Abstract
Extensins are cell wall basic glycoproteins with a polypeptide backbone that is extremely rich in hydroxyproline. In this paper, the function of extensins in embryo development was studied in Nicotiana tabacum. By using Western blot and immunohistochemistry, the extensin JIM20 epitopes were found to express in different developmental stages of embryos, and specifically in the top of the embryo proper (EP) and the suspensor of the late globular embryos. In order to clarify the functions of extensins, a potent hydroxyproline synthesis inhibitor, 3,4-dehydro-L-proline (3,4-DHP), was used in ovule and embryo culture. The results showed that the addition of 3,4-DHP caused abnormal embryos with single, asymmetry and supernumerary cotyledon primordia, and continuous culture led to cotyledon defects in the germinated seedlings. Histological sections showed that the shoot apical meristem (SAM) of the abnormal seedlings was dissimilar from the controls, especially in the seedlings with cup-shaped cotyledons. Furthermore, the vasculature of the abnormal cotyledons was in an out-of-order format and contained at least two main veins. Finally, both the hydroxyproline assay and fluorescent immunolocalization confirmed that 3,4-DHP treatment reduced the level of extensins in the cultured ovules and embryos. These results indicate that extensins may play important roles in the cotyledon primordium formation, SAM activity, and vasculature differentiation during embryo development.
Keywords: Cotyledon; embryo development; extensin; Nicotiana tabacum; shoot apical meristem; 3,4-DHP
Introduction
Extensins are hydroxyproline-rich glycoproteins found in many plant cell walls as a major protein component (Chen and Varner, 1985). They are composed of a polypeptide backbone which is extremely rich in hydroxyproline (Hyp) and also contains much serine, lysine, tyrosine, and valine residues, and oligosaccharides of arabinose and galactose (Wilson et al., 1986). This structure is the result of considerable post-translational processing of the polypeptide gene product. Extensin family members exhibit a high degree of post-translational modification (PTM) as more than 50% of the amino acids were glycosylated (Kieliszewski and Lamport, 1994). Among the proteins which are present in the plant cell wall, the extensins are implicated in nearly all aspects of plant development. It was found that the expression of extensins and their mRNAs occurred in response to a varieties of biotic and abiotic stresses, such as resistance to pathogens (Deepak et al., 2007), wounding (Ahn et al., 1996), ozone (Schneiderbauerf et al., 1995), and superoxide and hydrogen peroxide (Wisniewski et al., 1999). There is also some evidence showing that extensins participate in many events during plant development, such as pollen recognition and fertilization (Wu et al., 2001), cell division and differentiation, cessation of cell elongation (Ito et al., 1998), abscission and senescence (Merkouropoulos and Shirsat, 2003), and flower organ formation (Neale et al., 1990).
In order to provide direct evidence for the functions of extensins, researchers adopted different methods to alter the level of extensins in plant cell walls. A transgenic approach was one of the methods used by some researchers. Memelink et al. (1993) studied the extensin gene pCNT1 in Nicotiana tabacum L. with sense and antisense constructs under the control of the CaMV35S promoter. In studying the Atext1 gene in transgenic Arabidopsis, it was found that increasing the extensin level not only decreased the pathogen invasiveness (Wei and Shirsat, 2006), but also affected the inflorescence stem thickness and height (Roberts and Shirsat, 2006). Simultaneously, other methods such as treatment with chemical reagents were also adopted. In hydroxyproline-rich glycoproteins (HRGPs), most of the Pro residues are hydroxylated by prolyl hydroxylases and the resulting hydroxyproline (Hyp) residues serve as major sites for oligosaccharide decoration (Kieliszewski and Lamport, 1994). Because of this unique biosynthetic pathway, the use of specific inhibitors of prolyl hydroxylases could provide a valuable approach for studying the functions of Hyp-rich wall polymers. Such inhibitors should be particularly effective for HRGPs because hydroxylase inhibitors can also block the attachment of carbohydrate side chains, which are shown to be partial determinants of the structure of HRGPs (van Holst and Vamer, 1984; Stafstrom and Staehelin, 1986). As strongly basic HRGPs, extensins are extremely rich in Hyp, so the treatment with hydroxylase inhibitors will mostly influence the level of extensins in plant cell walls among all types of HRGPs. The chemical 3,4-DHP (3,4-dehydro-L-proline) was a potent inhibitor of prolyl hydroxylase and functions at micromole concentrations to inactivate prolyl hydroxylase rapidly and irreversibly (Cooper and Vamer, 1983). Previous studies showed that 3,4-DHP inhibited cell wall assembly and cell division in Nicotiana tabacum mesophyll protoplasts (Cooper et al., 1994), and in tomato seedlings, the treatment with 3,4-DHP shortened the root hairs (Bucher et al., 1997).
In sexual plant reproduction, there are several complex, precise, and key events: pollination, fertilization, and embryogenesis. Previous studies have indicated that hydroxyproline-rich glycoproteins are potential candidates for mediating pollen–pistil interactions in conjunction with partner molecules in female tissues. It has been shown that the reproductive tissues are a rich source of hydroxyproline-rich glycoproteins. For example, Pex1 (pollen extensin-like) protein mainly locates in the pollen tube wall and plays a role in tube growth (Rubinstein et al., 1995). CELP (Cys-rich extensin-like protein) has a possible role in the process of pollination and fertilization (Wu et al., 1993). PELPIII (Class III pistil-specific extensin-like proteins) abundantly and specifically localize in the intercellular matrix (IM) of tobacco style transmitting tissues, and translocates from IM to the pollen tube wall after pollination, which indicates that the biological function of the glycoprotein is related to pollen tube growth (Bosch et al., 2003). It was also found that a style-specific 120 kDa protein, which is located in the pistil-grown pollen tubes, was required for S-specific pollen rejection in Nicotiana (Lind et al., 1996; Hancock et al., 2005). Most glycoproteins have been shown to play roles in pollination, but a few HRGPs have been reported to be involved in embryo development. Up to now, only the RSH (Root–Shoot–Hypocotyl–Defective) extensin protein has been proved to be essential in regulating the accurate positioning of the cell plate during embryogenesis of Arabidopsis (Hall and Cannon, 2002). Because embryos reside deep in the ovules, it is difficult to observe the functions of extensins during embryo development in vivo. Therefore, very few studies have provided direct and unequivocal evidence for the functions of extensins during embryogenesis of angiosperms. In this paper, an in vitro embryo micro-culture system was used to solve this problem. By using an immunofluorescence labelling technique, the temporal and spatial distribution of extensins was detected at different embryonic stages. The biological functions of extensins were also investigated by examining the effects of 3,4-DHP on embryo development. The results showed that the inhibition of extensin synthesis increased the abnormal differentiation of embryos and caused defects of cotyledon and the shoot apical meristem development. These indicate that extensins may play important roles in cotyledon primordial formation and shoot apical meristem activity. This is the first paper that implicates extensins in the development of tobacco embryos.
Materials and methods
Plant materials
Nicotiana tabacum L. (cv. SR1) plants were grown in greenhouse of Wuhan University. Conditions were a 16/8 h light/dark cycle at 28±1 °C and the humidity was 65–70%. Flowers were artificially pollinated during anthesis.
Total protein extraction of ovules
The total proteins of tobacco ovules were extracted as described by Qin and Zhao (2006). One gram fresh weight of ovules at different developmental stages (1, 3, 5, 6, 7, 8, 9 days after pollination, DAP) were taken from the ovaries and ground to a fine powder in liquid nitrogen. The ground tissues were placed into 2 ml extraction buffer (0.1 M K3PO4, pH 7.0). After incubation at 4 °C for 3 h, the mixture was centrifuged at 12 000 rpm for 20 min. The supernatant was precipitated with 5 vols of cold acetone at –20 °C overnight and the precipitate was re-suspended by vortex-mixing in 0.5 ml 50 mM TRIS-HCl, pH 8.0 and then centrifuged. Finally, the supernatant (total proteins) was retained and stored at –80 °C until use.
SDS-PAGE and immunoblot assay
The total proteins from the ovules at different stages were analysed by SDS-PAGE using a 12.5% acrylamide separating gel and a 5% acrylamide stacking gel in a Mini-Protean II electrophoresis cell (Bio-Rad). Equal amounts of total proteins were loaded in each well. Gels were electroblotted (88 V, 3 h) onto nitrocellulose transfer membranes using electro-transfer buffer (20 mM TRIS-base, 150 mM glycine, 20% methanol). The nitrocellulose membrane blots were blocked with 5% non-fat dried milk in TBST buffer (20 mM TRIS-base, 500 mM NaCl, 0.05% Tween-20, pH 7.5) overnight at 4 °C. The membranes were then incubated with the primary monoclonal antibodies (Mb) JIM11, JIM12, JIM19, and JIM20 (1:50) respectively for 2 h at room temperature, and washed with TBST for 3–10 min. The JIM11 and JIM20 antibodies recognize specific arabinosylation of HRGPs whereas JIM12 may recognize a protein epitope or a non-terminal oligosaccharide structure (Smallwood et al., 1994; a kind gift from Dr JP Knox, Centre for Plant Sciences, University of Leeds, UK). They were incubated with alkaline-phosphatase-conjugated goat anti-rat antibody (1:500; Sino-American Biotechnology Co.) for 1 h, washed with TBST three times, and then stained with nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Sino-American Biotechnology Co.). The pre-stained protein molecular weight marker mixes (Pierce) were myosin (223 kDa), phosphorylase B (110 kDa), bovine serum albumin (81.6 kDa), ovalbumin (46.8 kDa), carbonic anhydrase (31.8 kDa), trypsin inhibitor (24.8 kDa), and lysozyme (16.5 kDa).
Isolation of zygotes and proembryos
Tobacco ovules were dissected from ovaries at 3, 5, 6, 7, 8, and 9 DAP, placed into an enzyme solution containing 8–13% mannitol, 3 mM 2-[N-morpholino] ethanesulphonic acid (MES), 3 mM polyvinlypyrrolidone K30 (PVP K30), 1% cellulose R-10 and 0.8% macerozyme R-10, pH 5.7, and incubated for 30 min at 28 °C. The ovules were washed 3–4 times with a solution containing 8–13% mannitol, 3 mM 2-[N-morpholino] ethanesulphonic acid (MES), 3 mM polyvinylpyrrolidone K30 (PVP K30), and gently ground with a small glass pestle. They were then treated in a second enzyme solution supplemented with 8–13% mannitol, 0.25% cellulose R-10, and 0.2% macerozyme R-10, pH 5.7, for 10–15 min. After this the zygotes and proembryos were isolated from the ovules and embryo sacs using microneedles under an inverted microscope (OLYMPUS CK30) (Qin and Zhao, 2006).
Immunolocalization techniques
For immunoenzyme localization, the isolated ovules were embedded using a Technovit 7100 Embedding kit (Heraeaus Kulzer, Wehrheim, Germany), described by Leroux et al. (2007). Five millimetre thick transverse sections were cut with a microtome (Sorvall MT-6000 ultramicrotome), and dried on object glasses. Immunoenzyme detection of extensins in the sections used the SABC (streptavidin and biotinylated horseradish peroxidase complex) method. The experiments were performed as described by Yuan et al. (2008) with some modification. The sections were incubated in 3% H2O2 (15 min at room temperature, RT) to block the endogenous peroxidase activity. After three 5 min washes with distilled water, the sections were incubated in 10 mM PBS buffer containing 5% BSA (20 min at RT) to block non-specific binding. Then the sections were incubated with 1:5 dilutions (10 mM PBS, 1% BSA, pH 7.2) of extensin antibody at 4 °C overnight, rinsed three times with PBS, and incubated with biotin-labelled goat anti-rat IgG antibody for 20 min at 37 °C. After that, the sections were rinsed three times with PBS and allowed to react with the SABC reagent for 20 min at 37 °C. After an extensive washing in PBS supplemented with 0.02% (v/v) Tween 20 (four times) and PBS (twice), the sections were stained with the AEC kit at RT. The control sections were treated similarly except that the primary antibody was substituted with PBS/BSA solution. Sections were then washed in distilled water and immediately examined under a microscope (Olympus IX-70).
For fluorescence inmunolocalization, the isolated embryos were fixed in 4% paraformaldehyde in 50 mM PIPES buffer, pH 6.7, 2 mM MgSO4, 2 mM EGTA, 8–13% mannitol (PIPES buffer) for 5 h at RT. The samples were rinsed three times with the PIPES buffer, once with 100 mM PBS, pH 7.4, and then incubated in the primary mono-antibody JIM20 diluted 1/5 with 100 mM PBS for 3 h at room temperature. The samples were rinsed three times with 100 mM PBS, and then incubated with the secondary antibody, anti-rat-IgG-FITC conjugate (Sigma) diluted at 1/100 with 100 mM PBS for 1 h at RT in the dark or at 4 °C overnight. The samples were rinsed three times with 100 mM PBS before microscopic examination (Zhao et al., 2004). Control samples were incubated in 100 mM PBS instead of the primary antibody. Green immunofluorescence of the samples was visualized under an inverted microscope (Olympus IMT-2).
For subcellular immunogold localization, the late globular stage embryos at 7 DAP were fixed in 2% glutaraldehyde, 4% paraformaldehyde, and 100 mM phosphate buffer (PBS pH 7.2), for 2.5 h, then washed in 100 mM PBS, pH 7.2, three times, each for 30 min, and dehydrated in a series of ethanol gradient at 4 °C. The samples were infiltrated with Lowicryl K4M via three intermediate steps at 2:1, 1:1, and 1:2 mixture of ethanol:Lowicryl K4M (12 h each time). Finally, the mixture was replaced by pure Lowicryl K4M, kept for 12 h at –20 °C, and then changed once with fresh Lowicryl K4M and kept for 1 d at –20 °C. The samples were transferred to capsules with fresh Lowicryl K4M, and cured under two 15 W ultraviolet lamps (360 nm) for at least 24 h at –20 °C, and then continued curing for 2 d at RT. Ultrathin sections (60 nm) were cut using a Sorvall MT-6000 ultramicrotome and collected onto Formvar-coated nickel grids. The grids were floated on PBST buffer (60 mM PBS, 0.1% Tween-20, 0.02% NaN3, pH 7.2) containing 0.2 M glycine and 1% BSA to block non-specific binding. The grids were floated in a drop of the primary antibody JIM20 for 3 h at 37 °C. They were rinsed with PBST three times, and then floated on 1:100 of goat anti-rat IgG antibody conjugated to 10 nm gold particles (Sigma) for 1 h at 37 °C. After washing in PBST three times and in distilled water three times, the grids were air-dried and stained with saturated uranyl acetate for 30 min. The control sections were treated similarly except that primary antibody was substituted with PBS. The samples were examined and photographed under a HT650 transmission electron microscope.
For histological sections, the shoot apexes of seedlings which germinated from the cultured ovules were fixed in 4% paraformaldehyde and 100 mM phosphate buffer, pH 7.2, and embedded in Epon 812 resin. 1 μm semi-sections were sliced under an ultramicrotome (Sorvall MT-6000), stained with toluidine blue, examined under an inverted microscope (Olympus IMT-2), and photographed with a Cool SNAP CCD (Photometric, USA).
Ovule culture
Ovules at the early globular embryo stage (5 DAP) were sterilized in 70% ethanol for 0.5 min and then in 2% NaClO for 4 min. After rinsing three or four times with sterile water, the ovules were cultured in MS medium (pH 5.8) supplemented with 6% sucrose, 0.25% phytagel, and different concentrations of 3,4-DHP (50, 100 and 200 μM) at 25 °C in the dark. After 10 d of culture, some ovules were fixed to detect the embryo development inside the ovules, and others were transferred to fresh half-strength MS medium supplemented with 3% sucrose and 0.25% phytagel, pH 5.8 and cultured at 25 °C in the dark until maturity. The seedlings originated from the cultured ovules were transplanted to soil in pots in order to track their continued growth. All experiments were repeated at least three times, and the standard deviation was calculated.
Embryo culture
Ovaries at the late globular embryo stage (7 DAP) were sterilized and the embryos were isolated as described above. The isolated embryos were first washed with 8% mannitol and then twice with KM8p medium (Kao and Michayluk, 1975), and cultivated in a millicell with a semi-permeable membrane (MILLICELL-CM 3.0 μm PICMO 1250, diameter 12 mm) placed in a 35 mm Petri dish with 1.5 ml KM8p medium (pH 5.8) containing 100–120 ovules (7.5 DAP) as feeder cells at 25 °C in the dark. In order to afford enough nutrition, 15 g l−1 glucose, 50 g l−1 sucrose, 15 g l−1 mannitol, 1 mg l−1 2, 4-D, 0.5 mg l−1 6-BA, 250 mg l−1 lactalbumin hydrolystate, 13 kinds of amino acids, and 10% coconut were added to the KM8p medium to form a rich organic chemical environment. In order to reduce the biosynthesis of extensins, 2, 5, 10, 50, and 100 μM 3,4-DHP (Sigma) were added to the medium, respectively. After 10 d of culture, the embryos were observed under an inverted microscope (Olympus IMT-2) and photographed with a Cool SNAP CCD (Photometric, USA). The differentiated embryos were then transferred to fresh half-strength MS medium supplemented with 30 g l−1 sucrose and 2.5 g l−1 phytagel, pH 5.8 and developed into seedlings. The experiments were repeated at least three times, and the standard deviation was calculated.
Hydroxyproline assay
The total Hyp content (μg mg−1 dry weight tissue) in the cultured tobacco ovules which were treated with or without 100 μM 3,4-DHP, as mentioned in the ovule culture, was measured using the method first described by Kivirikko and Liesmaa (1959) and then modified by Fry (1988). The experiments were repeated three times, and the standard deviation was given.
Cotyledon transparency technique
To investigate the cotyledon vascular bundles, a technique according to Bougourd et al. (2000) was used with minor modifications. The cotyledons were dehydrated through an ethanol series (15, 50, 70, 95, and two 100% ethanol steps) for 15 min at each step. The samples were left in fresh 100% ethanol at 4 °C overnight or at RT for at least 3 h. They were then rehydrated in an ethanol series for 15 min each step, and washed with distilled water for 15 min twice. The cotyledons were stained in 0.5% aniline blue and 0.2 M PBS, pH 6.5 for 30 min, rinsed gently and soaked in water for 15 min twice in order to remove unbound aniline blue. Finally, the samples were transferred into Hover's solution (200 g chloral hydrate, 20 g glycerol, and 50 ml water) until they became transparent. The samples were observed and photographed under an Olympus SZX 12 stereomicroscope with a RS photometric CCD.
Results
Expression of extensins during ovule development
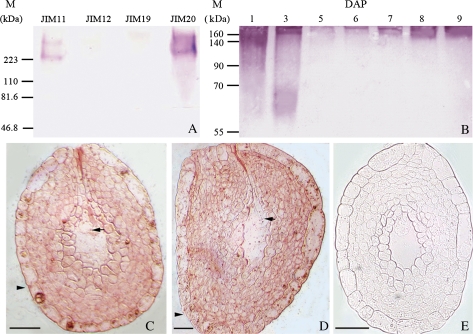
Western blots were used to detect the expression of extensins in the mixed tobacco ovules at different developmental stages using monoclonal antibodies (Mb) JIM11, JIM12, JIM19, and JIM20 (Fig. 1A). The results showed that the JIM11-labelled extensins were mainly found above 223 kDa, while JIM20 appeared to recognize more abundant and broader extensins from 60 kDa and mainly concentrated above 223 kDa. However, the JIM12 and JIM19-recognized extensins were hardly detectable in the ovules (Fig. 1A).
Fig. 1.
Western blot analysis of extensins recognized by Mb JIM11, JIM12, JIM19, and JIM20 and the localization of extensins in the ovules with an egg cell and zygote. (A) Labelling of JIM11, JIM12, JIM19, and JIM20 extensin binding epitopes in the tobacco mixed ovules at 1–9 DAP. (B) Labelling of JIM20 extensin binding epitopes in the ovules at 1–9 DAP. Molecular mass (kDa) was indicated on the left. (C) A strong extensin binding signal was detected in the integument and nucellar tissue of ovules with an egg cell. The egg cell and its integument are indicated by an arrow and an arrowhead, respectively. (D) Extensins labelled with JIM20 were found in the integument and nucellar tissue of ovules with a zygote. The zygote and its integument were indicated by an arrow and an arrowhead, respectively. (E) A control without treatment with JIM20 showed no signal in the ovule with an egg cell. Bar = 50 μm. (This figure is available in colour at JXB online.)
The expression of JIM20-labelled extensins during ovule development were examined further (Fig. 1B). The results showed that both of the level and the size of JIM20-labelled extensins changed dramatically during ovule development. Extensins ranging from 60 kDa to over 160 kDa were abundant in the unfertilized ovules (1 DAP) and the fertilized ovules (3 DAP). The level of detected extensins was low in ovules with early (5 DAP) and middle (6 DAP) globular embryos, but gradually increased in ovules with late globular embryos (7 DAP), heart embryos (8 DAP), and torpedo-shaped embryos (9 DAP), and the molecular weight was found to be from 140 kDa to over 160 kDa.
The JIM20-labelled extensins in ovules with an egg cell (Fig. 1C) and zygote (Fig. 1D) were examined by an immunoenzyme technique. A strong extensin signal was detected in the integument and nucellus tissue of ovules with an egg cell (Fig. 1C) and zygote (Fig. 1D). The control samples without the reaction with the JIM20 antibody showed no signal (Fig. 1D).
Immunolocalization of extensins in different developmental stages of embryos
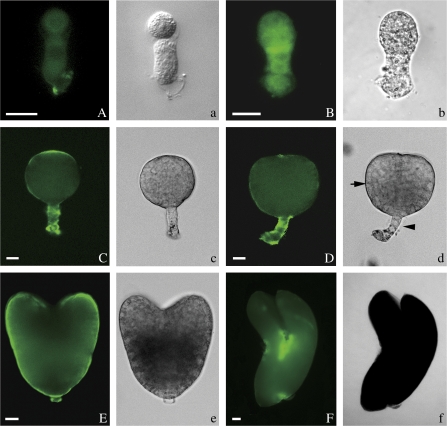
The expression of extensin epitopes was detected by JIM20 during tobacco embryo development. In the first cell of the embryo, there was almost no extensin immunofluorescence (data not shown), but the integument and nucellus tissue of ovules with a zygote showed a strong signal (Fig. 1D). A faint signal was detected in the two-celled embryos at the bottom of the based cell (Fig. 2A). And the signal gradually became stronger and more evenly distributed in the early globular embryos (Fig. 2B). In the late globular embryos, the extensin signal was concentrated mainly in the top of the embryo proper (EP) and in the suspensor (Fig. 2C). When the embryos began to differentiate, the signal was stronger in the suspensor of early and late heart embryos (Fig. 2D, E) and the shoot apical meristem of the torpedo embryos (Fig. 2F). In the control, no signal was detected in the embryos not labelled with JIM20 (see Supplementary Fig. 1E at JXB online).
Fig. 2.
Immunofluorescence localization of extensins in tobacco embryos. The extensins in the embryos at different development stages were immunostained with JIM20 monoclonal antibody. (A–F) Fluorescent images and (a–f) the corresponding bright-field micrographs. The embryo proper and suspensor were indicated respectively by an arrow and an arrowhead in (d). (A) A faint signal was detected in the two-celled embryos at the bottom of the based cell. (B) An early globular embryo with uniform fluorescence labelling. (C) A late globular embryo showing fluorescence labelling in the apex of embryo proper (EP) and suspensor cells. (D) The fluorescence labelling is strong in the suspensor cells, but disappears in the apex of embryo proper in an early heart-shaped embryo. (E) In a late heart-shaped embryo, the fluorescence is strong, especially in the shortened suspensor. (F) In the torpedo-shaped embryo, the fluorescence is mainly present in the shoot apical meristem. Bar=20 μm. (This figure is available in colour at JXB online.)
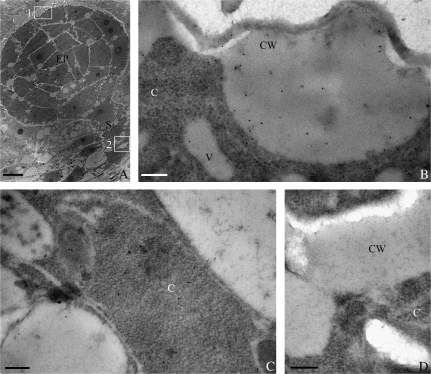
To detect the subcellular localization of extensins, immunogold labelling and TEM techniques were used. Numerous gold particles labelled with JIM20 were detected at the top of the EP in the late globular embryos (Fig. 3A), especially in the protruding cell walls of the EP (Fig. 3B). In the suspensor of late globular embryos (Fig. 3A), gold particles were also found to be distributed mainly in the cytoplasm (Fig. 3C). The control sections without JIM20 showed no gold particles (Fig. 3D). These results were consistent with those in the immunofluorescence experiments.
Fig. 3.
Immunogold localization of extensin epitopes recognized by JIM20 in the late globular embryos at 7 DAP of tobacco. (A) Ultrathin section of a late globular embryo. (B) A magnified images of the area indicated by square 1 in (A). Numerous gold particles appeared in the protruding cell wall at the top of the embryo proper. (C) A magnified image of the area indicated by square 2 in (A). The gold particles were seen in the cytoplasm. (D) A control image showed no gold particles. (A) Bar=10 μm; (B–D) bar=0.2 μm; C, cytoplasm; CW, cell wall; EP, embryo proper; S, suspensor; V, vacuole.
Effects of 3,4-DHP on the embryo development in ovule culture
To investigate the role of extensins in embryogenesis, ovules were chosen with early globular embryos at 5 DAP and cultured in MS medium supplemented with different concentrations of 3,4-DHP. After 10 d of culture, the embryos in the ovules developed into three types: undifferentiated, normal differentiated, and abnormal differentiated. In 3,4-DHP treated ovules, the frequencies of abnormal differentiated embryos obviously increased (Table 1) and appeared in a concentration-dependent manner (from 0 to 100 μM). But when the concentration was increased to 200 μM, there was no change in the frequency of abnormal differentiated embryos compared with the treatment with 100 μM. The total differentiation frequencies of embryos in the cultured ovules increased when the 3,4-DHP concentration was 100–200 μM, but these mainly resulted from the augmentation of abnormal embryo differentiation, normal embryo differentiation showed no visible contribution to the total differentiation frequencies. However, the 50 μM 3,4-DHP concentration showed a remarkably inhibitory effect on the normal embryo differentiation and resulted in the decrease of the total differentiation frequency, which was compared with the untreated control. In general, the normal differentiated embryos had two symmetrical cotyledon primordia (Fig. 4A), but the abnormal differentiated embryos had asymmetry (Fig. 4B), supernumerary (Fig. 4C) and single cotyledon primordia (Fig. 4D). These results indicate that extensins are important for embryo differentiation, and when their concentrations are lower than the natural level, the abnormal differentiated embryos increased.
Table 1.
Effects of 3,4-DHP on tobacco embryo development in culture of 5 DAP ovules
| Treatment of 3,4-DHP (μM) | No. of isolated embryos | Frequency of undifferentiated embryos (%) | Frequency of differentiated embryos (%)a |
||
| Total | Normal | Abnormal | |||
| 0 | 284 | 56.43±4.54 | 43.56±4.54 | 41.38±5.76 | 2.19 ±1.55 |
| 50 | 338 | 68.02±11.96 | 31.98±11.96** | 19.40±8.23** | 12.58±6.06** |
| 100 | 292 | 33.03±8.01** | 66.97±8.01** | 39.83±2.21 | 27.14±8.46** |
| 200 | 179 | 33.14±6.04** | 66.86±6.40** | 40.76±4.65 | 26.1±1.57** |
The results are shown by a value with a standard deviation (SD).** P <0.01 indicates a significant difference compared with the zero-DHP control.
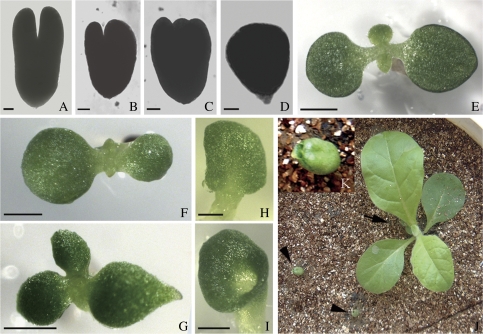
Fig. 4.
Effects of 3,4-DHP on the development of embryos in 5 DAP ovules after 10 d of in vitro culture and the phenotypes of germinated seedlings in tobacco. (A, E) Embryos isolated from ovules and seedlings cultured on MS medium without 3,4-DHP. (B–D, F–I, K) Embryos isolated from ovules and seedlings cultured on MS medium with 3,4-DHP. (A) A normal torpedo-shaped embryo with two symmetrical cotyledon primordia. (B) An abnormal torpedo-shaped embryo with two asymmetric cotyledon primordia. (C) An abnormal embryo with three cotyledon primordia. (D) An abnormal embryo with a single cotyledon primordium. (E) A normal germinating seedling with two symmetrical cotyledons. (F) An abnormal seedling with two asymmetric cotyledons. (G) An abnormal seedling with three cotyledons. (H) An abnormal seedling with a single cotyledon. (I) An abnormal seedling with a cup-shaped cotyledon. (J) A control seedling came from the ovules cultured without 3,4-DHP (arrow) and two cup-shaped seedlings from ovules cultured with 3,4-DHP (arrowheads) after being transplanted into soil for 20 d. The control seedling grew, while the cup-shaped seedlings stopped growing and gradually died. (K) A magnified image of a cup-shaped seedling in (J). (A–D) Bar=20 μm; (E–I) bar=2 mm. (This figure is available in colour at JXB online.)
When the cultured ovules were transferred into half-strength MS medium and continuously cultured until germination, the fate of embryos was observed longitudinally. The result showed that the frequencies of germinated seedlings with normal cotyledons decreased compared with the control, but the frequencies of seedlings with abnormal cotyledons increased when the ovules were treated with 3,4-DHP (50, 100, and 200 μM; Table 2). However, 3,4-DHP had no obvious effects on the total frequencies of seedling germination (Table 2). In the control, most seedlings had two normal symmetric cotyledons (Fig. 4E), but in the tests, the germinated seedlings displayed various cotyledon defects, including two asymmetric (Fig. 4F), supernumerary (Fig. 4G), single (Fig. 4H), and cup-shaped (Fig. 4I) cotyledons. These results indicated that adding 3,4-DHP to the culture medium probably inhibits the biosynthesis of extensins, thereby caused the defects in cotyledons. This suggests that extensins may play a role in the cotyledon differentiation of embryos.
Table 2.
Effects of 3, 4-DHP on the germination of cultured ovules in tobacco
| DHP (μM) | Frequencies of germination (%) |
||||||
| Total | Normal | Abnormal | Type of abnormal cotyledon |
||||
| SC | AC | SupC | CC | ||||
| 0 | 46.36±9.06 | 45.67±9.13 | 0.69±0.43 | 0 | 0.35±0.47 | 0.34±0.38 | 0 |
| 50 | 49.53±7.39 | 38.46±3.64* | 11.07±0.56** | 0.92±1.44** | 4.62±1.42 | 4.92±0.92 | 0.61±0.37** |
| 100 | 40.49±2.71 | 31.25±3.49** | 9.24±2.84** | 3.26±0.31** | 2.45±1.59 | 1.36±0.35 | 2.17±0.37** |
| 200 | 44.31±5.53 | 37.50±6.01** | 6.81±0.79** | 3.41±1.09** | 2.27±0.29 | 0.28±0.30 | 0.85±0.80** |
The results were listed by a value with a standard deviation (SD). *0.01<P<0.05 indicates distinct difference; **P<0.01 indicates significant distinct difference compared with the zero-DHP treated control. SC, Single cotyledon; AC, Asymmetry cotyledon; SupC, Supernumerary cotyledon; CC, Cup-shape cotyledon. The number of ovules cultured is about 280-380.
After the seedlings were transplanted into soil for 20 d, the control seedlings developed into normal plantlets (Fig. 4J, indicated by an arrow), flowered, and set seeds. The seedlings with cotyledon defects in Fig. 4F, G, and H could continuously develop into plantlets which had no obvious difference with the control plantlets (data not shown). However, seedlings with cup-shaped cotyledons (Fig. 4I) stopped growing and finally died (Fig. 4J, K, indicated by arrowheads). Therefore, it is speculated that the seedlings with cup-shaped cotyledons which did not grow were the result of a defect in the shoot apical meristem.
Effects of 3,4-DHP on the development of isolated embryos in culture
In order to investigate further the effect of 3,4-DHP on the differentiation of embryos, late globular embryos at 7 DAP were chosen and cultured in KM8p medium with different concentrations of 3,4-DHP. After 10 d of culture, the embryos also developed into three types: undifferentiated, normal differentiated, and abnormal differentiated (Table 3). In 3,4-DHP-treated embryos, the frequencies of the abnormal differentiated embryos significantly increased compared with the untreated control, and the configuration of the seedlings with defective cotyledons in the embryo culture was similar to that of the seedlings in the ovule culture (data not shown). The 5–100 μM 3,4-DHP concentrations could improve the total differentiation frequencies of embryos compared with the treated control, but these increases mainly come from the augmentation of abnormal embryo differentiation, the normal embryo differentiation had a weak contribution to the increase. However, the 2 μM 3,4-DHP showed the reverse effect and inhibited total embryo differentiation, specifically normal embryo differentiation. These results further indicated that extensins are important for embryo differentiation, which agrees with the results of ovule culture.
Table 3.
Effects of 3, 4-DHP on tobacco embryo development in culture
| Treatment of 3,4-DHP (μM) | No. of isolated embryos | Frequency of undifferentiated embryos (%) | Frequency of differentiated embryos (%)a |
||
| Total | Normal | Abnormal | |||
| 0 | 217 | 82.49±0.48 | 18.45±0.48 | 17.05±1.40 | 0.46±1.00 |
| 2 | 181 | 90.48±0.72 | 9.52±0.72** | 4.97±1.25** | 3.87±1.45** |
| 5 | 185 | 76.76±3.80 | 23.24±3.80 | 19.92±3.39 | 3.78±1.02** |
| 10 | 211 | 76.30±7.02 | 23.7±7.02 | 19.43±8.16 | 4.27±3.33** |
| 50 | 168 | 78.57±7.79 | 21.43±7.79 | 17.26±5.20 | 4.17±2.61** |
| 100 | 219 | 77.16±5.01 | 22.84±5.01 | 18.73±4.08 | 4.11±1.00** |
The results are shown by a value with a standard deviation (SD). ** P <0.01 indicates a significant difference compared with zero-DHP control.
Hydroxyproline level in the cultured ovules
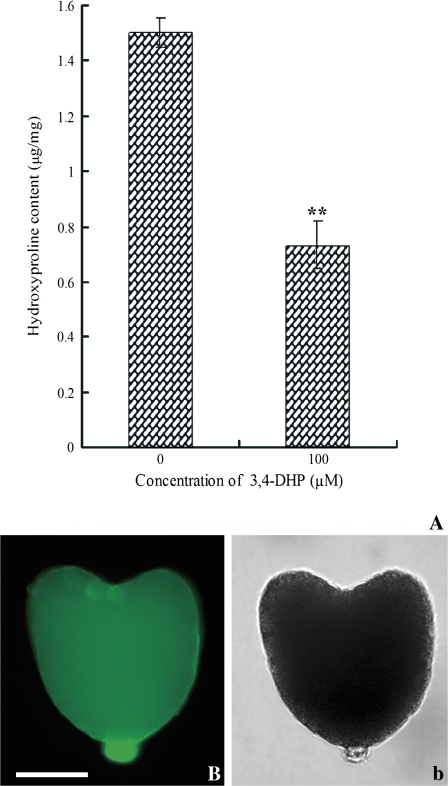
Total Hyp level in the cultured ovules treated with 100 μM 3,4-DHP was significantly reduced as compared with the control. The Hyp in cultured ovules treated with or without 3,4-DHP was 1.5 μg mg−1 dry weights and 0.731 μg mg−1 dry weights, respectively (Fig. 5A). The distribution of extensins in embryos isolated from cultured ovules was also detected by immunolocalization using the JIM20 antibody. The result showed that the signal in the heart-embryos (Fig. 5B) cultured without 3,4-DHP was similar to the embryos in vivo (Fig. 2E), whereas only a faint signal was detected in the embryos cultured with 3,4-DHP (see Supplementary Fig. 1C, D at JXB online).
Fig. 5.
Level of detection of extensins in the ovules treated with or without 3,4-DHP by hydroxyproline assays and the localization of extensins in embryos isolated from the treated ovules detected by JIM20 antibody. (A) Comparison of total Hyp content in the cultured ovules with or without 100 μM 3,4-DHP treatment. Measurements were taken in triplicate and the standard deviation (SD) was shown. **: P < 0.01 indicated a significant difference compared with zero-DHP control. Fluorescent images of immunolocalization were shown in (B) and the corresponding bright-field micrograph in (b). (B) A heart-shaped embryo isolated from the ovules treated without 3,4-DHP showed strong fluorescence in the suspensor. Bar=50 μm. (This figure is available in colour at JXB online.)
Effects of 3,4-DHP on the activity of shoot apical meristem and the formation of the cotyledon vasculature
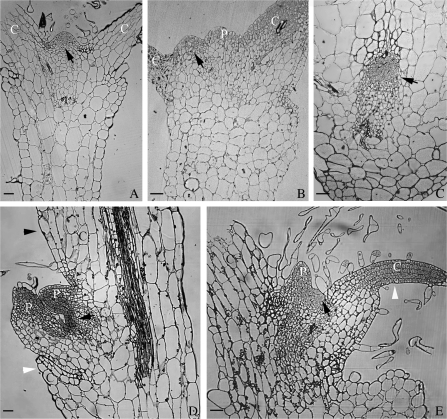
The characteristics of shoot apical meristems (SAM, indicated by arrows in Fig. 6) in various types of germinated seedlings after treatment with 100 μM 3,4-DHP were investigated using the semi-section technique. The position and morphology of the SAM in the supernumerary cotyledons (Fig. 6B) was similar to the control, which was located at the base of two separated cotyledons (Fig. 6A). The SAM in the cup-shaped (Fig. 6C), single (Fig. 6D), and asymmetrical cotyledon (Fig. 6E) plantlets had some dissimilarity compared with the control. The position of the SAM in the single (Fig. 6D) and asymmetrical (Fig. 6E) cotyledon plantlets was inclined towards the abnormal cotyledon. And the SAM in the cup-shaped cotyledon seedlings was encircled by one conjoint cotyledon (Fig. 6C), leading to a functional defect and no growth (Fig. 4J, K, indicated by arrowheads). However, the shape of the SAM in the single (Fig. 6D), asymmetrical (Fig. 6E), and the cup-shaped (Fig. 6C) cotyledon plantlets was flatter than that of the untreated control which looked like a cone (Fig. 4J, K, indicated by arrowheads), so it is presumed that the activity of the SAM was dependent on its position.
Fig. 6.
Effects of 3,4-DHP on the development of the tobacco shoot apical meristem (SAM, indicated by black arrows). (A) The SAM from a normal seedling cultured in MS medium without 3,4-DHP as a control. (B–E) The SAM from abnormal seedlings cultured in MS medium with 100 μM 3,4-DHP (A). The SAM of a control seedling located at the base of two separated cotyledons. (B) The SAM of an abnormal seedling with three cotyledons was similar to the control. One of the three cotyledons could not be shown in the picture. (C) The SAM of a cup-shaped seedling was encircled by a conjoint cotyledon. (D) The SAM in a single cotyledon plantlet. Only one cotyledon primordium developed in the normal cotyledon (indicated by a black arrowhead), while the other one degenerated (indicated by a white arrowhead). (E) The SAM of a seedling with two asymmetric cotyledons, the smaller one developed more slowly and stayed at an incompletely developed state (indicated by a white arrowhead). C, cotyledon; P, leaf primordium. Bar=50 μm.
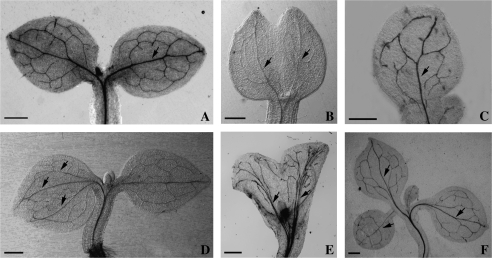
The transparency technique showed that the normal structure of the cotyledon venation in control seedlings was made up of one main vein and some branched veins (Fig. 7A). In the DHP-treated seedlings, a single-fused cotyledon contained two main veins (Fig. 7B), which indicates that it comes from the incomplete fusion of two cotyledons. The venation pattern of another single cotyledon type, in which only one cotyledon primordium could develop into a cotyledon, was similar to the untreated control (Fig. 7C). In seedlings with two asymmetrical cotyledons, the larger cotyledon had three main veins (indicated by three arrowheads), whereas the smaller one had a similar vasculature to the normal cotyledons (Fig. 7D). The cup-shaped cotyledon was completely fused and its vascular tissue was made up of more than two main veins and a large number of small branched veins (Fig. 7E). The venation of the supernumerary cotyledon (Fig. 7F) was similar to the normal cotyledons (Fig. 7A).
Fig. 7.
Effects of 3,4-DHP on the development of the cotyledon vasculature in tobacco. (A) The control cotyledon vasculature contained a main vein (indicated by an arrow) and small branched veins. (B) The vasculature contained two main veins (indicated by arrows) and small branched veins in the single cotyledon which originates from two cotyledons incompletely fused when treated with 100 μM 3,4-DHP. (C) The vasculature of another single cotyledon, in this situation, only one of the cotyledon primordia could develop into a normal cotyledon, therefore the vasculature was similar to the control, which contained a main vein (indicated by an arrow) and small branched veins. (D) The vasculature in two asymmetric cotyledons induced by 100 μM 3,4-DHP. The larger cotyledon has three main veins (indicated by three arrows), but the smaller cotyledon has a similar vasculature to the control. (E) In a cup-shaped cotyledon induced by 100 μM 3,4-DHP, the vasculature contained more than two main veins (indicated by arrows) and small branched veins. (F) The vasculature in the three cotyledon seedling induced by 100 μM 3,4-DHP contained a main vein (indicated by an arrow) and small branched veins. Bar=1 mm.
Discussion
Establishment of an effective system to study the function of extensins by using 3,4-DHP
Hydroxyproline-rich glycoproteins (HRGPs), of which extensins are a strongly basic protein presented in the plant cell wall, are characterized by a high level of the comparatively rare amino acid hydroxyproline (Hyp) (Chen and Varner, 1985). Because of the high abundance in cell walls and the extremely high level of Hyp, the use of specific inhibitors of prolyl hydroxylase which hydroxylated Pro into Hyp could provide a valuable approach for studying the function of extensins among all types of HRGPs. Unlike most other proline analogues, the potent inhibitor 3,4-DHP functions at micromole concentrations to inactivate prolyl hydroxylase in vivo rapidly and irreversibly (Cooper and Vamer, 1983). Studies with an Escherichia coli proline auxotroph demonstrated that 3,4-DHP was the only Pro analogue tested that could replace proline in the synthesis of enzymically active acid phosphatase (Morris and Schlesinger, 1972). For 3,4-DHP offers many advantages over other inhibitors, so it becomes a useful tool for studying the physiological and developmental functions of extensins among all types of HRGPs. In our test, the treatment with 100 μM 3,4-DHP significantly reduced the total hydroxyproline level in the cultured ovules about 51.3% (Fig. 5A). A similar result was observed in Allium cepa L. roots that were treated with 3,4-DL-dehydroproline (DP), a specific inhibitor of peptidyl-prolyl hydroxylase (Tullio et al., 1999). These results indicate that the use of 3,4-DHP or 3,4-DL-DP can offer good methods to study the functions of hydroxyproline-rich glycoproteins in specific tissues and development stages. So, using 3,4-DHP in culture tests primarily to inhibit the synthesis of extensins is an effective way to study their functions in embryo development.
Roles of extensins in embryo development
Embryogenesis is a critical stage of sporophytes in the plant life cycle, in which many genes and proteins play important roles. In our study, the dramatic change of extensins during tobacco embryo development was detected by Western blot and immunolocalization, and the results indicated that extensins may play roles in embryogenesis. In order to investigate the functions of extensins, the inhibitor of prolyl hydroxylase, 3,4-DHP, was applied to the in vitro culture of ovules and embryos. The results showed that addition of 100–200 μM 3,4-DHP to the ovule culture increased the total embryo differentiation frequencies, and the enhancement mainly reflected the increase of the abnormal differentiation frequencies. A similar result was also observed in embryo culture when the 3,4-DHP concentration was 5–100 μM. As 3,4-DHP inhibited the biosynthesis of Hyp and decreased the level of extensins in our tests, the in vitro culture results suggested that a low level of extensins could promote cell growth, which was in agreement with the result that removal of cell wall proteins increased extensibility in Avena sativa coleoptiles (Ruesink, 1969). In Allium cepa, the treatment of roots with a peptidylprolyl hydroxylase inhibitor caused a reduction in the level of hydroxyproline-rich glycoproteins that led to the early elongation of the developing root cells (Tullio et al., 1999). Conversely, increasing the level of extensins resulted in obviously shorter inflorescences in Arabidopsis (Roberts and Shirsat, 2006). Why does reducing the level of cell wall proteins promote cell growth? According to the previous studies, the cell wall comprised three interpenetrating networks: cellulose, pectins, and extensins and our results suggested that the changes to the extensins network may cause the cell wall to loosen and promote cell growth. Similarly, the decrease of the cellulose density stimulated pollen tube growth in Torenia fournieri and caused them to be slender and straighter (Wu et al., 2008).
Somewhat surprisingly in our work the frequency of total differentiated embryos decreased when treated with low concentration of 3,4-DHP (50 μM in ovule culture and 2 μM in embryo culture) (Tables 1, 3). In a widely accepted view, the structure of extensins was partially determined by the attachment of carbohydrate side chains (Cooper and Vamer, 1983), and then the regulation of cell wall extensibility by connecting different extensin structures via covalent and non-covalent cross-links (Cassab and Varner, 1988; Qi et al., 1995; Schnabelrauch et al., 1996). So in our experiment, the frequencies of the normal differentiated embryos that decreased in low concentrations of 3,4-DHP might be due to the partial inhibition of the activity of the prolyl hydroxylase that controlled the synthesis of extensins (Cooper and Vamer, 1983), thereby continuously changed the normal cross-link pattern of extensins into an abnormal pattern, which possibly then inhibited cell growth and differentiation. According to the data of in vitro ovule and embryo cultures, the increased frequencies of differentiated embryos induced by high concentrations of 3,4-DHP could be due to the total breakdown of the extensin network, which in turn induced cell wall loosening and promoted cell growth. Our results showed that the 3,4-DHP concentration point of 50 μM in ovules and 2 μM in embryos might be a significant point that influenced the balance of extensin synthesis and correct cross-links, and when the concentration increased, the balance was broken and the network was destroyed. This phenomenon had previously been observed in tomato; the inhibition of the extensin peroxidase activity depressed the hypocotyl height at a lower concentration, but promoted the hypocotyl height at a higher concentration (Brownleader et al., 2000). Together with our observations, these results suggest that the disturbance of the extensin level influenced embryo development under a special concentration effect: low concentrations of 3,4-DHP inhibited embryo differentiation, but high concentrations caused the increase of abnormal differentiated embryos.
The late globular embryos which were close to the start of differentiation showed an intense extensins signal in the apex of embryo proper (EP) and suspensor cells (Figs 2C, 3B, C). The polar localization of extensins may reflect the importance of the interaction between the suspensor and the EP, including cell communication, signal transduction, and material transportation. Previous studies indicated that PIN proteins which were putative auxin efflux carriers played important roles during Arabidopsis embryogenesis (Blilou et al., 2005). Moreover, recent studies have highlighted the roles of cell wall proteins in signal transduction and interactions with the plasma membrane proteins at the cell surface in determining cell fate during embryogenesis (Jamet et al., 2006). For example, the RSH glycoprotein AtEXT3 which was isolated from the cell cultures of wild-type Arabidopsis was critical for normal embryo development (Hall and Cannon, 2002; Cannon et al., 2008). In our tests, the globular embryos differentiate into heart embryos where bilateral symmetry of the cotyledon primordia is established, but aberrant differentiation of embryos was found in the ovule culture (Fig. 4B–D) when 3,4-DHP was applied. Strong evidence from the embryo culture further confirmed that extensins play important roles in the course of embryo differentiation, and the abnormal differentiated embryos appeared when the extensin synthesis was disturbed. The results of the hydroxyproline assay (Fig. 5A) and the immunolocalization of extensins in embryos isolated from the cultured ovules (see Supplementary Fig. 1 at JXB online) further validated that 3,4-DHP could reduce the level of extensins. So according to these results, it is suggested that extensins may play a role in embryo development. Taken together, our results also supported the hypothesis that cell wall proteins may play a function in the interaction between the suspensor and the EP.
The results from our studies indicated that extensins were essential for normal embryo differentiation, and when its level decreased, the differentiation frequency of the abnormal embryos increased. But, because of the lack of extensin mutants, the mechanism by which extensins play roles in embryo development is still unknown.
Roles of extensins in cotyledon formation and shoot apical meristem activity
In dicotyledonous plants, the just germinated seedlings exhibit bilateral symmetry, as evidenced by the two symmetrically located cotyledons and a shoot apical meristem (SAM) between the cotyledons. This symmetry can be traced back to early embryogenesis: the shoot meristem originates from the apical region of the radial symmetrical globular embryos, and subsequently the cotyledon primordia begin to form in the flank of the apical region (Long and Barton, 1998; Furutani et al., 2004), but the mechanism of how they initiate is still unknown. In our studies, extensins were found in the cotyledon primordia of the heart-shaped embryos and the SAM of the torpedo-shaped embryos (Fig. 2E, F), which indicated that extensins may play roles in cotyledon primordium formation and SAM activity. In ovule and embryo cultures, the embryos with abnormal cotyledon primordia and the young seedlings with abnormal SAM were observed in the treatment with 3,4-DHP. Seedlings normally germinate with two symmetrical cotyledons (Fig. 4E), but the germinated seedlings that had treatment with 3,4-DHP displayed various defects in the cotyledons (Fig. 4F–I). According to the results of semi-thin sections and transparency, the single cotyledon seedlings may have two origins: one is that only one cotyledon primordium developed into one normal cotyledon (Fig. 6D); the other is that two cotyledons formed that fused incompletely (Fig. 7B). Furthermore, a complete fusion of two cotyledons was found in the cup-shaped seedlings based on the results of venation patterns (Fig. 7E). The results showed that the seedlings from the ovules treated with 3,4-DHP had different extents of defects in the cotyledon primordia, and could not germinate when their primordia had extremely serious defects. Meanwhile, the abnormal cotyledons displayed a disorganized venation pattern, and this was also observed in the cotyledon of cuc1, cuc2 and pin1-3 pid1-2 mutants in Arabidopsis (Furutani et al., 2004). This indicates that the disorganized venation pattern in cotyledons may also come from the abnormality of the cotyledon primordia.
Further, the cup-shaped cotyledon seedlings did not mature, therefore, it is speculated that this was due to the interference of extensins, which resulted in the defects of SAM and the mislocalization of cotyledon-forming regions during the development of embryos. In our work, the position and morphology of the SAM in the abnormal cotyledon seedlings induced by 3,4-DHP was investigated and the difference was observed by comparison with the control (Fig. 6A). The position of the SAM in the cup-shaped cotyledon seedlings was seriously influenced, which was encircled by a conjoint cotyledon (Fig. 6C). So according to these results, it is presumed that the activity of SAM was dependent on the correct position. Therefore, these results indicated that the inhibition of extensins influenced the differentiation of embryos, which led to the unconventionality of cotyledon formation; and for the defects of the cotyledons, the correct positioning of the SAM was imvolved; and finally SAM activity was influenced.
The present study indicated that extensins play important roles in embryo differentiation, cotyledon primordium formation, and SAM activity, but how they function is still unknown. Further studies need to be done in the future.
Supplementary Material
Acknowledgments
The authors thank Dr JP Knox (Centre for Plant Sciences, University of Leeds, UK) for the generous gifts of the antibodies JIM11, JIM12, JIM19, and JIM20. The paper was supported by the National Natural Science Foundation of China (30770132, 30570103), the Special Doctorial Program Funds of the Ministry of Education of China (20050486015), and the Major State Basic Research Program of China (2007CB108704).
Glossary
Abbreviations
- 3,4-DHP
3,4-dehydro-l-proline
- DAP
days after pollination
- DP
3,4-dl-dehydroproline
- EP
embryo proper
- HRGPs
hydroxyproline-rich glycoproteins
- Mb
monoclonal antibody
- SAM
shoot apical meristem
- TEM
transmission electron microscopy
References
- Ahn JH, Choi Y, Kwon YM, Kim SG, Choi YD, Lee JS. A novel extensin gene encoding a hydroxyproline-rich glycoprotein requires sucrose for its wound-inducible expression in transgenic plants. The Plant Cell. 1996;8:1477–1490. doi: 10.1105/tpc.8.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heldstra R, Aida M, Plame K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Bosch M, Derksen J, Mariani C. A functional study of stylar hydroxyproline-rich glycoproteins during pollen tube growth. Sexual Plant Reproduction. 2003;16:87–98. [Google Scholar]
- Bougourd S, Marrison J, Haseloff J. An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis. The Plant Journal. 2000;24:543–550. doi: 10.1046/j.1365-313x.2000.00892.x. [DOI] [PubMed] [Google Scholar]
- Brownleader MD, Hopkins J, Mobasheri A, Dey PM, Jackson P, Trevan M. Role of extensin peroxidase in tomato (Lycopersicon esculentum Mill.) seedling growth. Planta. 2000;210:668–676. doi: 10.1007/s004250050058. [DOI] [PubMed] [Google Scholar]
- Bucher M, Schroeer B, Willmitzer L, Riesmeier JW. Two genes encoding extensin-like proteins are predominantly expressed in tomato root hair cells. Plant Molecular Biology. 1997;35:497–508. doi: 10.1023/a:1005869717158. [DOI] [PubMed] [Google Scholar]
- Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, Chen L, Lamport DTA, Chen Y, Kieliszewski MJ. Self-assembly of the plant cell wall requires an extensin scaffold. Proceeding of the National Academy of Sciences, USA. 2008;105:2226–2231. doi: 10.1073/pnas.0711980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassab GI, Varner JE. Cell wall proteins. Annual Review of Plant Physiology and Plant Molecular Biology. 1988;39:321–353. doi: 10.1146/annurev.arplant.49.1.281. [DOI] [PubMed] [Google Scholar]
- Chen J, Varner JE. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO Journal. 1985;4:2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JB, Heuser JE, Varner JE. 3,4-Dehydroproline inhibits cell wall assembly and cell division in tobacco protoplasts. Plant Physiology. 1994;104:747–752. doi: 10.1104/pp.104.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JB, Varner JE. Selective inhibition of proline hydroxylation by 3,4-dehydroproline. Plant Physiology. 1983;73:324–328. doi: 10.1104/pp.73.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak S, Shailasree S, Kini RK, Hause B, Shetty SH, Mithöfer A. Role of hydroxyproline-rich glycoproteins in resistance of pearl millet against downy mildew pathogen Sclerospora graminicola. Planta. 2007;226:323–333. doi: 10.1007/s00425-007-0484-4. [DOI] [PubMed] [Google Scholar]
- Fry SC. The growing plant cell wall: chemical and metabolic analysis. Essex: Longman; 1988. [Google Scholar]
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida1 M. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development. 2004;131:5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- Hall Q, Cannon MC. The cell wall hydroxyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. The Plant Cell. 2000;14:1161–1172. doi: 10.1105/tpc.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CN, Kent L, McClure BA. The stylar 120 kDa glycoprotein is required for S-specific pollen rejection in Nicotiana. The Plant Journal. 2005;43:716–723. doi: 10.1111/j.1365-313X.2005.02490.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Kodama H, Komamine A, Watanabe A. Expression of extensin genes is dependent on the stage of the cell cycle and cell proliferation in suspension-cultured Catharanthus roseus cells. Plant Molecular Biology. 1998;36:343–351. doi: 10.1023/a:1005913818129. [DOI] [PubMed] [Google Scholar]
- Jamet E, Canut H, Boudart G, Pont-LRF Cell wall proteins: a new insight through proteomics. Trends in Plant Science. 2006;11:1360–1385. doi: 10.1016/j.tplants.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Kao KN, Michayluk MR. Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta. 1975;126:105–110. doi: 10.1007/BF00380613. [DOI] [PubMed] [Google Scholar]
- Kieliszewski MJ, Lamport DTA. Extensin: repetitive motifs, functional sites, post-translational codes and phylogeny. The Plant Journal. 1994;5:157–172. doi: 10.1046/j.1365-313x.1994.05020157.x. [DOI] [PubMed] [Google Scholar]
- Kivirikko KI, Liesmaa M. A colorimetric method for determination of hydroxyproline in tissue hydrolysates. Scandinavian Journal of Clinical and Laboratory Investigation. 1959;11:128–133. [Google Scholar]
- Leroux O, Knox JP, Leroux F, Vrijdaghs A, Bellefroid E, Borgonie GT, Viane RLL. Intercellular pectic protuberances in Asplenium: new data on their composition and origin. Annals of Botany. 2007;100:1165–1173. doi: 10.1093/aob/mcm210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind JL, Bönig I, Clarke AE, Anderson MA. A style-specific 120-kDa glycoprotein enters pollen tubes of Nicotiana alata in vivo. Sexual Plant Reproduction. 1996;9:75–86. [Google Scholar]
- Long JA, Barton MK. The development of apical embryonic pattern in Arabidopsis. Development. 1998;125:3027–3035. doi: 10.1242/dev.125.16.3027. [DOI] [PubMed] [Google Scholar]
- Memelink J, Swords KM, De Kam RJ, Schilperoort RA, Hoge JHC, Staehelin LA. Structure and regulation of tobacco extensin. The Plant Journal. 1993;4:1011–1022. doi: 10.1046/j.1365-313x.1993.04061011.x. [DOI] [PubMed] [Google Scholar]
- Merkouropoulos G, Shirsat AH. The unusual Arabidopsis extensin gene atExt1 is expressed throughout plant development and is induced by a variety of biotic and abiotic stresses. Planta. 2003;217:356–366. doi: 10.1007/s00425-003-1002-y. [DOI] [PubMed] [Google Scholar]
- Morris H, Schlesinger MJ. Effects of proline analogs on the formation of alkaline phosphatase in Escherichia coli. Journal of Bacteriology. 1972;111:203–210. doi: 10.1128/jb.111.1.203-210.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale AD, Wahleithner JA, Lund M, Bonnett HT, Kelly A, Wagner DRM, Peacock WJ, Dennis ES. Chitinase, β-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. The Plant Cell. 1990;2:673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Behrens BX, West PR, Mort AJ. Solubilization and partial characterization of extensin fragments from cell walls of cotton suspension cultures. Plant Physiology. 1995;108:1691–1701. doi: 10.1104/pp.108.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhao J. Localization of arabinogalactan-proteins in egg cells, zygotes and two-celled proembryos and effects of β-D-glucosyl Yariv reagent on egg cell fertilization and zygote division in Nicotiana tabacum L. Journal of Experimental Botany. 2006;57:2061–2074. doi: 10.1093/jxb/erj159. [DOI] [PubMed] [Google Scholar]
- Roberts K, Shirsat AH. Increased extensin levels in Arabidopsis affect inflorescence stem thickening and height. Journal of Experimental Botany. 2006;57:537–545. doi: 10.1093/jxb/erj036. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL, Márque J, Cervera MS, Bedinger PA. Extensin-like glycoproteins in the maize pollen tube wall. The Plant Cell. 1995;7:2211–2225. doi: 10.1105/tpc.7.12.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesink AW. Polysaccharidases and the control of cell wall elongation. Planta. 1969;89:95–107. doi: 10.1007/BF00386978. [DOI] [PubMed] [Google Scholar]
- Schnabelrauch LS, Kieliszewski M, Upham BL, Alizedeh H, Lamport DT. Isolation of pI 4.6 extensin peroxidase from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. The Plant Journal. 1996;9:477–489. doi: 10.1046/j.1365-313x.1996.09040477.x. [DOI] [PubMed] [Google Scholar]
- Schneiderbauer BA, Back E, Sandermann H, Jr, Ernst D. Ozone induction of extensin mRNA in Scots pine, Norway spruce and European beech. New Phytologist. 1995;130:225–230. [Google Scholar]
- Stafstrom JP, Staehelin LA. The role of carbohydrate in maintaining extensin in an extended conformation. Plant Physiology. 1986;81:242–246. doi: 10.1104/pp.81.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio MCD, Paciolla C, Vecchia FD, Rascio N, D'Emerico S, Gara LD, Liso R, Arrigoni O. Changes in onion root development induced by the inhibition of peptidyl-prolyl hydro-xyllase and influence of the ascorbate system on cell division and elongation. Planta. 1999;209:424–434. doi: 10.1007/s004250050745. [DOI] [PubMed] [Google Scholar]
- van Holst GJ, Varner JE. Reinforced polyproline II conformation in a hydroxyproline-rich cell wall glycoprotein from carrot root. Plant Physiology. 1984;74:247–251. doi: 10.1104/pp.74.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Shirsat AH. Extensin over-expression in Arabidopsis limits pathogen invasiveness. Molecular Plant Pathology. 2006;7:579–592. doi: 10.1111/j.1364-3703.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- Wilson LG, Fry JC. Extensin, a major cell wall glycoprotein. Plant, Cell and Environment. 1986;9:239–260. [Google Scholar]
- Wisniewski JP, Cornille P, Agnel JP, Montillet JL. The extensin multigene family responds differentially to superoxide or hydrogen peroxide in tomato cell cultures. FEBS Letters. 1999;447:264–268. doi: 10.1016/s0014-5793(99)00315-4. [DOI] [PubMed] [Google Scholar]
- Wu H, de Graaf B, Marianid C, Cheung AY. Hydroxyproline-rich glycoproteins in plant reproductive tissues: structure, functions and regulation. Cellular and Molecular Life Sciences. 2001;58:1418–1429. doi: 10.1007/PL00000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Zou JT, May B, Gu Q, Cheung AY. A tobacco gene family for flower cell wall proteins with a proline-rich domain and a cysteine-rich domain. Proceedings of the National Academy of Sciences, USA. 1993;90:6829–6833. doi: 10.1073/pnas.90.14.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JZ, Lin Y, Zhang XL, Pang DW, Zhao J. IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. Journal of Experimental Botany. 2008;59:2529–2543. doi: 10.1093/jxb/ern119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Chen D, Ren YJ, Zhang XL, Zhao J. Characteristic and expression analysis of a metallothionein gene, OsMT2b, down-regulated by cytokinin suggests functions in root development and seed embryo germination of rice. Plant Physiology. 2008;146:1637–1650. doi: 10.1104/pp.107.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Mollet JG, Lord EM. Lily (Lilium longiflorum L.) pollen protoplast adhesion is increased in the presence of the peptide SCA. Sexual Plant Reproduction. 2004;16:227–233. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.