Abstract
Yersinia enterocolitica has three type three secretion systems, the flagellar, the plasmid Ysc type III secretion system (T3SS), and the chromosomal Ysa T3SS. The Ysc T3SS, through the proteins it secretes (Yops), prevents phagocytosis of Y. enterocolitica and is required for disease processes in the mouse host. Recent data demonstrate a role for the Ysa T3SS during initial colonization of the mouse via secretion of Ysps (Yersinia secreted proteins). This work characterizes the discovery of a newly identified Ysa type III secreted protein, YspM. Expression of yspM is regulated by temperature, NaCl concentration, and other known regulators of the ysa system. In addition, YspM is translocated into host cells via the Ysa T3SS. YspM is homologous to proteins classified as GDSL bacterial lipases, which possess a catalytic triad of amino acids (Ser, Asp, and His) located in three of five blocks of amino acid identity. Sequence analysis of the JB580v strain of Y. enterocolitica shows that, due to a premature stop codon, it no longer encodes the fifth block of amino acid identity containing the predicted catalytic histidine. However, seven other biotype 1B strains sequenced did possess the domain. A functional difference between the forms was revealed when YspM was expressed in Saccharomyces cerevisiae. Yeast growth was uninhibited when YspM from JB580v was expressed but greatly inhibited when YspM from Y295 (YspMY295) was expressed. Site-directed mutagenesis of the histidine of YspMY295 ablated the toxic effects. These results indicate that YspM is secreted by the Ysa T3SS and that, possibly due to lipase activity, it targets eukaryotic cellular component(s).
Yersinia enterocolitica is a gram-negative, enteric pathogen that infects humans through ingestion of contaminated food or water. Infected individuals can develop several disease manifestations including self-limiting enteritis, diarrhea, lymphadenitis, and, in severe cases, septicemia leading to death (6, 22). Orally infected mice develop a similar disease and serve as a useful tool to study Y. enterocolitica pathogenesis (8, 16, 17). The mouse model has been used to demonstrate that upon infection, Y. enterocolitica transverses the stomach and colonizes the small intestine, where the bacteria invade and replicate within Peyer's patches (2, 8, 28). The ability to survive within Peyer's patches is due, in part, to the presence of a plasmid-encoded Ysc-Yop type III secretion system (T3SS) (39). Several of the secreted effector Yops prevent phagocytosis of Y. enterocolitica by macrophages and polymorphonuclear cells due to their immune modulatory functions (10). This is a process of critical importance, demonstrated by the fact that Y. enterocolitica bacteria lacking specific Yops do not colonize the Peyer's patches, mesenteric lymph nodes, or spleens to levels seen with wild-type bacteria (39).
Biotype 1B strains of Y. enterocolitica, which is the most pathogenic of the species, possess a second T3SS, the chromosomally encoded Ysa (Yersinia secretion apparatus) T3SS (12, 15). The Ysa T3SS is responsible for secreting at least 15 Ysps, or Yersinia secreted proteins, when cultures are grown at 26°C in the presence of 290 mM NaCl (23, 47). While the exact role the Ysa T3SS plays in pathogenicity has yet to be determined, there is a decrease in virulence when a commonly used biotype 1B strain, JB580v, lacking a functional Ysa T3SS is used to infect mice. These Ysa T3SS mutant strains have a 10-fold attenuation, based on 50% lethal dose analysis, following oral inoculation and are deficient in their ability to colonize the terminal ileum of the mouse during the first 24 h of oral infection (15, 23). Several Ysps have been shown to have a role in the early colonization events of the gastrointestinal tract, as demonstrated by competitive index analysis following oral inoculation (23). Interestingly, strains of bacteria lacking some Ysps have a greater colonization defect than a ysa apparatus mutant alone. Two Ysps that had a defect in colonization of the mouse, YspP and YspK, have been further characterized to have enzymatic properties. YspK has demonstrated protein kinase activity, and YspP has demonstrated phosphatase activity (23).
Expression of the Ysa T3SS and secretion of its cognate Ysps are regulated by both environmental (high NaCl and lower temperatures) and genetic factors. Genetic factors involved in regulation of the ysa T3SS include the putative phosphorelay systems encoded by ysrRS and rcsC-yojN-rcsB as well as the araC-like regulator YsaE and the chaperone SycB (42, 43). Genes encoding the ysa apparatus, the chaperone sycB, and four genes (yspBCDA) that encode proteins with homology to proteins involved in host cell pore formation are located in a single genetic locus, and expression is controlled by two promoters. The genetic organization of the locus reveals that the ysaE promoter drives transcription of a long transcript that encodes the structural genes that make up the ysa secretion apparatus, sycB, and yspBCDA. Upon expression, YsaE and SycB act in concert to further activate transcription of the sycB-yspBCDA operon (43). YsrRS and RcsC-YojN-RcsB are believed to be responsible for sensing changes in the environment and then activating expression of the ysa operon. In support of this model, it has been shown that not only YsaE and SycB but also YsrRS and RcsB are required for secretion of Ysps by the Ysa T3SS (42, 43). Interestingly, with the exception of yspBCDA, the other Ysps are encoded elsewhere throughout the chromosome, and little is known about the regulation of their expression.
As the ysp genes are not located together on the Y. enterocolitica chromosome, it remains possible that there are additional, unidentified proteins secreted by the Ysa T3SS. Comparative phylogenomic analysis of Y. enterocolitica by Howard et al. identified an open reading frame (ORF), YE3614, with homology to a type III secreted protein of Salmonella (18). Work in our laboratory also identified YE3614 as a potential type III secreted protein (unpublished data). In this work, we examined the ability of YE3614 to be secreted and translocated by the Ysa T3SS. We also investigated the regulation of expression of YE3614 compared to other genes of the ysa regulon. Finally, we examined the effects of expression of YE3614 in eukaryotic cells using the Saccharomyces cerevisiae model system.
MATERIALS AND METHODS
Strains and growth conditions.
The bacterial strains, yeast strains, and plasmids used in this study are presented in Table 1 and further described below. Unless indicated, bacterial cultures were grown overnight in Luria broth (LB) (170 mM NaCl) at 26°C for Y. enterocolitica or 37°C for Escherichia coli. For promotion of protein secretion via the Ysa T3SS, bacteria were grown overnight in LB lacking NaCl at 26°C and subcultured into LB plus 290 mM NaCl. For examination of protein secretion via the Ysc T3SS, bacteria were grown overnight in LB at 26°C and subcultured into LB containing 20 mM Na2C2O4 and 20 mM MgCl2+ and grown at 37°C. Antibiotics were used as needed at the following concentrations: carbenicillin, 50 μg/ml; kanamycin, 100 μg/ml; and nalidixic acid, 20 μg/ml.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Bacteria strains | ||
| JB580v | Y. enterocolitica 8081v ΔyenR(r− m+); wild type | 19 |
| JB580c | JB580v cured of plasmid pYVe8081; lacks Ysc/Yop T3SS | 43 |
| Y295 | Y. enterocolitica, biotype 1B, O:8 | 26 |
| 657-83 | Y. enterocolitica, biotype 1B, O:20 | 4,26 |
| 658-83 | Y. enterocolitica, biotype 1B, O:21 | 4,26 |
| 655-83 | Y. enterocolitica, biotype 1B, O:18 | 4,26 |
| 634-83 | Y. enterocolitica, biotype 1B, O:4,32 | 4,26 |
| 9286-78 | Y. enterocolitica, biotype 1B, O:20 | 3,26 |
| 9287-78 | Y. enterocolitica, biotype 1B, O:20 | 3 |
| 2440-87 | Y. enterocolitica, biotype 1B, O:8 | 4,26 |
| YVM1374 | JB580v ΔyscC | This study |
| YVM1178 | JB580v ΔysaC | This study |
| YVM932 | JB580v ΔysaE | 43 |
| YVM981 | JB580v ΔsycB | 43 |
| YVM1006 | JB580v ΔysrR | 43 |
| YVM969 | JB580v ΔysrS | 43 |
| YVM1236 | JB580v ΔrcsB | This study |
| E. coli S17-1 λpir | Tpr StrrrecA thi pro hsdR hsdM+ RP4::2-Tc::Mu::Km Tn7 λpir lysogen | 25 |
| Yeast strains | ||
| SCW01 | S. cerevisiae MATaleu2Δ0 met15Δ0 ura3Δ0 | Gift of Vogel lab |
| SCW06 | SCW01 containing pDEST52 | This study |
| SCW07 | SCW01 containing pSW13 | This study |
| SCW08 | SCW01 containing pSW16 | This study |
| SCW11 | SCW01 containing pSW46 | This study |
| Plasmids | ||
| pSIF003-R1 | Used for PCR amplification of cyaA | 41 |
| pENTR-SD-TOPO | Kanr cloning vector | Invitrogen |
| pDEST52 | GAL1 promoter, C-terminal V5 epitope tag, 2μm ori URA3 | Invitrogen |
| pKW44 | pWKS130 backbone; for construction of C-terminal cyaA translation fusions | This study |
| pSW11 | yspM8081v cloned into pENTR-SD-TOPO | This study |
| pSW12 | yspMY295 cloned into pENTR-SD-TOPO | This study |
| pSW13 | GAL1 promoter driving expression of YspM8081v-V5 fusion protein | This study |
| pSW16 | GAL1 promoter driving expression of YspMY295-V5 fusion protein | This study |
| pSW29 | yspMY295(H304N) cloned into pENTR-SD-TOPO | This study |
| pSW41 | yopE promoter and yspM8081v codons 1 to 150 cloned into pKW44 | This study |
| pSW42 | 500-bp yspM8081v promoter region cloned into pKW44 | This study |
| pSW43 | 500-bp yspM8081v promoter region and yspM8081v codons 1 to 150 cloned into pKW44 | This study |
| pSW45 | 500-bp yspMY295 promoter region and yspMY295 codons 1 to 150 cloned into pKW44 | This study |
| pSW46 | GAL1 promoter driving expression of YspMY295(H304N)-V5 fusion protein | This study |
| pSW47 | 500-bp flanking regions of ysaC ORF in pSR47S; for construction of ΔyscC strain | This study |
| pKW43 | 500-bp flanking regions of ysaC ORF in pSR47S; for construction of ΔysaC strain | This study |
| pKW53 | 500-bp flanking regions of rcsB ORF in pSR47S; for construction of ΔrcsB strain | This study |
| pWKS130 | Kanr; low-copy-number cloning vector | 44 |
| pSR47S | KanrmobRP4 oriR6K; cloning vector | 24 |
S. cerevisiae was grown in YPD (yeast extract, peptone, and dextrose) medium at 30°C unless otherwise indicated. For protein induction studies, S. cerevisiae was grown at 30°C in synthetic complete medium [500 ml of medium contains 2.5g (NH4)2SO4, 0.85 g of yeast nitrogen base, and 1 g of dropout mix minus uracil for plasmid maintenance (US Biological)] containing either 2% glucose or 2% galactose.
Plasmid construction.
The plasmids used to detect secretion of YE3614 under various conditions were generated as follows. The primers used in this study are listed in Table 2. All PCR fragments generated in this study were sequenced to confirm that no undesired mutations were incorporated into the DNA and that the desired mutations were incorporated. The ORF of the cyaA gene of Bordetella pertussis was amplified using primers KW104 and KW105 from plasmid pSIF003-R1 (41) (a gift from the laboratory of J. Vogel, Washington University). The fragment was digested with XbaI and BamHI and cloned into the same sites of pWKS130, resulting in plasmid pKW44. Primers P1 and P2 were used to amplify an approximately 1,000-bp product containing 500 bp upstream of the start codon and 500 bp of the ORF of YE3614 from JB580v to include the first 150 amino acids of YE3614. This fragment was digested with NotI and XbaI and cloned into the same sites of pKW44, resulting in pSW43. Primers P1 and P3 were used to amplify the 500-bp region upstream of YE3614, including the start codon. This fragment was digested with NotI and XbaI and cloned into pKW44, resulting in pSW42. Plasmid pSW45, for detection of secretion of YspM from clinical isolate Y295, was constructed in a similar fashion using primers P1 and P2. For detection of secretion of YE3614 via the Ysc T3SS, primers P124 and P125 were used to amplify the yopE promoter region from JB580v, and the resulting fragment was digested with NdeI. Next, the first 500 bp of YE3614 were amplified with P126 and P2, and the product was digested with NdeI. Primers P125 and P126 were engineered to include an NdeI site as well as a start codon. The yopE promoter and YE3614 ORF digested fragments were ligated together overnight at 16°C. This mixture was then used in a PCR with primers P124 and P2, and the resulting product was digested with NotI and XbaI and cloned into pKW44 digested with the same enzymes, generating plasmid pSW41.
TABLE 2.
Primers
| Name | Sequence (5′ to 3′) |
|---|---|
| P1 | AAG GAA AAA AGC GGC CGC TAT ACT CAG TAT CAG GTG TTC |
| P2 | GCT CTA GAA CCA AGG GAT ACA ATA GCC AAA TC |
| P3 | GCC GCC CTA GTC TAG ACA TAA TTT CCT CTG GAT T |
| P27 | CCG CTC GAG ATG AGT ATT AAT TTT AAC CAC AAC |
| P67 | CAG TGT TGG TTT ACT GAA TTT TTC GG |
| P68 | CAC CAT GGG AAG TAT TAA TTT TAA CCA CAA C |
| P69 | GGA ACA TAT CCA GAT ATG ATA GGC GTG TTC |
| P70 | CAC CAT GGG AAT TAT TAA TTT AAA CCA CAA C |
| P81 | TCT TTT AAT GAC AAT TCA AAT CCA TCC CAA GAA TTG CAT GCC |
| P82 | GGC ATG CAA TTC TTG GGA TGG ATT TGA ATT GTC ATT AAA AGA |
| P124 | ATA AGA ATG CGG CCG CGA ATT CCC CAA CTT TGA CAC |
| P125 | GGG AAT TCC ATA TGT ATT TAT TCC CTT GGC |
| P126 | GGA ATT CCA TAT GAG TAT TAA TTT TAA CCA CAA CAG |
| P128 | GCT CTA GAC ATG ACT ATT TAT TCC CTT GGC |
| P135 | ACG CGT CGA CAT GCA AAA TTT ACT AAA AAA CTT GGC |
| P136 | CGC GGA TCC CGG AAA AGC CAT ATT ACT TTA ATT CC |
| P137 | CGC GGA TCC AAG CGT GGC GTA TTG TGA |
| P138 | ATA AGA ATG CGG CCG CGC TAA AGA AAC CTC AAT TCC |
| KW104 | GCT CTA GAC AGC AAT CGC ATC AGG CTG GTT AC |
| KW105 | CGG GAT CCT TAA TAG CCG GAA TCC TGG CGT TCC AC |
| KW154 | GGT GAG GTC AGG AAA TCA ACC C |
| rcsB-delA | GCG TCG ACG ATG TGA TGG TGA CTG ATA ACC C |
| rcsB-delB | CGG GAT CCT ACA TTA AGG TTG TTC ATG GTT ATG GG |
| rcsB-delC | CGG GAT CCC AAG AGA AAG AAT AAC TTC TGT TC |
| rcsB-delD | ATA AGA ATG CGG CCG CTC AAT TGA CAG CTT TAG GTT ATC G |
| ysaC-delA | GCG TCG ACC CCT TGA TGC ACC AGA TGA TGC GAC |
| ysaC-delB | CGG GAT CCA GCG ACG GCA TCA AAC AGC TTG CC |
| ysaC-delC | CCG GGA TCC CGT CAG TTC TCT GAA ACT GAA CGC |
| ysaC-delD | ATA AGA ATG CGG CCG CCG CAA TGC TTC CAG TTG TTC GAG C |
The plasmid (pSW47) to construct the in-frame ΔyscC null strain used in this study was generated as follows. Primers P135 and P136 were used to amplify an approximately 500-bp region upstream and including the start codon of yscC from JB580v. Primers P137 and P138 were used to amplify an approximately 500-bp region downstream and including the stop codon of yscC. These two fragments were digested with BamHI and ligated together. This product was then used in a PCR with primers P135 and P138, and the resulting product was digested with SalI and NotI and cloned into the same sites of pSR47S, resulting in plasmid pSW47.
Plasmid pKW43 used to construct the in-frame ΔysaC null strain was generated in a similar manner using primers ysaC-delA and ysaC-delB to amplify the fragment 500 bp upstream of ysaC, and primers ysaC-delC and ysaC-delD were used to amplify the 500-bp region downstream. Plasmid pKW53 used to generate the in-frame ΔrcsB null strain was constructed in a similar fashion, with primers rcsB-delA and rcsB-delB amplifying 500 bp upstream of the rcsB ORF and primers rcsB-delC and rcsB-delD amplifying the 500 bp downstream.
The plasmids used to detect protein production in yeast were generated using the Gateway system from Invitrogen. Briefly, primers P68 and P69 were used to amplify the ∼900-bp coding region of YE3614 from JB580v. This product was cloned into pENTR-SD-TOPO (Invitrogen) per the manufacturer's protocol, generating pSW11. Next, pSW11 and pDEST52 (Invitrogen) were combined using the enzyme Gateway LR Clonase II (Invitrogen), according to the manufacturer's instructions, resulting in pSW13, a C-terminal V5-tagged expression vector for YspM from strain JB580v (YspMJB580v). pSW16 was generated in the same fashion using primers P70 and P67 by first cloning the yspM ORF from clinical isolate Y295 into pENTR-SD-TOPO (pSW12) and recombining pSW12 with pDEST52 to generate the C-terminal V5-tagged fusion protein. Briefly, the H304N site-directed mutant in YspMY295 was generated as follows: primers P70 and P82, which contained the H304N mutation in P82, were used to amplify the first ∼700 bp of yspMY295 from pSW12 that contained the histidine at position 304 replaced with an asparagine. Next, primers P81 and P67, which contained the H304N mutation in P81, were used to generate a ∼200-bp product downstream of and including the H304N mutation. These two products contained ∼35 bp of overlap at their 3′ or 5′ ends, respectively, and were combined and used in a PCR along with primers P70 and P67. The resulting product was cloned into pENTR-SD-TOPO to generate pSW29. The C-terminal V5-tagged yeast expression vector for YspM in the Y295 strain carrying the H304N mutation [YspMY295(H304N)], pSW46, was generated using techniques described above.
In-frame deletion construction.
The yscC in-frame deletion (YVM1374) was constructed as follows. A total of 500 μl of E. coli S17-1 λpir carrying pSW47 was mixed with 500 μl of JB580v, plated onto LB agar, and incubated overnight at 26°C. The resulting lawn of cells was scraped and added to 1 ml of phosphate-buffered saline and plated at various dilutions onto LB agar containing nalidixic acid to select against E. coli and on kanamycin to select for plasmid integration. The resulting colonies were plated onto LB agar containing nalidixic acid and 5% sucrose to select for colonies that had undergone recombination and lost the plasmid. The resulting colonies were checked for kanamycin sensitivity and confirmed for the presence of the yscC deletion by PCR. Absence of Yop secretion was confirmed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis for separation of trichloroacetic acid-precipitated supernatants followed by Coomassie staining (S. E. Witowski and V. L. Miller, unpublished results). The ΔysaC in-frame deletion (YVM1178) and the ΔrcsB (YVM1236) in-frame deletion were constructed in a similar fashion.
Protein secretion preparations, SDS-PAGE, and Western blot analysis.
Secreted proteins were collected as described previously (47). Briefly, for detection of secretion via the Ysa T3SS, saturated cultures grown overnight in LB without NaCl at 26°C (noninducing conditions) were back-diluted to an optical density at 600 nm (OD600) of 0.2 into LB containing 290 mM NaCl (inducing conditions) and grown for 6 h at 26°C. For detection of secretion via the Ysc T3SS, saturated cultures grown overnight in LB medium at 26°C were back-diluted to an OD600 of 0.2 into LB containing 20 mM Na2C2O4 and 20 mM MgCl2+ (inducing conditions) and grown for 6 h at 37°C. Bacterial cells were removed from 5 ml of culture by centrifugation for 1 min at 13,000 rpm; this process was repeated. The supernatant was then passed through a 0.22-μm-pore-size syringe filter. Cold trichloroacetic acid was added to a final concentration of 10% (vol/vol) and incubated at 4°C overnight. Samples were centrifuged for 10 min at 13,000 rpm at 4°C. Supernatants were discarded, and the pellet was washed with cold acetone and resuspended in 1 M Tris, pH 9.0. Samples were boiled for 5 min in 1× sample buffer; OD600 equivalents were loaded onto SDS-polyacrylamide gels (31). Proteins were transferred to a nitrocellulose membrane for Western analysis. Blots were blocked for 1 h at room temperature in 1× Tris-buffered saline and 0.1% Tween-20 (TBST) containing 5% milk. Primary antibody against CyaA (purchased from List Biologicals and a gift from the laboratory of Erik Hewlett) was added at a dilution of 1:5,000 and incubated at room temperature for 2 h. Membranes were washed several times with 1× TBST and goat anti-mouse immunoglobulin G-horseradish peroxidase (Sigma) secondary antibody diluted 1:15,000 in TBST containing 5% milk and incubated for 30 min at room temperature. Membranes were washed again in TBST, and proteins were detected by chemiluminescence (ECL; Amersham).
Translocation assays.
The following protocol was adapted from Sory et al. (37). Chinese hamster ovary (CHO) cells were passaged in F12 medium containing 10% fetal bovine serum, and cells were grown at 37°C in the presence of 5% CO2 unless otherwise indicated. For translocation assays, CHO cells were seeded into 24-well tissue culture plates at a density of 4 × 105 to 5 × 105 cells/well 24 h prior to the start of the translocation assay. Thirty minutes before infection with Y. enterocolitica, cells were washed and covered with F12 medium plus 5% fetal bovine serum containing 1 μg/ml cytochalasin D. For analysis of protein translocation via the Ysa T3SS, Y. enterocolitica was grown at 26°C overnight in LB lacking NaCl and subcultured into LB containing 290 mM NaCl at an OD600 of 0.2 and grown for 2 h at 26°C. Bacteria were added in triplicate to CHO cells at a multiplicity of infection of 100:1 and incubated at 26°C in 5% CO2 for 2 h. For analysis of protein translocation via the Ysc T3SS, Y. enterocolitica was grown overnight in LB at 26°C, subcultured into LB to an OD600 of 0.2, and grown for 2 h at 37°C. Bacterial strains were added in triplicate to CHO cells at a multiplicity of infection of 100:1 and incubated at 37°C in 5% CO2 for 2 h.
After infection, CHO cells were washed three times with phosphate-buffered saline and lysed under denaturing conditions (50 mM HCl and boiling for 5 min). The resulting lysate was neutralized with NaOH, and cyclic AMP (cAMP) was extracted with ethanol. Lysate was then centrifuged at 13,000 rpm for 5 min, and the supernatant was removed and dried in a Speed Vac; intracellular cAMP levels were assayed by a commercially available enzyme immunoassay system (Biotrak, Amersham).
RNA extraction and Northern blot analysis.
Total cellular RNA was extracted from cell pellets that had been stored at −80°C using Trizol-LS reagent (Invitrogen). Trizol was added to the frozen cell pellet to a density of 10 OD units/ml, and RNA was extracted per the manufacturer's instructions. Total RNA was treated with DNase using DNA free, following the manufacturer's protocol (Ambion). For Northern blotting, 10 μg of RNA was separated in a 1% formaldehyde agarose gel and transferred to nitrocellulose membranes by capillary action (31). For dot blots, 5 μg of RNA was mixed with 50 μl of denaturing buffer (50% formamide, 7% formaldehyde, 1× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) and transferred to nitrocellulose membranes by vacuum suction (31). The yspM gene-specific probe was generated using primers P27 and KW154 and labeled with [α-32P]dATP by random-primed labeling per the manufacturer's protocol (Roche). Next, the radiolabeled PCR probe was hybridized to blots in 50% formamide buffer. To control for equivalent loading, the 16S RNA was probed. An oligonucleotide probe (KW157) was labeled with [γ-32P]ATP by T4 polynucleotide kinase. Hybridization and washes were conducted in a sodium pyrophosphate buffer (hybridization buffer containing 0.1% sodium pyrophosphate, 4× SSC, 5× Denhardt's reagent, 0.1% SDS, and 100 μg/ml salmon sperm DNA; wash buffer containing 0.1% sodium pyrophosphate, 4× SSC, and 0.1% SDS) (S. Strand, personal communication). The yspM gene-specific signal was normalized by dividing it by the 16S signal. Triplicate values were averaged and normalized to the peak of expression (for time course analysis) or to the wild-type levels (for analysis of regulators). Specific mRNA signals were quantified using an FLA-5000 phosphorimager and ImageGauge, version 4.22, software (Fuji Film Medical Systems, Stamford, CT).
Analysis of YspM toxicity in S. cerevisiae.
Yeast cells containing pDEST52 (strain SCW06), pSW13 (SCW07), pSW16 (SCW08), or pSW46 (SCW11) were grown overnight at 30°C in synthetic complete medium lacking uracil with 2% glucose. Yeast was subcultured to an OD600 of 0.2 into synthetic complete medium lacking uracil with 2% galactose and grown at 30°C. Samples were taken every hour, and OD600 measurements were taken over the course of growth.
To examine expression of tagged protein, Western blot analysis to detect the V5 epitope was performed. The following protocol was adapted from Sato et al. (33). Briefly, at the end of the induction growth curve, OD equivalents of yeast were collected by centrifugation, washed one time with water, immediately frozen by a dry ice-ethanol bath, and stored at −80°C. Cell pellets were thawed and resuspended in 100 μl of breaking buffer (40 mM Tris, pH 7.0, 8 M urea, 5% SDS, 0.1 M EDTA, 10% glycerol, 0.4 mg/ml bromophenol blue, 140 mM β-mercaptoethanol, and protease inhibitor cocktail [Roche Diagnostics]). The mixture was boiled for 5 min and vortexed with an equal volume of glass beads for 1 min before SDS-PAGE separation of proteins and Western blot analysis using primary mouse anti-V5 antibody (Sigma) diluted 1:2,000 in TBST and goat anti-mouse immunoglobulin G-horseradish peroxidase (Sigma) secondary antibody diluted 1:12,000 in TBST.
RESULTS
Identification of a potential Y. enterocolitica type III secreted protein.
Matsumoto and Young identified 15 proteins (Ysps) that are secreted via the Ysa T3SS. Interestingly, most of these proteins are encoded in locations distinct from the ysa locus in the Y. enterocolitica JB580v genome (23). A genetic screen, unrelated to type III secretion, conducted in our laboratory brought to our attention a potential 16th Ysa type III secreted protein, YE3614, encoded on the Y. enterocolitica JB580v chromosome (P. Revell and V. L. Miller, unpublished results). The results of this initial screen were complex, and thus YE3614 was not followed up on in that context. However, the similarity of YE3614 to a type III secreted protein became of interest to us and was studied further. The hypothesis that YE3614 could be a type III secreted protein was based on protein sequence analysis. One of the closest homologs of YE3614 is the SPI2 (Salmonella pathogenicity island 2) secreted effector, SseJ, from Salmonella enterica. Additionally, like the Ysa T3SS, YE3614 is present only in biotype 1B strains of Y. enterocolitica (18). Furthermore, a YE3614 homolog is not found in Yersinia pestis and Yersinia pseudotuberculosis, which also lack a Ysa T3SS homolog. Based on this knowledge, we hypothesized that YE3614 would most likely be a Ysa type III secreted protein rather than a Ysc type III secreted protein. Examination of the chromosomal region surrounding YE3614 reveals insight into the evolution of this region of the chromosome (Fig. 1A). Thomson et al. have identified a region that is hypervariable among Y. enterocolitica biotypes, spanning from YE3450 to YE3644, which they have termed the plasticity zone (38). The authors speculate that the plasticity zone did not arise from a single acquisition but, rather, from multiple independent insertion events. Additionally, surrounding YE3614 is a region containing remnants of an ancestral enteric flagellum cluster termed flgII, (YE3610, YE3610A, YE3611, and YE3614A) (38); many of these ORFs appear to be pseudogenes. Interestingly, YE3614 does not have homology to a flagellar protein. However, downstream of YE3614 is a region termed IS1330. This region is classified as a highly degenerative copy of the Y. enterocolitica IS10-like transposase. Therefore, it is possible that YE3614 was inserted into the genome in an event distinct from the acquisition of the flgII. Two pieces of evidence lead us to believe that YE3614 and YE3614A are not in an operon. First, they are separated by 228 bp. Second, the GC content of the genes within the flgII remnant, YE3610, YE3610A, YE3611, and YE3614A, is 55%, 41%, 41%, and 38.7%, respectively, while the GC content for YE3614 is only 31%. This information, combined with the data collected by Thomson et al., supports the hypothesis that YE3614 was acquired more recently via a horizontal transfer event (38).
FIG. 1.
Genomic location of YE3614 and sequence alignment of the GDSL lipase, SseJ, from S. enterica, and YE3614 from Y. enterocolitica JB580v. (A) Chromosomal location of YE3614 and its surrounding ORF. YE3610, homology to a lipoprotein; YE3610A, homology to a flagellar protein, pseudogene; YE3611, homology to a regulator, pseudogene; YE3614, homology to a type III secreted protein; YE3614A, homology to a flagella regulator, pseudogene; IS1330, disrupted ORF with homology to the IS10-like transposase from Y. enterocolitica. Gray arrows indicate ORFs with homology to ancestral flagellum cluster. (B) An amino acid alignment of YE3614 with SseJ from S. enterica. The five blocks of amino acid identity found in GDSL lipases are underlined. Arrowheads indicate catalytic residues (Ser, Asp, and His), black boxes indicate identical amino acids, and gray boxes indicate similar amino acids.
Both SseJ and YE3614 are classified as bacterial GDSL lipases based on protein sequence. Members of this family possess five blocks of amino acid identity, and lipase activity is dependent on the presence of three blocks that contain residues comprising a catalytic triad (Ser, Asp, and His) of amino acids (27, 40). In vitro studies reveal that SseJ plays a role in Salmonella-containing vacuole maintenance within infected host cells (5, 13, 30). In vivo analyses have shown that SseJ is required for full virulence in a competitive index model of infection in the mouse model (27). Additionally, mutations in the predicted catalytic residues of the protein SseJ ablate demonstrated in vitro lipase activity, as well as render the mutant strains unable to compete to wild-type levels in the competitive index mouse model of infection (27). Analysis of the protein sequence of YE3614 revealed that two of the three (Ser, His, and Asp) catalytic residues are present; however, the histidine-containing block of amino acid identity is absent from the protein sequence (Fig. 1B). A histidine present at the end of the protein potentially could function as the required catalytic residue; however, this histidine is not encoded in the block of conserved amino acids where this residue is typically found.
YE3614 is secreted by the Ysa T3SS.
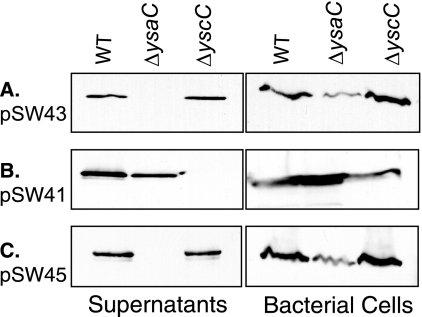
The homology of YE3614 to a SPI2-secreted effector suggested that YE3614 might be secreted by Y. enterocolitica. While we hypothesized that YE3614 was a Ysa type III secreted protein, it was necessary to determine if YE3614 was indeed secreted and, if so, if it was dependent on the Ysa or the Ysc T3SS. To test this, a CyaA reporter fusion was constructed containing the predicted promoter and the first 150 amino acids of YE3614 from Y. enterocolitica strain JB580v fused to the B. pertussis adenylate cyclase gene, cyaA (pSW43). To examine secretion by the Ysa T3SS, pSW43 was transformed into JB580v (wild type), and bacteria were grown under Ysa-inducing conditions at 26°C in the presence of 290 mM NaCl. Bacterial cells and culture supernatants were harvested after 6 h and analyzed by Western blotting using an antibody against CyaA. Under these conditions, a CyaA signal was present in culture supernatants (Fig. 2A). Thus, YE3614 was renamed YspM in keeping with the nomenclature established for the Ysa T3SS. To determine which T3SS was responsible for this event, pSW43 was transformed into JB580v lacking a functional Ysc T3SS (ΔyscC; strain YVM1374) and a strain lacking a functional Ysa T3SS (ΔysaC; YVM1178). YscC encodes the secretin for the Ysc T3SS, and YsaC is predicted to be the secretin for the Ysa T3SS based on homology to other T3SS secretins (12, 20). Cultures were again grown under Ysa-inducing conditions, and bacterial cells and supernatants were harvested. Protein secretion was seen in bacteria lacking a Ysc T3SS but not in a strain lacking a Ysa T3SS (Fig. 2A).
FIG. 2.
YE3614 is secreted into culture supernatants. (A) pSW43 (JB580v yspM ORF and promoter) was transformed into the wild-type (WT; JB580v), ΔysaC (YVM1178), and ΔyscC (YVM1374) strains. Bacteria were grown under Ysa T3SS-inducing conditions. Samples were harvested after 6 h, and samples were prepared for Western analysis with anti-CyaA antibody as described in Materials and Methods. (B) pSW41 (JB580v yspM ORF and yopE promoter) was transformed into the strains listed above. Bacteria were then grown under Ysc T3SS-inducing conditions (37°C and absence of Ca2+), and samples were prepared as described in panel A. (C) pSW45 (Y295 yspM ORF and promoter) was transformed into the strains listed above and grown under Ysa T3SS-inducing conditions, and supernatants and cells were analyzed as described in panel A.
To determine if YspM was also secreted by the Ysc T3SS, wild-type, ΔyscC, and ΔysaC strains of bacteria carrying pSW43 were grown under Ysc-inducing conditions, at 37°C in the absence of Ca2+, and bacterial cells and culture supernatants were examined for the presence of CyaA signal by Western blotting. Under these conditions, no CyaA signal was present in either cells or supernatants in any strain examined (data not shown). Together, these data support the model that YspM is a type III secreted protein and is natively secreted by the Ysa T3SS but not the Ysc T3SS. However, the lack of secretion of YspM by the Ysc T3SS may be due to lack of expression under Ysc-inducing conditions.
To test the possibility that the lack of YspM secretion by the Ysc T3SS is due to a lack of protein production and not the specificity of the apparatus itself, expression of the YspM-CyaA fusion protein was placed under the control of the yopE promoter (pSW41), a promoter that is active under Ysc inducing conditions. This promoter was chosen as it is a promoter native to Y. enterocolitica that is strongly expressed under Ysc T3SS-inducing conditions. When pSW41 was transformed into JB580v and bacteria were grown under Ysc-inducing conditions, CyaA signal was detected in the culture supernatants (Fig. 2B). To determine if this secretion was via the Ysa or the Ysc T3SS, pSW41 was transformed into the ΔysaC and the ΔyscC strains. CyaA signal was detected in culture supernatants of the ΔysaC strain but not in the ΔyscC strain (Fig. 3B). This demonstrates that the YspM protein is capable of being secreted by Ysc T3SS as well as the Ysa T3SS, and the presence or absence of protein secretion is dependent on protein production rather than specificity of the apparatus.
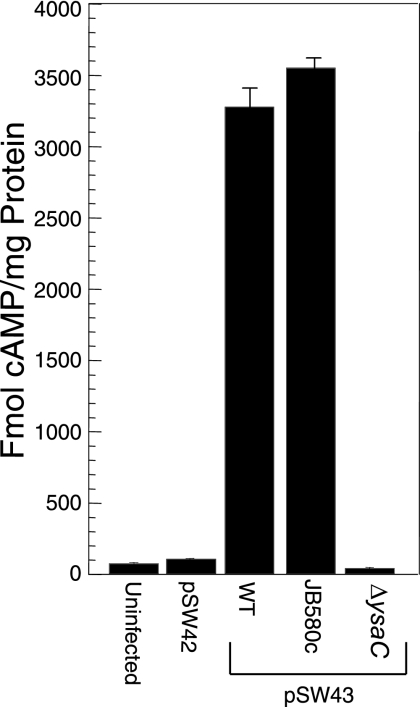
FIG. 3.
YspM is translocated into CHO cells by the Ysa T3SS. pSW43 (JB580v yspM ORF and promoter) was transformed into the wild-type (WT; JB580v), pYVe8081v-cured (JB580c), and ΔyscC (YVM1374) strains. Strains were used to infect CHO cells in triplicate at 26°C for 2 h. Cytosolic levels of cAMP were measured as described in Materials and Methods and compared to uninfected CHO cells and CHO cells infected with JB580v carrying the negative control plasmid, pSW42 (JB580v yspM promoter). Results of a representative assay are shown.
YspM is translocated into host cells via the Ysa T3SS.
To determine if YspM could be translocated into mammalian cells, the CyaA reporter fusion used to detect secretion, plasmid pSW43, was also used to examine translocation. Upon translocation of CyaA into the host cell cytosol, calmodulin present there can bind CyaA and activate its ability to catalyze the conversion of ATP to cAMP, which can subsequently be measured by enzyme-linked immunosorbent assay (37). Thus, an increase in cAMP levels corresponds with a protein that is translocated by the bacterium into the host cell. CHO cells were infected for 2 h at 26°C with the wild type, a strain lacking the pYVe8081 virulence plasmid that contains the entire ysc locus and thus is deficient for secretion via the Ysc T3SS (JB580c), and ΔysaC, all containing pSW43. Under these conditions there was a significant increase in intracellular cAMP levels in both wild-type and JB580c strains but not in the ΔysaC mutant (Fig. 3). No increase in cAMP levels was detected in the negative control strain carrying a plasmid with the predicted yspM promoter alone driving cyaA expression, pSW42. This indicates that YspM is translocated into CHO cells under these conditions, and this event requires a functional Ysa T3SS. When these assays were repeated under Ysc T3SS-inducing conditions, no rise in cAMP levels was detected (data not shown).
Expression of yspM is regulated similarly to the ysa operon.
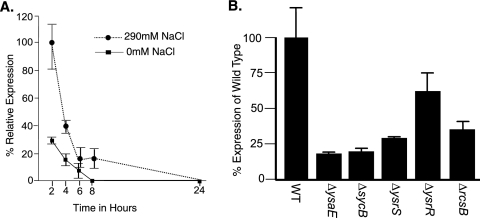
As yspM is encoded outside of the ysa locus, we sought to determine if the factors that control transcription of the ysa locus also affect expression of yspM. Northern blot analysis demonstrated that yspM was maximally expressed 2 h into the growth curve when bacteria were grown at 26°C in the presence of 290 mM NaCl. There was a 3.3-fold induction of yspM expression in the presence of NaCl as opposed to the absence of NaCl (Fig. 4A). Next, we looked for the presence of yspM transcript in mutants of known ysa regulators: ΔysaE (strain YVM932), ΔsycB (YVM981), ΔysrS (YVM969), ΔysrR (YVM1006), and ΔrcsB (YVM1236). For these experiments wild-type and mutant strains of bacteria were grown at 26°C in the presence of 290 mM NaCl, and RNA was extracted at 2 h as this is when maximal yspM transcript is present in wild-type cells. Expression of yspM was 16% of the wild-type level in ΔysaE, 18% in ΔsycB, 27% in ΔysrS, 60% in ΔysrR, and 33% in ΔrcsB (Fig. 4B). Together, these data indicate that yspM expression is controlled by the same factors as the ysa apparatus; however, the mechanism of this regulation remains to be determined.
FIG. 4.
yspM is regulated in a manner similar to the ysa operon. (A) Triplicate saturated cultures of wild-type bacteria were grown in LB and subcultured into LB containing either 290 mM or 0 mM NaCl and grown at 26°C for the indicated times. RNA was extracted and used for Northern blot analysis. Data are represented as a relative percentage of the maximal expression. (B) Triplicate cultures of wild-type (WT), ΔysaE, ΔsycB, ΔysrS, ΔysrR, and ΔrcsB strains were grown as described in panel A, and RNA was extracted 2 h into the growth curve. Expression in the wild type is set to 100%, and expression of yspM in the various mutants is represented as a relative percentage of the expression seen in the wild type.
YspM from Y. enterocolitica JB580v lacks a catalytic domain required for yeast growth inhibition.
As many type III secreted proteins have been shown to exert an effect on eukaryotic cells, we sought to determine if YspM also had any effect. The use of an S. cerevisiae system as a tool to assess protein functionality has been described for other type III secreted effectors (21, 33, 35, 36). To determine if YspM was toxic for eukaryotic cell growth, a YspM fusion protein under the control of the GAL1 promoter was constructed with a V5 tag at the C terminus (plasmid pSW13). This plasmid was then used to examine the effects of YspM on the yeast S. cerevisiae. The GAL1 promoter is repressed when yeast cultures are grown in glucose and activated when grown in galactose, thus allowing for control of expression of potentially toxic proteins. When grown under noninducing conditions (2% glucose), yeast containing pSW13 (strain SCW07) had growth rates similar to the vector-only control, which does not encode a tagged protein (SCW06) (data not shown). When yeast was grown under inducing conditions (2% galactose), growth rates of strains SCW06 and SCW07 were similar (data not shown). Protein production was confirmed for SCW07 using Western blot analysis against the V5 tag (data not shown). Knowing that YspM from Y. enterocolitica JB580v lacked the conserved histidine domain common to GDSL lipases, these data raised the possibility that absence of toxicity toward eukaryotic cells may be due to the lack of the conserved His catalytic residue.
After this result was observed, the sequence of yspM from Y. enterocolitica JB580v was analyzed further, and it was discovered that the catalytic His residue-containing domain is present in a different reading frame but not translated due to a frameshift mutation (Fig. 5). As it was discovered that yspM is present in other biotype 1B strains, we sequenced the corresponding gene from eight additional biotype 1B clinical isolates to determine the prevalence of this frameshift mutation. Seven of these strains had sequences of yspM that encoded proteins containing all three catalytic residue-containing domains found in GDSL lipases; however, one strain, 634-83, was truncated within the first half of the protein (Fig. 5A). Strain Y295 was chosen for further analysis as this is also an O:8 serotype strain, as is JB580v. Comparison of yspM from JB580v (yspMJB580v) and yspM from Y295 (yspMY295) revealed the location of the frameshift mutation responsible for the truncation of yspMJB580v (Fig. 5B). To determine if YspMY295 was also secreted by the Ysa T3SS, a CyaA reporter fusion was constructed containing 500 bp of the promoter region and the first 150 amino acids of YspMY295 (pSW45). This construct was transformed into a wild-type, ΔyscC, and ΔysaC strains. CyaA signal was detectable by Western blot analysis in the wild type and ΔyscC only when cultures were grown under Ysa-inducing conditions, demonstrating that the yspM promoter from strain Y295 is active in our laboratory strain (JB580v) and that this protein can be secreted by the Ysa T3SS (Fig. 2C).
FIG. 5.
Sequence alignment of YspM from nine biotype 1B isolates of Y. enterocolitica. (A) Amino acid alignment of YspM from these strains demonstrates the presence of the fifth conserved domain found in GDSL lipases (underlined) in all but two isolates, JB580v and 634-83, and the location of the predicted catalytic histidine (arrowhead). The alignment also reveals the presence of various amino acid changes among the different YspM isolates examined. Unique amino acids are in black boxes, and similar amino acids are in gray boxes. (B) The DNA sequences of YspMJB580v and YspMY295 reveal the insertion of an adenosine (bold) which shifts the reading frame to encode a stop codon (italicized). The reading frame of each sequence is noted.
YspMY295 is toxic for yeast growth, and this toxicity is dependent on the predicted catalytic His.
Based on the discovery of YspM containing the predicted catalytic His residue, YspMY295, we sought to determine if the presence of this domain conferred toxicity for yeast growth. As with YspMJB580v, a YspMY295-V5 fusion protein was constructed under the control of the GAL1 promoter (pSW16). When yeast carrying pSW16 (SCW08) was grown in 2% glucose (repressing conditions), there was no effect on growth compared to control strain SCW06 or to SCW07 (Fig. 6A). However, when SCW08 was grown in 2% galactose (inducing conditions), yeast growth was completely inhibited compared to strains carrying either vector alone (SCW06) or YspMJB580v-V5 (SCW07) (Fig. 6B). These data suggest that YspM possesses toxic activity toward eukaryotic cells and that this activity requires the presence of the histidine-containing domain found in GDSL lipases. To determine if, indeed, the predicted catalytic His was responsible for the toxicity seen in S. cerevisiae, a site-directed mutant was constructed changing the histidine at position 304 to an aspargine, YspMY295(H304N). A V5 fusion protein was constructed with this mutant, YspMY295(H304N)-V5 (pSW46) and transformed into S. cerevisiae (SCW11). Analysis of yeast growth under inducing conditions revealed that mutation of this His ablates the toxic effects of YspMY295 expression as growth rates were similar to both vector control (SCW06) and YspMJB580v-V5 (SCW07) (Fig. 6B). Protein production was confirmed for SCW07 and SCW11 using Western blot analysis against the V5 tag, but there was no signal detected for SCW08 (Fig. 6C). One possible explanation for the lack of signal for strain SCW08 is that due to the toxic nature of expression of YspMY295, yeast cells are never able to enter a growth phase where detectable amounts of YspMY295 are produced. Another possibility is that surviving yeast cells have developed mutations within plasmid pSW46 such that YspMY295 is no longer produced. Together, these data demonstrate that YspM toxicity is likely due to possession of the catalytic triad of amino acids common to GDSL lipases.
FIG. 6.
YspMY295 is toxic for yeast growth. Yeast carrying plasmids pDEST-52 (strain SCW06), pSW13 (YspMJB580v-V5, strain SCW07), pSW16 (YspMY295-V5, strain SCW08), and pSW46 (YspMY295H304N-V5, strain SCW11) were analyzed for growth under induction conditions (A) and noninduction conditions (B). Samples were taken over the course of the growth curve and OD600 readings were measured. Representative assays are shown. (C) Western blot analysis on cellular lysates of SCW07, SCW08, and SCW11 using anti-V5 antibody. Samples were prepared as described in Materials and Methods.
DISCUSSION
The Ysa T3SS was previously shown to secrete 15 different proteins into culture supernatants (23). Here, we identify a new protein from Y. enterocolitica that is natively secreted by the Ysa T3SS, named YspM. The yspM ORF is expressed under the same conditions as the ysa locus and requires for full expression the known ysa regulators, YsaE, SycB, YsrRS, and RcsB. In addition to being secreted by the Ysa T3SS, YspMJB580v is translocated into host cells by the Ysa T3SS when cultures are grown under Ysa-inducing conditions. When YspM is expressed from a promoter active under Ysc secretion conditions, YspM can be secreted by the bacterium via the Ysc T3SS. Other studies in Y. enterocolitica have shown that YopE, YopN, and YopP are secreted by the Ysa and the Ysc T3SS, and YplA, natively a flagellar type III secreted protein, can be secreted by both the Ysc and Ysa T3SS (45-47). As it is still unclear what signals within the host activate the various T3SSs, these data suggest that during infection (at least for a subset of type III secreted proteins), if YspM is expressed, then it potentially could be secreted by any T3SS. This theory is further supported by work showing that deletion of some individual ysp genes had a greater impact on virulence than deletion of the entire Ysa apparatus (23). One possible explanation for this apparently anomalous observation is that when the Ysa T3SS is not present, these Ysps are being secreted by alternative means during infection of the host, such as the Ysc T3SS.
Based on sequence homology, YspM is characterized as a bacterial lipase. These proteins have the ability to cleave fatty acid chains from the glycerol backbone and may also have cholesterol acyltransferase activity (40). Much work has been done to characterize a close homolog, SseJ, an SPI2 secreted effector from S. enterica, and to demonstrate its role in Salmonella-containing vacuole maintenance within infected cells and to show that the catalytic domains of the protein are required for this activity. The SPI2 T3SS is crucial to the ability of Salmonella to replicate within host cells and to achieve full virulence within the mouse (reviewed in references 11, 14, and 9). The similarity between SseJ and YspM would lead one to think that YspM might also play a role in the survivability of Y. enterocolitica within infected host cells. While Y. enterocolitica is typically thought of as an extracellular pathogen, there is some evidence to support the idea that the bacterium can replicate intracellularly. Two studies have shown an outgrowth of Y. enterocolitica within infected host cells. In unactivated J774.1 macrophages, there was a 30-fold increase in the number of bacteria obtained 25 h postinfection (29). Also, another study reported a 110-fold increase in bacteria recovered from infected J774 macrophages at 24 h postinfection (7). While the role of intracellular survival remains poorly understood in the context of Y. enterocolitica pathogenesis, it is possible that YspM, similar to SseJ, may play a role in that process.
While there is little sequence similarity, another possibility is that YspM acts like the phospholipase ExoU, a type III secreted protein from Pseudomonas aeruginosa. ExoU is an important virulence factor in the mouse model of infection and a potent cytotoxin causing multiple effects on a variety of cell types, including yeast, and these toxic effects are due, in part, to the phospholipase activity of ExoU (1, 33; also reviewed in reference 34). Like Y. enterocolitica, P. aeruginosa is an extracellular pathogen that exerts its effects on eukaryotic cells, in part, through a T3SS. It has been shown that ExoU requires the eukaryotic Cu2+/Zn2+ superoxide dismutase (SOD1) as a cofactor (32). Efforts to date in our laboratory have failed to demonstrate lipase activity for purified recombinant YspM (S. E. Witowski and V. L. Miller, unpublished results). Perhaps this is due to the requirement of a specific eukaryotic cofactor, similar to ExoU. Continued analysis of the enzymatic properties of YspM may help to determine if this protein functions to aid in either intracellular survival, like SseJ, or extracellular survival, like ExoU.
Analysis of yspM in strain JB580v of Y. enterocolitica reveals the presence of a naturally occurring mutation. This mutation truncates the protein such that the catalytic His-containing domain is no longer encoded by yspM. It was determined that this mutation was not acquired by passage of JB580v through our laboratory as a strain of JB580v from the laboratory of B. Wren also contained the same point mutation (Witowski and Miller, unpublished). When yspM from eight other clinical isolates was sequenced, seven of these strains did not have the mutation, and the yspM sequence did encode the His-containing domain. The eighth strain sequenced (634-83) contained a point mutation that truncated the protein within the first half of the protein. The strains sequenced were all biotype 1B strains and comprised several different serotypes. When a representative isolate, YspMY295, was assayed in yeast cells, its expression was toxic for yeast growth. Mutational analysis revealed the predicted catalytic His to be required for this toxicity, suggesting that YspM has potent biological activity and that this activity is dependent on the His-containing domain found in all GDSL bacterial lipases. Further investigation of the mechanism of YspM lethality in yeast may help elucidate a role for this protein in Y. enterocolitica pathogenesis.
Howard et al. in their comparative phylogenomic study of the evolution of Y. enterocolitica also found that yspM was present only in the eight highly pathogenic strains they examined (18). The information gathered in their work provides excellent insight into the evolution of the pathogenicity of Y. enterocolitica. Their discovery that only 20.8% of the total genome of all strains examined (including strains of high and low degrees of pathogenicity and nonpathogenic strains) was conserved as core genes indicates that the majority of the genome is distinct between the various strains (18). These data, combined with the location of a transposase-like element upstream of yspM, indicates that the gene was possibly acquired via horizontal transfer. The discovery of the inactive form of yspM in a strain of Y. enterocolitica (JB580v) that is still highly pathogenic to both people and mice is intriguing. These observations raise questions about when these mutations arose as well as about the interplay of YspM with other Ysa type III secreted effectors and their role in pathogenesis.
Acknowledgments
We sincerely thank Joe Vogel (Washington University, St. Louis, MO) for the gift of the yeast strain SCW01 used in this study as well as the plasmid, pSIF003-R1, used for amplification of the cyaA ORF. We also thank Erik L Hewlett (University of Virginia, Charlottesville, VA) for the generous gift of the anti-CyaA antibody used in these studies and Brendan Wren (Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine) for the other Y. enterocolitica JB580v strain. We also thank the members of the Miller laboratory for providing support and discussion regarding the completion of the manuscript.
This research was supported by the National Institutes of Health grant AI42736 awarded to V. L. Miller.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Allewelt, M., F. T. Coleman, M. Grout, G. P. Priebe, and G. B. Pier. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 683998-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44285-294. [DOI] [PubMed] [Google Scholar]
- 3.Baker, P. M., and J. J. Farmer, 3rd. 1982. New bacteriophage typing system for Yersinia enterocolitica, Yersinia kristensenii, Yersinia frederiksenii, and Yersinia intermedia: correlation with serotyping, biotyping, and antibiotic susceptibility. J. Clin. Microbiol. 15491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer, K. B., and V. L. Miller. 1992. Amino acid substitutions in naturally occurring variants of ail result in altered invasion activity. J. Bacteriol. 1741360-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birmingham, C. L., X. Jiang, M. B. Ohlson, S. I. Miller, and J. H. Brumell. 2005. Salmonella-induced filament formation is a dynamic phenotype induced by rapidly replicating Salmonella enterica serovar Typhimurium in epithelial cells. Infect. Immun. 731204-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouza, E., A. Dominguez, M. Meseguer, L. Buzon, D. Boixeda, M. J. Revillo, L. de Rafael, and J. Martinez-Beltran. 1980. Yersinia enterocolitica septicemia. Am. J. Clin. Pathol. 74404-409. [DOI] [PubMed] [Google Scholar]
- 7.Brzostek, K., A. Raczkowska, and A. Zasada. 2003. The osmotic regulator OmpR is involved in the response of Yersinia enterocolitica O:9 to environmental stresses and survival within macrophages. FEMS Microbiol. Lett. 228265-271. [DOI] [PubMed] [Google Scholar]
- 8.Carter, P. B. 1975. Pathogenecity of Yersinia enterocolitica for mice. Infect. Immun. 11164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30175-188. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 2002. The Yersinia Ysc-Yop “type III” weaponry. Nat. Rev. Mol. Cell Biol. 3742-752. [DOI] [PubMed] [Google Scholar]
- 11.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foultier, B., P. Troisfontaines, S. Muller, F. R. Opperdoes, and G. R. Cornelis. 2002. Characterization of the ysa pathogenicity locus in the chromosome of Yersinia enterocolitica and phylogeny analysis of type III secretion systems. J. Mol. Evol. 5537-51. [DOI] [PubMed] [Google Scholar]
- 13.Freeman, J. A., M. E. Ohl, and S. I. Miller. 2003. The Salmonella enterica serovar Typhimurium translocated effectors SseJ and SifB are targeted to the Salmonella-containing vacuole. Infect. Immun. 71418-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groisman, E. A., and H. Ochman. 1997. How Salmonella became a pathogen. Trends Microbiol. 5343-349. [DOI] [PubMed] [Google Scholar]
- 15.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 361436-1446. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, G. E., R. W. Schaedler, and T. T. MacDonald. 1986. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect. Immun. 5326-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handley, S. A., P. H. Dube, P. A. Revell, and V. L. Miller. 2004. Characterization of oral Y. enterocolitica infection in three different strains of inbred mice. Infect. Immun. 721645-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard, S. L., M. W. Gaunt, J. Hinds, A. A. Witney, R. Stabler, and B. W. Wren. 2006. Application of comparative phylogenomics to study the evolution of Yersinia enterocolitica and to identify genetic differences relating to pathogenicity. J. Bacteriol. 1883645-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O8 and construction of a transformable R-M+ mutant. Gene 136271-275. [DOI] [PubMed] [Google Scholar]
- 20.Koster, M., W. Bitter, H. de Cock, A. Allaoui, G. R. Cornelis, and J. Tommassen. 1997. The outer membrane component, YscC, of the Yop secretion machinery of Yersinia enterocolitica forms a ring-shaped multimeric complex. Mol. Microbiol. 26789-797. [DOI] [PubMed] [Google Scholar]
- 21.Lesser, C. F., and S. I. Miller. 2001. Expression of microbial virulence proteins in Saccharomyces cerevisiae models mammalian infection. EMBO J. 201840-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks, M. I., C. H. Pai, L. Lafleur, L. Lackman, and O. Hammerberg. 1980. Yersinia enterocolitica gastroenteritis: a prospective study of clinical, bacteriologic, and epidemiologic features. J. Pediatr. 9626-31. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto, H., and G. M. Young. 2006. Proteomic and functional analysis of the suite of Ysp proteins exported by the Ysa type III secretion system of Yersinia enterocolitica biovar 1B. Mol. Microbiol. 59689-706. [DOI] [PubMed] [Google Scholar]
- 24.Merriam, J. J., R. Mathur, R. Maxfield-Boumil, and R. R. Isberg. 1997. Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect. Immun. 652497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, V. L., and S. Falkow. 1988. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect. Immun. 561242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, V. L., J. J. Farmer III, W. E. Hill, and S. Falkow. 1989. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect. Immun. 57121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohlson, M. B., K. Fluhr, C. L. Birmingham, J. H. Brumell, and S. I. Miller. 2005. SseJ deacylase activity by Salmonella enterica serovar Typhimurium promotes virulence in mice. Infect. Immun. 736249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 634837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pujol, C., and J. B. Bliska. 2005. Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clin. Immunol. 114216-226. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Albert, J., X. J. Yu, C. R. Beuzon, A. N. Blakey, E. E. Galyov, and D. W. Holden. 2002. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol. Microbiol. 44645-661. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 32.Sato, H., J. B. Feix, and D. W. Frank. 2006. Identification of superoxide dismutase as a cofactor for the Pseudomonas type III toxin, ExoU. Biochemistry 4510368-10375. [DOI] [PubMed] [Google Scholar]
- 33.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 222959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitkiewicz, I., K. E. Stockbauer, and J. M. Musser. 2007. Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends Microbiol. 1563-69. [DOI] [PubMed] [Google Scholar]
- 35.Skrzypek, E., T. Myers-Morales, S. W. Whiteheart, and S. C. Straley. 2003. Application of a Saccharomyces cerevisiae model to study requirements for trafficking of Yersinia pestis YopM in eukaryotic cells. Infect. Immun. 71937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slagowski, N. L., R. W. Kramer, M. F. Morrison, J. LaBaer, and C. F. Lesser. 2008. A functional genomic yeast screen to identify pathogenic bacterial proteins. PLoS Pathog 4e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sory, M. P., and G. R. Cornelis. 1994. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol. Microbiol. 14583-594. [DOI] [PubMed] [Google Scholar]
- 38.Thomson, N. R., S. Howard, B. W. Wren, M. T. Holden, L. Crossman, G. L. Challis, C. Churcher, K. Mungall, K. Brooks, T. Chillingworth, T. Feltwell, Z. Abdellah, H. Hauser, K. Jagels, M. Maddison, S. Moule, M. Sanders, S. Whitehead, M. A. Quail, G. Dougan, J. Parkhill, and M. B. Prentice. 2006. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trulzsch, K., T. Sporleder, E. I. Igwe, H. Russmann, and J. Heesemann. 2004. Contribution of the major secreted Yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect. Immun. 725227-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Upton, C., and J. T. Buckley. 1995. A new family of lipolytic enzymes? Trends Biochem. Sci. 20178-179. [DOI] [PubMed] [Google Scholar]
- 41.Valinsky, L., I. Nisan, X. Tu, G. Nisan, I. Rosenshine, E. Hanski, I. Barash, and S. Manulis. 2002. A host-specific virulence protein of Erwinia herbicola pv. gypsophilae is translocated into human epithelial cells by the type III secretion system of enteropathogenic Escherichia coli. Mol. Plant Path. 397-101. [DOI] [PubMed] [Google Scholar]
- 42.Venecia, K., and G. M. Young. 2005. Environmental regulation and virulence attributes of the Ysa type III secretion system of Yersinia enterocolitica biovar 1B. Infect. Immun. 735961-5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker, K. A., and V. L. Miller. 2004. Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J. Bacteriol. 1864056-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100195-199. [PubMed] [Google Scholar]
- 45.Warren, S. M., and G. M. Young. 2005. An amino-terminal secretion signal is required for YplA export by the Ysa, Ysc, and flagellar type III secretion systems of Yersinia enterocolitica biovar 1B. J. Bacteriol. 1876075-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young, B. M., and G. M. Young. 2002. Evidence for targeting of Yop effectors by the chromosomally encoded Ysa type III secretion system of Yersinia enterocolitica. J. Bacteriol. 1845563-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young, B. M., and G. M. Young. 2002. YplA is exported by the Ysc, Ysa, and flagellar type III secretion systems of Yersinia enterocolitica. J. Bacteriol. 1841324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]