Abstract
The co-ordination of cell wall synthesis with plant cell expansion is an important topic of contemporary plant biology research. In studies of cell wall synthesis pathways, cellulose synthesis inhibitors are broadly used. It is demonstrated here that ancymidol, known as a plant growth retardant primarily affecting gibberellin biosynthesis, is also capable of inhibiting cellulose synthesis. Its ability to inhibit cellulose synthesis is not related to its anti-gibberellin action and possesses some unique features never previously observed when conventional cellulose synthesis inhibitors were used. It is suggested that ancymidol targets the cell wall synthesis pathway at a regulatory step where cell wall synthesis and cell expansion are coupled. The elucidation of the ancymidol target in plant cells could potentially contribute to our understanding of cell wall synthesis and cell expansion control.
Keywords: Ancymidol; cell expansion; cell wall; cellulose synthesis; 2,6-dichlorobenzonitrile; gibberellin; isoxaben; microtubules; vesicle trafficking
Introduction
The control of polarity establishment and morphogenesis of plant cells and tissues is a complex process, which relies on many mechanisms. In this process, a crucial role is played by the cell wall. Plant cell walls are composed mainly of polymers of cellulose, hemicelluloses, and pectins. The co-ordination of cell expansion and cell wall synthesis is a topic of great interest to plant biologists. Cell expansion is a complicated process which includes controlled loosening of the cell wall and deposition of new material (Cosgrove, 2005). Cellulose, the main component of the cell wall, is synthesized by cellulose synthase complexes embedded in the plasma membrane. It forms long rigid polymers which surround the cell, endowing the cell wall with the strength against turgor pressure. Our understanding of the synthesis, deposition, and loosening of the cell wall has improved recently but many details still remain to be elucidated (for reviews see Somerville, 2006; Taylor, 2008). The disruption of cellulose synthesis by isoxaben, a specific inhibitor of some cellulose synthases, led to the loss of polarity of cultured tobacco BY-2 cells (Fisher and Cyr, 1998), which demonstrates a key role of cellulose in plant cell shape and polarity determination. It has also been shown in dark-grown hypocotyls of Arabidopsis that elongation is a biphasic process with a slower first phase and a faster second phase. The deposition of cellulose during the slow first phase is an important prerequisite for accelerated growth. The impairment of cellulose deposition during the slow growth phase, for example, by mutations affecting cellulose synthesis or treatment with isoxaben, resulted in the absence of the fast growth phase (Refregier et al., 2004).
The exact mechanism of co-ordination of cellulose synthesis and cell expansion remains to be elucidated. Cortical microtubules (MTs) are known as a key structure which participates in the control of cellulose deposition. Their polymers are often found aligned in parallel with cellulose microfibrils in expanding cells (Ledbetter and Porter, 1963). This led to the hypothesis that the orientation of cellulose deposition is controlled by microtubules, which co-ordinate cellulose synthase movement in the plasma membrane. It has been shown recently in several studies that the involvement of MTs in the orientation of cellulose deposition is a complex process. Microtubules undoubtedly play an important role in cellulose deposition, guiding the movement of cellulose synthases in the plasma membrane (Paredez et al., 2006). The interaction between MTs and the cell wall is bi-directional, because both MTs and the cell wall influence each other's organization. However, they both still possess a certain, but not well understood, level of independence in their influence on cell growth and morphogenesis (for recent reviews see Wasteneys, 2004; Wasteneys and Fujita, 2006). In addition, vesicle transport and endosomal cycling play more than just a transport role in cell wall building and represent another key factor in plant cell growth. Some recently described proteins such as COBRA (Roudier et al., 2005) and KOR1 (Robert et al., 2005), whose functions are clearly linked to the control of cellulose orientation in the cell wall, or to cellulose synthesis, have been shown to be associated with the Golgi apparatus (COBRA) or with early endosomes and the tonoplast (KOR1). This indicates that unperturbed vesicle trafficking is needed for cell wall synthesis and for the control of cellulose deposition.
Cellulose synthesis inhibitors are often used to investigate cellulose synthesis pathways. They constitute a group of structurally diverse drugs, which impair certain steps in the cellulose synthesis and deposition processes. Surprisingly, although very often used, the specific site of action of some of them is still not known. Examples of broadly used cellulose synthesis inhibitors are isoxaben and 2,6-dichlorobenzonitrile (DCB). Isoxaben inhibits the cellulose synthase isoforms CESA 3 (Scheible et al., 2001) and CESA 6 (Desprez et al., 2002) by binding to the enzyme and disrupting its function. The target of DCB in plant cells is not known. Nevertheless, several authors have concluded that it is not identical to that of isoxaben.
In the current work, evidence is presented that ancymidol is another compound which inhibits cellulose synthesis. Ancymidol is known as a plant growth retardant, the primary mode of action of which is the inhibition of the enzyme ent-kaurene oxidase, a cytochrome P450 mono-oxygenase that controls the oxidation of ent-kaurene (Coolbaugh et al., 1978). Inhibition of ent-kaurene activity interferes with gibberellin (GA) biosynthesis. Plants treated with ancymidol display decreased growth and their GA content is reduced (Shive and Sisler, 1976). The effect of ancymidol on higher plants is eliminated by simultaneous GA application (Shive and Sisler, 1976; Coolbaugh et al., 1982). However, Coolbaugh et al. (1982) showed that this was true only for ancymidol applied at concentrations lower than 100 μM. The inhibitory effect of 100 μM ancymidol or higher concentrations could not be overcome by externally applied GA. This suggests that the effects of ancymidol on plants at least partly involve mechanisms not associated with its anti-GA role.
To study possible GA-independent mechanisms of action of ancymidol on plant cells, the tobacco cell line BY-2 was used (Nagata et al., 1992). The aim of this study was to characterize the effect of ancymidol on cultured BY-2 tobacco cells and to determine its relation to the anti-GA effects observed in plants. In a previous study (Boříková et al., 2003) and in our present work it is shown that GA has no effect on the growth of BY-2 cells; despite this, it is demonstrated here that ancymidol induces extensive cell shape changes in these cells, followed by eventual death. This suggests that the effect of ancymidol is not GA-related. The effect of ancymidol has been compared with the effect of isoxaben and DCB and it was found that their effects were similar. It is shown here that ancymidol is a potent cellulose synthesis inhibitor, higher doses of which completely inhibit cell elongation and the deposition of new cell wall. Therefore, ancymidol might represent a new cellulose synthesis inhibitor, acting at an unknown step in the synthesis pathway. The mode of action is different from isoxaben and DCB and the elucidation of the ancymidol target could contribute to a better understanding of the co-ordination between cellulose synthesis and plant cell expansion.
Materials and methods
Plant material and cultivation conditions
The tobacco cell line BY-2 (Nicotiana tabacum L. cv. Bright Yellow 2; Nagata et al., 1992) was cultured in medium containing 4.3 g l−1 of Murashige–Skoog salts (Sigma, St Louis, Mo, USA), 1 mg l−1 thiamine, 200 mg l−1 KH2PO4, 100 mg l−1 myo-inositol, 30 g l−1 sucrose, and 0.9 μM 2.4-dichlorophenoxyacetic acid (2.4-D), pH 5.8. The transgenic BY-2 cell line GT16 expressing tobacco GFP-α-tubulin (Kumagai et al., 2001) was maintained on the same medium supplemented with 100 μg ml−1 kanamycin and 100 μg ml−1 cefotaxime. Every 7 d, 1.5 ml of cell suspension were transferred to 30 ml of fresh medium and cultured in darkness at 25 °C on an orbital shaker (IKA KS501; IKA Labortechnik, Staufen, Germany; 120 rpm; orbital diameter, 30 mm). Nicotiana benthamiana seeds were surface-sterilized, immediately sown on inclined agar growth medium (4.3 g l−1 Murashige–Skoog salts, 2.5 mg l−1 thiamine, 2.5 mg l−1 nicotinic acid, 100 mg l−1 inositol, 2.5 mg l−1 pyridoxine, 10 mg l−1 glycine, 1 g l−1 casein, 30 g l−1 sucrose, and 6 g l−1 agar, pH 5.8) and cultivated for 7 d under a long-day photoperiod (16 h of light) at 25 °C. For inhibitor or GA treatment, a liquid growth medium with an inhibitor at its double final concentration was poured onto plates containing the same volume of agar growth medium without the inhibitor. The plates were incubated for at least 5 h to allow inhibitors to diffuse evenly in the whole volume of the medium, decreasing its concentration to the required one. After this time, the liquid medium was discarded and 7-d-old plants were transplanted onto the agar and grown in the vertical position for 4 weeks.
Chemicals
Stock solutions of 100 mM ancymidol (α-cyclopropyl-α-[4-methoxyphenyl]-5-pyrimidine-methanol; Sigma), 1 mM isoxaben (Pestanal; Sigma), 100 mM DCB (Sigma), 2.53 mM latrunculin B (Sigma), 10 mM taxol (Paclitaxel; MP Biomedicals, Irvine, California, USA), 10 mM oryzalin (Surflan; Elanco Products Co., USA) in DMSO and stock solution of 20 mM brefeldin A (Sigma) in ethanol were prepared and appropriate volumes were added directly to the growth media to obtain the final concentrations required. Stock solution of 20 mM gibberellic acid (GA3, MP Biomedicals) in H2O was prepared and appropriate volumes were added directly to growth media to obtain the final concentrations. After the addition of GA3, the pH of the growth medium was adjusted. All chemicals were obtained from Sigma unless stated otherwise.
Viability and cell shape assessment of changes
Cell viability was assessed with fluorescein diacetate (FDA) according to the method of Widholm (1972). 40 μl of 0.2% (w/v) FDA stock solution in acetone were diluted with 7 ml of culture medium, and an aliquot mixed 1:1 (v/v) with cell suspension on a microscopic slide. The viability was determined from at least 10 optical fields on each of three separate slides as a percentage of fluorescing cells (about 400 cells were counted in each sample in total). Malformed cells were counted in at least 10 optical fields on each of three separate slides and expressed as a percentage of malformed cells (at least 400 cells were counted in each sample in total).
Cell wall visualization
The cell wall was visualized using 10 μM Calcofluor White M2R (Sigma, stock solution 1 mM in H2O).
Protoplasts preparation
The cell wall of 3-d-old BY-2 cells was removed by digestion in 1% cellulase and 0.1% pectolyase Y-23, supplemented with 0.45 M mannitol. After 3–4.5 h of digestion, protoplasts were overlaid onto the growth medium supplemented by 0.4 M sucrose and centrifuged at 200 g for 10 min. Floating protoplasts were collected, filtered through a nylon mesh (mesh diameter 100 μm), resuspended in the growth medium supplemented by 0.4 M sucrose, and cultivated at 25 °C without shaking.
Microscopy and image processing
An epifluorescence microscope (Olympus Provis AX 70; Olympus Optical Co., Ltd., Japan) equipped with appropriate filter sets for the detection of Calcolfuor White fluorescence (excitation at 330–385 nm, barrier filter at 420 nm) was used for the observation of cell walls in regenerating protoplasts. A LUMPlan Fl objective lens was used (magnification ×40; numerical aperture 0.80). The fluorescence signal was grabbed with a monochromatic integrating charge coupled-device camera Cohu 4910 (Cohu, Inc., Poway, California, USA). Nomarski differential interference contrast (DIC) images were taken with a Nikon digital camera DXM1200 (Nikon Inc., Tokyo, Japan) and images were stored and analysed using the Lucia image analysis software (Laboratory Imaging, Prague, Czech Republic).
Optical sections were obtained with a confocal laser scanning microscope (Leica TCS SP2). For GFP observation, excitation at 488 nm (Ar/ArKr laser) and emission at 515–545 nm was used. For Calcofluor White observations, the excitation was at 405 nm (HeNe laser) and the emission was at 410–480 nm. An objective lens Plan Apo (magnification ×63; numerical aperture 1.2, water immersion) was used for all observations.
Images of N. benthamiana plants were taken with a Canon DS6041 photo camera (Canon, Tokyo, Japan). Plates with N. benthamiana plants cultivated on the inclined agar were photographed at the end of the experiment and the length of hypocotyls was measured using the Lucia image analysis software. At least 30 plants were analysed in each variant. Experiments were repeated three times.
Results
Ancymidol induces changes of the cell shape in a GA-independent manner
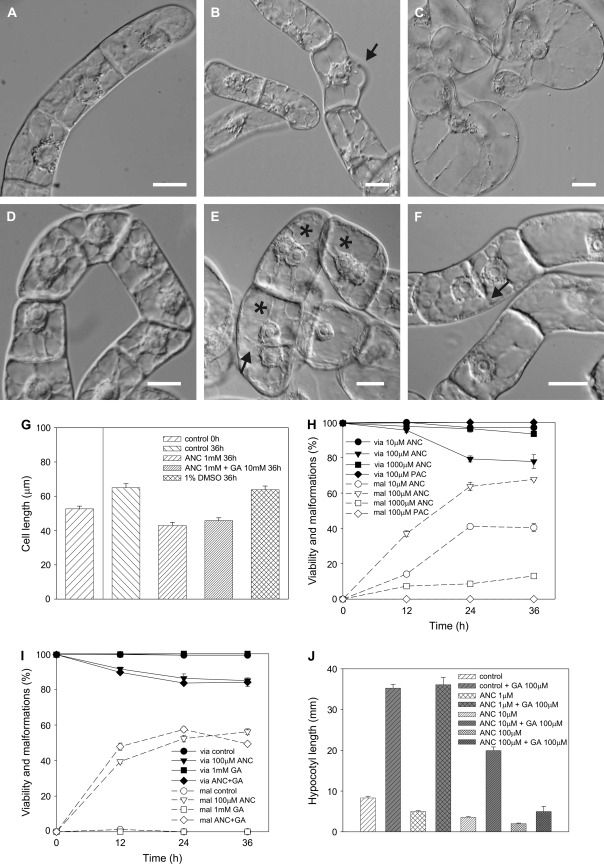
The application of ancymidol to 3-d-old BY-2 cells resulted in cell malformations in the form of bulges (Fig. 1A–C). Control BY-2 cells showed a characteristic polar phenotype (Fig. 1A). The first changes in cell shape were detected 12 h after treatment (Fig. 1B) and the number of malformed cells was shown to be concentration- and time-dependent (Fig. 1H). Bulges started to form as small local protrusions of the cell wall (Fig. 1B). These gradually increased in size and the nucleus often moved into them. Prolonged exposure to ancymidol (36 h) resulted in nearly spherical cells (Fig. 1C) resembling protoplasts. These cells often burst when covered by a coverslip and observed under the microscope. The malformation effect of ancymidol was shown to be concentration-dependent, increasing from 10 μM to the most effective concentration of 100 μM. An increase in the number of malformed cells was always accompanied by a slight decrease in cell viability (Fig. 1H). By contrast, a higher concentration of ancymidol (1 mM) prevented cell elongation (Fig. 1D, G), but had little effect on cell shape or on cell viability (Fig. 1H). Whereas untreated control cells elongated during the 36 h of cultivation, the length of cells treated with 1 mM ancymidol actually decreased when compared with their lengths at the beginning of the experiment (Fig. 1G). Further, the cultivation of cells in 1 mM ancymidol resulted in the incidence of bi-nuclear cells (Fig. 1E) or cells with incomplete transverse cell walls (Fig. 1F), which comprised about 4% of the whole population. It appeared that nuclear division was not arrested by 1 mM ancymidol, but that cell elongation and new cell wall synthesis during the cell division was affected. Cell-malforming effects or increased cell lethality were never observed when paclobutrazol (PAC), an inhibitor of ent-kaurene oxidase with a similar mode of action to ancymidol (Rademacher, 2000), was applied to BY-2 cells (Fig. 1H).
Fig. 1.
GA-reversible cell-malforming effect of ANC in 3-d-old tobacco BY-2 cells and the GA-irreversible inhibitory effect of ANC on tobacco hypocotyl elongation. (A–F) Nomarski DIC. (A) Control cells. (B, C) Cells treated with 100 μM ANC for 12 h (B) and 48 h (C). (D–F) Cells treated with 1 mM ANC for 48 h (D) and 72 h (E, F). Asterisks in (E) show bi-nuclear cells, arrows in (E) and (F) show incomplete transverse cell walls. (G) Cell length of BY-2 cells after 36 h treatment with 1 mM ANC. (H) Viability (via) and malformations (mal) of BY-2 cells treated with various concentrations of ANC or 100 μM PAC. (I) Viability and malformations of BY-2 cells treated with 100 μM ANC and/or 1 mM GA. Scale bars 20 μm. Error bars=SE, n=3, at least 400 cells were counted in each variant. (J) The average length of the hypocotyl of N. benthamiana plants treated with 1, 10, and 100 μM ANC and/or 100 μM GA. Error bars=SE, n=30.
To address whether ancymidol malformation effects could be reversed by GA application, GA was applied to 3-d-old BY-2 cells. At all concentrations tested, GA had no effect on cell viability and did not induce any cell shape malformations itself (Fig 1I, only 1 mM GA, the highest GA concentration tested, is shown). Application of 1 mM GA together with 100 μM ancymidol did not reverse the effect of ancymidol (Fig. 1I) suggesting that the effect of ancymidol was GA-independent. Ancymidol-retarding and GA-stimulating effects on elongation growth were further tested using hypocotyls of N. benthamiana seedlings grown for 4 weeks on inclined agar plates with various concentrations of ancymidol (1, 10, and 100 μM) and/or 100 μM GA. As shown on Fig. 1J, an increasing concentration of ancymidol had an increasingly inhibitory effect on the growth of the plants. GA significantly stimulated the growth of control seedlings as well as the growth of seedlings treated with 1 μM and 10 μM ancymidol, suggesting that the effect of ancymidol in these concentrations was reversed by GA. The inhibitory effect of 100 μM ancymidol was reversed much less by GA; the average length of the hypocotyls was smaller than that in the control seedlings (Fig. 1J).
Together, these results show that up to a concentration of around 10 μM, GA and ancymidol have opposite effects on hypocotyl elongation in N. benthamiana plants. This is consistent with the view that, at lower concentrations, ancymidol functions as an anti-GA. However, neither the inhibition of hypocotyl growth by 100 μM ancymidol in N. benthamiana plants nor its cell-malformation effect on tobacco BY-2 cells is reversed with GA. It is demonstrated below that GA-independent effects leading to cell malformation, the inhibition of cell elongation, and the formation of incomplete transverse cell walls in BY-2 cells were connected with a defect in cell wall synthesis.
The cell-malforming effect of ancymidol is similar to the effect of cellulose synthesis inhibitors
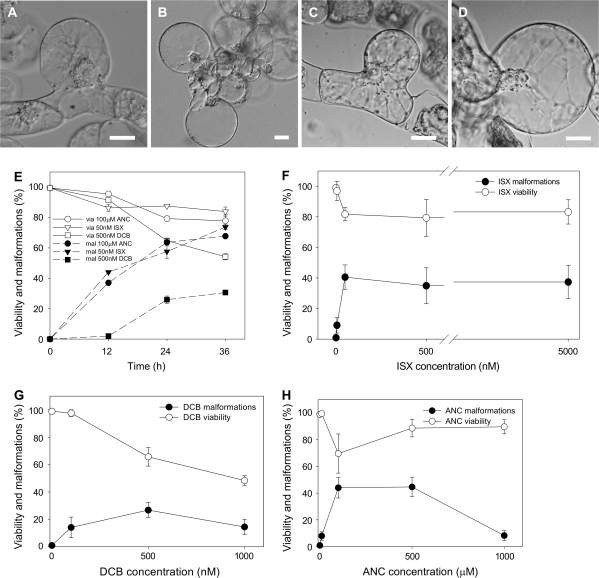
The inhibition of cellulose synthesis is known to induce cell shape malformations in cultured cells similar to those observed following ancymidol treatment. Therefore, isoxaben and DCB, widely used inhibitors of the cellulose synthesis, were applied in a broad concentration range to BY-2 cell suspension. Isoxaben and DCB induced cell malformations and decreased the cell viability in a similar way to ancymidol (Fig. 2A–D, cf. to Fig. 1A–C). The lowest effective concentrations were found to be 50 nM for isoxaben (Fig. 2A, B) and 500 nM for DCB (Fig. 2C, D). Therefore, these concentrations were used in all other experiments unless stated otherwise. The decrease in cell viability and increased incidence of cells with malformations during 36 h of treatment with 50 nM isoxaben and 500 nM DCB was comparable with the effect of 100 μM ancymidol (Fig. 2E). The effect of DCB on the cell viability was slightly more severe, and the malforming ability of DCB was lower when compared with ancymidol and isoxaben.
Fig. 2.
The comparison of cell-malforming effects of ANC, ISX, and DCB in 3-d-old tobacco BY-2 cells. (A–D) Nomarski DIC. (A, B) Cells treated with 50 nM ISX for 24 h (A) and 36 h (B). (C, D) Cells treated with 500 nM DCB for 24 h (C) and 36 h (D). (E) Viability (via) and malformations (mal) of BY-2 cells treated with 100 μM ANC, 50 nM ISX, and 500 nM DCB for 36 h. (F–H) Viability and malformations of BY-2 cells after 24 h treatment with 5 nM, 50 nM, 500 nM, and 5000 nM ISX (F), 100 nM, 500 nM, and 1000 nM DCB (G), and 10 μM, 100 μM, 500 μM, and 1000 μM ANC (H). 1% DMSO was used as the control. Scale bars 20 μm. Error bars=SE, n=3, at least 400 cells were counted in each variant.
To compare the effect of three inhibitors, isoxaben, DCB, and ancymidol were applied in a broad concentration range to BY-2 cells and cell viability and the number of malformed cells was assessed after 24 h of cultivation. Dose-dependent effects of the three compounds differed. The lowest effective concentration of isoxaben was 50 nM, but its effect was not increased or decreased when higher concentrations were applied (Fig. 2F). By contrast, the effect of DCB was more severe when higher concentrations were applied (Fig. 2G). The decrease in cell malformations at the highest concentration tested (1 μM) was probably associated with a severe effect on cell viability. The most effective concentration range of ancymidol was 100–500 μM, but higher concentration (1 mM) had only little effect (Fig. 2H).
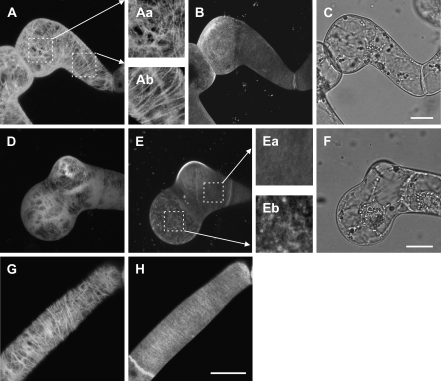
Major determinants of cell morphogenesis, namely microtubules, cellulose microfibrils of the cell wall, and the protein secretion machinery, were studied in malformed cells. Only the effects of ancymidol and isoxaben have been compared, because the mode of action of DCB is not well understood. In a BY-2 cell line expressing tobacco GFP-tubulin (Kumagai et al., 2001) microtubules were shown to form typical transverse cortical arrays in elongating interphase cells (Fig. 3G). However, cortical microtubules were always disoriented in malformed regions of cells treated with ancymidol or isoxaben. Disoriented microtubules were often observed only in the cortical region of the bulge, whereas the rest of the cell retained transversally oriented microtubules (Fig. 3A, D; for details of the orientation of cortical microtubules see Fig. 3Aa and Ab). The structure of the cell wall was investigated using the fluorescent probe Calcofluor White, which binds specifically to cellulose microfibrils. The cellulose structure of the cell wall in control cells was easily detectable as transverse and parallel cellulose microfibrils, particularly in elongated cells (Fig. 3H). The structure of the cell wall was strongly affected in cell bulges induced by ancymidol or isoxaben. The cellulose distribution was not continuous, some parts of the bulge displaying regions with a very thin layer of cellulose or being devoid of Calcofuor White fluorescence at all (Fig. 3B, E; for details of the cell wall see Fig. 3Ea, Eb). Some bulges had cellulose deposited in patches and dots on their surface. The cell wall in the non-malformed region of the cells was stained evenly by the fluorescent probe and displayed no disturbance of the structure. Therefore, the defect in cell wall deposition was a highly localized event, occurring only in the malformed part of the cell, and not affecting the rest of the cell.
Fig. 3.
(A–H) Confocal laser scanning microscopy: projections of optical confocal sections through the cortical cytoplasm and the cell wall of transgenic BY-2 GT16 cells expressing tobacco GFP–α-tubulin, cell wall visualized by Calcofluor White. (A–C) Microtubules (A) and the cell wall (B) of BY-2 GT16 cell treated with 100 μM ANC for 16 h, developing a typical bulge (C). Details show disoriented cortical microtubules in the region of the bulge (Aa) and transverse microtubules in the non-malformed part of the same cell (Ab). (D–F) Microtubules (D) and the cell wall (E) in BY-2 GT16 cells treated with 50 nM ISX for 16 h developing a typical bulge (F). Details show the organization of cellulose in the cell wall in the non-malformed part of the cell (Ea) and disturbed deposition of cellulose in the form of patches in the region of the bulge (Eb). (G, H) Cortical microtubules (G) and cell wall (H) in control BY-2 GT16 cells. Scale bars 20 μm.
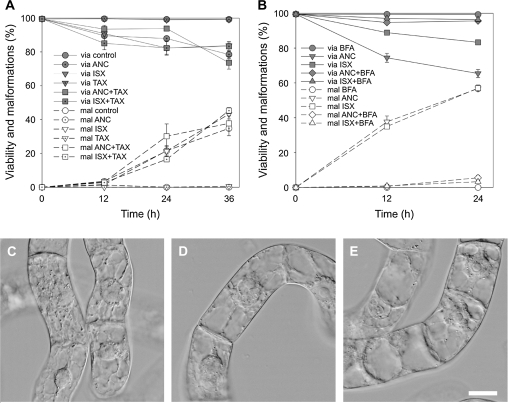
Inhibitor studies using the microtubule-stabilizing drug taxol and the anterograde vesicle trafficking inhibitor brefeldin A were performed to test further for possible targets for ancymidol. Microtubule stabilization with 10 μM taxol had no effect on either 100 μM ancymidol- or 50 nM isoxaben-induced cell malformations (Fig. 4A). Cell viability and the incidence of malformed cells were comparable to cells treated only with isoxaben or ancymidol. By contrast, 1 μM brefeldin A applied along with 50 nM isoxaben or 100 μM ancymidol completely abolished the formation of cell bulges (Fig. 4B–E). Moreover, the decrease in the cell viability characteristic following treatment with ancymidol or isoxaben was not observed (Fig. 4B). The application of taxol or brefeldin A alone did not influence the cell viability during the time-course of our experiments and induced no cell shape changes (Fig. 4A, B).
Fig. 4.
Taxol and brefeldin A effects on the incidence of cell malformations induced by ANC and ISX in 3-d-old tobacco BY-2 cells. (A) Viability (via) and malformations (mal) of BY-2 cells treated with 100 μM ANC, 50 nM ISX, and 10 μM taxol for 36 h. (B) Viability and malformations of BY-2 cells treated with 100 μM ANC, 50 nM isoxaben, and 1 μM brefeldin A. (C–E) Nomarski DIC: BY-2 cells treated with 1 μM brefeldin A (C), 1 μM brefeldin A and 100 μM ANC (D), and 1 μM brefeldin A and 50 nM ISX (E) for 24 h. Scale bars 20 μm. Error bars=SE, n=3, at least 400 cells were counted in each variant.
A comparison of the effects of 100 μM ancymidol, 50 nM isoxaben, and 500 nM DCB on BY-2 cells revealed that their effects seem to be similar, inducing cell malformations and eventual cell death. Since isoxaben and DCB are known as cellulose synthesis inhibitors, ancymidol action is also likely to be connected with cellulose synthesis disruption. The effect of higher concentrations of all three inhibitors differed, suggesting different modes of actions. The cell malformations were accompanied by local disruption of the cellulose deposition in the cell wall and disorientation of microtubules in malformed parts of cells. The malforming effect of 50 nM isoxaben and 100 μM ancymidol was not dependent on microtubular cytoskeleton dynamics, but was completely prevented by BFA, the inhibitor of vesicle trafficking.
Ancymidol disrupts cellulose synthesis
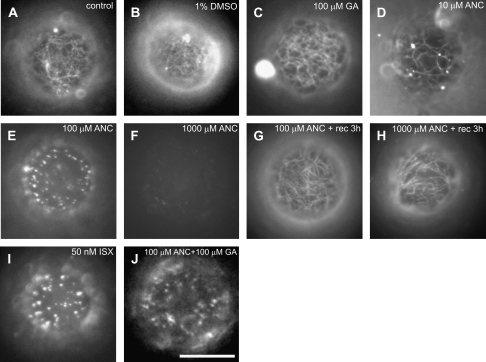
To test further the hypothesis that ancymidol acts as a cellulose synthesis inhibitor, the effects of ancymidol and isoxaben on the process of the de novo cell wall synthesis in regenerating BY-2 protoplasts were compared. After removing the cell wall by enzymatic digestion, protoplasts were allowed to regenerate cell walls for 24 h in a medium containing 10 μM, 100 μM, and 1 mM ancymidol or 50 nM isoxaben. As described previously by Fisher and Cyr (1998), cellulose was deposited in patches on the surface of protoplasts in the presence of isoxaben (Fig. 5I). This contrasted with non-treated control protoplasts which regenerated a dense net of cellulose microfibrils (Fig. 5A). Protoplasts treated with 10 μM ancymidol regenerated a sparse net of filamentous microfibrils (Fig. 5D) when compared with control protoplasts. 100 μM ancymidol inhibited cellulose regeneration in the same manner as isoxaben, inducing the formation of patches of cellulose during protoplast regeneration (Fig. 5E). Interestingly, protoplasts treated with 1 mM ancymidol failed to deposit any cellulose at all; after 24 h of regeneration no Calcofluor White fluorescence was observed on the surface of treated protoplasts (Fig. 5F). The effect of ancymidol was reversible; the regeneration of cellulose in protoplasts, which was inhibited by ancymidol treatment for 24 h, was fully activated when ancymidol was removed by washing from the regeneration medium (Fig. 5G, H). The effect of 100 μM ancymidol was not reversed by 1 mM GA (Fig. 5J), confirming our previous results that this effect of ancymidol is GA-independent. GA alone (Fig. 5C) or the solvent DMSO alone (Fig. 5B) had no effect on cellulose deposition.
Fig. 5.
ANC and ISX disruption of cellulose deposition during regeneration of protoplasts from tobacco BY-2 cells. Calcofluor White-visualized cell walls of BY-2 cell protoplasts regenerated for 24 h observed by epifluorescence microscopy. Optical sections through the cortical cytoplasm of the protoplast regenerated in control medium (A), and in the medium supplemented by 1% DMSO (B), 0.1 mM GA (C), and ANC at concentrations of 10 μM (D), 100 μM (E), or 1000 μM (F). (G, H) Optical sections through the cortical cytoplasm of protoplasts regenerated for 24 h in the medium supplemented with ANC at concentrations of 100 μM (G) or 1000 μM (H) and then regenerated for another 3 h in the medium without ANC. (I, J) Optical sections through the cortical cytoplasm of protoplasts regenerated in the medium supplemented and with 50 nM ISX (I), and 100 μM ANC and 0.1 mM GA (J). Scale bar 20 μm.
Additional evidence supporting the cellulose inhibitory action of ancymidol was obtained using Fourier transform infrared (FTIR) spectroscopy on Arabidopsis thaliana hypocotyls grown in the dark and treated with ancymidol and GA (Mouille et al., 2003). The clustering analysis of the FTIR spectrotypes clearly showed that plants treated with 100 μM ancymidol and 100 μM GA clustered with the cellulose-deficient mutant rsw1-2 as well as with wild-type seedlings treated with isoxaben and DCB (Mouille and Schwarzerová, unpublished results).
Discussion
GA-independent ancymidol action
The anti-GA action of ancymidol is based on the inhibition of ent-kaurene oxidase, a key enzyme of the GA biosynthetic pathway (Coolbaugh et al., 1978). Later, Coolbaugh et al. (1982) showed that the anti-GA action of ancymidol on plants, which is manifested by a growth reduction, and which is reversible by externally applied GA, occurs at concentrations of ancymidol that are lower than 100 μM. Effects of ancymidol at concentrations greater than 100 μM were not reversed by GA application. Coolbaugh et al. (1982) therefore concluded that ancymidol in this concentration had another unknown mode of action. In our experiments with BY-2 tobacco cells, it has been demonstrated that 100 μM ancymidol inhibits cellulose synthesis, which is followed by changes in cell shape, and that this action is not the result of the inhibition GA biosynthesis.
Three observations indicate that the effect of 0.1–1 mM ancymidol is not associated with the GA synthesis inhibition. First, the inhibition of tobacco seedling growth by 100 μM ancymidol is not reversible by the external application of GA. Second, application of a broad range of GA concentrations did not induce any growth or cell shape changes in BY-2 cells, but the application of ancymidol in concentrations around 100 μM induced severe changes in cell shape, accompanied by eventual cell death. This effect of ancymidol was not reversible by GA. This indicates that BY-2 cells do not react to GA by changing their growth properties, however, they are very sensitive to ancymidol. Third, and the strongest evidence, paclobutrazol, an inhibitor of GA biosynthesis acting at the same step of GA biosynthesis as ancymidol (Rademacher, 2000), has no effect on BY-2 cells at any concentration tested.
The fact that externally applied GA and paclobutrazol do not influence the growth parameters of BY-2 cells indicate that these cells are as non-sensitive to GA in respect of their growth or cell shape response. The possibility remains, however, that unknown signalling pathways could be activated by GA in these cells, but that this activation does not result in changes in growth. Apart from a recent study by Hussain et al. (2005) of GA signalling in BY-2, no information or systematic analysis on the metabolism of GA in the BY-2 cell line is currently available in the literature. These authors showed that in BY-2 cells stably transformed with Arabidopsis RGL2 protein (belonging to DELLA proteins, whose degradation is triggered by GA), the introduced RGL2 protein undergoes degradation upon GA treatment. This suggests that the activation of a GA signalling pathway (or some components of such a pathway) is not necessarily reflected in growth or cell shape changes, even though some molecular components of GA signalling are active in BY-2. This is perhaps not surprising considering the pleiotropic effects of GA on plant growth and development.
Ancymidol inhibits cellulose synthesis
The effect of 100 μM ancymidol in BY-2 is very similar to the effects of the widely used cellulose synthesis inhibitors, isoxaben (Fisher and Cyr, 1998) and DCB (Hogetsu et al., 1974). Similar to the effects of cellulose synthesis inhibitors, cells treated with ancymidol created local bulges in the cells wall, which expanded during prolonged cultivation. The effect was clearly localized. It is hypothesized that the site of bulge formation probably represented the site where cell wall remodelling, including cell wall loosening and new cell wall material synthesis and deposition, was most active at the moment of cellulose inhibitor addition. Following the partial inhibition of cellulose synthesis, the cell wall was locally not strengthened by filamentous cellulose deposition, resulting in bulge formation.
Since the mode of action of isoxaben, but not DCB, is well known, the effects of ancymidol have been further compared with isoxaben only. Bulges induced by both ancymidol and isoxaben displayed local disorganization of microtubules and the deposition of cellulose material was not continuous, in contrast to non-malformed parts of cells. Furthermore, it is shown that the deposition of cellulose was disturbed in protoplasts treated by isoxaben as well as ancymidol. As before, ancymidol-induced inhibition of cellulose synthesis was not GA-dependent. From these results, it is concluded that 100 μM ancymidol induces effects that are indistinguishable from the effects of isoxaben and DCB and that ancymidol can be considered as a true cellulose synthesis inhibitor. The cellulose synthesis inhibitory action of 100 μM ancymidol was further confirmed using FTIR spectroscopy of etiolated Arabidopsis thaliana hypocotyls (see Mouille et al., 2003). Plants treated with 100 μM ancymidol and 100 μM GA clustered clearly with mutants known to be defective in cellulose synthesis (such as rsw1-2) or with wild-type plants treated with isoxaben or DCB. Therefore, despite the fact that the anti-GA action of ancymidol was suppressed by the simultaneous application of GA in this experiment, the cellulose deficiency occurred in treated plants.
Mechanism of ancymidol action on cellulose synthesis
What step of the cellulose synthesis pathway is targeted by ancymidol? Isoxaben is known to inhibit the cellulose synthase isoforms 3 (Scheible et al., 2001) and 6 (Desprez et al., 2002). The target of DCB in plant cells is not known, but several authors have concluded that it is not identical to that of isoxaben. For example, DeBolt et al. (2007) reported recently that, in Arabidopsis plants expressing YFP-labelled cellulose synthase 6 (CESA6), treatment with isoxaben resulted in the disappearance of YFP-CESA6 from the cortical region, whereas DCB treatment inhibited the motility of YFP-CESA6 and caused hyperaccumulation of CESAs in the plasma membrane. Our results also support the hypothesis that isoxaben and DCB target different steps in cellulose synthesis. When applied in higher concentrations, all inhibitors tested differed in their action. The dose-dependent influence of isoxaben on cell viability and malformation is consistent with its mode of action. Reaching the saturation concentration, which is around 50 nM in BY-2 cells, most target CESAs were probably inhibited by isoxaben. Because of the limited amount of target CESAs in the plasma membrane, higher concentrations did not substantially increase the effect. Higher concentrations of DCB resulted in more severe effect of the inhibitor and no saturating concentration was observed. The reduced malformation effect of the highest concentration could be assigned to the rapid death of the cell population. The concentration curve of ancymidol action showed very different characteristics. At 1 mM, ancymidol was much less effective than at lower concentrations (100 μM) in its ability to induce cell malformation. Cells did not develop any bulges and remained alive, but cell elongation was significantly inhibited and new cell wall formation between dividing cells was impaired in some cells. Both cell elongation arrest and new cell wall formation defects imply that the effect of 1 mM ancymidol is also connected with the inhibition of cell wall synthesis.
It remains to explain why the effect of various concentrations of ancymidol differed, and why an effect similar to that of 1 mM ancymidol was never observed when isoxaben and DCB in various concentrations were used. Isoxaben, and possibly also DCB, inhibit partly, but not fully, the process of cellulose deposition. Cellulose synthases comprise a multiple family (Doblin et al., 2002), from which only CESA3 and 6 are targeted by isoxaben (Scheible et al., 2001; Desprez et al., 2002, respectively). Therefore, low rates of cellulose synthesis could be maintained during treatment with isoxaben due to the activity of isoxaben-non-sensitive CESAs. In cultured cells of Zinnia elegans, isoxaben and DCB were shown to inhibit cellulose synthesis activity by approximately 70% (Kiedaisch et al., 2003). In our experiments, 50 nM isoxaben (or 100 μM ancymidol)-treated protoplasts deposited the cellulose in the form of patches or as a sparse net, also indicating that cellulose synthesis was not fully inhibited. The formation of bulges could be the result of insufficient synthesis of cellulose in walled cells resulting in localized reductions in wall strength. This, in turn, would locally affect the proper co-ordination of cell wall synthesis and polarized cell expansion. At a concentration around 1 mM, ancymidol did not induce cell malformation, but clearly inhibited cell elongation and impaired the formation of a new cell wall during cell division. This suggests that cell expansion was inhibited together with cellulose synthesis. The question arises why 100 μM ancymidol inhibits only cellulose deposition, and 1 mM ancymidol inhibits cellulose deposition together with cell expansion. Ancymidol may inhibit a step possibly located near the regulation point that couples cellulose synthesis and cell expansion. It is suggested that concentrations of ancymidol around 100 μM partially block cell wall synthesis, resulting in the formation of bulges in walled cells and the deposition of cellulose patches in regenerating protoplasts. Concentrations of ancymidol around 1 mM completely block the cell wall synthesis, which leads to the cessation of growth processes such as elongation in walled cells and the complete inhibition of cellulose deposition in regenerating protoplasts. In this respect ancymidol is an inhibitor with a unique mechanism of action and therefore could represent a useful tool for studies of cell expansion. It would be very interesting to find out if the cellulose synthesis inhibitory action of ancymidol is based on the inhibition of an enzyme structurally related to ent-kaurene oxidase, which functions in an unknown cell wall synthesis pathway, or if ancymidol influences cell wall synthesis by another mechanism.
It is important to mention that although most effects of ancymidol on cellulose synthesis were induced by concentrations of 100 μM or higher, the GA-independent cellulose synthesis inhibitory action of ancymidol could be observed in BY-2 cells as well as in tobacco seedlings at concentrations as low as 10 μM (Fig. 1J, H). The possibility cannot be excluded that the cellulose synthesis could be partly disturbed in plants treated with sub-micromolar concentrations of ancymidol. Therefore, although sub-micromolar concentrations of ancymidol mainly act by inhibiting GA biosynthesis in plants, even at these low concentrations, a possible dual action of ancymidol on cellulose synthesis should be taken into consideration.
The relationship between cellulose synthesis inhibition and cell death
Besides bulge formation, ancymidol and isoxaben also induce a significant decrease in cell viability. Two different types of cell death were detected. One of them was clearly related to the malformation of the cell shape; most cells died due to bursting of the very thin cells wall in the bulge. Protoplasts cultivated in the presence of ancymidol for 24 h, in contrast to walled cells, showed no decrease in viability (data not shown). Moreover, cellulose synthesis was quickly restored in these protoplasts when ancymidol was removed from the cultivation medium. This suggests that cellulose synthesis is the main target of ancymidol and probably no other essential processes are influenced by ancymidol in BY-2 cells. However, a certain proportion of walled dying cells did not display malformations of their shape. Such cells died mostly during the first day of cultivation. These cells possibly died due to the signal associated with an increased concentration of cell wall-derived fragments, which are known to act as specific elicitors of programmed cell death.
The role of vesicle trafficking and the cytoskeleton in cell wall formation
In our experiments, the cell malforming effect of cellulose inhibitors was completely inhibited by brefeldin A, an inhibitor of vesicle trafficking. Several genes that participate in cell wall synthesis have been identified in screens for cellulose-deficient mutants (for a review see Hématy and Höfte, 2006). KORRIGAN1, a membrane-bound cellulase, was shown to cycle through various intracellular compartments (Robert et al., 2005). The intracellular cycling was sensitive to isoxaben treatment and was microtubule-dependent. Furthermore, a protein involved in the control of cellulose orientation in the cell wall, COBRA (Roudier et al., 2005), was shown to be associated with the Golgi apparatus. These facts, together with our results with BFA, suggest that intracellular cycling is one of the essential processes needed for correct cell wall building. Our results also suggest that bulge formation in cells treated with cellulose synthesis inhibitors was an active process, to which vesicle trafficking contributed substantially. The inhibition of vesicle trafficking by brefeldin A suppressed bulge formation in cells treated with cellulose synthesis inhibitors and was accompanied by increased cell viability when compared with cells treated with cellulose synthesis inhibitors only. This can be explained by the fact that most cells died due to bursting of the bulge, where the cell wall was too weak to resist the turgor pressure. Apparently, inhibition of both cellulose synthesis and vesicle trafficking was temporarily less harmful than inhibition of cellulose synthesis alone.
The role of microtubules in cell wall formation and cellulose deposition is still a matter of debate. Whereas cellulose microfibrils and microtubules are often aligned with each other and microtubules are believed to guide the movement of cellulose synthase complexes in the plasma membrane, strong evidence exists that the orientation of microfibrils can also be independent of microtubules (Himmelspach et al., 2003; for a review see Wasteneys and Fujita, 2006). Microtubules were locally disoriented in bulges induced by cellulose synthesis inhibitors, whereas they remained in transverse orientation in the rest of the cell. The same observation was made by Fisher and Cyr (1998) in cells treated with isoxaben. Microtubular reorganization clearly had a local character and was closely connected with the inhibition of cellulose synthesis. It is also shown that microtubule stabilization by taxol did not influence the process of bulge formation. Therefore, microtubules did not play an active role in the formation of bulges, supporting the hypothesis that microtubules only have an effect on cell shape in close co-operation with active cellulose synthesis (Wasteneys, 2004).
Conclusion
In conclusion, a dual action of ancymidol, a known inhibitor of GA biosynthesis in plants, is reported here. In addition to its anti-GA effect a new, GA-independent activity of ancymidol, has been identified, which leads to the inhibition of cellulose synthesis. At the high concentration of 1 mM, ancymidol induced distinctly unique effects that were never observed when other cellulose synthesis inhibitors were used. We suggest that 1 mM ancymidol completely inhibits cell wall synthesis or metabolism. It is possible that ancymidol targets the cell wall synthesis pathway at a regulatory step where cellulose synthesis is coupled to the control of cell expansion. Therefore, ancymidol seems to be a good tool for studies of the cell wall synthesis pathway and plant cell expansion.
Acknowledgments
The authors thank Professor Seichiro Hasezawa for the kind gift of the transgenic BY-2 cell line GT16, and Dr Gregory Mouille (INRA, Laboratoire de Biologie Cellulaire, France) for FTIR spectroscopy analysis. This work was supported by the Czech Science Foundation (project no. 522/06/1030) and research programs of the Ministry of Education of the Czech Republic, projects LC06034 and MSM 0021620858.
Glossary
Abbreviations
- ANC
ancymidol
- DCB
2,6-dichlorobenzonitrile
- FTIR
Fourier transform infrared
- GA
gibberellin
- ISX
isoxaben
- MTs
microtubules
- PAC
paclobutrazol
References
- Boříková P, Pokorná J, Opatrný Z. Is the lethal and malforming effect of the potential anti-gibberellin retardant ANC on the tobacco BY-2 cell line mediated by the cytoskeleton? Cell Biology International. 2003;27:175–176. doi: 10.1016/s1065-6995(02)00299-8. [DOI] [PubMed] [Google Scholar]
- Coolbaugh RC, Hirano SS, West CA. Studies on the specificity and site of action of α-cyclopropyl-α-[p-methoxyphenylj-5-pyrimidine methyl alcohol (ancymidol), a plant growth regulator. Plant Physiology. 1978;62:571–576. doi: 10.1104/pp.62.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbaugh RC, Swanson DI, West CA. Comparative effects of ancymidol and its analogs on growth of peas and ent-kaurene oxidation in cell-free extracts of immature Marah macrocarpus endosperm. Plant Physiology. 1982;69:707–711. doi: 10.1104/pp.69.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Growth of the plant cell wall. Nature Reviews Molecular Cell Biology. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- DeBolt S, Gutierrez R, Ehrhardt DW, Somerville C. Nonmotile cellulose synthase subunits repeatedly accumulate within localized regions at the plasma membrane in Arabidopsis hypocotyl cells following 2,6-dichlorobenzonitrile treatment. Plant Physiology. 2007;145:334–338. doi: 10.1104/pp.107.104703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez T, Vernhettes S, Fagard M, Refregier G, Desnos T, Aletti E, Py N, Pelletier S, Hofte H. Resistance against herbicide isoxaben and cellulose deficiency caused by distinct mutations in same cellulose synthase isoform CESA6. Plant Physiology. 2002;128:482–490. doi: 10.1104/pp.010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblin MS, Kurek I, Jacob-Wilk D, Delmer DP. Cellulose biosynthesis in plants: from genes to rosettes. Plant and Cell Physiology. 2002;43:1407–1420. doi: 10.1093/pcp/pcf164. [DOI] [PubMed] [Google Scholar]
- Fisher DD, Cyr RJ. Extending the microtubule/microfibril paradigm: cellulose synthesis is required for normal cortical microtubule alignment in elongating cells. Plant Physiology. 1998;116:1043–1051. doi: 10.1104/pp.116.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Höfte H. Cellulose and cell elongation. In: Verbelen JP, Vissenberg K, editors. The expanding cell. Springer Verlag; 2006. pp. 33–56. [Google Scholar]
- Himmelspach R, Williamson RE, Wasteneys GO. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. The Plant Journal. 2003;36:565–575. doi: 10.1046/j.1365-313x.2003.01906.x. [DOI] [PubMed] [Google Scholar]
- Hogetsu T, Shibaoka H, Shimokor M. Involvement of cellulose synthesis in actions of gibberellin and kinetin on cell expansion: gibberellin–coumarin and kinetin–coumarin interactions on stem elongation. Plant and Cell Physiology. 1974;15:265–272. [Google Scholar]
- Hussain A, Cao DN, Cheng H, Wen ZL, Peng JR. Identification of the conserved serine/threonine residues important for gibberellin-sensitivity of Arabidopsis RGL2 protein. The Plant Journal. 2005;44:88–99. doi: 10.1111/j.1365-313X.2005.02512.x. [DOI] [PubMed] [Google Scholar]
- Kiedaisch BM, Blanton RL, Haigler CH. Characterization of a novel cellulose synthesis inhibitor. Planta. 2003;217:922–930. doi: 10.1007/s00425-003-1071-y. [DOI] [PubMed] [Google Scholar]
- Kumagai F, Yoneda A, Tomida T, Sano T, Nagata T, Hasezawa S. Fate of nascent microtubules organized at the M/G(1) interface, as visualized by synchronized tobacco BY-2 cells stably expressing GFP-tubulin: time-sequence observations of the reorganization of cortical microtubules in living plant cells. Plant and Cell Physiology. 2001;42:723–732. doi: 10.1093/pcp/pce091. [DOI] [PubMed] [Google Scholar]
- Ledbetter MC, Porter KR. A microtubule in plant cell fine structure. Journal of Cell Biology. 1963;19:239–250. doi: 10.1083/jcb.19.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouille G, Robin S, Lecomte M, Pagant S, Hofte H. Classification and identification of Arabidopsis cell wall mutants using Fourier-Transform InfraRed (FT-IR) microspectroscopy. The Plant Journal. 2003;35:393–404. doi: 10.1046/j.1365-313x.2003.01807.x. [DOI] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S. Tobacco BY-2 cell line as the ‘HeLa’ cell in the cell biology of higher plants. International Review of Cytology. 1992;132:1–30. [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- Rademacher W. Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annual Review of Plant Physiology and Plant Molecular Biology. 2000;51:501–531. doi: 10.1146/annurev.arplant.51.1.501. [DOI] [PubMed] [Google Scholar]
- Refregier G, Pelletier S, Jaillard D, Hofte H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiology. 2004;135:959–968. doi: 10.1104/pp.104.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Bichet A, Grandjean O, Kierzkowski D, Satiat-Jeunemaitre B, Pelletier S, Hauser MT, Hofte H, Vernhettes S. An Arabidopsis endo-1,4-β-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. The Plant Cell. 2005;17:3378–3389. doi: 10.1105/tpc.105.036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, et al. COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. The Plant Cell. 2005;17:1749–1763. doi: 10.1105/tpc.105.031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C. Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proceedings of the National Academy of Sciences, USA. 2001;98:10079–10084. doi: 10.1073/pnas.191361598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive JB, Sisler HD. Effects of ancymidol (a growth retardant) and triarimol (a fungicide) on the growth, sterols, and gibberellins of Phaseolus vulgaris (L.) Plant Physiology. 1976;57:640–644. doi: 10.1104/pp.57.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. Cellulose synthesis in higher plants. Annual Review of Cell and Developmental Biology. 2006;22:53–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- Taylor NG. Cellulose biosynthesis and deposition in higher plants. New Phytologist. 2008;178:239–252. doi: 10.1111/j.1469-8137.2008.02385.x. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO. Progress in understanding the role of microtubules in plant cells. Current Opinion in Plant Biology. 2004;7:651–660. doi: 10.1016/j.pbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Fujita M. Establishing and maintaining axial growth: wall mechanical properties and the cytoskeleton. Journal of Plant Research. 2006;119:5–10. doi: 10.1007/s10265-005-0233-3. [DOI] [PubMed] [Google Scholar]
- Widholm JM. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technology. 1972;47:189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]