Abstract
This study was designed to investigate whether thermotolerant roots exhibit respiratory acclimation to elevated temperatures. Root respiratory acclimation traits in response to increasing temperatures were compared between two Agrostis species contrasting in heat tolerance: thermal A. scabra and heat-sensitive A. stolonifera. Roots of both species were exposed to 17, 27, or 37 °C. Root RGR declined with increasing temperatures from 17 °C to 37 °C in both species; however, root growth of A. scabra maintained a significantly higher RGR than A. stolonifera at 27 °C or 37°C. A. scabra exhibited a significantly higher respiration acclimation potential to elevated temperatures, both in the short term (60 min) and in the long term (7–28 d) as compared with A. stolonifera, when temperatures increased from 17 °C to 27 °C or from 27 °C to 37 °C. Thermal A. scabra also maintained a significantly lower maintenance cost than A. stolonifera as temperatures increased to 27 °C or 37 °C. The results suggested that root thermotolerance of thermal A. scabra was associated with both short-term and long-term respiratory acclimation to changes in temperatures. The superior ability of adjusting the rate of root respiration to compensate for increases in carbon demand during short- or long-term temperature increases in the heat-tolerant A. scabra may result in the reduction in carbon expenditure or costs for maintenance, leading to extended root survivability in high temperature soils.
Keywords: Acclimation, grass, heat tolerance, high temperature, root respiration
Introduction
High temperature is one of the most important environmental factors limiting growth and productivity of plant species from temperate climates. Shoot responses to high-temperature stress have been studied extensively. However, mechanisms of root growth and survival at high temperatures have been investigated much less than those pertaining to above-ground plant organs. Roots play a critical role in regulating plant adaptation to high temperature stress (Udomprasert et al., 1995; McMichael, 1999; Rachmilevitch et al., 2006b). The fundamental question about how roots maintain growth and function under high soil temperature conditions is not well understood.
Physiological processes that are essential for root growth include carbohydrate metabolism. Maintaining high carbon-use efficiency at high temperatures is of fundamental importance, because roots depend solely on shoots for the supply of assimilates. Carbon supply to roots typically decreases while root respiratory carbon consumption increases at high temperatures. Excessive consumption of assimilates for the maintenance of roots may be a significant factor limiting plant productivity, particularly under stressful environments (Penning de Vries, 1975). Maintaining low root respiration rates, particularly low maintenance cost, has been associated with high-temperature adaptation and root survival for various plant species (Hendrick and Pregitzer, 1993; Bouma et al., 1997; Gunn and Farrar, 1999; Rachmilevitch et al., 2006b).
Temperature is an important factor affecting respiratory rates of plants. Temperature sensitivity of respiration is often expressed as Q10, the ratio of respiration rates per 10 °C increase in temperature (Lambers, 1985). Root Q10 values vary from 1.1 (Higgins and Spomer, 1976) to 2.9 (Tjoelker et al., 1999), depending on plant species, growth conditions, and tissue physiological conditions. Long-term exposure to specific temperatures can result in respiratory acclimation, which is the adjustment of respiration rates to compensate for a change in temperature (Atkin et al., 2000). Thermal acclimation of root respiration has received far less attention than acclimation of above-ground processes (Xiong et al., 2000). The limited data on root acclimation to elevated temperatures suggest that the ability for root respiration acclimation varies with plant species. Some species such as Festuca ovina (Fitter et al., 1998) and Poa annua (Gunn and Farrar, 1999) exhibit full acclimation or homeostasis, while others such as Dactylis glomerata (Gunn and Farrar, 1999) show no ability for acclimation of root respiration. Plants with a large degree of acclimation show less variation in root growth rate with temperature than those with a low degree of acclimation, suggesting that acclimation in respiration coincides with acclimation in growth rate (Gunn and Farrar, 1999; Kurimoto et al., 2004b). Atkin et al. (2000) estimated that the amount of CO2 released per year through respiration by plants exhibiting respiration homeostasis is only half the amount of that released by plants lacking homeostasis when temperature increased. Therefore, the responses of root respiration to temperatures may be important for plant acclimation to long-term exposure to high temperatures in terms of carbon economy. However, it is unclear whether the variation in short-term or long-term respiratory response to elevated soil temperatures is associated with differences in root adaptation to high-temperature conditions.
A C3 perennial grass species, Agrostis scabra (‘thermal’ rough bentgrass), has recently been identified growing in geothermally heated areas in Yellowstone National Park (Stout and Al-Niemi, 2002). It survives or even thrives in the chronically hot soils with temperatures up to 45 °C (Tercek et al., 2003) and avoids further increases in temperatures by flowering early (Tercek and Whitbeck, 2004). In contrast, temperatures above 24 °C are detrimental for common C3 grasses used as turf or forage in cool climates (Dipaola, 1992). The fact that thermal A. scabra can grow in soil temperatures that are above lethally high temperatures for other C3 grass species suggest that these plants may possess some unique mechanisms enabling their survival of heat stress. The mechanisms of root survival up to such high temperatures for this C3 grass species are not well understood. Our previous studies suggest that efficient carbon utilization in thermal A. scabra may be involved in root survival under high soil temperatures (Rachmilevitch et al., 2006a). Heat tolerance of roots could be related to their ability to maintain a high respiratory efficiency by lowering their maintenance requirements (Rachmilevitch et al., 2006b, 2007), however, how root respiratory acclimation or dynamic changes in respiration responding to short-term or long-term temperature increases may be involved in root thermototlerance has not been studied. Thermotolerant roots may exhibit greater acclimation of their respiration in response to increasing soil temperatures, and thus control carbon expenditure for long-term survival.
The objective of this study was to investigate whether root tolerance to high soil temperature is associated with the capability of short-term and/or long-term respiratory acclimation in response to elevated temperature by comparing two Agrostis species contrasting in heat tolerance. Thermal A. scabra is adapted to geothermally heated soils in Yellowstone National Park, and A. stolonifera is a high-temperature sensitive cool-season perennial grass that is widely cultivated as turf or forage grass in cool-climate regions. Root respiration responses to short-term (minutes) and long-term (weeks) of exposure to high soil temperatures were examined in both species.
Materials and methods
Plant materials
A. stolonifera (cv. Penncross) plants were collected from mature field plots at Hort Farm II, Rutgers University, NJ, USA. A. scabra plants were propagated in a greenhouse at Rutgers University from seeds collected from geothermally heated areas in Yellowstone National Park, Wyoming. Plants (8–10 tillers) of each species were established in sand in polyethylene bags (5 cm in diameter and 40 cm in length) with holes at the bottom for drainage. Plants were watered daily and fertilized twice a week with a nutrient solution containing 1 mM KNO3, 0.2 mM CaSO4, 0.35 mM KH2PO4, 2 mM MgSO4, 0.2 g l−1 Fe-Na-EDTA, and micronutrients (Epstein, 1972). After 6 weeks of establishment in the greenhouse, plants were transferred to a controlled-environment growth chamber (Conviron, Winnipeg, Canada) with a constant temperature of 17 °C (day/night), a 14 h photoperiod, 60% relative humidity, and an irradiance of 450 μmol m−2 s−1 (photosynthetically active radiation) at canopy height. Plants were acclimated to growth-chamber conditions for 1 week prior to exposure to different temperature treatments.
Treatments and experimental design
Plants in polyethylene bags (total of 225), were placed in PVC tubes with an open bottom installed in specially designed water baths (40 cm deep, 30 cm wide, and 60 cm in length), and the tubes extended from the bottom of the water bath. The system was designed to enable plant growth in well-drained sand while allowing the root-zone temperature to be controlled. The entire root-zone (a 40 cm long sand column in a polyethylene bag) was kept in the water bath, while the turf canopy was kept approximately 1.0 cm above the water level. A detailed description of the water baths has been presented previously (Wang et al., 2003) and used in various previous studies (Rachmilevitch et al., 2006a, b).
Roots were exposed to a constant day/night temperature of 17, 27, or 37 °C for 28 d in water baths, while shoots were maintained at 17 °C day/night air temperatures. Each temperature treatment was repeated in five water baths. The water bath temperature was controlled with an immersion-circulating heater. Root-zone temperature was monitored daily using thermocouples located in the root-zone at a depth of 10 cm. The experiment was a split-plot design with temperature as main plot and grass species as sub-plot with five replicates for each species and temperature treatment.
Determination of root relative growth rate (RGR)
Roots of five replicates were washed free of sand. Root dry weight was determined at weekly intervals, from the five replicates for each specie and plot, following drying in an oven at 80 °C for 72 h. RGR was determined as the slope of the natural logarithm of root dry mass versus time and expressed as mg g−1 dw d−1 (Hunt, 1982).
Root respiration measurements
Root respiration rate was measured following the procedure described by Rachmilevitch et al. (2006a). At weekly intervals following the initiation of the temperature treatments, roots were washed free of sand and transferred to 500 ml Erlenmeyer flasks containing the nutrient solution used to fertilize the plants as described above. Shoots were sealed by a rubber stopper and vacuum grease around the bases into an Erlenmeyer flask maintaining a 150 ml air space with 400 ml nutrient solution. The hydroponic solution in each flask was aerated via a recirculating pump (Apollo Enterprises Inc. Oxnard, CA, USA), maintaining an open flow system. Flasks were submerged in water in a water bath to maintain root temperatures at 17, 27 or 37 °C, while shoots were exposed to ambient air temperature (17 °C) in the growth chamber. After a 24 h period, a closed flow system was created by connecting the exit air from the Erlenmeyer to a recirculating pump. The rubber stopper was sealed with vacuum grease and Teflon tape in order to create an air-tight condition. The system was tested for leaks before each sampling. The air was sampled via a septum valve from each flask for the measurements of O2 consumption, every 10 min for a total period of 60 min using 1 ml air-tight syringes. Following O2 sampling, roots were harvested and dried in an oven at 80 °C for 72 h. The air samples were analysed via gas chromatography as described below for respiration measurements.
The concentrations of O2 collected in the hydroponic system were measured using a thermal conductivity gas chromatograph (model GC-8AIT, Shimadzu Corporation, Kyoto, Japan) with an injector connected to an O2 column (model MR62827, Supelco, St Louis, MO, USA). The column temperature was set at 30 °C, and the detector was a thermal-conductivity detector set at 100 mA. The sampled air (0.5 ml) was injected into the injection port. Oxygen concentrations were plotted versus time, and root respiration rates were determined from the regressions of O2 concentration versus time. In addition to the closed-flow system for the measurements of root respiration via gas chromatography, CO2 evolution rate was also measured using an open-flow system with an infrared analyser (IRGA) (Li-Cor 6400, Li-Cor, Inc. Lincoln, NB, USA). The Erlenmeyer's exit was connected to the sample inlet of the IRGA. The results were compared with results obtained using the gas chromatography, and the differences between the two methods were found to be not significant (P >0.1). All the respiration measurements presented in the current study are from the closed-flow system described above.
Evaluation of short-term and long-term root respiration responses to elevated soil temperatures
Long-term respiratory response to temperature (LTR) was calculated as the ratio of respiration rate of roots grown at 37 °C to those grown at 27 °C for 7–28 d (LTR37/27) or the ratio of roots grown at 27 °C to those at 17 °C for 7–28 d (LTR27/17). Short-term respiratory response to temperature was expressed as Q10, which was calculated as the ratio of respiration rate of roots grown at 17 °C for 7–28 d and measured at 27 °C during a 60 min period, to those grown and measured at 17 °C [Q10(27/17)] or the ratio for roots grown at 27 °C for 7–28 d and measured at 37 °C during a 60 min period to those grown and measured at 27 °C [Q10(37/27)].
Maintenance respiration cost
Using a linear regression approach described by Scheurwater et al. (1998), specific respiratory cost for maintenance of biomass and specific respiratory cost for growth, including ion uptake of roots, were determined using the following equation:
The slope of the regression line of the total rate of root respiration (Rt, mmol O2 g−1 dw d−1) versus the RGR gives the specific respiratory cost for growth including ion uptake [C(g+u), mmol O2 g−1 dw]. All regression lines were R2 >0.8. The y-intercept of the regression line gives specific respiratory cost for maintenance of biomass (Rm, mmol O2 g−1 dw d−1).
Statistical analysis
Analysis of variance was conducted (PROC GLM using repeated measures in SAS 8.02, SAS Institute, Cary, NC, USA) to determine the effects of temperature and time and their interaction on RGR and root respiration rate. Treatment means were separated using Fisher's least significance tests at P = 0.05. The relationships between RGR and respiration were determined with linear regression equations. Significant differences between regression lines were tested using analysis of variance. Probabilities of less than 5% were deemed significant.
Results
Relative growth rate
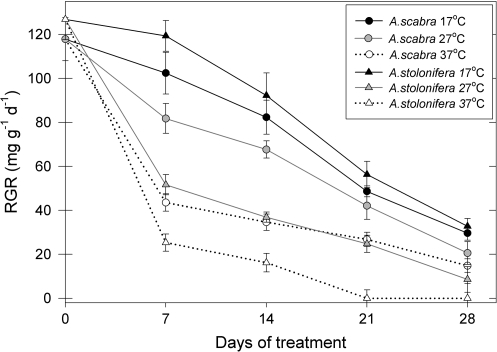
The relative growth rate (RGR) of roots of all plants exhibited similar growth rates (regardless of treatment designation) prior to the initiation of temperature treatments (0 d) (Fig. 1). Root RGR of control plants at 17 °C decreased during the 28-d experimental period, which was associated with root ageing. Increases in temperatures to 27 °C or 37 °C caused more dramatic reduction in RGR (Fig. 1). In A. stolonifera RGR decreased by 82% after exposure of roots to 37 °C for 14 d, compared with roots grown at 17 °C (control temperature), whereas RGR in thermal A. scabra decreased by 58% after the same time of treatment (Fig. 1). Root RGR of A. stolonifera declined to zero after 21 d and 28 d of exposure to 37 °C, whereas roots of A. scabra exhibited a RGR of 27 mg g−1 d−1 and 15 mg g−1 d−1 after 21 d and 28 d of treatment, respectively. The decline in RGR at 27 °C, compared with that at 17 °C, was also more pronounced for A. stolonifera than that for A. scabra throughout the entire treatment period.
Fig. 1.
Root relative growth rate (RGR) of A. scabra and A. stolonifera exposed to soil temperatures of 17, 27, and 37 °C. Error bars represent standard errors (n=5).
At 17 °C, no significant differences in root RGR were detected between the two species. At 27 °C and 37 °C, roots of A. scabra had significantly higher RGR than those of A. stolonifera throughout the entire treatment period.
Root respiration
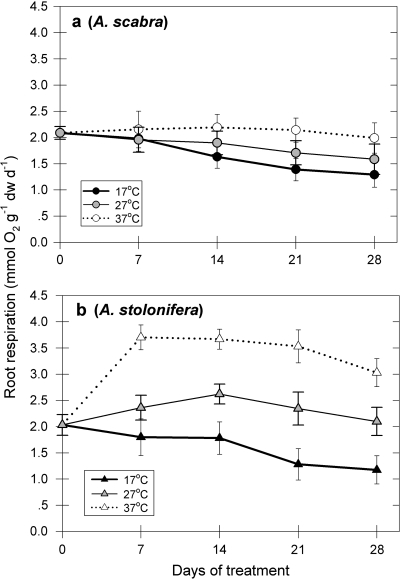
Root respiration rate was higher as temperature was elevated from 17 °C to 27 °C or 37 °C in A. stolonifera (Fig. 2A), while significant increases in root respiration rate for A. scabra occurred only at 37 °C (Fig. 2A). Root respiration rate in A. stolonifera at 27 °C was 31, 47, 83, and 79% higher than that at 17 °C at 7, 14, 21, and 28 d of treatment, respectively (Fig. 2B). Following 7 d of exposure of roots to 37 °C, root respiration rate in A. stolonifera was higher by 106% (Fig. 2B) as compared with 8.5% in A. scabra (Fig. 2A), and after 14 d of treatment, root respiration rate was higher by 260% in A. stolonifera, whereas the increase was only 54% in A. scabra.
Fig. 2.
Root respiration (mmol O2 g−1 dw d−1) of Agrostis scabra (a) and Agrostis stolonifera (b) exposed to soil temperatures of 17, 27, and 37 °C. Error bars represent standard errors (n=5).
Plants of both species maintained similar levels of root respiration rate under the control temperature at 17 °C and a moderate similar decline through time. As temperature was increased to 27 °C or 37 °C, root respiration rate was significantly (P <0.05) higher in A. stolonifera compared with that in A. scabra.
Specific respiratory cost for maintenance
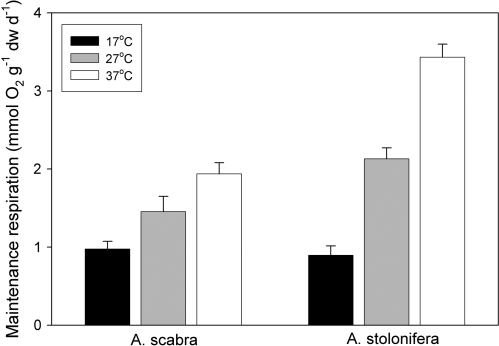
The correlation between O2-consumption rate and RGR was used to calculate specific respiratory cost for maintenance respiration. The Y-intercept of the regression line is an estimate of maintenance respiration cost. Specific respiratory cost for maintenance increased with elevated temperatures to 27 °C or 37 °C in both species (Fig. 3). Roots exposed to 37 °C had 300% higher maintenance cost in A. stolonifera and 98% higher cost in A. scabra, compared with their respective control at 17 °C. Maintenance costs at 27 °C were significantly (P <0.05) higher than at 17 °C, but lower than at 37 °C for both species.
Fig. 3.
Specific maintenance respiration rates (mmol O2 g−1 dw d−1) of Agrostis scabra and Agrostis stolonifera exposed to soil temperatures of 17, 27, and 37 °C. Error bars represent standard errors (n=5).
No differences in maintenance cost were detected between the two species exposed to 17 °C. However, at both 27 °C and 37 °C, the maintenance costs in A. stolonifera were significantly (P <0.05) higher than those in A. scabra (Fig. 3).
Short- and long-term responses of root respiration to elevated soil temperature
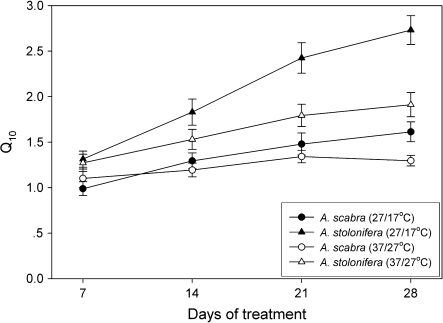
Short-term responses of root respiration to changes in root temperature were expressed as short-term Q10 values (Fig. 4). Short-term Q10 was calculated as the respiration rate of roots grown at 17 °C or 27 °C for 7, 14, 21, and 28 d, but measured at 27 °C or 37 °C during a 60 min period [Q10(27/17) and Q10(37/27], respectively. Q10 values of A. scabra were significantly (P <0.05) lower than those of A. stolonifera when grown at either 17 °C and measured at 27 °C [Q10(27/17)] or grown at 27 °C and measured at 37 °C [Q10(37/27)] (Fig. 4). The differences in Q10 between the two species were more pronounced with prolonged exposure to elevated temperatures. For A. stolonifera, Q10(27/17) was significantly (P <0.05) higher than Q10(37/27) at 14, 21, and 28 d of treatment. However, no significant differences in short-term Q10 were detected in A. scabra whether plants were grown at 17 °C and measured at 27 °C or grown at 27 °C and measured at 37 °C until 28 d of treatment.
Fig. 4.
Short-term Q10 values of Agrostis scabra and Agrostis stolonifera grown at soil temperatures of 17 °C and 27 °C for 7, 14, 21, or 28 d. Q10 was calculated as the ratio of root respiration rate grown at 17 °C and measured at 27 °C to those grown and measured at 17 °C (27/17 °C) or the ratio for roots grown at 27 °C and measured at 37 °C d to those grown and measured at 27 °C (37/27 °C). Error bars represent standard errors (n=5).
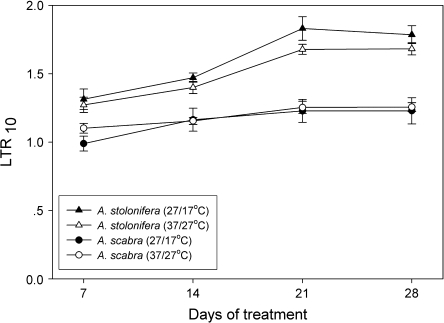
The long-term respiratory response to temperature (LTR) was calculated as the ratio of respiration rate of roots grown at 37 °C to those grown at 27 °C (LTR37/27) or of roots grown at 27 °C to those grown at 17 °C (LTR27/17) (Fig. 5). Both LTR27/17 and LTR37/27 were significantly (P <0.05) higher in A. stolonifera than in A. scabra during the 28 d treatment period. The differences in LTR27/17 and LTR37/27 between the two species became greater with prolonging the duration of the treatment from 7 d to 28 d. No significant differences were found between LTR37/27 and LTR27/17 for A. stolonifera or A. scabra.
Fig. 5.
Long-term respiration ratio (LTR10) of Agrostis scabra and Agrostis stolonifera grown at soil temperatures of 17, 27, and 37 °C for 7, 14, 21, and 28 d. LTR10 was the ratio of the respiration rate of roots grown at 37 °C to those grown at 27 °C (37/27 °C) or the ratio of roots grown at 27 °C to those at 17 °C (27/17 °C). Error bars represent standard errors (n=5).
Discussion
The optimum temperature for cool-season plant growth is generally between 10 °C and 24 °C; however, in many areas soil temperatures often reach injuriously high levels during summer which strongly influence shoot and root growth and the survival of whole plants (Paulsen, 1994). Plant species vary in heat tolerance, which is largely related to root thermotolerance. Soil temperatures of 23 °C or above are detrimental to root growth and other activities of A. stolonifera (Pote et al., 2006), while roots of thermal A. scabra can grow at soil temperatures up to 45 °C in geothermal areas (Tercek et al., 2003). Previous studies have reported that the superior tolerance to high soil temperatures of thermal A. scabra was manifested by its ability to maintain higher root viability, cell membrane stability, and nitrate uptake under prolonged exposure to high soil temperature, compared with heat-sensitive A. stolonifera (Rachmilevitch et al., 2006a, b). The present study found that root relative growth rate of thermal A. scabra was 1.6 times higher that that of A. stolonifera during 28 d of exposure to 27 °C. By 21 d and 28 d of exposure to 37 °C, thermal A. scabra still maintained a positive root growth (RGR=25 mg g−1 d−1), whereas root growth of A. stolonifera completely ceased (RGR=0). These results confirmed that roots of thermal A. scabra, adapted to chronically high soil temperatures in the geothermal areas in Yellowstone National Park, had superior thermotolerance, as compared with common temperate grass species, such as A. stolonifera. Many metabolic factors may be involved in root tolerance to high temperature, but this study suggested that root thermotolerance could be associated with the adjustment of root maintenance respiration and respiratory acclimation.
Respiration is sensitive to temperatures within the plant's physiological range. When roots were exposed to soil temperatures of 17, 27, and 37 °C, total respiration rate and maintenance respiration cost increased significantly with increasing soil temperatures from 17 °C to 27 °C or 37 °C; however, the relative change with temperature and the absolute values of total root respiration rate and maintenance cost were significantly less in thermal A. scabra than those in A. stolonifera. Respiration is a major sink for carbohydrates. Fast respiratory rates have long been proposed to be a primary factor responsible for root growth inhibition (Heichel, 1971; Barneix et al., 1984; Lambers et al., 1996). The results in this study suggest that the capacity to restrict the increase in root respiration rate and maintenance cost at high temperature conditions in thermal A. scabra contributes to its greater capacity to maintain root growth and survive at high soil temperatures. Down-regulation of maintenance processes and subsequent decreases in root respiration can conserve carbon and energy which may prolong root survival at high temperatures.
As discussed in the Introduction, root respiration acclimation may be an important factor determining root tolerance to high temperatures in terms of carbon economy. It is hypothesized that thermal A. scabra exhibits greater root respiration acclimation potential, and thus has a greater ability to maintain growth in high-temperature soils, as compared with heat-sensitive A. stolonifera. This hypothesis is supported by the data on both short-term (Q10) and long-term (LTR) respiratory responses to increasing soil temperatures when comparing the two species. Short-term Q10(27/17) and Q10(37/27) values for A. stolonifera were 2.7 and 1.9, respectively, as temperature increased from 17 °C to 27 °C or 27 °C to 37 °C, while Q10(37/27) values were 1.6 and 1.3 for A. scabra, respectively, over the same temperature range. The lower Q10 values for A. scabra indicate that root respiration in the thermal species was less affected by short-term increases in root temperature than root respiration in A. stolonifera, particularly at higher temperatures (from 27 °C to 37 °C). In addition, the short-term acclimation potential of A. stolonifera decreased significantly with time when grown under both low and high temperatures, whereas the short-term acclimation potential did not change with time for A. scabra when grown under high temperatures and was significantly higher under low temperatures only after 28 d of treatment (Fig. 4). Therefore, short-term acclimation potential in the heat-sensitive A. stolonifera depends on the exposure period to relatively high temperature, whereas in A. scabra the exposure period was insignificant under high temperatures and under low temperatures was even less sensitive to the exposure period than heat-sensitive A. stolonifera under high temperatures.
Long-term exposure to certain temperatures can result in respiratory acclimation, which may result in respiratory homeostasis, i.e. the maintenance of identical rates of respiration in plants grown at different temperatures (Lambers et al., 1998; Atkin and Tjoelker, 2003; Kurimoto et al., 2004a). Root LTR values in both species did not increase significantly between 21 d and 28 d of root exposure to high temperatures, indicating that roots of both Agrostis species exhibited a steady-state response to long-term exposure to high temperatures. However, the degree of long-term response was significantly different between the two species. Root LTR10 values for A. stolonifera averaged 1.6 at growth temperatures of 17 °C to 27 °C and from 27 °C to 37 °C, whereas LTR values for thermal A. scabra averaged 1.2 over both temperature ranges. These results suggest that root respiration of thermal A. scabra was less affected by increased temperatures, after either short-term (minutes to hours) or long-term (weeks) exposure to higher temperatures when compared with heat-sensitive A. stolonifera which could be an important metabolic factor contributing to its superior ability to tolerate high temperatures. It should be noted that the long-term response almost reached homeostasis in A. scabra.
In summary, the root thermotolerance of A. scabra is associated with short- and long-term respiratory acclimation, linked with lower energy requirements for cellular maintenance in response to increasing temperatures. Root respiration of thermal A. scabra plants was less responsive to short-term (minutes) or long-term (weeks) exposure to high soil temperatures than that of heat-sensitive A. stolonifera, and the long-term response was close to homeostasis in thermal A. scabra. This study demonstrates that respiratory acclimation that allows roots to adjust carbon expenditure in order to compensate for the increase in demand for respiratory energy with elevated temperatures could play important roles in the survival of grass roots in high temperature soils.
Acknowledgments
The authors wish to thank the United State Department of Agriculture (grant 2003-00759) and the United States Golf Association for providing funding for this research project. Thanks also go to Steve MaCann, Emily Merewitz, Jiang Tian, and Yan Xu for a critical review of the manuscript.
References
- Atkin OK, Holly C, Ball MC. Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in the capacity and temperature sensitivity of respiration. Plant, Cell and Environment. 2000;23:15–26. [Google Scholar]
- Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science. 2003;8:343–351. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Barneix AJ, Breteler H, van de Geijn SC. Gas and ion exchange in wheat roots after nitrogen supply. Physiologia Plantarum. 1984;61:357–362. [Google Scholar]
- Bouma TJ, Nielsen KL, Eissenstat DM, Lynch JP. Estimating respiration of roots in soil: interactions with soil CO2, soil temperature, and soil water content. Plant and Soil. 1997;195:221–232. [Google Scholar]
- DiPaola J. Physiological responses of turfgrasses to heat stress. In: Waddington DV, Carrow RN, Shearman RC, editors. Turfgrass. Madison, WI: Agronomy Society of America Inc; 1992. [Google Scholar]
- Epstein E. Mineral nutrition of plants: principles and perspectives. New York: John Wiley and Sons Inc; 1972. [Google Scholar]
- Fitter AH, Graves JD, Self GK, Brown TK, Bogie DS, Taylor K. Root production, turnover and respiration under two grassland types along an altitudinal gradient: influence of temperature and solar radiation. Oecologia. 1998;114:20–30. doi: 10.1007/s004420050415. [DOI] [PubMed] [Google Scholar]
- Gunn S, Farrar JF. Effects of a 4 °C increase in temperature on partitioning of leaf area and dry mass, root respiration and carbohydrates. Functional Ecology. 1999;13:12–20. [Google Scholar]
- Heichel GH. Confirming measurements of respiration and photosynthesis with dry matter accumulation. Photosynthetica. 1971;5:93–98. [Google Scholar]
- Hendrick RL, Pregitzer KS. Patterns of fine root mortality in two sugar maple forests. Nature. 1993;361:59–61. [Google Scholar]
- Higgins PD, Spomer GG. Soil-temperature effects on root respiration and ecology of alpine and subalpine plants. Botanical Gazette. 1976;137:110–120. [Google Scholar]
- Hunt R. Plant growth curves: the functional approach to plant growth analysis. Baltimore, MD: University Park Press; 1982. [Google Scholar]
- Kurimoto K, Day DA, Lambers H, Noguchi K. Effect of respiratory homeostasis on plant growth in cultivars of wheat and rice. Plant, Cell and Environment. 2004a;27:853–862. doi: 10.1093/pcp/pch116. [DOI] [PubMed] [Google Scholar]
- Kurimoto K, Millar AH, Lambers H, Day DA, Noguchi K. Maintenance of growth rate at low temperature in rice and wheat cultivars with a high degree of respiratory homeostasis is associated with a high efficiency of respiratory ATP production. Plant and Cell Physiology. 2004b;45:1015–1022. doi: 10.1093/pcp/pch116. [DOI] [PubMed] [Google Scholar]
- Lambers H. Respiration in intact plants and tissues: its regulation and dependence on environmental factors, metabolism and invaded organisms. In: Douce R, Day DA, editors. Encyclopedia of plant physiology. Berlin: Springer-Verlag; 1985. pp. 418–473. [Google Scholar]
- Lambers H, Chapin FSI, Pons TL. Plant physiological ecology. New York: Springer; 1998. [Google Scholar]
- Lambers H, Stulen I, Van Der Werf A. Carbon use in root respiration as affected by elevated atmospheric O2. Plant and Soil. 1996;187:251–263. [Google Scholar]
- McMichael BL. Temperature effects on root growth. In: Waisel YE, Kafkafi A, Kafkafi U, editors. Plant roots: the hidden half. New York: Marcel Dekker; 1999. [Google Scholar]
- Paulsen GM. High temperature responses of crop plants. In: Boote KJ, Bennett JM, Sinclair TR, Paulsen GM, editors. Physiology and determination of crop yield. Madison, WI: ASA, CSSA, SSA; 1994. pp. 365–389. [Google Scholar]
- Penning de Vries FWT. The cost of maintenance processes in plant cells. Annals of Botany. 1975;39:77–92. [Google Scholar]
- Pote J, Wang ZL, Huang BR. Timing and temperature of physiological decline for creeping bentgrass. Journal of the American Society for Horticultural Science. 2006;131:608–615. [Google Scholar]
- Rachmilevitch S, Huang BR, Lambers H. Assimilation and allocation of carbon and nitrogen of thermal and nonthermal Agrostis species in response to high soil temperature. New Phytologist. 2006a;170:479–490. doi: 10.1111/j.1469-8137.2006.01684.x. [DOI] [PubMed] [Google Scholar]
- Rachmilevitch S, Lambers H, Huang BR. Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. Journal of Experimental Botany. 2006b;57:623–631. doi: 10.1093/jxb/erj047. [DOI] [PubMed] [Google Scholar]
- Rachmilevitch S, Xu Y, Gonzalez-Meler MA, Huang B, Lambers H. Cytochrome and alternative pathway activity in roots of thermal and non-thermal Agrostis species in response to high soil temperature. Physiologia Plantarum. 2007;129:163–174. [Google Scholar]
- Scheurwater I, Cornelissen C, Dictus F, Welschen R, Lambers H. Why do fast- and slow-growing grass species differ so little in their rate of root respiration, considering the large differences in rate of growth and ion uptake. Plant, Cell and Environment. 1998;21:995–1005. [Google Scholar]
- Stout RG, Al-Niemi TS. Heat-tolerant flowering plants of active geothermal areas in Yellowstone National Park. Annals of Botany. 2002;90:259–267. doi: 10.1093/aob/mcf174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tercek MT, Hauber DP, Darwin SP. Genetic and historical relationships among geothermally adapted Agrostis (bentgrass) of North America and Kamchatka: evidence for a previously unrecognized, thermally adapted taxon. American Journal of Botany. 2003;90:1306–1312. doi: 10.3732/ajb.90.9.1306. [DOI] [PubMed] [Google Scholar]
- Tercek MY, Whitbeck JL. Heat avoidance life history strategy controls the distribution of geothermal Agrostis in Yellowstone. Ecology. 2004;85:1955–1966. [Google Scholar]
- Tjoelker MG, Oleksyn J, Reich PB. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Global Change Biology. 1999;5:679–691. [Google Scholar]
- Udomprasert N, Li PH, Davis DW, Markhart AH. Root cytokinin level in relation to heat tolerance of Phaseolus acutifolius and Phaseolus vulgaris. Crop Science. 1995;35:486–490. [Google Scholar]
- Wang Z, Pote J, Huang B. Responses of cytokinins, antioxidant enzymes, and lipid peroxidation in shoots of creeping bentgrass to high root-zone temperatures. Journal of the American Society for Horticultural Science. 2003;128:648–655. [Google Scholar]
- Xiong FSS, Mueller EC, Day TA. Photosynthetic and respiratory acclimation and growth response of antarctic vascular plants to contrasting temperature regimes. American Journal of Botany. 2000;87:700–710. [PubMed] [Google Scholar]