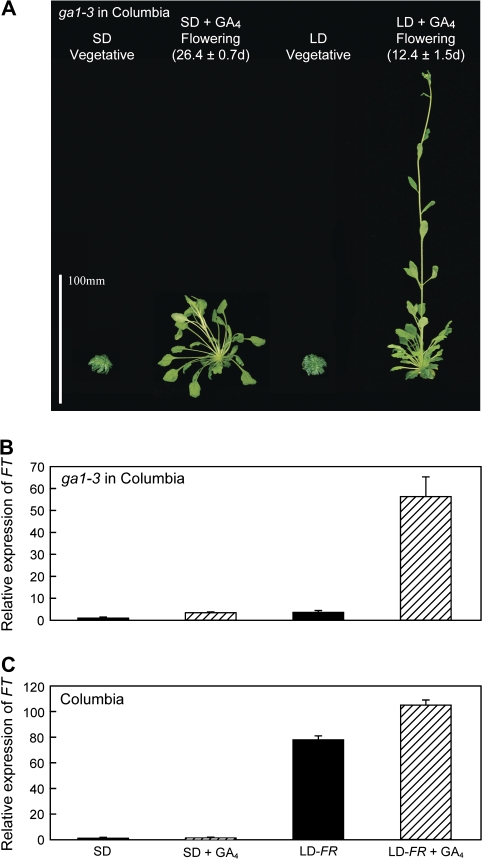
In the present studies, ga1-3 in Columbia was vegetative and severely dwarfed after 6 months in 8 h SD (cf. Fig 1). It also failed to flower when exposed at 3 months to 30 LD either from FR-rich incandescent lamps or at a high intensity from R-rich fluorescent lamps (data not shown). However, for plants grown for 3 months in SD, the non-flowering, dwarf phenotype of ga1-3 was completely and rapidly reversed by applying GA4 twice over consecutive days with plants both held in SD and transferred to one or two LD (Fig 1). The same response was obtained after a single GA4 application (not shown). Within 16 h of the first GA applications, the stem, petioles, and leaf blades began to elongate (not shown) and flower buds were visible within 7–9 d in LD and at 15 d in SD (photographed at 10 d in Fig. 1). This rapid response contrasts with findings with a ga1 T-DNA mutant in Columbia which took 90 d to flower in SD when treated twice weekly with GA (Ericksson et al., 2006).
Fig. 1.
GA4 applied to ga1-3 shows an FT-independent effect on flowering in SD and a permissive effect involving FT expression in LD. A 10 μl drop of GA4 [1 mM in 20% ethanol:water (v:v)] was applied to each of three leaves on consecutive days either in SD or at the start of a far-red-rich LD (LD-FR). Plants of ga1-3 flowered, bolted, and leaves grew (A). Its FT expression increased most after GA treatment in LD (B), and (C) shows the effect of GA4 on FT expression in Columbia. Prior to treatment, the plants of ga1-3 had been grown in SD for 12 weeks and those of Columbia for 5 weeks. The low intensity FR-rich LD exposure was for 2 d. GA4 was applied 8 h after starting the day, and leaf blades were harvested 19.5 h later for assays of FT expression (leaves harvested at 16 h showed similar increases; not shown). There was no effect of solvent application on flowering or gene expression (not shown). All FT expression was normalized to the value in SD without GA application. The means and SE were based on three replicates for FT assays and 10 replicates for flowering time.
The present results also contrast with the rapid, GA-independent flowering of ga1-3 exposed to LD from germination (Koorneef and van der Veen 1980; Wilson et al., 1992; Putterill et al., 1995; Reeves and Coupland, 2001; Rieu et al., 2008). GA3 applied for germination of ga1-3 may carry over to the plant (Y Kamiya, Riken, Kanagawa, Japan, personal communication) but probably not for the less stable GA4 used here for germination. Furthermore, Reeves and Coupland (2001) and Rieu et al. (2008) showed that carryover was not important when they used seed coat removal, not GA, for germination. All the responses reported here for ga1-3 were completely reproducible and there does not appear to be an explanation for the non-flowering in LD, but this may relate to environmental differences and the age of plants when first treated with GA or exposed to LD.
To examine the effect of GA on FT expression, leaf blades were harvested 19.5 h after GA4 application, a time which matches high LD expression of FT in Columbia (see the companion paper). In LD, GA treatment increased FT expression 15-fold (Fig. 1B) and the plants flowered. In SD, flowering induced by GA was associated with a much smaller increase in FT (3.5-fold). Comparable responses were found for harvests at 16 h (not shown)
A crucial clue to explaining the GA effects on FT in LD is provided by comparison of its expression in ga1-3 with that in Columbia (Fig. 1B versus C). LD up-regulation of FT in ga1-3 required GA application, but an LD alone was sufficient for Columbia. The quite small increase in FT when GA was applied to Columbia in LD (35% increase) contrasts with the large increase in ga1-3 (15-fold). Apparently, the high endogenous GA level in Columbia (>10 times that in ga1-3; Zeevaart and Talon, 1992; Xu et al., 1997) permits FT expression in LD whereas the low GA level in ga1-3 almost completely blocks FT expression.
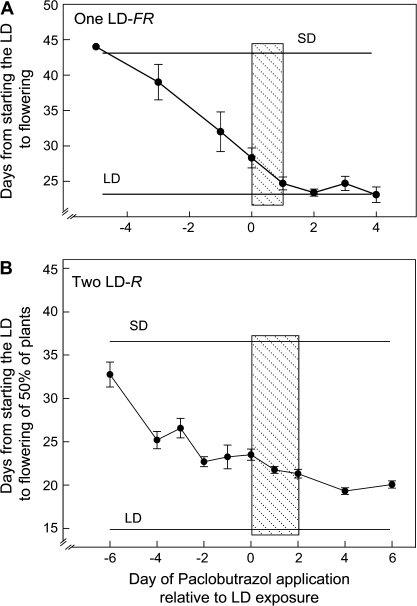
In further support of a permissive role for GA in LD-regulated FT expression in Columbia (Fig. 1), a single application of paclobutrazol, an inhibitor of GA biosynthesis (Rademacher, 2000), completely blocked flowering in an FR-rich LD (Fig. 2). Paclobutrazol action was GA specific as its inhibition of flowering was completely reversed by a simultaneous application of GA4 (data not shown).
Fig. 2.
Flowering is blocked by a GA biosynthesis inhibitor, paclobutrazol (PAC), if it is applied prior to an LD exposure. PAC was applied once as a soil drench at various times before or after the plants were exposed to: (A) a single FR-rich LD from incandescent lamps (LD-FR); or (B) two LD at high intensity from fluorescent lamps (LD-R). The shaded bar shows the LD exposure. The horizontal lines indicate flowering times of untreated plants exposed to one or two LD. The SD plants were vegetative when the experiment was terminated. The means and SE were based on 14 replicates in (A) and 16 in (B).
Flowering was only inhibited when paclobutrazol was applied before the LD (Fig. 2) so GA is required for flowering; however, this evidence does not imply an LD increase in GA biosynthesis. Of the two LD light conditions used in this experiment, only the FR-rich LD increases GA biosynthesis (Hisamatsu et al., 2005); however, flowering in high light, R-rich LD was also substantially inhibited by paclobutrazol yet this LD does not increase GA biosynthesis (Hisamastsu et al., 2005) but acts by photosynthetic amplification of FT expression in the leaf blade (cf. companion paper).
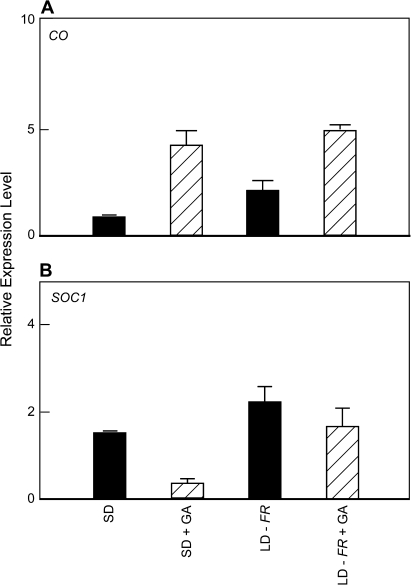
As an aside, for a harvest of ga1-3 at the same time that it was found that GA4 increased FT expression (Fig. 1), there was no promotion of SOC1 expression in the leaf blade (Fig. 3). Compared with the substantial GA/LD effect on FT, there were only small GA-dependent increases in CONSTANS (CO) expression and they were similar across all daylength and light quality conditions (Fig. 3 and data not shown). Nevertheless, the positive GA responsiveness of CO is consistent with its role in activation of FT. Circadian regulation of CO message and protein abundance may influence the extent of this GA regulation,c but such a study was beyond the scope of this work.
Fig. 3.
Effect of daylength and GA on expression of CO and SOC1 in the leaf blade of the Arabidopsis ga1-3 mutant. A 1 mM solution of GA4 was applied to the leaf blade of ga1-3. These assays were from the same experiment reported in Fig. 1. Comparable results were obtained in a second sample harvested at 16 h (not shown).
FT-independent regulation of flowering by GA
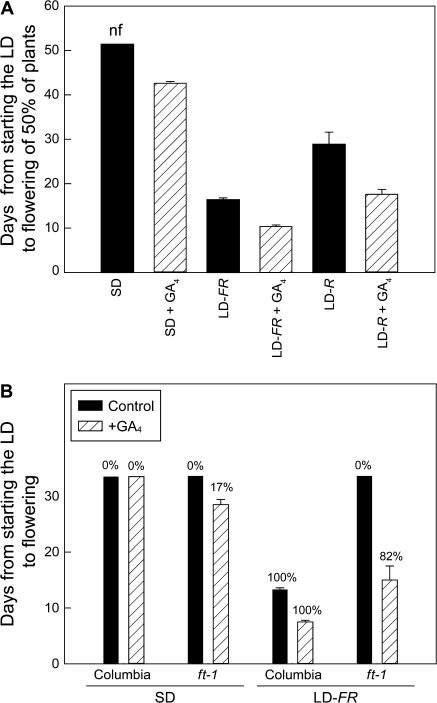
GA4 promoted flowering of Columbia in all daylengths (Fig. 4). In SD, whereas 50% of the GA4-treated plants had flowered after 42 d, only 12% of untreated plants had flowered by 52 d (Fig. 4A).This GA4-reguated flowering in SD should be independent of FT because FT levels are low and GA4 had little immediate effect on FT expression (Fig. 1C). The enhanced flowering with GA4 treatment in LD (Fig. 4) might also be FT independent because there was only a small GA-induced FT increase (35%; Fig. 1). A more compelling argument for FT-independent action of GA is seen in the GA4 reversal of the block to flowering in ft-1. In SD, 17% of GA4-treated ft-1 plants had flowered by 33 d, whereas all untreated plants were still vegetative (Fig. 4B). In LD, the effect of GA4 on flowering of ft-1 was even more dramatic; all treated plants had flowered by 15 d but none of the untreated ft-1 controls in LD had flowered by 33 d. It is not clear why in SD GA4-treated ft-1 plants flowered earlier than GA4 -treated plants of Columbia.
Fig. 4.
Flowering of Columbia or the ft-1 mutant after a single GA treatment to the leaves of plants in SD or exposed to LD. GA4 (1 mM in 20% ethanol) or the solvent alone was applied as a spray to run off. There were 12–17 plants per treatment. In (A) only 12% of the Columbia plants had flowered in SD at 52 d when the experiment was terminated compared with 50% flowering 42 d after GA treatment. In LD, all Columbia plants flowered after exposure to a single low-intensity, FR-rich LD (LD-FR) or two cycles of a high intensity R-rich LD (LD-R). In (B) the experiment was terminated at 3s d when the only flowering in SD was 17% for GA-treated ft-1 plants. In LD, all Columbia plants had flowered after 33 d, 82% of the GA-treated ft-1 plants, and none of the untreated ft-1 plants.
Taken together, these studies along with those with ga1-3 (Fig. 1) highlight a complex coupling between daylength, GA, FT, and flowering. Below, to examine this coupling further, lines with restricted GA biosynthesis were used to examine LD-specific GA input.
Endogenous GA contributes to flowering in LD
To define the link between GA biosynthesis and flowering in an FR-rich LD, two LD-specific GA 20-OXIDASE2 gene silencing lines were used and, as a negative control, a daylength-insensitive GA 20-OXIDASE1 T-DNA mutant was used. These 20-oxidases control an important step in GA biosynthesis (Thomas and Hedden, 2006).
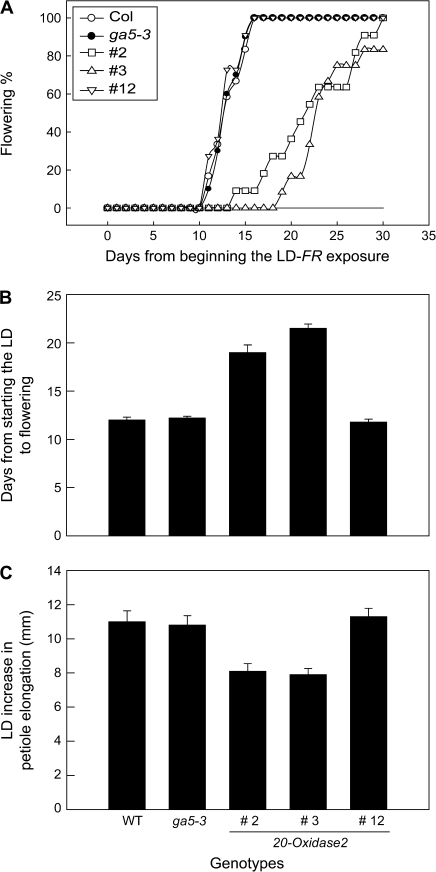
In the two GA 20-OXIDASE2 RNAi silencing lines, flowering was delayed on exposure to two LD from FR-rich incandescent lamps (Fig. 5A, B). These two lines show ∼55% (hpAtGA20ox2#2) and 90% (hpAtGA20ox2#3) reduction in 20-OXIDASE2 expression (Hisamatsu et al., 2005) and, in parallel, they inhibit a GA-regulated, LD increase in petiole elongation (Fig. 4C; Hisamatsu et al., 2005). An additional RNAi line (hpAtGA20ox2#12) was included as a control for transformation effects; it showed normal 20-OXIDASE2 expression (Hisamatsu et al., 2005) and there was neither a delay in its flowering nor a reduction in LD promotion of its petiole elongation (Fig. 5). The GA 20-OXIDASE1 null mutant, ga5-3, although dwarfed in its growth (not shown, but see Rieu et al., 2008), showed normal LD flowering and LD increase in petiole elongation (Fig. 5).
Fig. 5.
Silencing GA 20-OXIDASE2 expression delays flowering of Arabidopsis exposed to a single FR-enriched LD from incandescent lamps. Comparisons involve two GA 20-OXIDASE2 silencing lines, hpAtGA20ox2#2 (open square) and #3 (open triangle); the wild type, Columbia (open circle); a non-silenced transgenic line #12 (inverted open triangle); and ga5-3 (filled circle), a null mutant recently renamed as ga20ox1-3. There was no flowering in SD plants at 30 d as indicated by the horizontal line. In (B) days to flowering is shown as the mean and SE at 50% flowering (n=10–14). The LD effect on petiole elongation of the same plants is redrawn with the permission of Hisamatsu et al. (2005).
A repeat study with T4 progeny of the most effective RNAi silencing line (hpAtGA20ox2#3-6) confirmed the delay of flowering in LD-FR. Columbia flowered after 16.8±0.6 d but hpAtGA20ox2#3-6 flowered significantly later at 20.1±0.6 d (P <0.001). As a negative test for up-regulation of GA biosynthesis, a high light intensity R-rich LD does not increase GA biosynthesis (Hisamatsu et al., 2005), and LD flowering of Columbia and hpAtGA20ox2#3-6 was not significantly different (Columbia 20.2±1.4 d; hpAtGA20ox2#3-6 22.5±0.9 d; ga5-3 21.0±0.6 d, and the SD controls were still vegetative at 40 d). Recently Rieu et al. (2008) reported a very slight delay of flowering in a T-DNA mutant of 20-OXIDASE2, and this supports the present findings; however, it is difficult to draw any conclusions from their study. The LD was at a high light intensity from lamps with an R/FR output of ∼2.2, so flowering of both the mutant and wild type will be affected by photosynthesis, along with uncertain effects of lamp spectral composition on GA 20-OXIDASE2 expression in the non-mutant line.
In parallel with delayed flowering and reduced petiole elongation in the GA 20-OXIDASE2 silencing lines (Fig. 5), expression of this 20-oxidase increased when plants of Columbia were transferred to LD (Fig. 6). In other studies, the increase in gene expression in the petiole was 10-fold to 100-fold over the first 2–3 h of starting the LD, and then expression declined (Hisamatsu et al., 2005).
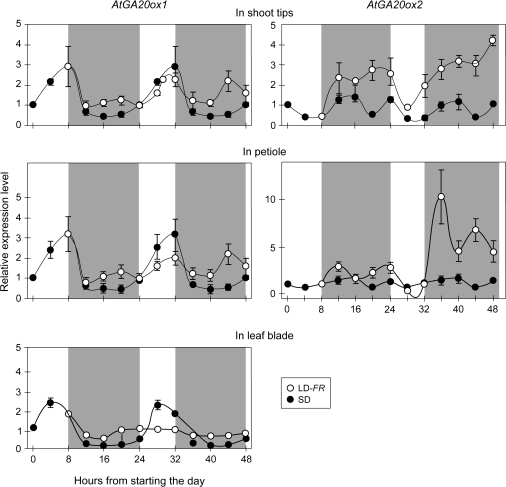
Fig. 6.
Effect of LD on expression of two GA 20-oxidase genes in the leaf blade, petiole, and shoot tip of Arabidopsis. Gene expression was analysed for plants of Columbia held in SD (filled circle) or shifted to LD (open circle). The values of the second SD cycle are those of the first day as previously very little difference across days was found (Hisamatsu et al., 2005). The shaded areas show when the ‘overnight’ 16 h light or dark treatments were imposed. There was no detectable expression of GA 20-OXIDASE2 in the leaf blade. All values are means ±SE (n=3). Error bars when not evident were smaller than the symbol.
On a point of technique, it can be expected that, in precisely controlled conditions, the oscillation in gene expression over any one diurnal cycle of an SD will be the same as for the next day. This has been confirmed (Hisamatsu et al., 2005) in a parallel study where petiole GA 20-OXIDASE1 expression was followed for 48 h (i.e. over two SD). Therefore, here, the gene expression patterns found over an SD have been extended to indicate the probable oscillation over the following SD.
Surprisingly, the 20-OXIDASE2 gene is only expressed in the petiole and shoot tip and not in the leaf blade (Fig. 6). In contrast, all three tissues clearly expressed the closely related GA 20-OXIDASE1 gene (Fig. 6), and its absolute level of expression was comparable in all three tissues although slightly lower than for 20-OXIDASE2 in the shoot tip and petiole (not shown). In addition to 20-OXIDASE1, ACTIN was effectively detected in all leaf blade samples where 20-OXIDASE2 was not detected. Thus, the possibility of failed assays can be excluded; there is a true lack of expression of the 20-OXIDASE2 gene in the leaf blade. Differences in tissue expression patterns of GA 20-oxidases have been reported previously for rice (Kaneko et al., 2003).
The diurnal periodicity shown for GA 20-OXIDASE1 expression (Fig. 6) reveals circadian regulation based on cycling continuing over 48 h in constant conditions involving high intensity white light (Hisamatsu et al., 2005). Thus, in much the same way as the circadian rhythm in CO expression (Suarez-Lopez et al., 2001) modulates the effect of light on FT (Valverde et al., 2004), GA synthesis could be regulated by a circadian clock. Specifying how light and rhythms regulate flowering is tangential to the analysis, but the characterization of diurnal changes in gene expression is important for any integrated analysis of responses to a LD.
In contrast to 20-OXIDASE2, FT expresses most in the leaf blade (∼70-fold more than in the petiole: data not shown). Previous studies showed a similar pattern, with the highest FT promoter::GUS expression in the leaf blade, very little in petioles (Yamaguchi et al., 2005; Yoo et al., 2005), and, based on in situ expression assays, none in the shoot apex (Kardailsky et al., 1999).
Overall, because of their non-sympatric expression, GA and FT might act as independent LD signals but with a dominant role for FT.
Discussion
Floral signalling in LD plants may involve leaf to shoot apex transport of the FT protein (Turck et al., 2008) and/or the GA class of plant hormones (King and Evans, 2003). The role of the FT protein as a transported floral signal has been highlighted in a number of recent genetic/molecular studies with Arabidopsis, rice, and cucumber (Corbesier et al., 2007; Jaeger and Wigge, 2007; Lin et al. 2007; Mathieu et al., 2007; Tamaki et al., 2007). Although the response to ft mutants shows that FT plays a dominant role in LD flowering, GA contributes to flowering of Arabidopsis in LD (Figs 5, 6) and in SD (see Ericksson et al., 2006).
Based on evidence presented here and in the companion paper, Fig. 7 summarizes the ways that LD light might regulate FT, GA, and flowering. The complete block by ft-1 of flowering of Arabidopsis exposed to a high light intensity, R-rich LD shows the dominance of photosynthesis in FT up-regulation. In contrast, at low, non-photosynthetic intensities involving FR-rich LD, phytochrome is the primary step of regulation of flowering and of FT expression (Goto et al., 1991; Reed et al., 1994; Bagnall and King, 2001; Cerdän and Chory 2003; Halliday et al., 2003). Interestingly, ft-1 incompletely blocked flowering in response to an FR-rich LD (see companion paper). Therefore, there could be an additional FT-independent LD input and, potentially, via GA since FR-rich conditions up-regulate GA biosynthesis in Arabidopsis plants (Xu et al., 1997; Gocal et al., 2001; Hisamatsu et al., 2005) as also in other species (reviewed in García–Martínez and Gil, 2002; King and Evans, 2003).
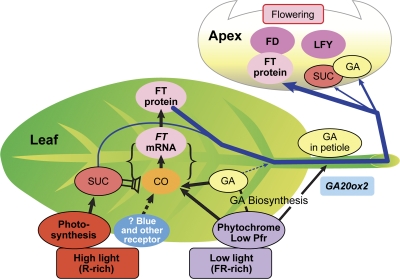
Fig. 7.
Summary of findings here and in the companion paper of positive effects (arrows) on flowering and CO/FT for two commonly used LD photoresponses. This schematic incorporates effects on FT and flowering of: mutants; gene silencing; change in light intensity; and a block to photosynthesis. Predominantly, in LD, photosynthetic sucrose amplifies CO/FT expression (see companion paper) while phytochrome acts directly and also via GA, which plays a permissive and, often, non-limiting role. There is also a direct but lesser LD-mediated increase in GA supply via the petiole response to FR-rich light. A dashed arrow indicates a potential step of regulation, and weaker responses are indicated by thinner arrows. The electronics symbol for a speaker is used to show sucrose amplification of CO/FT expression.
Two potential actions of GA on flowering are indicated in Fig. 7, namely that GA acts on FT signalling in LD and that LD increases GA content in the petiole, this GA acting as a second floral signal. Based on the present findings, the extremely low levels of GA in ga1-3 (see Zeevaart and Talon, 1992; Xu et al., 1997) allowed the demonstration of GA regulation of FT expression (Fig. 1) whereas with Columbia its endogenous GA levels were apparently close to sufficient for LD up-regulation of FT expression and flowering. Thus, GA plays an important permissive role for FT up-regulation and LD flowering.
Previously the possibility of GA induction of flowering by up-regulation of FT was discounted because plants of ecotype Landsberg erecta flowered early when ga1-3 was crossed with a line overexpressing FT under the control of the 35S promoter (Blázquez et al., 2002). However, the FT promoter contains three GA response elements and a nearby pyrimidine box which could be sufficient for GA to regulate FT transcriptionally. Use of the constitutively expressed 35S promoter to control FT would not reveal such potential for GA regulation of FT.
Considering the role of GA as a second LD floral signal, flowering was inhibited when GA biosynthesis was blocked in ga1-3 or by application of paclobutrazol (Figs 1, 2). Conversely, GA application caused rapid flowering and reversed the dwarfing effect of ga1-3. Predominantly these responses to applied GA involve FT up-regulation (see above). However, GA enhanced flowering even in SD where FT is only weakly expressed (Fig. 1) and, more cogently, GA dependent FT-independent flowering was demonstrated by application of GA to the ft-1 mutant in SD or LD (Fig. 4).
This claim that GA can act endogenously as a floral signal is supported by earlier evidence that an FR-rich LD up-regulates GA biosynthesis in the petiole via a specific GA 20-OXIDASE2 gene (Hisamatsu et al., 2005) and that there are associated increases in endogenous GA content (Gocal et al., 2001). Interestingly, this 20-OXIDASE2 is not expressed in the leaf blade (Fig. 6) and, conversely, FT is expressed in the leaf blade and not the petiole (Kardailsky et al., 1999; Yamaguchi et al., 2005; Yoo et al., 2005; Hisamatsu unpublished data). Lastly, the inhibition of LD flowering on silencing GA 20-OXIDASE2 expression confirms that endogenous GA plays a small role in flowering in an FR-rich LD (Fig. 5).
Although the effect of applied GA on flowering in SD or LD is only weak (Figs 1, 4; and see Gocal et al., 2001; Ericksson et al., 2006), a role for GA is consistent with recent evidence of a large increase in endogenous GAs associated with very late SD flowering (Ericksson et al., 2006). However, the site of action of LD-generated GA is unclear. Despite evidence for GA4 transport from the leaf blade to the shoot tip of Arabidopsis (Ericksson et al., 2006), GA sourced from the LD petiole could be transported to and act in either or both the leaf blade and the shoot apex.
At the molecular level, in the leaf blade GA acts in an as yet unknown way on FT expression. At the shoot apex there is evidence that GA activates a GAMYB (Blázquez and Weigel, 2000; Gocal et al., 2001) which up-regulates expression of the floral regulator gene, LEAFY (Blázquez et al., 1998). Although the focus in the present study was on early response to GA, it also enhances later, visible, stem elongation (bolting) of Arabidopsis (Xu et al., 1997). Such GA action on later steps of floral development/stem bolting might explain the more rapid visible flowering after GA treatment (Fig. 4A; 6–10 d earlier). An equally plausible explanation, but one not generally considered, involves a common action of GA on both floral initiation and later floral development. Some common actions are likely since, within 48 h of exposure of Arabidopsis to a single LD, there are large increases in shoot apex height (Gocal et al., 2001).
Although direct GA regulation of flowering is weak in Arabidopsis, its extent varies across plant species and, possibly, inversely with the role played by FT. Unlike Arabidopsis, in L. temulentum, leaf-applied GA causes substantial and rapid flowering in SD despite the low level of expression of LtFT in SD (King et al., 2006). More cogently, GA is an important floral signal in L. temulentum because it is also effective when supplied directly to isolated shoot tips in culture (reviewed in King and Evans, 2003). Evidence of rapid increases in endogenous GAs first in the LD leaf blade and then in the shoot apex further supports direct GA signalling, as does evidence of a relationship between GA dose, flowering, and transport of intact tetradeuterated GA from the leaf to the apex (King et al., 2001, 2006). In these studies there was also little or no effect of increased or decreased GA on the LD increase in FT expression (King et al., 2006). This latter result contrasts with evidence for Arabidopsis where flowering and FT expression are restricted when GA synthesis is blocked in ga1-3 (Fig. 1) or, probably, with the use of paclobutrazol to inhibit flowering (Fig. 2).
Overall, the focus of the present study was on FT and GA, but the findings emphasize the importance of treating the photoperiodic regulation of flowering as a complex of interacting responses. As summarized in Fig. 7, FT plays a dominant role in floral signalling in Arabidopsis, and its protein (An et al., 2004; Corbesier et al., 2007; Turck et al., 2008) or some closely linked factor is the primary leaf-sourced factor transported to the shoot apex where it evokes flowering. LD up-regulate FT expression whether by phytochrome or by photosynthesis, but in the latter instance FT expression may involve an additional action of a blue or red photoreceptor. However, although it is considered that GA and photosynthetically generated sucrose up-regulate FT expression, they may also play direct, albeit small, roles as mobile floral signals.
Acknowledgments
Dr Tai-ping Sun provided seed of ga1-3. Drs Lloyd Evans and Masumi Robertson (CSIRO) provided valuable comment on the manuscript.
Glossary
Abbreviations
- CO
CONSTANS
- FR
far red light
- FT
FLOWERING LOCUS T
- GA
gibberellin
- LD
long day conditions
- R
red light
- RNAi
RNA interference
- SD
short day conditions
References
- An HL, Roussot C, Suarez-Lopèz P, et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development. 2004;131:3615–3626. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- Bagnall DJ, King RW. Phytochrome and flowering of Arabidopsis thaliana: photophysiological studies using mutants and transgenic lines. Australian Journal of Plant Physiology. 2001;28:401–408. [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. The Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Trenor M, Weigel D. Independent control of gibberellin biosynthesis and flowering time by the circadian clock in Arabidopsis. Plant Physiology. 2002;130:1770–1775. doi: 10.1104/pp.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Weigel D. Integration of floral inductive signals in Arabidopsis. Nature. 2000;404:889–902. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C. Multiple pathways in the decision to flower: enabling, promoting, and resetting. The Plant Cell. 2004;16:S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdän PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang SH, et al. FT protein movement contributes to long-distance signalling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Bohlenius H, Moritz T, Nilsson O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. The Plant Cell. 2006;18:2172–2181. doi: 10.1105/tpc.106.042317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez JL, Gil J. Light regulation of gibberellin biosynthesis and mode of action. Journal of Plant Growth Regulation. 2002;20:354–368. doi: 10.1007/s003440010033. [DOI] [PubMed] [Google Scholar]
- Gocal GFW, Sheldon C, Gubler F, Moritz T, Bagnall D, Song FL, Parish RW, Dennis ES, Weigel D, King RW. GAMYB-like genes and gibberellin in Arabidopsis. Plant Physiology. 2001;127:1682–1693. [PMC free article] [PubMed] [Google Scholar]
- Goto N, Kumagi T, Koornneef M. Flowering responses to light breaks in photomorphogenic mutants of Arabidopsis thaliana, a long day plant. Physiologia Plantarum. 1991;83:209–215. [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. The Plant Journal. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- Hisamatsu T, King RW, Helliwell CA, Koshioka M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiology. 2005;138:1106–1116. doi: 10.1104/pp.104.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Kay SA. Photoperiodic control of flowering not only by coincidence. Trends in Plant Science. 2006;11:550–558. doi: 10.1016/j.tplants.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Current Biology. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Itoh H, Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. Where do gibberellin biosynthesis and gibberellin signaling occur in rice plants? The Plant Journal. 2003;35:104–115. doi: 10.1046/j.1365-313x.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- King RW, Evans LT. Gibberellins and flowering of grasses and cereals: prizing open the lid of the ‘florigen’ black box. Annual Review of Plant Biology. 2003;54:307–328. doi: 10.1146/annurev.arplant.54.031902.135029. [DOI] [PubMed] [Google Scholar]
- King RW, Moritz T, Evans LT, Junttila O, Herlt AJ. Long day induction of flowering in Lolium temulentum involves sequential increases in specific gibberellins at the shoot apex. Plant Physiology. 2001;127:624–632. [PMC free article] [PubMed] [Google Scholar]
- King RW, Moritz T, Evans LT, Martin J, Andersen CH, Blundell C, Kardailsky I, Chandler PM. Regulation of flowering in the long day grass, Lolium temulentum L., by gibberellins and the gene, FLOWERING LOCUS T (FT) Plant Physiology. 2006;141:498–507. doi: 10.1104/pp.106.076760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Hisamatsu T, Goldschmidt EE, Blundell C. The nature of floral signals in Arabidopsis I Photosynthesis and a Far-Red independently regulate flowering by increasing expression of FLOWERING LOCUS T (FT) Journal of Experimental Botany. 2008 doi: 10.1093/jxb/ern231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Weigel D. Move on up, it's time for change—mobile signals controlling photoperiodic-dependent flowering. Genes and Development. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theoretical and Applied Genetics. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Lin MK, Belanger H, Lee YJ, et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the Cucurbits. The Plant Cell. 2007;19:488–1506. doi: 10.1105/tpc.107.051920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Kuttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Current Biology. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Rademacher W. Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:501–531. doi: 10.1146/annurev.arplant.51.1.501. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatini A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiology. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PH, Coupland G. Analysis of flowering time control in Arabidopsis by comparison of double and triple mutants. Plant Physiology. 2001;126:1085–1091. doi: 10.1104/pp.126.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu R, Ruiz-Rivero O, Fernandez Garciaa N, et al. The gibberellin-biosynthetic genes AtGAa20ox1 and AtGAa20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. The Plant Journal. 2007;53:488–504. doi: 10.1111/j.1365-313X.2007.03356.x. [DOI] [PubMed] [Google Scholar]
- Searle I, Coupland G. Induction of flowering by seasonal changes in photoperiod. EMBO Journal. 2004;23:1217–1222. doi: 10.1038/sj.emboj.7600117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316:1033–1036. doi: 10.1126/science.1141753. [DOI] [PubMed] [Google Scholar]
- Thomas SG, Hedden P. Gibberellin metabolism and signal transduction. In: Hedden P, Thomas SG, editors. Plant hormone signalling. Oxford: Blackwell Publishing; 2006. pp. 147–184.l. [Google Scholar]
- Turck F, Formara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves centre stage. Annual Reviews of Plant Biology. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiology. 2004;135:1008–1019. doi: 10.1104/pp.104.039578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiology. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Gage DA, Zeevaart JAD. Gibberellins and stem growth in Arabidopsis thaliana. Effects of photoperiod on the GA4 and GA5 loci. Plant Physiology. 1997;114:1471–1461. doi: 10.1104/pp.114.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant and Cell Physiology. 2005;46:1175–1189. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiology. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Talon M. Gibberellins and mutants in Arabidopsis thaliana. In: Karstens CM, Loon LC, van Vreugdenhil D, editors. Plant growth regulation. Amsterdam: Kluwer; 1992. pp. 34–42. [Google Scholar]