Abstract
DNA adenine methylase (dam) mutants of Salmonella enterica serovar Typhimurium grown under laboratory conditions express the std fimbrial operon, which is tightly repressed in the wild type. Here, we show that uncontrolled production of Std fimbriae in S. enterica serovar Typhimurium dam mutants contributes to attenuation in mice, as indicated by the observation that an stdA dam strain is more competitive than a dam strain upon oral infection. Dam methylation appears to regulate std transcription, rather than std mRNA stability or turnover. A genetic screen for std regulators showed that the GATC-binding protein SeqA directly or indirectly represses std expression, while the poorly characterized yifA gene product serves as an std activator. YifA encodes a putative LysR-like protein and has been renamed HdfR, like its Escherichia coli homolog. Activation of std expression by HdfR is observed only in dam and seqA backgrounds. These data suggest that HdfR directly or indirectly activates std transcription. Since SeqA is unable to bind nonmethylated DNA, it is possible that std operon derepression in dam and seqA mutants may result from unconstrained HdfR-mediated activation of std transcription. Derepression of std in dam and seqA mutants of S. enterica occurs in only a fraction of the bacterial population, suggesting the occurrence of either bistable expression or phase variation.
DNA adenine methylase (Dam) catalyzes postreplicative methylation of adenosine moieties located in 5′-GATC-3′ sites, using S-adenosyl-methionine as a methyl donor (6, 31, 53). Methylation of daughter DNA strands occurs shortly, but not immediately, after passage of the replication fork. As a consequence, all GATC sites go through a stage called “hemimethylation” in which one DNA strand is methylated and the other is nonmethylated (53). Because Dam trails the replication machinery at a relatively small distance, hemimethylated DNA is usually short-lived (31, 53).
Whenever a GATC site is embedded within a protein-binding sequence, its methylation state can affect DNA-protein interactions (53). For instance, the mismatch repair endonuclease MutH is active only on hemimethylated or nonmethylated GATC sites, while the replication protein DnaA binds more efficiently to the chromosome replication origin when its GATC sites are methylated (31, 53). The methylation state of specific GATC sites can also influence promoter activity. For instance, transient hemimethylation can activate or repress transcription in a cell cycle-coupled fashion (31, 32). Furthermore, in the regulatory regions of certain promoters, binding of proteins can prevent DNA methylase activity, giving rise to stably undermethylated (hemimethylated or nonmethylated) GATC sites. Undermethylation patterns can be maintained beyond cell division, thereby permitting epigenetic inheritance of transcriptional states (6, 32).
dam mutants of Salmonella enterica are severely attenuated in the mouse model: lack of DNA adenine methylation causes a 10,000 increase in the oral 50% lethal dose, and a 1,000-fold increase in the intraperitoneal 50% lethal dose (17, 19, 21). This extreme attenuation reflects the pleiotropy of dam mutations, which cause reduced invasion of epithelial cells (17), reduced cytotoxicity after infection of M cells (17), inefficient colonization of Peyer's patches and mesenteric lymph nodes (17, 21), sensitivity to bile (22, 40, 41), envelope instability accompanied by leakage of proteins (41), reduced motility (3), and probably additional defects still to be discovered. This plethora of virulence-related alterations, combined with the long persistence of dam mutants in infected animals, makes Dam-deficient strains highly suitable as live vaccines (13, 14, 22). On the other hand, the essential role played by Dam methylation in the virulence of Salmonella and other bacterial pathogens (24) has raised the possibility of using Dam inhibitors as antibacterial drugs (34).
Some of the virulence-related defects so far described in Salmonella dam mutants involve alterations in gene expression (3). For instance, the reduced capacity of dam mutants to invade epithelial cells (17) seems to be caused by lowered expression of Salmonella pathogenicity island I (3), which in turn reflects the existence of reduced levels of the main Salmonella pathogenicity island I activator, HilD (J. López-Garrido and J. Casadesus, unpublished data). Altered expression patterns of flagellar and chemotaxis genes may also contribute to deficient invasion (3). Other virulence-related genes with anomalous expression in dam mutants are the Braun lipoprotein gene, lppB (3), and the spv operon of the virulence plasmid (21).
This study deals with an additional virulence-related locus under Dam methylation control: the std fimbrial operon, initially identified in serovar Typhi (46) and later found in other Salmonella enterica serovars, including Typhimurium (2, 7, 27, 38). Std fimbriae belong to the π group (37) and play a role in the adhesion of Salmonella to specific intestinal sections, as indicated by the fact that deletion of the std operon causes reduced intestinal persistence (51). Synthesis of Std fimbriae is tightly repressed under laboratory conditions, and several lines of evidence suggest that derepression occurs in the intestine of infected animals (9, 51).
The mechanisms that prevent std expression outside the animal environment remain unknown, but recent studies have identified the cellular functions involved. Lack of RosE, a protein with homology to the Escherichia coli ArgR repressor, permits std expression under laboratory conditions (9). Derepression of the std operon also occurs in the absence of Dam methylation: dam mutants were found among Std-expressing isolates induced by transposon mutagenesis (9). This finding was in agreement with a proteomic study that identified StdA as one of the most abundant proteins in dam mutants (1) and with the >100-fold increase in std mRNA detected by transcriptomic analysis of dam mutants (3). The addition of std to the list of Dam-regulated genes strengthens the curious observation that genes under Dam methylation control often encode surface structures such as fimbriae, nonfimbrial adhesins, type III secretion systems, and conjugative pili (32).
Below, we provide evidence that uncontrolled expression of Std fimbriae may be listed as an additional defect contributing to virulence attenuation in Dam mutants of S. enterica serovar Typhimurium. Furthermore, we show that the GATC-binding protein SeqA is a repressor of the std operon and that the poorly known protein HdfR, a putative member of the LysR family of transcriptional regulators (29), is an activator of std expression whose activity may be antagonized by SeqA.
MATERIALS AND METHODS
Bacterial strains, media, chemicals, and culture conditions.
The strains of S. enterica used in this study (Table 1) belong to serovar Typhimurium and derive from the mouse-virulent strain ATCC 14028. For simplicity, S. enterica serovar Typhimurium is often abbreviated as serovar Typhimurium. Luria-Bertani (LB) broth was used as standard liquid medium. Carbon sources were either 0.2% glucose or 0.2% arabinose. Solid LB medium contained agar at a 1.5% final concentration. Green plates were prepared according to Chan et al. (8), except that methyl blue (Sigma-Aldrich, St. Louis, MO) was substituted for aniline blue. Antibiotics were used at concentrations described previously (45). Transductional crosses using phage P22 HT 105/1 int201 (43; also G. Roberts, unpublished data) were used for strain construction operations involving chromosomal markers. The transduction protocol was previously described elsewhere (18). To obtain phage-free isolates, transductants were purified by streaking on green plates. Phage sensitivity was tested by cross-streaking with the clear-plaque mutant P22 H5.
TABLE 1.
Strain list
| Strain designation | Genotype or description | Reference or source |
|---|---|---|
| SV4248 | traB1::MudJ zge-6313::Tn10dCm (50% linked to dam) | Laboratory stock |
| SV4536 | Δdam-230 | 40 |
| SV4752 | ΔseqA1 | 39 |
| SV4783 | dam-201::Tn10dTc ΔseqA1 | Laboratory stock |
| SV5031 | ΔstdA::Kmr | This study |
| SV5032 | Δdam-230 ΔstdA::Kmr | This study |
| SV5139 | stdB::Cmr | This study |
| SV5206 | stdA::lacZ | This study |
| SV5208 | Δdam-230 stdA::lacZ | This study |
| SV5637 | ΔseqA1 ΔhdfR | This study |
| SV5638 | Δdam-230 ΔhdfR | This study |
| SV5643 | seqA::Tn10dTc stdA::lacZ | This study |
| SV5645 | Δdam-230 seqA::Tn10dTc stdA::lacZ | This study |
| SV5651 | ΔstdAB::Kmr | This study |
| SV5652 | Δdam-230 ΔstdAB::Kmr | This study |
| SV5657 | pBAD(stdA stdB::Cmr) ΔstdAB::Kmr | This study |
| SV5658 | pBAD(stdA stdB::Cmr) Δdam-230 ΔstdAB::Kmr | This study |
| SV5716 | ΔhdfR::Cmr | This study |
| SV5717 | Δdam-230 ΔhdfR::CmrstdA::lacZ | This study |
| SV5718 | ΔseqA1 ΔhdfR::CmrstdA::lacZ | This study |
| SV5719 | ΔseqA1 stdA::lacZ | This study |
β-Galactosidase assays.
Levels of β-galactosidase activity were assayed as described by Miller (36), using the CHCl3-sodium dodecyl sulfate permeabilization procedure.
Primer extension.
Strain SV4536 was grown in LB medium until late exponential phase; total RNA was then extracted. The oligonucleotide 5′-ACC TGA GCC GAA CGG GCC TG-3′, complementary to an internal region of the stdA gene of serovar Typhimurium (GenBank accession number AE008839), was end labeled with [γ32P]ATP and annealed to 20 μg of RNA. For annealing, 106 cpm of oligonucleotide was used. The end-labeled primer was extended with avian myeloblastosis virus reverse transcriptase (Roche, Basel, Switzerland) under the conditions described by Camacho et al. (5). The extension products were separated in a polyacrylamide gel containing 6% urea. For autoradiography, gels were exposed to X-ray film.
DNA sequencing.
Sequencing reactions were carried out with a Sequenase, version 2.0, sequencing kit (USB Corporation, Cleveland, OH). The manufacturer's instructions were followed. Additionally, 1 μl of unlabeled 10 μM dATP was added to the reaction mixtures. Sequencing gels were prepared in Tris-borate-EDTA buffer containing 6% acrylamide and 500 g/liter urea. Gels were run in a Sequi-Gen GT System (Bio-Rad, Hercules, CA), dried in a Slab Gel Dryer, model SE1160 (Hoefer Scientific Instruments, Holliston, MA), and developed by exposure to X-ray film.
Construction of strains carrying stdA, stdB, stdAB, and yifA (hdfR) deletions.
All deletions were generated by the method of Datsenko and Wanner (11). Kanamycin resistance cassettes introduced during construction were excised by recombination with plasmid pCP20 (11). Elimination of 351 bp in the putative stdA coding sequence (from position +150 to position +506) was achieved with primers 5′-CGC CAG GAG TTG CGG CAT CTG TCA GGG CTA TCA GGC GGG CGT GTA GGC TGG AGC TGC TTC-3′, and 5′-TTT CAC TGG TAC CAT CAC CAA CTC ACC CTG TGA TAT CGC AAT TCC GGG GAT CCG TCG ACC-3′. The resulting deletion eliminates the entire stdA gene except for 129 bp at its 5′ end and 81 bp at its 3′ end. PCR amplification using primers from both sides of the stdA locus identified kanamycin-sensitive isolates that carried the desired deletion. The sequences of these primers were 5′-GTG GAC GGC TTC TCC CTG TC-3′ and 5′-GCC GCC GAT ACT ACA CCC AC-3′. An internal 2,416-bp deletion in the stdB open reading frame (ORF) was created with the primers 5′-CGG AGC CTG CTG GAC AGC GGG AAC CTG TCT AAC GTG GAC CGT GTA GGC TGG AGC TGC TTC-3′ and 5′-GTG CGA GTA AAA TCA CAC GGC TTC TTC TGC TGT TTT TCA CAT GAA TAT CCT CCT TAG TTC-3′. Primers for stdB deletion verification by PCR amplification were 5′-CCA TTC TGA TTA CCC TGA CA-3′ and 5′-TAC GGG TCC GGT CAA CAT TG-3′. A 3,310-bp deletion that removed DNA from both stdA and stdB was obtained with primers 5′-CGG GTC CGG TCA ACA TTG ACG GCC GCC GGG CTG TAC TGG CGT GTA GGC TGG AGC TGC TTC-3 and 5′-GAG TTG TTT TCA GCC TTT GCA AAA TAA TTC TCA TTC ACC CAT TCC GGG GAT CCG TCG ACC-3′. Primers for deletion verification were 5′-CAT ACG AAT CTT TTC TGA AC-3′ and 5′-GGC CAC CGT TTT CTG CGG CG-3′. A 692-bp deletion in the putative yifA ORF was generated with primers 5′-AGA AGC ACT TTA CCT GAC GCA ATC CGC GGT GAG CTT TCG TGT GTA GGC TGG AGC TGC TTC-3′ and 5′-TCA TTG TTC ATC CAG CAC ATC CGT TTT TAA CAG ATC GCA GAT GAA TAT CCT CCT TAG TTC-3′ and verified with primers 5′-GGA GAG CAC AGT GGA TAC GG-3′ and 5′-GAT TAT CTG ATC AGG TAA TC-3′.
Construction of a strain carrying an stdA::lacZ translational fusion in the Salmonella chromosome (SV5206).
The FLT recombinase target site created by stdA gene disruption (ΔstdA::Kmr) in the immediate ancestor of strain SV5031 was used to integrate plasmid pCE40 (15), thereby generating a translational stdA::lacZ fusion.
Construction of strains expressing std from the arabinose-dependent pBAD promoter (SV5657 and SV5658).
Construction of strains expressing std from the arabinose-dependent pBAD promoter followed a procedure similar to that reported by Figueroa-Bossi et al. (16). The chromosomal gene araB of strain SV5651 (ΔstdAB) was replaced with a DNA fragment that included the start site of the std transcript, a complete stdA gene, and a portion of the stdB gene, using the λ Red technology (11). For this purpose, we generated a PCR product using primers with 40-nucleotide extensions homologous to regions adjacent to araB. As a template, we used genomic DNA of strain SV5139 (stdB::Cmr). The primers used to amplify stdA stdB::Cmr (with extensions homologous to araB boundaries) were 5′-TTA GCA TTT TTG TCC ATA GGA TTA GCG GAT CCT GCC TGA CTA TGC GTA ATA AAA TAA TAC TTG CC-3′ and 5′-GAT GAC GGT TAA TGC ACG GAT CGA GTT CAT CAA AGA AGC TCG CCA GTG CGA GTA AAA TCAC-3′. The resulting PCR product was used to transform derivatives of SV5651 and SV5652 (dam+ ΔstdAB and dam ΔstdAB, respectively) containing the λ Red recombinase-expressing pKD46 plasmid (11). Chloramphenicol-resistant transformants were selected and tested for the presence of the desired gene construct. This was the origin of strains SV5657 and SV5658 (pBAD-stdA stdB::Cmr ΔstdAB and pBAD-stdA stdB::Cmr ΔstdAB Δdam-230, respectively).
Protein extracts and Western blot analysis.
Total protein extracts were prepared from bacterial cultures grown at 37°C in LB medium until stationary phase (final optical density at 600 nm of ∼1.2 to 1.4). Bacterial cells contained in 1 ml of culture were collected by centrifugation (20,000 × g for 5 min at 4°C), washed in phosphate-buffered saline (PBS), pH 7.4, and suspended in the appropriate volume of Laemmli sample buffer (1.3% sodium dodecyl sulfate, 10% [vol/vol] glycerol, 50 mM Tris-HCl, 1.8% β-mercaptoethanol, 0.02% bromophenol blue, pH 6.8). Proteins were resolved by Tris-Tricine-polyacrylamide gel electrophoresis, using 10% gels. Conditions of protein transfer and optimal dilutions of primary (anti-StdA) and secondary antibodies have been described elsewhere (3). Proteins recognized by the antibodies were visualized by chemiluminescence using luciferin-luminol reagents.
Quantitative reverse transcriptase PCR (real-time PCR) and calculation of relative expression levels.
Salmonella RNA was extracted from stationary phase cultures using an SV Total RNA Isolation System (Promega Corporation, Madison, WI). The quantity and quality of the extracted RNA were determined using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). To diminish genomic DNA contamination, the preparation was treated with DNase I (Turbo DNA free; Applied Biosystems/Ambion, Austin, TX). An aliquot of 0.5 μg of DNase I-treated RNA was used for cDNA synthesis using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real-time PCRs were performed in an Applied Biosystems 7500 Fast Real-Time PCR System. Each reaction was carried out in a total volume of 15 μl on a 96-well optical reaction plate (Applied Biosystems) containing 7.5 μl of Power Sybr Green PCR Master Mix (Applied Biosystems), 6.9 μl of cDNA (1/10 dilution), and two gene-specific primers at a final concentration of 0.2 μM each. Real-time cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and at 60°C for 1 min. No-template controls and controls lacking reverse transcriptase were included for each primer set and template. Melting curve analysis verified that each reaction contained a single PCR product. Reported gene expression levels were normalized to transcripts of ompA, a housekeeping gene that served as an internal control. Gene-specific primers, designed with PRIMER3 software (http://primer3.sourceforge.net), were as follows: for ompA, 5′-TGT AAG CGT CAG AA CCG ATA CG-3′ and 5′-GAG CAA CCT GGA TCC GAA AG-3′; for stdA, 5′-CGG CTG CCG GTA TGA TGT-3′ and 5′-GGG CCT GCT GTG GGT GTA-3′; and for stdB, 5′-CTG CCG CCC TCT CTT CAG-3′ and 5′-GAC GGT GAC CTG TGC ATT ACT G-3′.
Cloning and molecular characterization of Tn10dTc inserts.
Amplification of DNA sequences close to Tn10dTc insertions was achieved by inverse PCR. Genomic DNA from each Tn10dTc-carrying isolate was digested with SmaI and PstI. The resulting fragment was autoligated and used as a template in two serial PCR amplifications with the primer 5′-ATT TGA TCA TAT GAC AAG ATG TGT-3′ (49). The final PCR product was purified and cloned onto pGEM-T (Promega Corporation, Madison, WI). Plasmid inserts were sequenced at the facilities of Sistemas Genómicos SL, Parque Tecnológico de Valencia, Paterna, Valencia, Spain, using the M13L and M13R universal primers.
Flow cytometry.
Approximately 5 × 108 cells were incubated with an equal volume of 4% paraformaldehyde (EM Science, Fort Washington, PA) at room temperature for 20 min. Cells were washed twice with 0.5 ml of 0.02% gelatin in PBS (PBS-gel). To block nonspecific binding, cells were harvested and resuspended in 0.5 ml of filter-sterilized 2% normal goat serum (Sigma) and incubated at room temperature for 30 min on a tabletop rotator. Polyclonal rabbit anti-StdA serum was added to the cells at a final dilution of 1:250 for detection of StdA, and cells were incubated at room temperature for 60 min on a tabletop rotator. After the cells were washed three times in PBS-gel, bacteria were resuspended in 0.5 ml of a solution of 0.04 mM propidium iodide in 2% normal goat serum with secondary antibody (fluorescein isothiocyanate [FITC]-conjugated goat anti-rabbit immunoglobulin G [IgG]) (Jackson ImmunoLabs, West Grove, PA), added at a dilution of 1:250. The mixture was rotated at room temperature for 1 h in the dark. Samples were washed three times with PBS-gel, and bacteria were resuspended in PBS to a final concentration of 5 × 106 cells/ml. For each sample, the fluorescence of 10,000 particles (bacterial cells) was measured by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA).
CI virulence assays.
Eight-week-old female BALB/c mice (Charles River Laboratories, Santa Perpetua de Mogoda, Spain) were used for virulence tests. Groups of three to four animals were inoculated with a 1:1 ratio of two strains. Bacteria were grown overnight at 37°C in LB medium without shaking. Oral inoculation was performed by feeding the mice with 25 μl of saline (0.9% NaCl) containing 0.1% lactose and 108 bacterial CFU. Bacteria were recovered from the mouse spleen 6 days after inoculation, and CFU were enumerated on appropriate medium. A competitive index (CI) for each mutant was calculated as the ratio between the wild type and the mutant strain in the output divided by their ratio in the input (4). To compare the virulence of a double mutant with that of a single mutant, a “cancelled-out” competitive index (COI) was calculated. A COI is the ratio between the double mutant and the single mutant in the output divided by their ratio in the input (4). Assays were carried out in triplicate. A Student's t test was used to analyze CIs and COIs. The null hypothesis was that CIs were not significantly different from 1. COIs were analyzed with two null hypotheses: (i) mean COI is not significantly different from 1; (ii) mean COI is not significantly different from the CI of the corresponding single mutant. P values of 0.01 or less were considered significant.
RESULTS
Overexpression of Std fimbriae contributes to virulence attenuation in S. enterica serovar Typhimurium dam mutants.
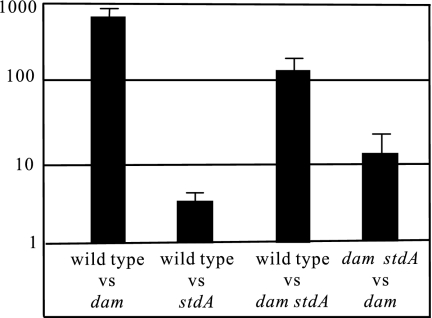
dam mutants of S. enterica serovar Typhimurium display a plethora of virulence-related defects whose basis is only partially understood (17, 40, 41). The recent observation that overexpression of Std fimbriae in a rosE mutant caused attenuation of serovar Typhimurium virulence (9) led us to examine whether Std overproduction, a conspicuous phenotype of Salmonella dam mutants (1, 3), might likewise contribute to their attenuation. To examine this hypothesis, BALB/c mice were inoculated with pairs of strains at a 1:1 ratio. Infections were performed by the oral route, and a CI was calculated. An stdA mutation caused a small but significant reduction of virulence by the oral route (Fig. 1). In contrast, an stdA mutation increased the COI in a dam background (Fig. 1). The latter observation indicates that ectopic expression of Std fimbriae does contribute to attenuation in Salmonella dam mutants and thus may be added to the list of virulence-related defects caused by lack of Dam methylation. On the other hand, the contribution of std overexpression to attenuation in dam mutants is in agreement with the view that ectopic expression of Std fimbriae is more detrimental than their absence during the intestinal stage of Salmonella infection (9).
FIG. 1.
CI and COI analysis of dam, std, and dam std strains after oral infection of BALB/c mice. The mixed infections performed were with the following strains: wild type (ATCC 14028) and dam (SV4536) (CI), wild type (ATCC 14028) and stdA (SV5031) (CI), wild type (ATCC 14028) and dam stdA (SV5032) (COI), and (d) dam stdA (SV5032) and dam (SV4536) (COI). The CIs and COI's represented are the means from three infections. Error bars represent the standard deviations. vs, versus.
Identification of the std operon start site.
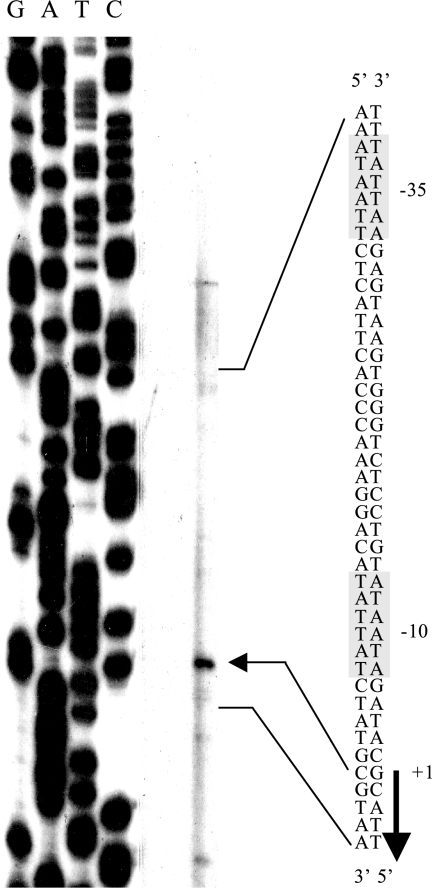
Primer extension was used to map the 5′ terminus of the std transcript (Fig. 2). Because the std operon is not expressed in wild-type serovar Typhimurium under laboratory conditions, a dam mutant (strain SV4536) was used. A sequencing reaction was run in parallel and used as a size marker. DNA sequencing was primed by the same oligonucleotide employed for primer extension. The std transcript was found to start 21 bp upstream of the putative start codon of the stdA gene. In silico analysis of the DNA sequence upstream of the +1 site identified DNA sequences with features similar to those of canonical, σ70-dependent promoters (25): (i) a putative −10 module including the motif 5′-TGTATAAT-3′, which has 6/8 matches with the consensus sequence; (ii) a putative spacer 19 nucleotides long; (iii) a 5′-TTATTTAAG-3′ sequence defining a putative −35 module, with 7/11 matches with the consensus sequence. Some of the DNA sequences found in the std promoter region are also compatible with the existence of an RpoS-dependent promoter (50); however, this possibility was judged unlikely since a previous study showed that std is expressed in dam derivatives of LT2 (3), a serovar Typhimurium strain known to be lacking RpoS (52). Upstream from the std promoter, a potential regulatory region containing three GATC motifs in a 25-bp interval was found, as previously described (3, 9).
FIG. 2.
Extension of an std mRNA primer with avian myeloblastosis virus reverse transcriptase. The extension product is indicated by an arrow. The DNA sequence of the std promoter region, the putative −10 and −35 modules, and the transcription start site are shown.
The identification of a promoter upstream of the stdA gene does not rule out the possibility that internal promoters may also exist. However, transcriptomic analysis has provided evidence that the five genes that are part of the std cluster (stdA, stdB, stdC, STM3026, and STM3025) undergo coordinate expression (3). Hence, we propose that the cluster formed by stdA, stdB, stdC, STM3026, and STM3025 constitutes a polycistronic operon, which is transcribed from the promoter identified in this study. The decreasing gradient of mRNA levels detected in downstream std genes (3) is typical of polycistronic operons with natural polarity (30).
Evidence that regulation of std expression by Dam methylation is transcriptional.
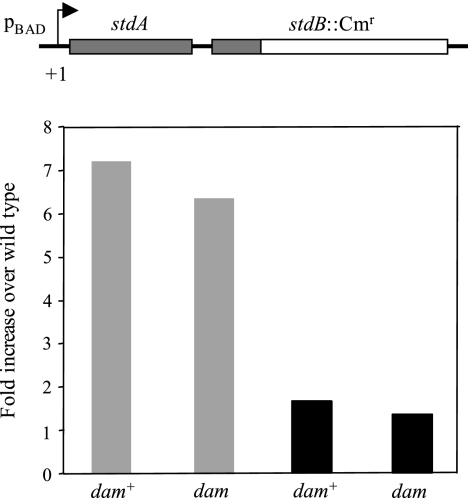
To determine whether Dam methylation-mediated control of std expression was transcriptional or posttranscriptional, we constructed dam+ and dam strains (SV5657 and SV5658, respectively) in which the first gene of the std operon, stdA, was expressed from the araBAD promoter. In these constructs, the transcription start site of stdA was conserved (described in Materials and Methods). The effect of Dam methylation on std expression from the heterologous pBAD promoter was examined by quantitative real-time PCR. Strains SV5657 and SV5658 were grown overnight in LB medium containing either 0.2% arabinose or 0.2% glucose. Total RNA was then extracted and retrotranscribed. The resulting cDNA preparations were analyzed by quantitative reverse transcriptase PCR. As expected, expression from the pBAD promoter was dependent on the presence or absence of arabinose (Fig. 3). However, the total amount of retrotranscribed DNA was similar in dam+ and dam strains, indicating that transcription from the pBAD promoter is insensitive to the presence or absence of Dam methylation (Fig. 3). Because the construct conserves the +1 site of the std operon, the 5′ end of the std mRNA can be expected to remain unaltered when transcription occurs from the pBAD promoter. Thus, these experiments provide evidence that Dam-dependent control of std expression requires its native promoter. This observation is consistent with the hypothesis that Dam-dependent control of std expression is transcriptional.
FIG. 3.
(Top) Diagram of the construction that permits std expression under the control of pBAD. (Bottom) Relative amounts of stdA mRNA transcribed from the heterologous pBAD promoter, normalized to ompA mRNA. Each bar represents the average from three independent experiments. Strains were grown in LB-arabinose (gray histograms) and LB-glucose (black histograms) media. Strains were SV5657 (dam+) and SV5658 (dam).
Genetic screens for transcriptional regulators of std operon expression.
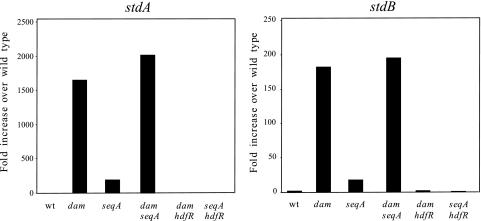
Tn10dTc insertions that altered the expression of the std operon were initially sought in a dam+ strain carrying a stdA::lacZ translational fusion (SV5206). Because the operon is not expressed in wild-type serovar Typhimurium, this fusion is Lac−. SV5206 was transduced with 10 pools of Tn10dTc insertions, each containing around 3,000 independent inserts. Tcr transductants were selected on LB plates containing tetracycline and 5-bromo-4-chloro-3-indolyl-β“(X-Gal), and Lac+ (blue) colonies were visually identified. Tn10dTc insertions in the dam gene (as well as upstream insertions polar on dam) were identified by cotransduction analysis. For this purpose, phage-free derivatives of the initial isolates were transduced with a lysate of SV4248, a strain that carries a Tn10dCm element 50% linked to dam. Whenever ∼50% of the Cmr transductants were Tcs, the isolate was judged to carry a Tn10dTc insertion in or near dam. Such mutants were the major class among the candidates analyzed (24/30). Six additional candidates whose insertions did not map in the aroB-damX-dam region were subjected to further study. Reverse PCR cloning and sequencing of one Tn10dTc boundary indicated that all six insertions were in seqA. Use of the previously characterized ΔseqA1 allele (39) confirmed that lack of SeqA derepressed std expression (Fig. 4, 5, and 6).
FIG. 4.
Relative amounts of stdA mRNA and stdB mRNA in various genetic backgrounds, normalized to ompA mRNA. The strains used were as follows: wild type (ATCC 14028), dam (SV4536), seqA (SV4752), dam seqA (SV4783), dam hdfR (SV5638), and seqA hdfR (SV5637). Each bar represents the average from three independent experiments. wt, wild type.
FIG. 5.
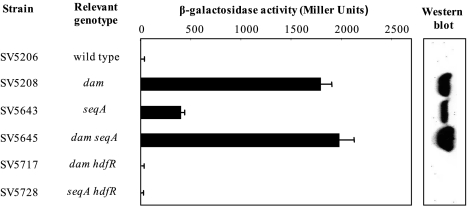
Expression of the stdA gene in S. enterica serovar Typhimurium strains carrying std regulatory mutations. The left panel shows β-galactosidase activities of a stdA::lacZ translational fusion constructed on the Salmonella chromosome. The right panel shows the levels of StdA protein in protein extracts from the same collection of strains, detected by Western blotting with anti-StdA serum.
FIG. 6.
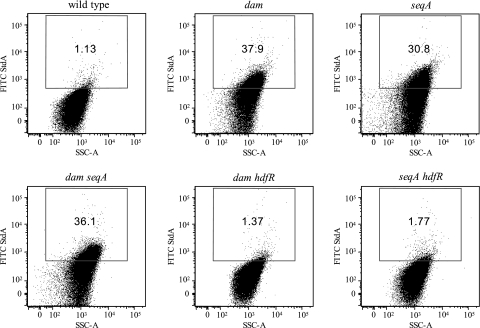
Flow cytometry analysis of std expression. Rabbit anti-StdA antiserum and FITC-conjugated goat anti-rabbit IgG were used for the detection of StdA antigen (y axes). Propidium iodide was used for the detection of DNA (x axes).The gate for the detection of StdA expression was set such that cells of the wild type (ATCC 14028) were considered positive for expressing StdA antigen when their FITC fluorescence intensity exceeded that of all but a small fraction (<2%) of the control population of the stdA mutant (not shown). The strains used were as follows: wild type (ATCC 14028), dam (SV4536), seqA (SV4752), dam seqA (SV4783), dam hdfR (SV5638), and seqA hdfR (SV5637). SSC-A, side scatter area.
A second screen involved use of a SeqA− strain carrying an stdA::lacZ translational fusion (SV5719). Because the std operon is expressed in a SeqA− background, this fusion is Lac+. Strain 5719 was transduced with 10 pools of Tn10dTc insertions, each containing some 3,000 independent fusions. Tcr transductants were selected on LB plates containing tetracycline, kanamycin, and X-Gal, and Lac− (white) colonies were visually identified. Two candidates of this kind were analyzed as above. Both contained a Tn10dTc insertion in yifA. This locus is part of the uncharacterized yifA-yifE-yifB region, which lies between trpT and ilvL at centisome 85 in the Salmonella genome map (35). In E. coli, the yifA homolog encodes a putative LysR-like transcriptional regulator called HdfR (29). Because 82% identity is found between the E. coli HdfR and Salmonella YifA ORFs (data not shown), the serovar Typhimurium yifA gene was renamed hdfR.
To rule out potential artifacts associated with the Tn10dTc insertion (e.g., caused by the existence of outward promoters), we constructed an hdfR deletion (described in Materials and Methods). Insertion and deletion mutations caused identical effects on std expression (data not shown), indicating that the hdfR::Tn10dTc insertion alleles described above were null.
Effects of dam, seqA, and hdfR mutations on std operon expression.
The amounts of std mRNA in strains carrying dam, seqA, and hdfR mutations were analyzed by quantitative reverse transcription-PCR. Levels of mRNA were monitored for the first gene of the operon, stdA, and for the second gene (stdB) as well. Data shown in Fig. 4 can be summarized as follows: (i) neither stdA nor stdB was expressed in the wild type, as previously reported (3, 27); (ii) extremely high levels of stdA mRNA (and, to a lesser extent, of stdB mRNA) were detected in a dam strain, again in agreement with previous observations (3); (iii) stdA and stdB mRNAs were also detected in a seqA background, but their amounts were smaller than in dam mutants; (iv) similar amounts of stdA and stdB mRNAs were found in seqA dam and dam mutants, indicating that a dam mutation is epistatic over a seqA mutation; (v) an hdfR mutation suppressed stdAB expression in both seqA and dam strains. Altogether, these observations suggest that Dam methylation and SeqA repress std operon expression and that HdfR activates std expression if either SeqA is absent or the genome lacks N6-methyl-adenosine. Lack of HdfR does not alter std expression in a seqA+ dam+ background (data not shown). The epistatic effect of a dam mutation over a seqA mutation is consistent with the well-known incapacity of SeqA to bind nonmethylated DNA (53).
The effects of dam, seqA, and hdfR mutations on std operon expression were also examined by β-galactosidase assays using a translational stdA::lacZ fusion and by Western blotting with polyclonal anti-StdA antibody. The results, shown in Fig. 5, were fully consistent with those described above: Dam methylation and the GATC-binding protein SeqA are std repressors, and HdfR is an std activator in the absence of either Dam methylation or SeqA.
Flow cytometry analysis of StdA production.
To monitor expression of StdA in individual serovar Typhimurium cells, cultures of strains ATCC 14028 (wild type) and its isogenic derivatives SV4536 (Δdam), SV4752 (ΔseqA), SV4783 (Δdam ΔseqA), SV5638 (Δdam ΔhdfR), and SV5637 (ΔseqA ΔhdfR) were subjected to flow cytometry analysis, using rabbit anti-StdA antiserum and FITC-conjugated goat anti-rabbit IgG for the detection of StdA antigen and propidium iodide for the detection of DNA. Only 1.3% of wild-type cells produced StdA (Fig. 6). In contrast, expression of StdA was detected in 37.9% of cells in the dam mutant, 30.8% in the seqA mutant, and 36.0% in the double mutant dam seqA. Knockout of hdfR abolished StdA expression: only 1.37% of cells expressed StdA in the dam hdfR double mutant, and 1.77% of cells expressed StdA in the seqA hdfR double mutant. These observations confirm the regulatory patterns described above. Furthermore, individual cell analysis shows that the std derepression observed in dam and seqA mutants does not involve a massive, uniform response of the bacterial population but the formation of an Std-expressing subpopulation.
DISCUSSION
The genome of S. enterica serovar Typhimurium contains 13 fimbrial loci that constitute a potential arsenal of antigens and attachment factors for the interaction with host tissues (26, 28). One such locus is the std operon (35). Like the majority of S. enterica serovar Typhimurium fimbrial operons, std is not expressed under laboratory conditions (27). In contrast, Std fimbriae are synthesized during infection and play a role in virulence, as indicated by the following observations: (i) StdA, the major protein of Std fimbriae, is detected upon infection of bovine ileal loops (27); (ii) mice infected with serovar Typhimurium seroconvert to StdA (26); and (iii) std deletion reduces intestinal persistence of serovar Typhimurium infection (51). However, synthesis of Std fimbriae inside the animal host must remain under tight control, as indicated by the observation that uncontrolled std expression in rosE mutants reduces their ability to colonize both the cecum and the spleen of mice (9). In this study, we provide additional evidence that massive synthesis of Std fimbriae is detrimental for Salmonella virulence: the extreme attenuation of Dam mutants upon oral infection (17, 21) is partially suppressed by deletion of std. Hence, optimal Salmonella infection may require either restrained levels or std expression or formation of an Std-expressing bacterial subpopulation (see below).
Our genetic screens for the identification of mutations that derepressed std operon expression in vitro identified Tn10dTc insertions in or upstream of dam, as previously described (3), and in seqA. Expression of std was found to be identical in dam and dam seqA mutants, indicating that SeqA is unable to repress std in the absence of Dam methylation. Because seqA mutations are less pleiotropic than dam mutations (and seqA mutants are healthier than dam mutants), the screen for mutations that suppressed std expression was performed in a seqA strain instead of a dam mutant as initially planned. This second screen identified HdfR (previously, YifA) as a function needed for std expression in dam and seqA mutants. Hence, HdfR appears to be an std activator whose action is antagonized by both Dam methylation and SeqA.
The mechanisms underlying SeqA- and HdfR-mediated regulation of the std operon remain to be investigated. However, because SeqA is a DNA-binding protein (33), it seems reasonable to suspect that it might be a transcriptional repressor of std. This view is supported by the involvement of SeqA in transcriptional regulation of other genes, such as the lambda PR promoter (44) and the agn43 gene of E. coli (10). In the wild type, SeqA binding to methylated and hemimethylated GATCs could endlessly maintain repression under laboratory conditions, thereby explaining why std is not expressed in batch cultures of serovar Typhimurium. In dam mutants, however, the well-known inability of SeqA to bind nonmethylated DNA (53) would permit std derepression. This hypothesis is supported by the observation that dam mutations are epistatic over seqA mutations regarding std operon derepression. With respect to HdfR, its E. coli counterpart has been characterized as an LysR-like transcriptional regulator that represses flhDC transcription (29). However, many LysR-like proteins are transcriptional activators (42). It is thus conceivable that HdfR might be a transcriptional activator of std transcription.
Although previous studies had detected enormous amounts of std mRNA and StdA protein in extracts from serovar Typhimurium dam strains (1, 3), examination of std expression in individual cells provided the noteworthy observation that synthesis of Std fimbriae occurs in only a fraction (≥30%) of dam and seqA Salmonella cells. The possibility that std undergoes either bistable expression (12) or phase variation (47) in dam and seqA mutants of serovar Typhimurium can thus be considered. Additional, intriguing questions concern the mechanisms that derepress std transcription inside the animal host. The observation that excess Std synthesis by rosE and dam mutants is detrimental in vivo (9; also above) argues in favor of self-limited Std expression in the animal environment, perhaps involving bistable or phase-variable std expression, as observed under laboratory conditions in dam and seqA mutants. An attractive (albeit speculative) model is that competition between a transcriptional activator (HdfR) and transcriptional repressors (SeqA and perhaps RosE) might create lineages of std+ and std cells, in a manner reminiscent of the self-propagating states described for phase-variable loci like pap (23) and agn43 (20, 48). However, std regulation presents unique features. One is that the operon is fully repressed under laboratory conditions, and subpopulation formation is observed only upon derepression by dam and seqA mutations. Another specific trait of std regulation is the involvement of poorly understood cell functions such as those of RosE and HdfR, whose study might unveil novel mechanisms of fimbrial control.
Acknowledgments
This work was supported by grants BIO2004-3455-CO2-02 and BIO2007-67457-CO2-02 from the Spanish Ministry of Education and Science and the European Regional Fund (to J.C.) and U.S. Public Health Service grants AI040124, AI044170, and AI079173 (to A.J.B.). M.J. was supported by an FPI predoctoral fellowship from the Spanish Ministry of Education and Science.
Preliminary experiments showing Dam-dependent regulation of the std operon were carried out by Eva M. Camacho. We are grateful to Marjan van der Woude, Francisco Ramos-Morales, Roberto Balbontín, Meritxell García-Quintanilla, Clara García-Calderón, Javier López Garrido, and Ignacio Cota for helpful discussions.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Alonso, A., M. G. Pucciarelli, N. Figueroa-Bossi, and F. García-del Portillo. 2005. Increased excision of the Salmonella prophage ST64B caused by a deficiency in Dam methylase. J. Bacteriol. 1877901-7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum, M. F., C. Marooney, M. Fookes, S. Baker, G. Dougan, A. Ivens, and M. J. Woodward. 2005. Identification of core and variable components of the Salmonella enterica subspecies I genome by microarray. Infect. Immun. 737894-7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balbontin, R., G. Rowley, M. G. Pucciarelli, J. López-Garrido, Y. Wormstone, S. Lucchini, F. Garcia-del Portillo, J. C. Hinton, and J. Casadesus. 2006. DNA adenine methylation regulates virulence gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1888160-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuzón, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 31345-1352. [DOI] [PubMed] [Google Scholar]
- 5.Camacho, E. M., A. Serna, C. Madrid, S. Marques, R. Fernandez, F. de la Cruz, A. Juarez, and J. Casadesus. 2005. Regulation of finP transcription by DNA adenine methylation in the virulence plasmid of Salmonella enterica. J. Bacteriol. 1875691-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadesus, J., and D. A. Low. 2006. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 70830-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, K., S. Baker, C. C. Kim, C. S. Detweiler, G. Dougan, and S. Falkow. 2003. Genomic comparison of Salmonella enterica serovars and Salmonella bongori by use of an S. enterica serovar Typhimurium DNA microarray. J. Bacteriol. 185553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, R. K., D. Botstein, T. Watanabe, and Y. Ogata. 1972. Specialized transduction of tetracycline by phage P22 in Salmonella typhimurium. II. Properties of a high frequency transducing lysate. Virology 50883-898. [DOI] [PubMed] [Google Scholar]
- 9.Chessa, D., M. G. Winter, S. P. Nuccio, C. Tukel, and A. J. Bäumler. 2008. RosE represses Std fimbrial expression in Salmonella enterica serotype Typhimurium. Mol. Microbiol. 68573-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correnti, J., M. Munster, T. Chan, and M. van der Woude. 2002. Dam-dependent phase variation of Ag43 in Escherichia coli is altered in a seqA mutant. Mol. Microbiol. 44521-532. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 906640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubnau, D., and R. Losick. 2006. Bistability in bacteria. Mol. Microbiol. 61564-572. [DOI] [PubMed] [Google Scholar]
- 13.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect. Immun. 697950-7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2003. Salmonella DNA adenine methylase mutants prevent colonization of newly hatched chickens by homologous and heterologous serovars. Int. J. Food Microbiol. 80153-159. [DOI] [PubMed] [Google Scholar]
- 15.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290153-161. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa-Bossi, N., S. Lemire, D. Maloriol, R. Balbontin, J. Casadesus, and L. Bossi. 2006. Loss of Hfq activates the sigmaE-dependent envelope stress response in Salmonella enterica. Mol. Microbiol. 62838-852. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Del Portillo, F., M. G. Pucciarelli, and J. Casadesus. 1999. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc. Natl. Acad. Sci. USA 9611578-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garzon, A., D. A. Cano, and J. Casadesus. 1995. Role of Erf recombinase in P22-mediated plasmid transduction. Genetics 140427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacomodonato, M. N., S. H. Sarnacki, R. L. Caccuri, D. O. Sordelli, and M. C. Cerquetti. 2004. Host response to a dam mutant of Salmonella enterica serovar Enteritidis with a temperature-sensitive phenotype. Infect. Immun. 725498-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haagmans, W., and M. van der Woude. 2000. Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol. Microbiol. 35877-887. [DOI] [PubMed] [Google Scholar]
- 21.Heithoff, D., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284967-970. [DOI] [PubMed] [Google Scholar]
- 22.Heithoff, D. M., E. I. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 696725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernday, A., M. Krabbe, B. Braaten, and D. Low. 2002. Self-perpetuating epigenetic pili switches in bacteria. Proc. Natl. Acad. Sci. USA 9916470-16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heusipp, G., S. Fälker, and M. A. Schmidt. 2007. DNA adenine methylation and bacterial pathogenesis. Int. J. Med. Microbiol. 2971-7. [DOI] [PubMed] [Google Scholar]
- 25.Huerta, A. M., and J. Collado-Vides. 2003. Sigma70 promoters in Escherichia coli: specific transcription in dense regions of overlapping promoter-like signals. J. Mol. Biol. 333261-278. [DOI] [PubMed] [Google Scholar]
- 26.Humphries, A., S. Deridder, and A. J. Bäumler. 2005. Salmonella enterica serotype Typhimurium fimbrial proteins serve as antigens during infection of mice. Infect. Immun. 735329-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphries, A. D., M. Raffatellu, S. Winter, E. H. Weening, R. A. Kingsley, R. Droleskey, S. Zhang, J. Figueiredo, S. Khare, J. Nunes, L. G. Adams, R. M. Tsolis, and A. J. Bäumler. 2003. The use of flow cytometry to detect expression of subunits encoded by 11 Salmonella enterica serotype Typhimurium fimbrial operons. Mol. Microbiol. 481357-1376. [DOI] [PubMed] [Google Scholar]
- 28.Humphries, A. D., S. M. Townsend, R. A. Kingsley, T. L. Nicholson, R. M. Tsolis, and A. J. Bäumler. 2001. Role of fimbriae as antigens and intestinal colonization factors of Salmonella serovars. FEMS Microbiol. Lett. 201121-125. [DOI] [PubMed] [Google Scholar]
- 29.Ko, M., and C. Park. 2000. H-NS-dependent regulation of flagellar synthesis is mediated by a LysR family protein. J. Bacteriol. 1824670-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Y., and S. Altman. 2004. Polarity effects in the lactose operon of Escherichia coli. J. Mol. Biol. 33931-39. [DOI] [PubMed] [Google Scholar]
- 31.Løbner-Olesen, A., O. Skovgaard, and M. G. Marinus. 2005. Dam methylation: coordinating cellular processes. Curr. Opin. Microbiol. 8154-160. [DOI] [PubMed] [Google Scholar]
- 32.Low, D. A., and J. Casadesus. 2008. Clocks and switches: bacterial gene regulation by DNA adenine methylation. Curr. Opin. Microbiol. 11106-112. [DOI] [PubMed] [Google Scholar]
- 33.Lu, M., J. L. Campbell, E. Boye, and N. Kleckner. 1994. SeqA: a negative modulator of replication initiation in E. coli. Cell 77413-426. [DOI] [PubMed] [Google Scholar]
- 34.Mashhoon, N., C. Pruss, M. Carroll, P. H. Johnson, and N. O. Reich. 2006. Selective inhibitors of bacterial DNA adenine methyltransferases. J. Biomol. Screen. 11497-510. [DOI] [PubMed] [Google Scholar]
- 35.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413852-856. [DOI] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Nuccio, S. P., and A. J. Bäumler. 2007. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 71551-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porwollik, S., E. F. Boyd, C. Choy, P. Cheng, L. Florea, E. Proctor, and M. McClelland. 2004. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J. Bacteriol. 1865883-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prieto, A. I., M. Jakomin, I. Segura, M. G. Pucciarelli, F. Ramos-Morales, F. Garcia-del Portillo, and J. Casadesus. 2007. The GATC-binding protein SeqA is required for bile resistance and virulence in Salmonella enterica serovar Typhimurium. J. Bacteriol. 1898496-8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prieto, A. I., F. Ramos-Morales, and J. Casadesus. 2004. Bile-induced DNA damage in Salmonella enterica. Genetics 1681787-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pucciarelli, M. G., A. I. Prieto, J. Casadesus, and F. Garcia-del Portillo. 2002. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 1481171-1182. [DOI] [PubMed] [Google Scholar]
- 42.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47597-626. [DOI] [PubMed] [Google Scholar]
- 43.Schmieger, H. 1972. Phage P22 mutants with increased or decreased transducing abilities. Mol. Gen. Genet. 11975-88. [DOI] [PubMed] [Google Scholar]
- 44.Slominska, M., A. Wegrzyn, A. Konopa, K. Skarstad, and G. Wegrzyn. 2001. SeqA, the Escherichia coli origin sequestration protein, is also a transcription factor. Mol. Microbiol. 401371-1379. [DOI] [PubMed] [Google Scholar]
- 45.Torreblanca, J., S. Marques, and J. Casadesus. 1999. Synthesis of FinP RNA by plasmids F and pSLT is regulated by DNA adenine methylation. Genetics 15231-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend, S. M., N. E. Kramer, R. Edwards, S. Baker, N. Hamlin, M. Simmonds, K. Stevens, S. Maloy, J. Parkhill, G. Dougan, and A. J. Bäumler. 2001. Salmonella enterica serovar Typhi possesses a unique repertoire of fimbrial gene sequences. Infect. Immun. 692894-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Woude, M. W., and A. J. Bäumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldron, D. E., P. Owen, and C. J. Dorman. 2002. Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. Mol. Microbiol. 44509-520. [DOI] [PubMed] [Google Scholar]
- 49.Way, J. C., and N. Kleckner. 1984. Essential sites at transposon Tn10 termini. Proc. Natl. Acad. Sci. USA 813452-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber, H., T. Polen, J. Heuveling, V. F. Wendisch, and R. Hengge. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 1871591-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weening, E. H., J. D. Barker, M. C. Laarakker, A. D. Humphries, R. M. Tsolis, and A. J. Bäumler. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 733358-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilmes-Riesenberg, M. R., J. W. Foster, and R. Curtiss, 3rd. 1997. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 65203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wion, D., and J. Casadesus. 2006. N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. Nat. Rev. Microbiol. 4183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]