Abstract
Extracellular ATP (eATP) is a novel signalling agent, and nitric oxide (NO) is a well-established signal molecule with diverse functions in plant growth and development. This study characterizes NO production induced by exogenous ATP and examines its relationship with other important signalling agents, Ca2+ and H2O2 in Salvia miltiorrhiza hairy root culture. Exogenous ATP was applied at 10–500 μM to the hairy root cultures and stimulated NO production was detectable within 30 min. The NO level increased with ATP dose from 10–100 μM but decreased from 100–200 μM or higher. The ATP-induced NO production was mimicked by a non-hydrolysable ATP analogue ATPγS, but only weakly by ADP, AMP or adenosine. The ATP-induced NO production was blocked by Ca2+ antagonists, but not affected by a protein kinase inhibitor. ATP also induced H2O2 production, which was dependent on both Ca2+ and protein kinases, and also on NO biosynthesis. On the other hand, ATP induced a rapid increase in the intracellular Ca2+ level, which was dependent on NO but not H2O2. The results suggest that NO is implicated in ATP-induced responses and signal transduction in plant cells, and ATP signalling is closely related to Ca2+ and ROS signalling.
Keywords: Ca2+, extracellular ATP, hairy roots, nitric oxide, reactive oxygen species, Salvia miltiorrhiza
Introduction
ATP is the ubiquitous energy source in all living organisms, and also plays other important roles in several physiological processes. In animal systems, extracellular ATP (eATP) is well-established as a signal molecule implicated in a number of cellular responses such as neurotransmission, the immune response, and apoptosis (Zheng et al., 1991; Bours et al., 2006). The eATP signal is transmitted across the plasma membrane through specific receptors, namely the P2 family of purinoceptors including the ligand-gated ion channel P2X receptors and the G-protein-coupled P2Y receptors (Ralevic and Burnstock, 1998).
The role of eATP as a signal agent in plant cells had not drawn much attention until recently, however, it was first proposed by Demidchik et al. (2003) based on the finding that exogenous ATP applied to Arabidopsis roots induced rapid and transient increase in the cytosolic Ca2+ concentration. Two later studies in Arabidopsis seedlings (Jeter et al., 2004; Song et al., 2006) showed several other important events in stress response and signalling induced by exogenous ATP, including the production of reactive oxygen species (ROS), the transcription of mitogen-activated protein kinases (MAPKs), lipoxygenase (LOX, a key enzyme for JA biosynthesis), and ACS6 (a key enzyme for ethylene biosynthesis). Earlier, Tang et al. (2003) had shown that exogenous ATP at millimolal levels could strongly affect gravitropic growth and auxin distribution in Arabidopsis roots, suggestive of the role of eATP as a regulatory signal in plant growth. Extracellular ATP has been found to be essential for maintaining plant cell viability in both cell cultures and whole plants of Arabidopsis (Chivasa et al., 2005). Kim et al. (2006) detected the presence of eATP in Medicago truncatula root hairs, localizing in the interstitial spaces between epidermal cells, and found that ATP release was a calcium-dependent process. These studies strongly suggest that eATP plays a regulatory role in plant growth and development, and a signal role in plant stress response (Roux and Steinebrunner, 2007). Our recent study has shown that a polysaccharide elicitor from yeast extract induces the transient release of ATP from Salvia miltiorrhiza hairy roots to the culture medium, and Ca2+ is required for activating elicitor-induced ATP release and signal transduction (Wu et al., 2008).
Nitric oxide (NO) is a free radical gas formed endogenously and has multiple functions in both animal and plant systems (Neill et al., 2003). Although its physiological functions in plants remain to be characterized, NO is a well-established second messenger in plant stress signalling (Delledonne et al., 1998; Beligni and Lamattina, 2001; Lamotte et al., 2004). Nitric oxide synthase (NOS) or its analogue, the major enzyme for NO biosynthesis in animals, is also regarded as a major NO producer in plants (del-Rio et al., 2004; Zemojtel et al., 2006). Nitrate reductase (NR) is another possible enzyme for NO synthesis in plants (Xu and Zhao, 2003). An important characteristic of NO signalling in plant stress responses is its interplay or cross-talk with the reactive oxygen species (ROS). It has been shown that NO and ROS are produced concomitantly in plants in response to pathogen and stress challenges, and the two types of signal compounds can act co-operatively in mediating the defence responses such as hypersensitive cell death, expression of defence genes and secondary metabolite accumulation (Delledonne et al., 2001; Wang and Wu, 2005; Bright et al., 2006; Zaninotto et al., 2006).
In animal systems, NO production has been characterized as a quick response to exogenous ATP stimulation (Silva et al., 2006), and NO has been shown to play a role in ATP-induced Ca2+ signalling (Shen et al., 2005). When this study began, however, there had been no reported study on ATP-induced NO biosynthesis and its relationship with Ca2+ or any other signalling element in the plant systems. In a very recent short correspondence, Foresi et al. (2007) reported exogenous ATP-induced NO production in tomato cell suspensions. In this study, ATP-induced NO production in Salvia miltiorrhiza Bunge (Lamiaceae) hairy root cultures was characterized further, and its dependence on the membrane receptors analogous to mammalian purinoceptors, and its relationship with the membrane Ca2+ influx, protein kinase and H2O2 biosynthesis was examined.
Materials and methods
Plant hairy root culture
Salvia miltiorrhiza hairy root culture was derived after the infection of plantlets with a Ri T-DNA bearing Agrobacterium rhizogenes (ATCC15834), maintained in a liquid, hormone-free MS medium with 30 g l−1 sucrose but without ammonium nitrate at 25 °C in the dark. The hairy root culture was incubated in 125 ml Erlenmeyer flasks, each filled with 25 ml liquid medium on an orbital shaker at 110–120 rpm (shake-flask cultures, as described in Ge and Wu, 2005).
Treatment of hairy roots with ATP, other purine nucleotides and various inhibitors
ATP and the purine nucleotides, ADP, AMP, and adenosine (A), and a non-hydrolysable ATP analogue, ATPγS (sodium salts from Sigma-Aldrich, St Louis, MO) were tested in parallel to discern the effect of the ATP molecule from its hydrolysed derivatives. The involvement of various signal agents in a response was examined through gain-and-loss of function experiments using their specific antagonists as shown in Table 1. For example, reaction blue (RB) and suramin are two specific inhibitors of purinoceptors which were originally used for mammalian cells, and have also been shown to be effective for blocking the exogenous ATP responses in plant cells (Ralevic and Burnstock, 1998; Demidchik et al., 2003; Song et al., 2006). The concentration range of all inhibitors was selected based on the literature and our preliminary tests, which showed no inhibitory effect on root growth and viability. The ATP relatives (as inducers) and inhibitors were all predissolved in distilled water as 100× concentrated stock solutions and filter-sterilized through a 0.2 μm membrane.
Table 1.
ATP, Ca2+, NO and protein kinase antagonists employed in the experiments and their putative functions in plant cells
| Targeted responses | Chemical name (abbreviation): function |
| Perception of extracellular ATP signal | Reactive blue (RB) and suramin: inhibitors of plasma membrane purinoceptors |
| Ca2+ influx through membrane | 1,2-bis(2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (EGTA): Ca2+ chelator; verapamil: Ca2+ channel blocker |
| NO production and release | L-ω-nitro-Arg-methyl-ester (L-NAME) and S,S’-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea (PBITU): NOS inhibitor; sodium azide (SoA): NR inhibitor; 2-phenyl-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (PTIO): NO scavenger |
| Protein phosphorylation | Staurosporine (ST): protein kinase inhibitor |
| Calmodulin (CaM) activation | N-(6-aminohexyl)-5-chloro-1-naphthalenesulphonamide (W-7) and trifluoperazine (TRF): CaM Inhibitor |
All treatment experiments were carried out in 50 ml Erlenmeyer flasks, each filled with 15 ml fresh MS medium and inoculated with 1.5 g fresh weight of the hairy roots from the shake-flask cultures which had been incubated for 18–21 d. After an initial incubation for 4 d, ATP was applied to the hairy root cultures at selected doses. The various inhibitors, when needed, were added to the hairy root cultures 30 min before the addition of ATP and relatives. All treatments were performed in triplicate flasks and the results were represented by their mean plus standard deviation; all experiments were performed at least twice to confirm the treatment effects.
Quantification and observation of NO production in hairy root cultures
The concentration change or relative level of NO in the hairy root culture medium was quantified by a fluorometric method using the NO-sensitive fluorescence probe 4,5-diaminofluorescein diacetate (DAF-2DA) (Sigma Aldrich) (Lamotte et al., 2004; Hu et al., 2005; Wang and Wu, 2005). The DAF-2DA reagent was predissolved in fresh MS medium and added to the culture medium at a final concentration of 10 μM 2 h prior to treatment with ATP or its relatives. After the treatment, 50 μl of medium was withdrawn from each culture flask at selected time intervals and mixed with 250 μl TRIS-KCl (10 mM, pH 7.2), and the fluorescence intensity was measured on a luminescence spectrometer (LS50B, Perkin–Elmer, Shelton, CT) at 495 nm excitation and 515 nm emission.
NO accumulation in the hairy roots was visualized by fluorescence microscopy. The root samples for the microscopy were stained with 10 μM DAF-2-DA in 10 mM TRIS-KCl buffer (pH 7.2) for 30 min, and then rinsed with 10 mM TRIS-KCl buffer for 10 min (Hu et al., 2005). The specimen for fluorescence microscopy was taken from the elongation part of the root near the root tip in which the cells are usually more active in NO biosynthesis and permissible to the DAF-2-DA dye than the older and mature root cells (Stöhr and Ullrich, 2002). The root specimen was pressed into a thin layer and placed between the slides, and the photo image was taken by Axionvert 200 inverted microscopy connected with a laser confocal scanner (LSM 510 meta; Carl Zeiss, Oberkochen, Germany) (excitation: 488 nm; emission: 515–560 nm).
Measurement of H2O2
Hydrogen peroxide (H2O2) in the culture medium was measured by luminol chemiluminescence as described by Wang and Wu (2005). In brief, 50 μl of sample medium was mixed with 750 μl of phosphate buffer (0.05 M, pH 7.9), followed by auto-injection of 200 μl luminol (0.3 mM in phosphate buffer) and 100 μl of K3[Fe(CN)6] (14 mM in water). Fluorescence intensity was recorded after the last injection at an integration time of 5 s, and the intensity value was calibrated to actual H2O2 concentration with pure H2O2 liquid (30 wt% in water from Junsei Chemical Co., Ltd., Tokyo, Japan).
Measurement of intracellular Ca2+ in hairy roots
Intracellular Ca2+ concentration change in the hairy roots after various treatments was measured with the Ca2+-sensitive probe Fluo-3-AM (Sigma, Cat F6142). Before various treatments, the hairy roots were incubated at 4 °C for 2 h in 10 mM MES-TRIS loading buffer (pH 6.1) containing 0.2 mM CaCl2, 50 mM sorbitol, and 20 μM Fluo-3AM. The hairy roots loaded with the fluorescence probe were incubated in the MS medium at 25 °C for another 2 h, and then subject to various treatments (Zhang et al., 1998; Ma et al., 2002). After the treatments, the hairy root samples were collected and examined by laser-confocal scanning at 480 nm excitation and with signal collection from 515 nm and above (Zhang et al., 1998; Ma et al., 2002). The integration of fluorescence intensity over area on the laser image of each sample was performed to show the relative intracellular Ca2+ concentration level.
Results
ATP-induced NO production in S. miltiorrhiza hairy roots
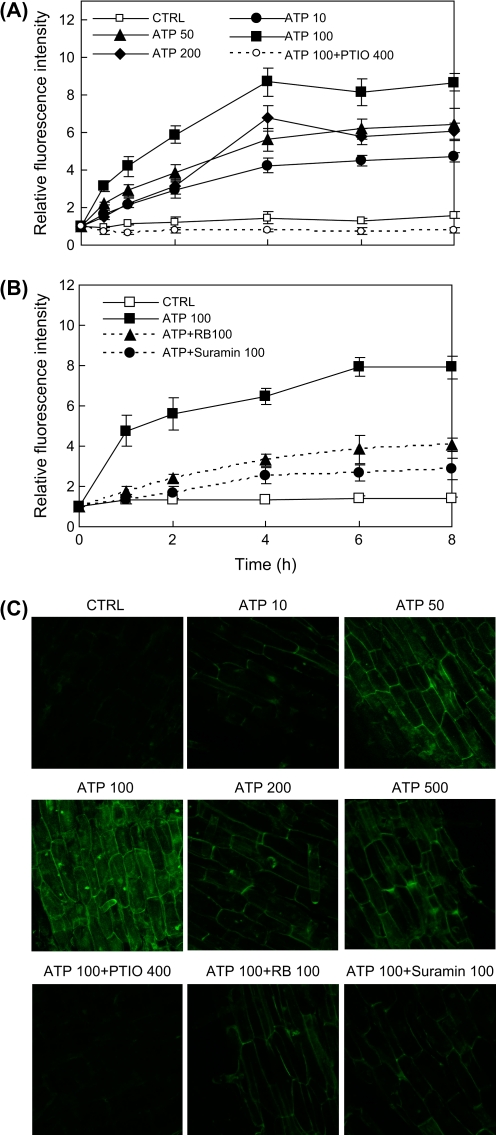
As shown in Fig. 1A, the fluorescence intensity of the culture medium began to increase within 30 min after the addition of ATP to the hairy root culture at various concentrations from 10 μM to 200 μM. At most of the ATP doses applied, the fluorescence intensity increase occurred between 0–4 h and then reached a plateau or a maximum level, which increased gradually with the increase in the ATP dose from 10 μM to 100 μM but dropped significantly from 100 μM to 200 μM (and 500 μM, not shown). There was only a slight or negligible change in the fluorescence intensity in the control culture or the culture supplied with the specific NO scavenger PTIO (at 0.4 mM) throughout the test period, which confirmed that the fluorescence intensity increase in the ATP-treated cultures was due to NO production induced by ATP. The results showed that ATP induced rapid and dose-dependent NO production in the hairy root cultures, and the optimal and most effective dose was about 100 μM. In addition, the ATP-induced NO production was significantly suppressed by both RB and suramin (Fig. 1B), suggesting that the requirement of purinoceptors for ATP signal transmission across the plasma membrane was to activate the NO production inside the cell.
Fig. 1.
NO production in S. miltiorrhiza hairy root cultures induced by exogenously-applied ATP at various concentrations. (A) Time-course of NO concentration (relative fluorescence intensity) in the culture medium after ATP application. (B) Effects of purinoceptor inhibitors RB and suramin on ATP-induced NO production. (C) NO accumulation in hairy roots after ATP treatment (2 h) at various concentrations (laser confocal images of roots stained by NO-fluorescence probe DAF-2DA; scale bar=100 μm). CTRL stands for control, and the number after each agent represents the concentration in μM; error bars for standard deviations, n=3; root specimen for microscopy were taken from the elongation part of the hairy root. (This figure is available in colour at JXB online.)
In addition to the fluorescence intensity of the culture medium, NO accumulation in the hairy root cells after ATP treatment can be observed from the microscopic images of hairy roots stained with the NO-specific fluorescence probe, DAF-2DA (Fig. 1C). These images were all taken from epidermal cells in the elongation part of the hairy root, while the pericycle cells also exhibited strong fluorescence in response to the ATP treatment (data not shown). Strong fluorescence was found in the roots treated with ATP at 50 μM and 100 μM doses, and weak or no fluorescence was observed in the roots of the control culture or the culture treated with ATP plus the NO scavenger PTIO.
NO production induced by ATP analogue and derivatives
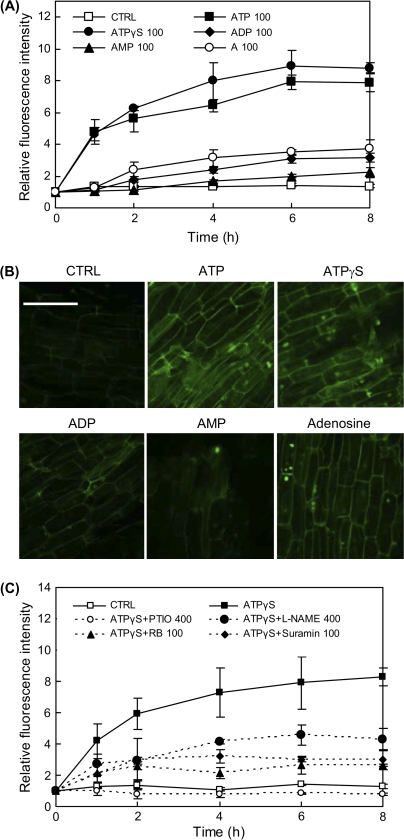
As shown by Fig. 2A, at an equal dose of 100 μM, the non-hydrolysable ATP analogue ATPγS induced a similar level of NO production to that by ATP, but the ATP derivatives ADP, AMP, and adenosine (A) induced much lower levels of NO in the hairy root culture. Among the ATP relatives, AMP had the weakest activity for NO induction, and was even weaker than ADP and adenosine. The fluorescence microscopic images of the hairy roots (Fig. 2B) also show the similar changes in NO accumulation induced by various ATP relatives. The results suggest that the hydrolysis of ATP is neither essential nor favourable for ATP activation of NO production. A further inference drawn from the results is that the membrane receptors are specific to the ATP molecule and its partial or complete hydrolysis would significantly weaken the signal and response. Similar to the ATP-induced NO production (Fig. 1B), the ATPγS-induced NO production was strongly suppressed by purinoceptor inhibitors (RB and suramin), and also by NO scavengers (L-NAME and PTIO) (Fig. 2C).
Fig. 2.
Effects of ATP analogue and ATP derivatives (A for adenosine) on NO production in the hairy root cultures. (A) Time-course of NO concentration change (relative fluorescence intensity) in the hairy root culture medium after treatment with ATP and relatives. (B) NO accumulation in hairy roots observed by laser confocal microscopy after treatment (for 2 h) by ATP and relatives (laser confocal microscopic images of roots stained by NO-fluorescence probe DAF-2DA; scale bar=100 μm). (C) Inhibition of ATPγS-induced NO production by NO scavenger, NOS inhibitor, and purinoceptor inhibitors (PTIO and L-NAME at 400 μM and all other agents at 100 μM in culture; error bars for standard deviations, n=3). (This figure is available in colour at JXB online.)
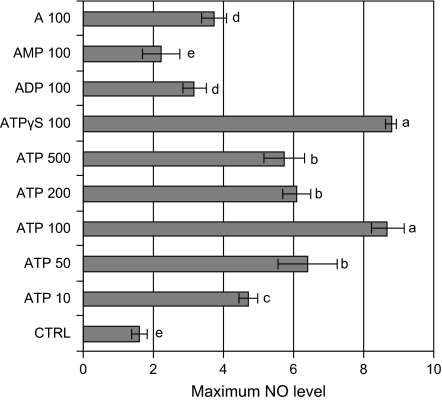
Figure 3 shows the maximum NO levels induced by ATP at various doses (10–500 μM) and by various ATP relatives, which provides a direct comparison of their NO-inducing activities.
Fig. 3.
Maximum NO levels in hairy root cultures after treatment (8 h) with ATP at various concentrations and with ATP relatives (A for adenosine) at a fixed concentration of 100 μM. The number after each agent represents the concentration in μM. In cultures treated with ATP+PTIO, ATP+RB, and ATP+suramin, the inhibitors were added 30 min before ATP (ATP fixed at 100 μM). Error bars for standard deviations, n=3; different letters (a–e) on top of columns indicate significant difference among the treatment effects, P <0.05, LSD (least significant difference)=0.91.
Dependence of ATP-induced NO on NOS, NR, Ca2+ and protein kinase
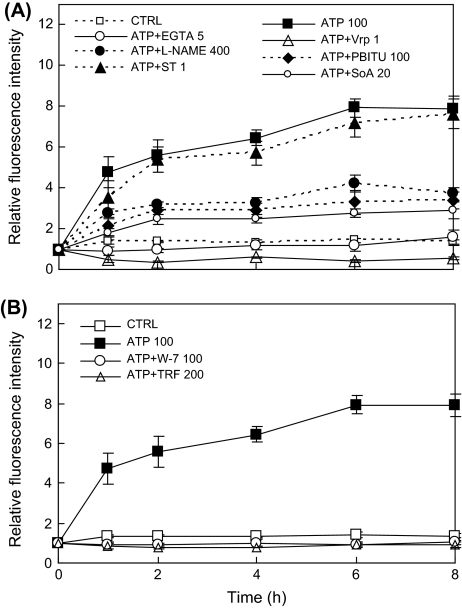
The ATP-induced NO production was significantly suppressed by the NOS inhibitors L-NAME and PBITU, and the NR inhibitor sodium azide (Fig. 4A), suggesting that both NOS and NR contributed to the ATP-induced NO-biosynthesis in this plant hairy roots.
Fig. 4.
Inhibition of ATP-induced NO production by (A) NO biosynthesis inhibitors (L-NAME at 400 μM; PBITU at 100 μM; SoA at 20 μM), protein kinase inhibitor (ST at 1 μM), and Ca2+ antagonists (EGTA at 5 mM, verapamil at 1 mM) and (B) CaM antagonists (W-7 at 100 μM, trifluoperazine, TRF, at 200 μM).
The ATP-induced NO production was also effectively blocked by both EGTA (an external Ca2+ chelator) and verapamil (a Ca2+ channel blocker) (Fig. 4A), suggestive of a strong dependence on the Ca2+ membrane influx. The ATP-induced NO production was also blocked by both W-7 and trifluoperazine (TRF), two specific inhibitors of calmodulin (CaM) (Fig. 4B). Ca2+ influx through the plasma membrane and the subsequent increase in cytosolic Ca2+ concentration are early steps in the ATP signalling cascade, and CaM activation is associated with several downstream enzyme activities in the Ca2+ signalling pathway. Therefore, the results here indicate the involvement of Ca2+ signalling in activating the ATP-induced NO biosynthesis. On the other hand, staurosporine (ST), a general inhibitor for a broad range of protein kinases (Lamotte et al., 2004; Menke et al., 1999), had no significant influence on ATP-induced NO production (Fig. 4A), suggesting that protein kinases were not involved in activating the ATP-induced NO biosynthesis.
ATP-induced NO and H2O2 production
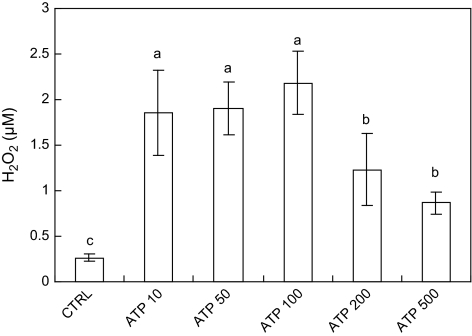
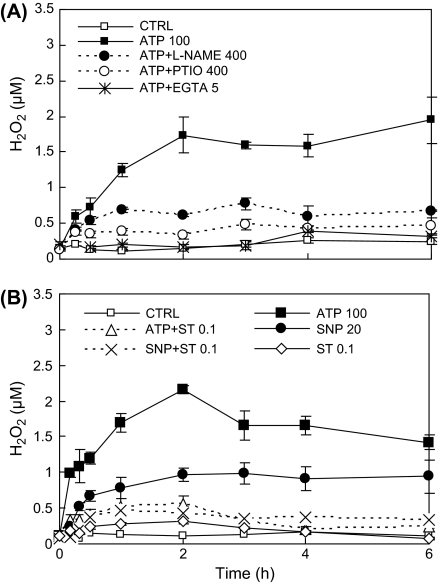
Exogenous ATP induced H2O2 production in a dose-dependent manner, with the highest H2O2 level attained at 100 μM (Fig. 5). The result suggests that an optimum exogenous ATP concentration exists for the stimulation of H2O2 biosynthesis in hairy roots. The ATP-induced H2O2 biosynthesis was strongly suppressed by the NO scavenger PTIO and the NOS inhibitor L-NAME (Fig. 6A), and the NO donor SNP (sodium nitroprusside) at 20 μM also induced H2O2 production (Fig. 6B). The results suggest that NO biosynthesis induced by ATP was closely associated with H2O2 biosynthesis. In addition, the H2O2 production induced by both ATP and SNP was inhibited by the protein kinase inhibitor staurosporine (Fig. 6B). Notice that the concentration of staurosporine 0.1 μM for effectively inhibiting the H2O2 production was only one-tenth of that tested for the NO production of 1 μM which showed no significant effect (Fig. 4).
Fig. 5.
The effect of ATP at various concentrations on H2O2 biosynthesis in S. miltiorrhiza hairy roots (ATP treatment for 1 h). Different letters (a–c) on top of columns indicate significant difference among the treatment effects, n=3, P <0.05, LSD=0.61.
Fig. 6.
(A) Suppression of ATP-induced H2O2 production by NO and Ca2+ antagonists. (B) Effect of protein kinase inhibitor (ST) on ATP- and SNP-induced H2O2 production. The number after each agent represents the concentration in μM or mM for EGTA only.
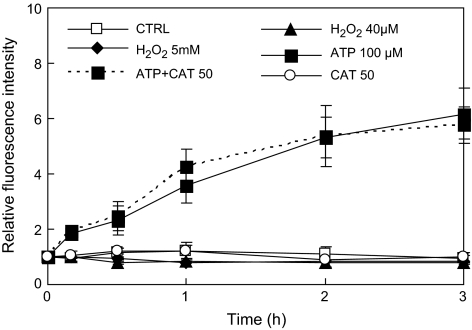
However, exogenous H2O2 applied to the hairy root culture did not stimulate NO biosynthesis (H2O2 40 μM and 5 mM versus the control), and the addition of the H2O2 scavenger catalase (CAT) did not suppress ATP-induced H2O2 (ATP+CAT 50 versus ATP) (Fig. 7). In agreement with our results, Laxalt et al. (2007) have also found that NO can stimulate H2O2 biosynthesis but ROS cannot induce NO synthesis.
Fig. 7.
Effect of H2O2 manipulation on NO and exogenous ATP-induced NO biosynthesis in S. miltiorrhiza hairy roots. The unit for CAT (catalase) is U ml−1.
ATP-induced intracellular Ca2+ increase and dependence on NO and H2O2
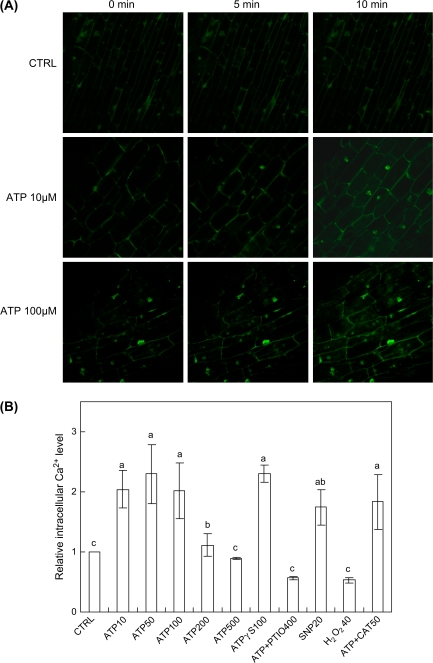
Exogenous ATP at 10–100 μM induced a significant increase in the intracellular Ca2+ level of the hairy roots, which was detected within 5 min of the ATP treatment (Fig. 8). Similar to the induction of H2O2 production (Fig. 5), exogenous ATP at the much higher concentrations of 200 μM and 500 μM induced a smaller or no intracellular Ca2+ increase, which again suggests an optimal ATP dose for the induction (Fig. 8B). The non-hydrolysable ATP analogue ATPγS at 100 μM induced a similar level of intracellular Ca2+ to that by 100 μM ATP. ATP (100 μM) in combination with PTIO (0.4 mM) (ATP+PTIO 400) failed to induce the intracellular Ca2+ increase, suggesting the requirement of NO signalling for the ATP-induced intracellular Ca2+ increase (Fig. 8B). It has also been found that NO biosynthesis was required for osmotic stress-induced intracellular Ca2+ concentration increase in tobacco cells based on the PTIO inhibitor test (Lamotte et al., 2006). In addition, a significant increase in the intracellular Ca2+ level of hairy roots was induced by the NO donor SNP (20 μM) but not by exogenous H2O2 (40 μM). Similarly, Foreman et al. (2003) reported that H2O2 did not induce a Ca2+ concentration increase in plant roots. However, H2O2 did induce the Ca2+ concentration increase in guard cells (Pei et al., 2000). These contrasting results could be attributed to the organ-specific response of plants to ROS. In addition, the responses of plant cells to H2O2 may be dependent upon the age and location of cells in plant roots. As shown in a recent study (Demidchik et al., 2007), the H2O2-induced Ca2+ influx in the elongation part of A. thaliana roots was much more pronounced than in the older and mature part of roots.
Fig. 8.
Changes of intracellular Ca2+ level in the hairy roots after treatment by exogenous ATP, NO, and H2O2. (A) Time-course of intracellular Ca2+ level after ATP treatment, represented by the fluorescence intensity of roots loaded with the Ca2+-specific fluorescence probe Fluo-3AM (laser-confocal images taken from epidermal cells in the elongation part of roots; scale bar=100 μm). (B) Effects of various inhibitors on the induced Ca2+ concentration increase (different letters on top of the columns indicating significant difference among the treatment effects, n=3, P <0.05, LSD=0.54). The number after each agent represents the concentration in μM or U ml−1 for CAT only, and ATP was fixed at 100 μM in ATP+PTIO 400 and ATP+CAT 50. (This figure is available in colour at JXB online.)
Discussion
Our experimental results have shown that exogenous ATP induces rapid and dose-dependent NO production in S. miltiorrhiza hairy root cultures. The ATP action was inhibited by suramin and RB, two reagents capable of blocking the combination of extracellular ATP with purinoceptors in animal cells, suggesting the involvement of similar purinoceptors in the extracellular ATP signal transmission across the plant cell membrane. The induction of NO by ATP could be mimicked by the non-hydrolysable ATP analogue ATPγS but not by its hydrolysed derivatives ADP and AMP. These results are similar to those in tomato cell suspensions reported by Foresi et al. (2007), but some different characteristics have also been found in our study. Firstly, an optimum ATP dose (about 100 μM) was observed in our experiments for maximum NO induction, while in Foresi et al. (2007), the NO increased constantly with the ATP concentration from 0.01 to 1 mM and then levelled off from 1 mM to 5 mM. This difference may be attributed to the different plant species or culture systems. Our results have also shown an optimum ATP dose or range for the induction of H2O2 production (about 100 μM) and intracellular Ca2+ increase (10–100 μM). Similarly, an optimum ATP concentration (or range) about 50 μM was suggested for effective induction of ROS production in Arabidopsis cells (Song et al., 2006). The lower levels of NO and ROS production induced by excessive ATP may be attributed to an adverse or negative effect of ATP on plant cell growth and metabolism. Roux and Steinebrunner (2007) have also suggested that extracellular ATP is beneficial at suitable concentrations to plant cell growth but becomes inhibitory at excessive concentrations. Another possible cause for the weaker responses induced with excessive ATP is a stronger interaction of ATP with some components of the culture medium such as Ca2+ chelation.
Another new finding from our study is the strong dependence of the exogenous ATP-induced NO biosynthesis on Ca2+ signalling and the independence on protein kinase activities. Our present study and previous studies (Demidchik et al., 2003; Jeter et al., 2004) have shown the activation of Ca2+ influx and the elevation of intracellular Ca2+ levels within a few minutes after ATP treatment in plant cells. It has also been reported that Ca2+ influx and the activation of CaM are prerequisites for the activation of NOS-like enzymes (del-Rio et al., 2004; Kondo et al., 1999). As a NOS-like enzyme has been detected as a possible source of ATP-induced NO production in hairy roots, the dependence of NO production on Ca2+ signalling is consistent with the requirement of Ca2+ signalling for NOS-like enzyme activation. The insignificant effect of the protein kinase inhibitor staurosporine on ATP-induced NO biosynthesis detected in our experiments suggests that protein phosphorylation is not a prerequisite for activating NO biosynthesis. Likewise, the protein kinase inhibitor could not block the intracellular Ca2+ increase in the S. miltiorrhiza hairy roots induced by ATP or the NO donor SNP as observed from our supplementary tests (data not shown). This may further suggest that ATP induces Ca2+ influx and signalling, leading to NO biosynthesis earlier than protein kinase activation and protein phosphorylation. Such a signal cascade is in agreement with the findings from previous studies (Lamotte et al., 2006; Lanteri et al., 2006; Courtois et al., 2008), that Ca2+ and CaM were required for activating the NO response, osmotic stress-induced activation of 42 kDa protein kinase NtOSAK was dependent on NO, and the activation of a 50 kDa CDPK by NO was essential for NO-induced adventious root formation.
The independence of protein kinase activity for the ATP-induced NO biosynthesis found in our study may be explained as follows. As shown in animal cells (Ralevic and Burnstock, 1998), exogenous ATP treatment induces the rapid activation (within a few milliseconds) of a non-selective flux of cations (Na+, K+, Ca2+) across the cell membrane, which is mediated by purinoceptors, perhaps via G-proteins. The ATP-induced cation ion flux leads to a significant increase in intracellular Ca2+, which activates NO biosynthesis in the cytosol. The ATP-induced Ca2+ influx and the following NO biosynthesis can be accomplished without the involvement of protein kinases in the plasma membrane or cytosol. By contrast, the activation of Ca2+ flux and NO biosynthesis in plant cells by other signal agents such as elicitors may depend on protein kinase activities and protein phosphoryaltion. For example, Gelli et al. (1997) have shown that the activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors is modulated by phosphorylation of the channel protein; Lamotte et al. (2004) have shown that the activation of protein kinases is required for fungal elicitor-induced NO biosynthesis. Future discovery and characterization of eATP-binding proteins in plant cells will be most helpful for elucidating the role of Ca2+ channel and protein kinases in the eATP signalling pathway.
On the other hand, the strong dependence of H2O2 production induced by exogenous ATP and the NO donor SNP on protein kinase activity found in our study suggests the requirement of protein phosphorylation for the activation of H2O2 production. This is in agreement with the general consensus that protein kinases are required for the activation of NADPH oxidases, the major enzymes responsible for ROS production in plant cells (Mehdy, 1994; Yoshioka et al., 2003). This also implies that H2O2 production is located downstream of protein phosphorylation in the eATP signal pathway, as that in animal cells (Gertsberg et al., 2004). NO may be an important element of the eATP signalling pathway for activating the protein kinases and the release of Ca2+ from the intracellular stores. NO has been found to induce the expression of MAPKs, while ATP treatment also induced the expression of MAPKs (Jeter et al., 2004; Grun et al., 2006). Therefore, NO biosynthesis may be an event upstream of protein kinase activation and protein phosphorylation in the extracelluar ATP signalling pathway. In Panax ginseng cells, Hu et al. (2004) suggested that H2O2 production is downstream of MAPK activation induced by a chitosan elicitor, based on the suppressed H2O2 production with a MAPK inhibitor. As NO can induce the MAPK pathways, it may activate the H2O2 biosynthesis through the MAPK signal pathways.
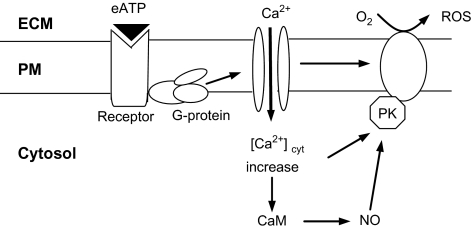
Based on our experimental results and the above discussion, a signal pathway leading to exogenous ATP-induced NO and ROS biosynthesis in S. miltiorrhiza hairy roots, as shown in Fig. 9, is proposed. In addition to the signal components detected in our study, the involvement of NADPH oxidases in eATP-induced H2O2 biosynthesis has been detected by Song et al. (2006) in Arabidopsis cells, and the role of NO for activating the Ca2+ release from intracellular stores is well-established in the plant response to biotic and abiotic elicitors (Garcia-Mata et al. 2003; Courtois et al., 2008). The simultaneous and balanced production of NO and ROS in plants is a common event in the plant defence response, and the NO and ROS signals co-regulate the defence response (Bright et al., 2006; Zaninotto et al., 2006). The two signal elements can exert reciprocal regulation over each other, and their signalling functions can be complementary, synergistic or parallel. A recent study (Laxalt et al., 2007) showed that the inhibition of NO production with NO antagonists also led to the inhibition of H2O2 production, suggesting that NO regulates H2O2 biosynthesis. NO may regulate H2O2 production through the lipid signalling system which activates ROS biosynthesis (Laxalt et al., 2007). The lipid signalling system has been recognized as an integral part of extracellular ATP signalling in animal cells (Ralevic and Burnstock, 1998), but remains to be further characterized in plant signalling cascades. In plants, NO can also facilitate the accumulation of ROS by directly inhibiting the enzymes eliminating ROS such as catalase and ascorbate peroxidase (Clark et al., 2000). Alternatively, NO can act as an antioxidant to counteract the cytotoxic effect of excessive ROS in plants evoked by biotic and abiotic stress (Beligni and Lamattina, 2001).
Fig. 9.
A hypothetical extracellular ATP signal pathway leading to NO and ROS production in S. miltiorrhiza hairy roots (ECM, extracellular matrix; PM, plasma membrane; PK, protein kinase; CaM, calmodulin).
The rapid induction of NO by exogenous ATP found here in the S. miltiorrhiza hairy roots and previously in other plant cells provides another line of evidence for the role of extracellular ATP as a signal agent in plant cells. Ca2+ signalling has been found to be essential for activating the NO and ROS production induced by ATP in plant cells. These results suggest a strong interrelationship among several signalling elements including Ca2+, ROS, NO, and protein kinases in eATP signalling and activation of plant cell responses. Further investigation is needed to characterize and understand the respective signal pathways and their interrelationships.
Acknowledgments
This work was supported by grants from The Hong Kong Polytechnic University (G-U268, G-YF75 and 1-BB80).
References
- Beligni MV, Lamattina L. Nitric oxide: a non-traditional regulator of plant growth. Trends in Plant Science. 2001;6:508–509. doi: 10.1016/s1360-1385(01)02156-2. [DOI] [PubMed] [Google Scholar]
- Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signalling molecules in immunity and inflammation. Pharmacology and Therapeutics. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal. 2006;45:113–122. doi: 10.1111/j.1365-313X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Chivasa S, Ndimba BK, Simon WJ, Lindsey K, Stabas AR. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. The Plant Cell. 2005;17:3019–3034. doi: 10.1105/tpc.105.036806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Molecular Plant–Microbe Interactions. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Alain Pugin, Wendehenne D. Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases. Journal of Experimental Botany. 2008;59:155–163. doi: 10.1093/jxb/erm197. [DOI] [PubMed] [Google Scholar]
- del-Rio LA, Corpas FJ, Barroso JB. Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry. 2004;65:783–792. doi: 10.1016/j.phytochem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interaction between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proceedings of the National Academy of Sciences, USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM. Is ATP a signaling agent in plants? Plant Physiology. 2003;133:456–461. doi: 10.1104/pp.103.024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Davies JM. Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. The Plant Journal. 2007;49:377–386. doi: 10.1111/j.1365-313X.2006.02971.x. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell J, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Foresi NP, Laxalt AM, Claudia V, Tonon CV, Casalongue CA, Lamattina L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiology. 2007;145:589–592. doi: 10.1104/pp.107.106518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR. Nitric oxide regulates K+ and Cl– channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proceedings of the National Academy of Sciences, USA. 2003;100:11116–11121. doi: 10.1073/pnas.1434381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XC, Wu JY. Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Science. 2005;168:487–491. [Google Scholar]
- Gelli A, Higgins VJ, Blumwald E. Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiology. 1997;113:269–279. doi: 10.1104/pp.113.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsberg I, Hellman V, Fainshtein M, Weil S, Silberberg SD, Danilenko M, Priel Z. Intracellular Ca2+ regulates the phosphorylation and the dephosphorylation of ciliary proteins via the NO pathway. The Journal of General Physiology. 2004;124:527–540. doi: 10.1085/jgp.200409153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun S, Lindermayr C, Sell S, Durner J. Nitric oxide and gene regulation in plants. Journal of Experimental Botany. 2006;57:507–516. doi: 10.1093/jxb/erj053. [DOI] [PubMed] [Google Scholar]
- Hu XY, Zhang WQ, Fang JY, Cai WM, Tang ZC. Chitosan induced the activity of MAP kinase and saponin accumulation in ginseng cells. Science in China, Series C, Life Sciences. 2004;34:31–40. [Google Scholar]
- Hu XY, Neill SJ, Tang ZC, Cai WM. Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiology. 2005;137:663–670. doi: 10.1104/pp.104.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeter CR, Tang WQ, Henaff E, Butterfield T, Roux SJ. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. The Plant Cell. 2004;16:2652–2664. doi: 10.1105/tpc.104.023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Sivaguru M, Stacey G. Extracellular ATP in plants. visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiology. 2006;142:984–992. doi: 10.1104/pp.106.085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo R, Tikunova SB, Cho MJ, Johnson J. A point mutation in a plant calmodulin is responsible for its inhibition of nitric-oxide synthase. Journal of Biological Chemistry. 1999;274:36213–36218. doi: 10.1074/jbc.274.51.36213. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, Sequeira-Legrand A, Lebrun-Garcia A, Durner J, Pugin A, Wendehenne D. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiology. 2004;135:516–529. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Dobrowolska G, Besson A, Pugin A, Wendehenne D. Mechanisms of nitric-oxide-induced increase of free cytosolic Ca2+ concentration in Nicotiana plumbaginifolia cells. Free Radical Biology and Medicine. 2006;40:1369–1376. doi: 10.1016/j.freeradbiomed.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lanteri L, Pagnussat GC, Lamattina L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. Journal of Experimental Botany. 2006;57:1341–1351. doi: 10.1093/jxb/erj109. [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Raho N, Have AT, Lamattina L. Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. Journal of Biological Chemistry. 2007;282:21160–21168. doi: 10.1074/jbc.M701212200. [DOI] [PubMed] [Google Scholar]
- Ma QF, Rengel Z, Kuo J. Aluminium toxicity in rye (Secale cereale): root growth and dynamics of cytoplasmic Ca2+ in intact root tips. Annals of Botany. 2002;89:241–244. doi: 10.1093/aob/mcf017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke FLH, Parchmann S, Mueller MJ, Kijne JW, Memelink J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiology. 1999;119:1289–1296. doi: 10.1104/pp.119.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defence against pathogens. Plant Physiology. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytologist. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:414–491. [PubMed] [Google Scholar]
- Roux SJ, Steinebrunner I. Extracellular ATP: an unexpected role as a signaler in plants. Trends in Plant Science. 2007;12:522–527. doi: 10.1016/j.tplants.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Shen J, Harada N, Nakazawa H, Yamashita T. Involvement of the nitric oxide-cyclic GMP pathway and neuronal nitric oxide synthase in ATP-induced Ca2+ signalling in cochlear inner hair cells. European Journal of Neuroscience. 2005;21:2912–2922. doi: 10.1111/j.1460-9568.2005.04135.x. [DOI] [PubMed] [Google Scholar]
- Silva G, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension. 2006;47:563–567. doi: 10.1161/01.HYP.0000197954.93874.ef. [DOI] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang XZ, Stout SC, Roux SJ. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiology. 2006;140:1222–1232. doi: 10.1104/pp.105.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr C, Ullrich WR. Generation and possible roles of NO in plant roots and their apoplastic space. Journal of Experimental Botany. 2002;53:2293–2303. doi: 10.1093/jxb/erf110. [DOI] [PubMed] [Google Scholar]
- Tang WQ, Brady SR, Sun Y, Muday G, Roux SJ. Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiology. 2003;131:147–154. doi: 10.1104/pp.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, Wu JY. Nitric oxide is involved in methyl jasmonate-induced defence responses and secondary metabolism activities of Taxus cells. Plant and Cell Physiology. 2005;46:923–930. doi: 10.1093/pcp/pci098. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Liu YS, Wu JY. The signal role of extracellular ATP and its dependence on Ca2+ flux in elicitation of Salvia miltiorrhiza hairy root cultures. Plant and Cell Physiology. 2008;49:617–624. doi: 10.1093/pcp/pcn033. [DOI] [PubMed] [Google Scholar]
- Xu YC, Zhao BL. The main origin of endogenous NO in higher non-leguminous plants. Plant Physiology and Biochemistry. 2003;41:833–838. [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones J, Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. The Plant Cell. 2003;15:706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto F, Camera SL, Polverari A, Delledonne M. Cross-talk between reactive nitrogen and oxygen species during the hypersensitive disease resistance response. Plant Physiology. 2006;141:379–383. doi: 10.1104/pp.106.078857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemojtel T, Frohlich A, Palmieri MC, et al. Plant nitric oxide synthase: a never-ending story? Trends in Plant Science. 2006;11:524–525. doi: 10.1016/j.tplants.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang WH, Rengel Z, Kuo J. Determination of intracellular Ca2+ in cells of intact wheat roots: loading of acetoxymethyl ester of Fluo-3 under low temperature. The Plant Journal. 1998;15:147–151. [Google Scholar]
- Zheng LM, Zychlinsky A, Liu C, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. Journal of Cell Biology. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]