Abstract
4-Nitrophenol (4-NP) is a toxic product of the hydrolysis of organophosphorus pesticides such as parathion in soil. Rhodococcus sp. strain PN1 degrades 4-NP via 4-nitrocatechol (4-NC) for use as the sole carbon, nitrogen, and energy source. A 5-kb EcoRI DNA fragment previously cloned from PN1 contained a gene cluster (nphRA1A2) involved in 4-NP oxidation. From sequence analysis, this gene cluster is expected to encode an AraC/XylS family regulatory protein (NphR) and a two-component 4-NP hydroxylase (NphA1 and NphA2). A transcriptional assay in a Rhodococcus strain revealed that the transcription of nphA1 is induced by only 4-NP (of several phenolic compounds tested) in the presence of nphR, which is constitutively expressed. Disruption of nphR abolished transcriptional activity, suggesting that nphR encodes a positive regulatory protein. The two proteins of the 4-NP hydroxylase, NphA1 and NphA2, were independently expressed in Escherichia coli and purified by ion-exchange chromatography or affinity chromatography. The purified NphA2 reduced flavin adenine dinucleotide (FAD) with the concomitant oxidation of NADH, while the purified NphA1 oxidized 4-NP into 4-NC almost quantitatively in the presence of FAD, NADH, and NphA2. This functional analysis, in addition to the sequence analysis, revealed that this enzyme system belongs to the two-component flavin-diffusible monooxygenase family. The 4-NP hydroxylase showed comparable oxidation activities for phenol and 4-chlorophenol to that for 4-NP and weaker activities for 3-NP and 4-NC.
Nitroaromatic compounds are important building blocks for the synthesis of chemical compounds such as dyes, explosives, and herbicides and therefore are used in large quantities in chemical industries. However, they are generally toxic to living organisms and easily bioconverted into more mutagenic and carcinogenic aromatic amines under anaerobic environments (41). One such compound, 4-nitrophenol (4-NP), is known to be very toxic and has often been detected in soil as a hydrolysis product of organophosphorus pesticides, parathion and methylparathion, especially in developing countries. For instance, in India, more than 20 mg kg−1 of 4-NP was detected in soils of tea fields (R. K. Jain, Institute of Microbial Technology, India, personal communication). The U.S. Environmental Protection Agency has listed 4-NP as a priority pollutant (17). Therefore, much attention has been paid to its biodegradability in the environment and its biodegradation has been extensively studied for the last two decades.
4-NP can be degraded aerobically through two different pathways via 4-nitrocatechol (4-NC) or hydroquinone (HQ) (48). In the former pathway, 4-NP is first oxidized into 4-NC, which is further converted into 1,2,4-benzenetriol, followed by the cleavage of the aromatic ring. In the latter pathway, it is first oxidized into HQ, which is subjected to the cleavage of the aromatic ring. Then both resulting ring-cleaved compounds, γ-hydroxymuconic semialdehyde and maleylacetate, are metabolized into tricarboxylic acid cycle intermediates via β-ketoadipate. Arthrobacter sp. strain JS443, Arthrobacter aurescens TW17, Pseudomonas sp., Rhodococcus sp., and Serratia sp. were reported to employ the 4-NC pathway (11, 15, 28, 37, 38), while Moraxella sp., Nocardia sp., Burkholderia cepacia RKJ200, Arthrobacter protophormiae RKJ100, and Pseudomonas spp. were reported to use the HQ pathway (5, 11, 30, 40, 48). In these pathways, the initial degradation starts with hydroxylation (monooxygenation) of the 4-NP aromatic ring.
Earlier studies on 4-NP oxidation using cell extracts and partially purified cell fractions indicated that bacteria have monooxygenases capable of oxidizing NPs (25, 39). A membrane preparation of a Moraxella sp. oxidized 4-NP into HQ using molecular oxygen; the oxidation was dependent on the presence of NADPH (39), and the addition of flavin adenine dinucleotide (FAD) stimulated the reaction. In a different study, an extract of 4-NP-induced Nocardia sp. cells also oxidized 4-NP into 4-NC in the presence of molecular oxygen, NADH, and FAD (25). The enzymes catalyzing 4-NP oxidation, however, have not yet been purified. Later, a two-component 4-NP monooxygenase system, consisting of two proteins, an oxygenase component and a flavoprotein reductase component, was purified from Bacillus sphaericus JS905 (16). The reductase component reduced FAD with the concomitant oxidation of NADH, while the oxygenase component catalyzed two sequential oxygenations from 4-NP into 1,2,4-benzenetriol through 4-NC using the reduced flavin. Hence, this monooxygenase is a key enzyme in the 4-NC pathway of JS905; however, at present, the nucleotide and amino acid sequence data remain unpublished.
In our previous studies, we isolated a 4-NP-degrading bacterium, Rhodococcus sp. strain PN1, from activated sludge (43). This bacterium can degrade not only mono-NPs, including 4-NP, but also poly-NPs, such as 2,4-dinitrophenol and 2,4,6-trinitrophenol (picric acid) (1). Analyses of the metabolites in NP degradation revealed that PN1 degrades 4-NP through the 4-NC pathway, whereas it also degrades 2,4-dinitrophenol and picric acid via the corresponding hydride-Meisenheimer complexes (1, 13, 44). Thus, PN1 has at least two quite different pathways for NP degradation. The gene clusters involved in 4-NP degradation and picric acid degradation have been independently cloned from PN1 (13, 43). The gene cluster encoding 4-NP oxidation consists of three genes, nphR, nphA1, and nphA2, which were expected from the deduced amino acid sequences to encode an AraC/XylS family regulatory protein (9) and a 4-NP hydroxylase belonging to the two-component flavin-diffusible monooxygenase (TC-FDM) family (8, 43). Later, in a different study, another 4-NP degradation gene cluster (npcBAC), consisting of 4-NP monooxygenase genes (npcBA) and the subsequent hydroxyquinol 1,2-dioxygenase gene (npcC), was cloned from Rhodococcus opacus SAO101 and characterized (22). The conversion of 4-NP into maleylacetate via 4-NC was reported using the cell extracts of recombinant Escherichia coli including this gene cluster. In addition, a transcriptional assay by reverse transcription-PCR revealed that these three genes were transcribed in a unit and that the transcription was induced by 4-NP. Neither of these gene products has been purified, nor has the detailed regulation mechanism of the 4-NP oxidation been characterized. Very recently, Perry and Zylstra cloned a 4-NP monooxygenase gene cluster (npdBA1RXA2C) from Arthrobacter sp. strain JS443 (29) that showed significant homology with the chlorophenol 4-monooxygenase gene cluster of Arthrobacter chlorophenolicus A6 (27). In this cluster, a flavin reductase gene (npdA1) and a monooxygenase gene (npdA2) are separated by a large putative regulatory gene (npdR) and transcribed divergently. RT-PCR analysis revealed that npdA2 and npdB (encoding a hydroxyquinol 1,2-dioxygenase) are transcribed when JS443 is grown on 4-NP but not on a rich medium, suggesting that this gene cluster is involved in 4-NP degradation. The histidine-tagged product of npdA1 showed NADH-dependent FAD reductase activity, while E. coli lysates including the npdA2 gene product are capable of oxidizing 4-NP and a wide range of 4-substituted phenols, indicating that NpdA1 and NpdA2 also belong to the TC-FDM family in function as well as in sequence. This report suggested the presence of another novel 4-NP degradation pathway, in which 4-NP is converted into 1,2,4-benzenetriol via 1,4-benzoquinone and hydroxy-1,4-benzoquinone but not via HQ and 4-NC. Although the npd gene cluster has the putative regulatory gene, npdR, no analysis of the regulatory mechanism of gene expression has been performed.
To understand the mechanism of 4-NP oxidation in PN1 at both genetic and enzymatic levels, we first investigated the regulatory system for the expression of the 4-NP hydroxylase genes, nphA1A2. Then we purified each protein of the 4-NP hydroxylase using Escherichia coli expression systems and characterized their functions in 4-NP oxidation in vitro. The results showed that the hydroxylase oxidizes 4-NP and some phenolic compounds preferentially but its gene expression is specifically induced by 4-NP in the presence of a putative regulatory gene, nphR.
MATERIALS AND METHODS
Strains, plasmids, media, and growth conditions.
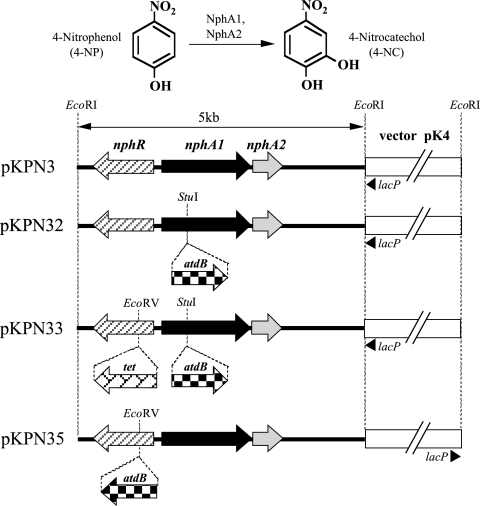
A Rhodococcus-E. coli shuttle vector, pK4 (12), was used to construct the pKPN series plasmids (Fig. 1), which were used for the transcriptional assay. Rhodococcus sp. strain PN1 (43) and Rhodococcus rhodochrous ATCC 12674 (12) were used as hosts for pK4-based recombinant plasmids. E. coli JM109 (Takara, Kyoto, Japan) was used as a host for propagating recombinant plasmids and purification of recombinant proteins. Luria-Bertani (LB) medium (36) was used as a rich medium, and kanamycin and ampicillin were added to the rich medium at final concentrations of 50 mg liter−1 and 100 mg liter−1, respectively, unless otherwise mentioned. Rhodococcus spp. and E. coli were grown at 30°C and 37°C, respectively.
FIG. 1.
Physical map of pKPN3 containing the 4-NP hydroxylase gene cluster of Rhodococcus sp. strain PN1 and its derivatives. A catechol 2,3-dioxygenase gene (atdB) from Acinetobacter sp. strain YAA (42, 45) was inserted into the StuI site and the EcoRV site of pKPN3 as a reporter gene to construct pKPN32 and pKPN35, respectively. For the disruption of nphR, a tetracycline resistance gene (tet) from pBR322 (4) was introduced into the EcoRV site of pKPN32 to construct pKPN33. These derivatives were used to investigate the transcriptional activity of nphA1 and nphR in R. rhodochrous ATCC 12674 in the presence or absence of various inducers.
Construction of pKPN series plasmids.
To assay transcriptional activity, pKPN series plasmids were constructed as follows (Fig. 1). First, to use as a reporter gene, a DNA fragment of approximately 1.0 kb containing a catechol 2,3-dioxygenase (C23O) gene (atdB) of Acinetobacter sp. strain YAA (42, 45) was amplified by PCR using KOD DNA polymerase (Toyobo, Osaka, Japan), which produces blunt ends on the amplified fragment. The PCR mixture contained 0.5 μl (1.25 U) of KOD DNA polymerase, 5 μl of KOD DNA polymerase buffer, 5 μl of deoxynucleoside triphosphate (dNTP) mixture (2 mM each), 2 μl of 25 mM MgCl2, 1 μl of primer atdBnds50F (10 μM [5′-TGAGTGCACAACAACTAAAGCAATGAGGTAATG-3′]), 1 μl of primer atdBnds50R (10 μM [5′-CTAATCCCTGCTTCATTCCTGCAGGATCATGTC-3′]), 1 μl of template DNA (10 ng [pAS16]) (42), and 34.5 μl of double-deionized water in a total volume of 50 μl. The atdBnds50F primer contained a putative ribosome-binding sequence (GAGG) but no possible promoter sequences. The PCR cycle employed was as follows: (i) 98°C for 5 min; (ii) 98°C for 15 s, 55°C for 5 s, and 74°C for 30 s for 25 cycles; and (iii) 74°C for 5 min. Then the amplified DNA fragment was introduced into the StuI site of pKPN3 (44) in the same transcriptional direction as nphA1 to construct pKPN32. Similarly, the same amplified fragment was inserted into the EcoRV site of pKPN3 in the same transcriptional direction as nphR to construct pKPN35. Furthermore, to disrupt nphR in pKPN32, a tetracycline resistance gene, which was amplified in the same way using another primer set, tetnds50F (5′-TAACGCAGTCGTGCACCGTGTATGAAATCTAAC-3′) and tetnds50R (5′-GTTAGCGAGGTCCTGCAGGCTTCCATTCAGGTC-3′), with pBR322 (4) as the template DNA, was introduced into the EcoRV site of pKPN32 to construct pKPN33. These plasmids were introduced into Rhodococcus hosts by electroporation as described previously (43).
Assay for transcriptional activity.
Recombinant R. rhodochrous ATCC 12674 harboring one of the pKPN series plasmids was incubated in LB medium containing glycine (0.5g liter−1) and kanamycin for 24 h in the presence or absence of various phenolic compounds (phenol, 2-NP, 3-NP, 4-NP, 2-hydroxyphenylacetate [2-HPA], 3-HPA, 4-HPA, or 4-NC) at 0.3 mM for induction. Then the cells were harvested by centrifugation (7,000 × g at 4°C for 10 min) and washed twice with 10 mM phosphate buffer (pH 7.0). The cells were resuspended with 5 ml of the same buffer and disrupted by ultrasonication using a TOMY UD-200 cell disrupter (TOMY, Kyoto, Japan) (output 6, 2 min, four times on ice). After centrifugation (24,900 × g at 4°C for 30 min), the supernatant was used as a cell extract. C23O activity in the cell extract was measured as transcriptional activity as described previously (45).
Preparation of E. coli cell extracts for protein purification.
Recombinant E. coli JM109 harboring pUPNH-A1 or pUPNH-A2 (43) was cultured in the LB medium containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and ampicillin at 37°C and 150 rpm on a rotary shaker for 20 h, harvested by centrifugation (7,000 × g at 4°C for 10 min), and washed with TD buffer (50 mM Tris-HCl, 1 mM dithiothreitol [DTT], pH 7.6) for NphA1 or lysis buffer (50 mM Tris-HCl, 10 μM FAD, 1 mM DTT, 1 mM MgSO4, 2.5% [vol/vol] glycerol, pH 7.6) for NphA2. The bacterial pellet was resuspended in 5 ml of the same buffer, sonicated with the cell disrupter (output 6, 1 min, six times, on ice), and then centrifuged (11,000 × g at 4°C for 30 min). The supernatant was used as a cell extract for protein purification.
For the preparation of a cell extract including histidine-tagged NphA2 (His-NphA2), recombinant E. coli JM109 harboring pQNPHA2 (pQE80L [Qiagen, Tokyo, Japan] plus nphA2 [see the Results section]) was cultured in LB medium containing ampicillin at 25°C and 150 rpm on a rotary shaker. IPTG was added at 1 mM after an 8-h incubation, and further cultivation was continued for 16 h. The cells were harvested by centrifugation (7,000 × g at 4°C for 10 min), washed and resuspended with TD buffer, and disrupted as described above.
Purification of NphA1 by ion-exchange chromatography.
The cell extract containing NphA1 was applied to a HiTrap Q-Sepharose column (column volume, 5 ml [GE Healthcare Bio-Science, Tokyo, Japan]) pre-equilibrated with 25 ml TD buffer, and then the column was extensively washed with TD buffer. Proteins on the column were eluted with a linear NaCl gradient from 100 mM to 400 mM in TD buffer. Fractions containing NphA1 were pooled and desalted using a PD-10 desalting column (Amersham Biosciences, Uppsala, Sweden) preequilibrated with TD buffer. Finally, the solution obtained was concentrated to an appropriate volume using a Vivaspin 6-ml concentrator (Vivascience AG, Hannover, Germany). Determination of protein concentration and relative molecular mass and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis were carried out as described previously (45).
Purification of NphA2 by ion-exchange chromatography and His-NphA2 by affinity chromatography.
The cell extract containing NphA2 was applied to a Q-Sepharose fast-flow column (resin from GE Healthcare Bio-Science; column size, 1.5 cm inside diameter by 30 cm) preequilibrated with TF buffer (lysis buffer without glycerol), and then the column was extensively washed with TF buffer. Proteins on the column were eluted with a linear NaCl gradient from 0 mM to 500 mM in TF buffer. Fractions containing NphA2 were pooled, desalted, and concentrated as described above.
For the purification of His-NphA2, binding buffer (20 mM sodium phosphate buffer containing 500 mM NaCl, pH 7.8) was used for the cell extract preparation instead of lysis buffer. To a 5-ml disposable polyethylene column, 2.5 ml of Ni-nitrilotriacetic acid (NTA) resin (GE Healthcare Bio-Science) was added, and the column was equilibrated with 7.5 ml binding buffer. Then 5 ml of the cell extract containing His-NphA2 was loaded onto the column, which was gently inverted several times and allowed to stand for 30 min at 4°C. After the solution was removed from the column, 15 ml of binding buffer and 10 ml of washing buffer (20 mM sodium phosphate buffer containing 500 mM NaCl, pH 6.0) were passed through the column. Finally, His-NphA2 was eluted with elution buffer (washing buffer including 50 mM or 200 mM imidazole). The eluted fractions were pooled, buffer exchanged with 50 mM potassium phosphate buffer (pH 7.5), and concentrated as described above.
Enzyme assay. (i) NAD(P)H-oxidizing activity.
The assay cuvette for the standard reaction contained 0.1 mM FAD (or flavin mononucleotide [FMN]) and 0.2 mM NAD(P)H in 50 mM Tris-HCl buffer (pH 7.5) in a final volume of 1 ml. After the addition of NphA2 (or His-NphA2) to the cuvette, the assay was run at 22°C during a controlled period of time. NAD(P)H-oxidizing activity was estimated by measuring the decrease in A320 for NADH (ɛ of 5,275 M−1 cm−1) or NADPH (ɛ of 4,898 M−1 cm−1) in the reaction mixture using a Hitachi spectrophotometer U-2900A (Hitachi, Tokyo, Japan).
(ii) Oxidizing activity toward phenolic compounds.
Oxidizing activity toward phenolic compounds (4-NP, 3-NP, 2-NP, phenol, 4-chlorophenol, 4-NC, 4-HPA, 3-HPA, and 2-HPA) was determined by measuring the decrease in substrate concentration by high-performance liquid chromatography (HPLC). The standard reaction mixture contained 1 mM NADH, 5 μM FAD, 1 mM DTT, NphA1 (approximately 0.5 mg of purified protein), and NphA2 (approximately 0.1 mg of purified protein) in 1 ml of 50 mM Tris-HCl buffer (pH 7.5). The reaction was initiated by the addition of substrate (0.3 mM); samples were taken at specific intervals and frozen immediately in liquid nitrogen to stop the reaction. After they were thawed on ice, the concentrations of substrates and putative metabolites in the samples were determined by HPLC using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) including an LC20AT pump, CBM-20A communication bus module, SPD-20A UV detector, CTO-20A column oven, DGU-20A5 degasser, and LC solution software and equipped with a Mightysil RP-18 GP Aqua column (Kanto Kagaku, Tokyo, Japan [4.6 mm inside diameter by 250 mm]) under the following conditions: column temperature, 40°C; flow rate, 0.8 ml min−1; injection volume, 15 μl; mobile phase, CH3CN-H2O-CH3COOH at 250:750:1 for 3-HPA, 150:850:1 for 4-HPA, and 350:650:1 for other compounds. The detection wavelength of the detector was set at 254 nm for 4-NP and 4-NC and at 277 nm for other compounds.
Nucleotide sequence accession number.
The nucleotide sequence of a 2.3-kb DNA fragment containing nphA1A2 had already been deposited in the DDBJ database under accession no. AB081773. As some errors were recently found in the sequence, they were corrected and a new DNA segment containing nphR was added to the deposited sequence. The final 3,606-bp sequence was newly registered in the DDBJ database under the same accession number.
RESULTS
Sequence analysis of nphRA1A2 gene products.
In our previous study, we isolated from PN1 a 5-kb EcoRI DNA fragment (the insert of pKPN3 in Fig. 1) that conferred the activity to oxidize 4-NP onto a host strain, R. rhodochrous ATCC 12674 (43, 44). Nucleotide sequence analysis revealed that it contained at least three genes named nphR, nphA1, and nphA2 (Fig. 1). BLAST searches (2) using the deduced amino acid sequences showed that the nphR gene product (NphR) shares quite high identities with the putative AraC/XylS family (hereafter, AraC type) transcriptional regulatory proteins (9) of Rhodococcus sp. strain RHA1 (99% identity, accession no. YP_703831) (24) and Nocardia farcinica IFM 10152 (74%, YP_119268) (14), respectively. Putative functions for these proteins were assigned during the annotation of the whole genome, but these functions have not been experimentally confirmed. NphR also shows moderate identities to some members of the AraC-type regulatory proteins, CadR (31%), NitR (28%), and HpaA (24%), which have been shown to be positive regulators for the expression of the 2,4-dichlorophenoxyacetic acid degradation genes (cadABKC) in Bradyrhizobium sp. strain HW13 (21), the nitrilase gene (nitA) in R. rhodochrous J1 (23), and the 4-hydroxyphenylacetate 3-hydroxylase genes (hpaBC) in E. coli W (ATCC 11105) (33, 35), respectively. NphR has a DNA-binding helix-turn-helix motif (pfam00165) conserved among the AraC-type regulators at its C-terminal region (at amino acids [aa] 284 to 328 in NphR).
The homology searches also revealed that NphA1 and NphA2 share significant identities with the large subunits and the small subunits of the putative two-component aromatic ring hydroxylases of Rhodococcus sp. strain RHA1 (99%, YP_703830; 98%, YP_703829) (24) and N. farcinica IFM 10152 (89%, YP_119267; 78%, YP_119266) (14), respectively. Their putative functions were also assigned during the annotation of the whole genome. The genes encoding these putative hydroxylases are clustered with the corresponding AraC-type regulator genes described above. This sequence analysis revealed that nphRA1A2-like gene clusters are well conserved in the genomes of these high-G+C-content, gram-positive bacteria. NphA1 also shows remarkable identities to the 4-coumarate 3-hydroxylase (Sam5 [78%, ABC88666]) of Saccharothrix espanaensis (3), the phenol hydroxylase large subunits (PheA [61%, AAC38324] and PheA1 [52%, AAF66546]) of Geobacillus thermoleovorans A2 (6) and Geobacillus thermoglucosidasius A7 (7), and the 4-HPA 3-hydroxylase large subunits (HpaB [53%, Q48440] and HpaB [52%, Q57160]) of Klebsiella oxytoca (10) and E. coli W ATCC 11105 (33, 46). These hydroxylases can oxidize phenol and/or 4-substituted phenols.
In contrast, NphA2 shows considerable identities to the hydroxylase component B (MocB [55%, BAD08312]) of Bacillus sp. strain JF8 (26), the naphthalene-inducible monooxygenase small subunit (NimA [46%, AAL61657]) of Rhodococcus aetherivorans I24 (31), the phenol hydroxylase component B (46%, YP_145483) of Thermus thermophilus HB8, and the phenol 2-hydroxylase component B (PheA2 [45%, AAF66547]) of G. thermoglucosidasius A7 (7, 20). PheA2 was reported to be an NADH/flavin oxidoreductase in the phenol hydroxylase system of A7 (20). Therefore, NphA2 was expected to enhance the hydroxylase activity of NphA1 by reducing flavins with the concomitant oxidation of pyridine nucleotides such as NAD(P)H and supplying the reduced flavins to NphA1.
In conclusion, these sequence analyses suggest that NphA1 and NphA2 are an oxygenase component and a flavin reductase component, respectively, in the 4-NP hydroxylase system of PN1, while NphR is likely an AraC-type regulatory protein for the expression of nphA1A2.
Mechanism of regulating 4-NP oxidation in PN1.
In our previous study, R. rhodochrous ATCC 12674 cells harboring pKPN3 (nphRA1A2) (Fig. 1) grown in the presence of 4-NP degraded 0.3 mM of 4-NP completely in only 1 h, whereas those grown in the absence of 4-NP took 8 h to achieve complete degradation of the same concentration with a 2-h lag period (43). This fact indicated that the DNA fragment on the plasmid encoded a regulatory system for 4-NP degradation. In order to understand the regulatory mechanism, the transcriptional activity of nphA1 was first measured using the cell extract of the recombinant Rhodococcus strain harboring pKPN32 with a reporter C23O gene in nphA1 (Fig. 1), which was grown in the presence of various phenolic compounds for induction (Table 1). When 4-NP was used as an inducer, the cell extract showed more than 120-fold-higher C23O activity than that without induction. In contrast, the transcriptional activity upon induction by other phenolic compounds was quite low and similar to that without induction. Therefore, of the compounds tested, 4-NP was found to be the only inducer for nphA1 expression in the presence of nphR.
TABLE 1.
Transcriptional activities for nphA1 and nphR in response to phenolic compounds
| Inducer | Relative transcriptional activitya
|
|
|---|---|---|
| nphA1 | nphR | |
| Phenol | 1.6 | 42.1 |
| 2-NP | 2.9 | 36.2 |
| 3-NP | 0.6 | 33.0 |
| 4-NP | 122.0 | 54.6 |
| 2-HPA | 0.8 | 39.1 |
| 3-HPA | 2.1 | 51.8 |
| 4-HPA | 1.4 | 39.7 |
| 4-NC | 3.0 | 50.5 |
| None (uninduced) | 1.0 | 44.4 |
The absolute C23O activity for a relative transcriptional activity of 1.0 (for nphA1 under the uninduced condition) was 0.43 mU mg protein−1. One unit of C23O activity was defined as the amount required to convert 1 μmol of catechol into 2-hydroxymuconic semialdehyde per min.
To examine the effect of nphR on the transcription of nphA1, we disrupted the gene by inserting a tetracycline resistance gene from pBR322 (4) into the EcoRV site of pKPN32 to construct pKPN33 (Fig. 1). The cell extract of a recombinant Rhodococcus strain harboring pKPN33 showed no detectable transcriptional activity irrespective of the presence of any inducers (data not shown). This result suggests that nphR encodes a positive regulatory protein. In turn, the transcriptional activity of nphR itself was evaluated using the cell extract of a recombinant Rhodococcus strain harboring pKPN35 with the reporter C23O gene in nphR (Fig. 1). Similar levels of the reporter activity were observed under all induction conditions (Table 1), suggesting that nphR is constitutively expressed in this strain. In this experiment, it is possible that self-regulation of nphR by NphR still remains unchecked, because the disruption of nphR by the reporter gene causes loss of NphR in the cell extract. Thus, pKPN35 was introduced into the wild-type strain PN1, in which mature NphR protein is produced from the intact nphR in its genome. In this strain, however, irrespective of the presence of any inducers, the activity levels detected were similar to those detected in the cell extracts of R. rhodochrous ATCC 12674 harboring pKPN35 (data not shown). This result supports our assertion that the transcription of nphR in PN1 is constitutive.
Purification of NphA1 and NphA2.
The NphA1 protein was purified up to electrophoretic homogeneity from cell extract of recombinant E. coli JM109 harboring pUNPH-A1 (43) by ion-exchange chromatography (Table 2), showing by SDS-PAGE a molecular mass of 53 kDa, which is slightly smaller than the size (58.8 kDa) calculated from the amino acid sequence. Gel filtration chromatography analysis using HPLC revealed that the native molecular mass of NphA1 was approximately 207 kDa (data not shown), indicating that it forms a tetrameric structure. We also tried to purify the NphA2 protein from cell extract of recombinant E. coli JM109 harboring pUNPH-A2 (43). The production of NphA2 was very poor in the cells, however, and the final amount purified by chromatography using a Q-Sepharose column was too small to carry out further studies. Thus, to improve the production, we attempted to replace the pUC18 vector used with some other expression vectors, but we were unfortunately unsuccessful. Hence, we decided to produce NphA2 as a histidine-tagged protein using the expression vector pQE80L (Qiagen). The nphA2 gene was introduced into the BamHI site of pQE80L to construct pQNPHA2, resulting in the addition of 12 aa residues (MRGSHHHHHHGS) to the N terminus of NphA2. This trial was very successful. That is, His-NphA2 was successfully purified in large amounts by passing the cell extract of E. coli JM109 harboring pQNPHA2 through an affinity column of Ni-NTA resin. The size estimated by SDS-PAGE was 20 kDa, which is consistent with the size calculated from the amino acid sequence (20.6 kDa). The native molecular mass estimated by gel filtration analysis was approximately 40 kDa (data not shown), indicating that it forms a dimeric structure. For convenience, His-NphA2 was used for further studies.
TABLE 2.
Purification of NphA1 and His-NphA2
| Step | Vol (ml) | Amt of protein (mg ml−1) | Total activity (mU)a | Sp act (mU mg−1)a,b | Yield (%) |
|---|---|---|---|---|---|
| NphA1 | |||||
| Cell extract | 20 | 4.68 | 1,048 | 11.2 ± 1.9 | 100.0 |
| HiTrap Q-Sepharose | 12 | 2.39 | 703 | 24.5 ± 3.1 | 67.1 |
| His-NphA2 | |||||
| Cell extract | 12 | 6.32 | 20,700 | 248 ± 49 | 100.0 |
| Ni-NTA | 10 | 0.24 | 8,150 | 3460 ± 820 | 39.4 |
One unit of 4-NP-oxidizing activity of NphA1 was defined as the amount required to convert 1 μmol of 4-NP into 4-NC per min, while 1 U of NADH-oxidizing activity of His-NphA2 was defined as the amount required to oxidize 1 μmol of NADH per min.
The specific activities shown here were determined from at least three independent experiments.
Substrate specificity and kinetic parameters of His-NphA2.
From sequence analysis, NphA2 was expected to be an NAD(P)H/flavin oxidoreductase in the 4-NP hydroxylase system. Thus, NAD(P)H-oxidizing activity was evaluated in the presence of FAD or FMN using the purified His-NphA2. Maximal activity for the oxidation of NADH was achieved in the presence of FAD (6.6 ± 0.2 μmol min−1 mg-protein−1). No activity was detected when NADPH was used instead of NADH. FMN was also unable to replace FAD. This result shows that NphA2 reduces FAD with the concomitant oxidation of NADH and that this enzyme recognizes these substrates strictly. The corresponding proteins of the 4-NP monooxygenases of B. sphericus JS905 (16) and Arthrobacter sp. strain JS443 (29) also prefer the combination of NADH and FAD. In contrast, the most efficient substrates for the flavin reductase component of the 4-HPA 3-monooxygenase of E. coli were NADH and FMN; this enzyme also reduces FAD and riboflavin with similar Km values to that for FMN in the presence of NADH (8). Other 4-NP monooxygenases have been reported to use NADPH as an electron donor (40, 47). The NADH-oxidizing activity of His-NphA2 was almost identical to that of the wild-type NphA2 (data not shown). More than 80% of the NADH-oxidizing activity could be kept for a week in the presence of 20% (vol/vol) glycerol at −20°C. The optimal pH for the activity was 7.5.
To determine the kinetic parameters for NADH and FAD, specific activities of His-NphA2 for NADH were measured at several concentrations of NADH (0.03 to 0.3 mM) in the presence of 0.5 mM FAD (for the determination of the kinetic parameters for NADH) or at several concentrations of FAD (0.05 to 0.4 mM) in the presence of 0.3 mM NADH (for the determination of kinetic parameters for FAD). The results are presented in Table 3. The high Km value of His-NphA2 for FAD, compared with those in nanomolar range that have been determined for other monooxygenases, suggests that this protein uses FAD as a substrate but not as a prosthetic group. In fact, the typical spectrum for flavins was not detected from the purified NphA2 and His-NphA2 (data not shown). In contrast, the phenol hydroxylase component B, PheA2, of G. thermoglucosidasius A7 displays a spectrum typical for a flavoprotein, with maxima at 376 nm and 455 nm and characteristic shoulders around 355 nm and 485 nm (20). This protein showed 38% amino acid sequence identity to NphA2, uses FAD as a substrate in addition to a prosthetic group, and has been suggested to employ the “ping pong bi bi” kinetic mechanism for FAD reduction (20).
TABLE 3.
Kinetic parameters of His-NphA2 for NADH and FADa
| Substrate | Km (μM) | Vmax (U mg−1)b | kcat (s−1) |
|---|---|---|---|
| NADH | 58.1 | 17.6 | 5.58 |
| FAD | 271 | 18.3 | 5.80 |
These kinetic parameters were calculated from the data on Lineweaver-Burk plots for NADH and FAD. Six independent measurements of NADH oxidation at each NADH or FAD concentration were made. The R2 values of the standard curves in the plots were 0.994 for NADH and 0.997 for FAD.
One unit of NADH-oxidizing activity was defined as the amount required to oxidize 1 μmol of NADH per min.
Oxidation activities of NphA1 toward 4-NP and other phenolic compounds.
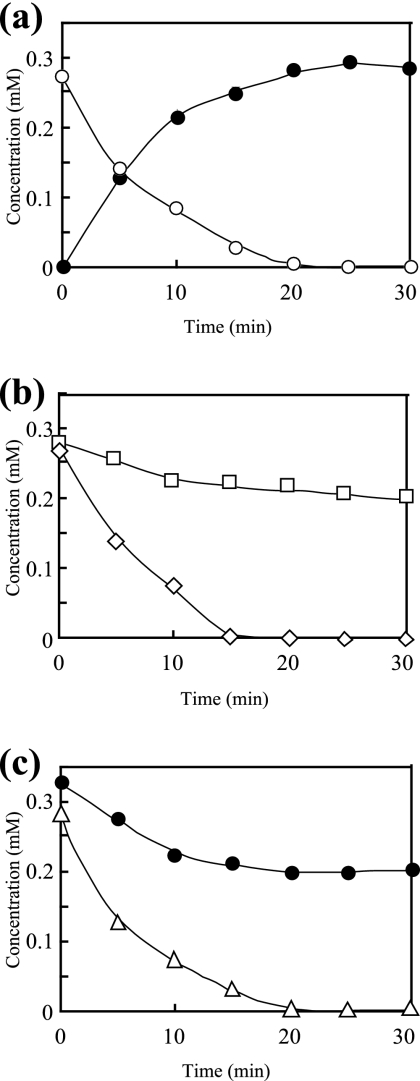
In order to confirm the function of NphA1, enzymatic conversion of 4-NP was carried out using the purified NphA1 in the presence of NADH, FAD, and His-NphA2. As shown in Fig. 2, 0.3 mM of 4-NP was almost quantitatively converted into 4-NC within 20 min. In contrast, 4-NP never decreased in the same reaction mixture without NphA1 or one of the other components (NADH, FAD, or His-NphA2) (data not shown). Hence, NphA1 is the oxygenase component in the 4-NP hydroxylase system. During the 4-NP oxidation experiment using NphA1, inhibition of 4-NP oxidation was observed at >10 μM FAD and 4-NP-oxidizing activity was completely lost at >50 μM. In contrast, concentrations below 1 μM were less effective, and thus the optimal concentration was determined to be 5 μM. More than 80% of the 4-NP-oxidizing activity of NphA1 could be kept for at least a week in the presence of 20% (vol/vol) glycerol at −20°C. The optimal pH for 4-NP oxidation was 8.0.
FIG. 2.
Enzymatic conversion of 4-NP (a) and other phenolic compounds (b and c) using NphA1 and His-NphA2. Enzymatic conversion was carried out in the standard reaction mixture, except for the buffer pH, which was 8.0. In 4-NP conversion (a), 4-NC was monitored as the product. Open circles, 4-NP; closed circles, 4-NC; squares, 3-NP; diamonds, phenol; triangles, 4-chlorophenol.
To determine the substrate specificity of NphA1, enzymatic conversion of several phenolic compounds (phenol, 2-NP, 3-NP, 2-HPA, 3-HPA, 4-HPA, and 4-NC) was carried out in the reaction mixture with the same composition. As shown in Fig. 2, NphA1 degraded phenol and 4-chlorophenol as rapidly as it did 4-NP, while it reduced 4-NC and 3-NP more slowly. No decreases in concentrations for 2-NP, 2-HPA, 3-HPA, and 4-HPA were observed (data not shown). In the phenol, 4-chlorophenol, and 3-NP oxidation reactions, major metabolites were detected by HPLC, whose retention times on the HPLC chromatograms were consistent with those of catechol, 4-chlorocatechol, and 4-NC, respectively. Some metabolites, probably being 1,2,4-benzentriol or its oxidized forms, were also detected in the 4-NC oxidation reaction, but we were unable to exactly identify them due to the overlapping of some other peaks originated from the reaction mixture components on the chromatogram.
DISCUSSION
In this study, we clarified the mechanism by which PN1 oxidizes 4-NP. In this process, a two-component aromatic ring hydroxylase encoded by nphA1 and nphA2 is produced when its expression is induced by 4-NP in the presence of nphR; this hydroxylase carries out the oxidation of 4-NP to 4-NC in the presence of NADH and FAD. The kinetic and spectral studies suggest that FAD is used as a substrate but not a prosthetic group. This is one of the characteristics of the TC-FDM family enzymes.
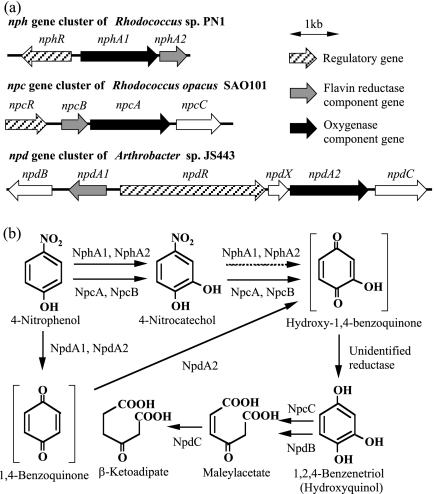
Figure 3a summarizes the gene clusters (nph, npc, and npd) for 4-NP oxidation that have been cloned from three bacteria, Rhodococcus sp. strain PN1, R. opacus SAO101, and Arthrobacter sp. strain JS443 (22, 29, 43). The genetic organizations of these clusters are quite different in each microorganism. The nph and npc gene clusters include an AraC-type regulatory gene (nphR) and a LysR-type regulatory gene (ORF1), respectively, while the npd gene cluster includes a large MalT-DnrI-fused-type regulatory gene (npdR). Although the npc and npd gene clusters were transcribed in the cells grown on 4-NP (22, 29), little is known about the regulatory systems for their expression, including the functions of ORF1 and npdR. We demonstrated in the present study that nphR encodes a positive regulatory protein for the expression of nphA1 and that 4-NP is the only molecule capable of functioning as an inducer (of the several compounds tested).
FIG. 3.
Genetic organization of the NP degradation gene clusters identified so far (a) and proposed NP degradation pathways (b). The genetic organization and the proposed pathways were constructed based on the following sequences and references: the nph gene cluster, AB081773 and this study; the npc gene cluster, AB154422 and reference 22; and the npd gene cluster, EF052871 and reference 29.
In the 4-NP oxidation reaction (Fig. 2a), the 4-NP hydroxylase converted 4-NP into 4-NC stoichiometrically. In contrast, in the 4-NC oxidation reaction (Fig. 2c), although it also showed weak oxidation activity for 4-NC, the decrease in 4-NC concentration almost stopped at 10 min, indicating that 4-NC inhibited the enzyme activity or deactivated the enzyme itself. Therefore, in the 4-NP oxidation, the 4-NC formed might inhibit further oxidation of 4-NC itself, resulting in the stoichiometric accumulation. As shown in Fig. 3b, the 4-NP monooxygenase of R. opacus SAO101 (NpcA and NpcB) (22) can carry out two sequential oxidations from 4-NP to 1,2,4-benzenetriol. In contrast, as shown here, the 4-NP hydroxylase of PN1 catalyzed the first oxidation mainly. Since PN1 doesn't accumulate large amounts of 4-NC in 4-NP degradation (43), it should have another enzyme capable of degrading 4-NC. In our previous study, we isolated a mutant strain from PN1 by chemical mutagenesis (43), which accumulated 4-NC from 4-NP almost stoichiometrically. It is probable that this mutant lost a gene encoding such a 4-NC-degrading enzyme. Recently, we found a gene encoding a functional hydroxyquinol dioxygenase in PN1 (data not shown), which cleaves the aromatic-ring of 1,2,4-benzenetriol to form maleylacetate in the 4-NC pathway (Fig. 3b). These facts strongly suggest that PN1 has enzymes involved in the 4-NC pathway in addition to the 4-NP hydroxylase.
In the TC-FDM family, the 4-HPA 3-hydroxylase system (HpaB and HpaC) of E. coli W ATCC 11105 (8, 32-35, 46) has been purified and characterized in detail at both the genetic and enzymatic levels. The gene cluster (hpaABC) that encodes this enzyme system also contains the regulatory gene hpaA, which encodes an AraC-type positive regulatory protein (33, 35). Therefore, hpaABC is very similar in its gene organization to nphRA1A2; however, hpaABC is transcribed in the same direction, whereas nphR and nphA1A2 are transcribed divergently. Generally, genes encoding the AraC-type regulatory proteins are located upstream of their target genes and transcribed in the same direction (9) like hpaABC. Thus, the genetic organization of nphRA1A2 is one of the minor cases.
The amino acid sequence identities of HpaA, HpaB, and HpaC to NphR, NphA1, and NphA2 are 24%, 52%, and 25%, respectively. HpaC was found to be able to reduce FMN, FAD, and riboflavin with concomitant oxidation of NADH or NADPH, but the most efficient cofactors were found to be a combination of NADH and FMN (8), as described above. In contrast, NphA2 uses only a combination of NADH and FAD and shows no NADPH-oxidizing activity and no FMN-reducing activity. The Km of NphA2 for NADH (58.1 μM) in the presence of FAD is comparable to that of HpaC (40 μM) in the presence of FMN, while that of NphA2 for FAD (271 μM) in the presence of NADH is much higher than those of HpaC for FMN (2.1 μM) or for FAD (3.1 μM). This value for FAD is also approximately 10 to 100 times higher than those of other NAD(P)H/flavin oxidoreductases listed by Kim et al. (19).
The oxygenase component, HpaB, was found to have a broad substrate range for phenolic compounds, since E. coli crude extracts containing both HpaB and HpaC were found to oxidize 4-HPA, 3-HPA, 3,4-dihydroxyphenylacetate, 2,5-dihydroxyphenylacetate, p-cresol, phenol, 4-chlorophenol, and others (32). Only 4-HPA, 3-HPA, and phenylacetate, however, can induce the expression of the hpaBC genes (35). These facts show that the 4-HPA 3-hydroxylase system have developed for HPA metabolism. Similar 4-HPA 3-hydroxylases and their genes can be found in many bacteria, because HPA metabolism is involved in aromatic amino acid metabolic pathways (http://www.genome.ad.jp/kegg/pathway/map/map01150.html). In contrast, NphA1, coupled with NphA2, oxidizes 4-NP, phenol, 4-chlorophenol, 3-NP, and 4-NC, suggesting that it too has broad substrate specificity. HPAs were not oxidized by the 4-NP hydroxylase, however, nor were they able to function as an inducer for the expression of nphA1A2 (Table 1). These differences in gene regulation and substrate specificity suggest that the 4-NP hydroxylase system might have been completely ramified for 4-NP oxidation from 4-HPA 3-hydroxylase systems, although both hydroxylase systems are expected to share the same origin, judging from their amino acid sequence identities. Very recently, the crystal structure of a different 4-HPA 3-monooxygenase (HpaB) of Thermus thermophilus HB8 was determined and its catalytic mechanism was discussed (18). To understand the catalytic mechanism of our enzyme, we are also trying to elucidate the structure through crystallographic study and X-ray analysis.
Acknowledgments
This research was financially supported by the Japan Society for Promotion of Science (JSPS) and Department of Science and Technology, India (DST) under the JSPS-DST Joint Research Program, by the Japan Science and Technology Agency (JST) and DST under the Strategic Japanese-Indian Cooperative program, and also by a Grant-in-Aid for Scientific Research (C) (no. 19560778) from JSPS.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Abe, Y., M. Takeo, A. Sakakibara, and S. Negoro. 2003. Characterization of picric acid degradation by Rhodococcus sp. PN1. Jpn. J. Water Treat. Biol. 3925-31. [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berner, M., D. Krug, C. Bihlmaier, A. Vente, R. Müller, and A. Bechthold. 2006. Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis. J. Bacteriol. 1882666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 295-113. [PubMed] [Google Scholar]
- 5.Chauhan, A., A. K. Chakraborti, and R. K. Jain. 2000. Plasmid-encoded degradation of p-nitrophenol and 4-nitrocatechol by Arthrobacter protophormiae. Biochem. Biophys. Res. Commun. 270733-740. [DOI] [PubMed] [Google Scholar]
- 6.Duffner, F. M., and R. Muller. 1998. A novel phenol hydroxylase and catechol 2,3-dioxygenase from the thermophilic Bacillus thermoleovorans strain A2: nucleotide sequence and analysis of the genes. FEMS Microbiol. Lett. 16137-45. [DOI] [PubMed] [Google Scholar]
- 7.Duffner, F. M., U. Kirchner, M. P. Bauer, and R. Muller. 2000. Phenol/cresol degradation by the thermophilic Bacillus thermoglucosidasius A7: cloning and sequence analysis of five genes involved in the pathway. Gene 256215-221. [DOI] [PubMed] [Google Scholar]
- 8.Gálan, B., E. Díaz, M. A. Prieto, and J. L. García. 2000. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J. Bacteriol. 182627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallegos, M.-T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibello, A., M. Suarez, J. L. Allende, and M. Martin. 1997. Molecular cloning and analysis of the genes encoding the 4-hydroxyphenylacetate hydroxylase from Klebsiella pneumoniae. Arch. Microbiol. 167160-166. [PubMed] [Google Scholar]
- 11.Hanne, L. F., L. L. Kirk, S. M. Appel, A. D. Narayan, and K. K. Bains. 1993. Degradation and induction specificity in actinomycetes that degrade p-nitrophenol. Appl. Environ. Microbiol. 593505-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto, Y., M. Nishiyama, F. Yu, I. Watanabe, S. Horinouchi, and T. Beppu. 1992. Development of a host-vector system in a Rhodococcus strain and its use for expression of the cloned nitrile hydratase gene cluster. J. Gen. Microbiol. 1381003-1010. [DOI] [PubMed] [Google Scholar]
- 13.Heiss, G., N. Trachtmann, Y. Abe, M. Takeo, and H.-J. Knackmuss. 2003. Homologous npdGI genes in 2,4-dinitrophenol and 4-nitrophenol-degrading Rhodococcus spp. Appl. Environ. Microbiol. 692748-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishikawa, J., A. Yamashita, Y. Mikami, Y. Hoshino, H. Kurita, K. Hotta, T. Shiba, and M. Hattori. 2004. The complete genomic sequence of Nocardia farcinica IFM 10152. Proc. Natl. Acad. Sci. USA 10114925-14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain, R. K., J. H. Dreisbach, and J. C. Spain. 1994. Biodegradation of p-nitrophenol via 1,2,4-benzenetriol by an Arthrobacter sp. Appl. Environ. Microbiol. 603030-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadiyala, V., and J. C. Spain. 1998. A two-component monooxygenase catalyzes both the hydroxylation of p-nitrophenol and the oxidative release of nitrite from 4-nitrocatechol in Bacillus sphaericus JS905. Appl. Environ. Microbiol. 642479-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith, L. H., and W. A. Telliard. 1979. Priority pollutants. I. A perspective view. Environ. Sci. Technol. 13416-423. [Google Scholar]
- 18.Kim, S. H., T. Hisano, K. Takeda, W. Iwasaki, A. Ebihara, and K. Miki. 2007. Crystal structure of the oxygenase component (HpaB) of the 4-hydroxyphenylacetate 3-monooxygenase from Thermus thermophilus HB8. J. Biol. Chem. 28233107-33117. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. H., T. Hisano, W. Iwasaki, A. Ebihara, and K. Miki. 2008. Crystal structure of the flavin reductase component (HpaC) of 4-hydroxyphenylacetate 3-monooxygenase from Thermus thermophilus HB8: structural basis for the flavin affinity. Proteins 70718-730. [DOI] [PubMed] [Google Scholar]
- 20.Kirchner, U., A. H. Westphal, R. Müller, and W. J. H. van Berkel. 2003. Phenol hydroxylase from Bacillus thermoglucosidasius A7, a two-component monooxygenase with a dual role for FAD. J. Biol. Chem. 27847545-47553. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa, W., S. Takami, K. Miyauchi, E. Masai, Y. Kamagata, J. M. Tiedje, and M. Fukuda. 2002. Novel 2,4-dichlorophenoxyacetic acid degradation genes from oligotrophic Bradyrhizobium sp. strain HW13 isolated from a pristine environment. J. Bacteriol. 184509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitagawa, W., N. Kimura, and Y. Kamagata. 2004. A novel p-nitrophenol degradation gene cluster from a gram-positive bacterium, Rhodococcus opacus SAO101. J. Bacteriol. 1864894-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komeda, H., Y. Hori, M. Kobayashi, and S. Shimizu. 1996. Transcriptional regulation of the Rhodococcus rhodochrous J1 nitA gene encoding a nitrilase. Proc. Natl. Acad. Sci. USA 9310572-10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLeod, M. P., R. L. Warren, W. W. Hsiao, N. Araki, M. Myhre, C. Fernandes, D. Miyazawa, W. Wong, A. L. Lillquist, D. Wang, M. Dosanjh, H. Hara, A. Petrescu, R. D. Morin, G. Yang, J. M. Stott, J. E. Schein, H. Shin, D. Smailus, A. S. Siddiqui, M. A. Marra, S. J. Jones, R. Holt, F. S. Brinkman, K. Miyauchi, M. Fukuda, J. E. Davies, W. W. Mohn, and L. D. Eltis. 2006. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. USA 10315582-15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitra, D., and C. S. Vaidyanathan. 1984. A new 4-nitrophenol 2-hydroxylase from a Nocardia sp. Biochem. Int. 8609-615. [PubMed] [Google Scholar]
- 26.Miyazawa, D., G. Mukerjee-Dhar, M. Shimura, T. Hatta, and K. Kimbara. 2004. Genes for Mn(II)-dependent NahC and Fe(II)-dependent NahH located in close proximity in the thermophilic naphthalene and PCB degrader, Bacillus sp. JF8: cloning and characterization. Microbiology 150993-1004. [DOI] [PubMed] [Google Scholar]
- 27.Nordin, K., M. Unell, and J. K. Jansson. 2005. Novel 4-chlorophenol degradation gene cluster and degradation route via hydroxyquinol in Arthrobacter chlorophenolicus A6. Appl. Environ. Microbiol. 716538-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pakala, S. B., P. Gorla, A. B. Pinjari, R. K. Krovidi, R. Baru, M. Yanamandra, M. Merrick, and D. Siddavattam. 2007. Biodegradation of methyl parathion and p-nitrophenol: evidence for the presence of a p-nitrophenol 2-hydroxylase in a Gram-negative Serratia sp. strain DS001. Appl. Microbiol. Biotechnol. 731452-1462. [DOI] [PubMed] [Google Scholar]
- 29.Perry, L. L., and G. J. Zylstra. 2007. Cloning of a gene cluster involved in the catabolism of p-nitrophenol by Arthrobacter sp. strain JS443 and characterization of the p-nitrophenol monooxygenase. J. Bacteriol. 1897563-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prakash, D., A. Chauhan, and R. K. Jain. 1996. Plasmid-encoded degradation of p-nitrophenol by Pseudomonas cepacia. Biochem. Biophys. Res. Commun. 224375-381. [DOI] [PubMed] [Google Scholar]
- 31.Priefert, H., X. M. O'Brien, P. A. Lessard, A. F. Dexter, E. E. Choi, S. Tomic, G. Nagpal, J. J. Cho, M. Agosto, L. Yang, S. L. Treadway, L. Tamashiro, M. Wallace, and A. J. Sinskey. 2004. Indene bioconversion by a toluene inducible dioxygenase of Rhodococcus sp. I24. Appl. Microbiol. Biotechnol. 65168-176. [DOI] [PubMed] [Google Scholar]
- 32.Prieto, M. A., A. Perez-Aranda, and J. L. Garcia. 1993. Characterization of an Escherichia coli aromatic hydroxylase with a broad substrate range. J. Bacteriol. 1752162-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prieto, M. A., and J. L. Garcia. 1994. Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J. Biol. Chem. 26922823-22829. [PubMed] [Google Scholar]
- 34.Prieto, M. A., E. Diaz, and J. L. Garcia. 1996. Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J. Bacteriol. 178111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto, M. A., and J. L. García. 1997. Identification of a novel positive regulator of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli. Biochem. Biophys. Res. Commun. 232759-765. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Schäfer, A., H. Harms, and A. J. Zehnder. 1996. Biodegradation of 4-nitroanisole by two Rhodococcus spp. Biodegradation 7249-255. [DOI] [PubMed] [Google Scholar]
- 38.Siddaramappa, S.-B., P. A. Wahid, and N. Sethunathan. 1978. Conversion of p-nitrophenol to 4-nitrocatechol by a Pseudomonas sp. Antonie van Leeuwenhoek 44171-176. [DOI] [PubMed] [Google Scholar]
- 39.Spain, J. C., O. Wyss, and D. T. Gibson. 1979. Enzymatic oxidation of p-nitrophenol. Biochem. Biophys. Res. Commun. 88634-641. [DOI] [PubMed] [Google Scholar]
- 40.Spain, J. C., and D. T. Gibson. 1991. Pathway for Biodegradation of p-nitrophenol in a Moraxella sp. Appl. Environ. Microbiol. 57812-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spain, J. C. 1995. Biodegradation of nitroaromatic compounds. Annu. Rev. Microbiol. 49523-555. [DOI] [PubMed] [Google Scholar]
- 42.Takeo, M., T. Fujii, K. Takenaka, and Y. Maeda. 1998. Cloning and sequencing of a gene cluster for the meta-cleavage pathway of the aniline degradation in Acinetobacter sp. strain YAA. J. Ferment. Bioeng. 85514-517. [Google Scholar]
- 43.Takeo, M., T. Yasukawa, Y. Abe, S. Niihara, Y. Maeda, and S. Negoro. 2003. Cloning and characterization of a 4-nitrophenol hydroxylase gene cluster from Rhodococcus sp. PN1. J. Biosci. Bioeng. 95139-145. [PubMed] [Google Scholar]
- 44.Takeo, M., Y. Abe, and S. Negoro. 2003. Simultaneous degradation of 4-nitrophenol and picric acid by two different mechanisms of Rhodococcus sp. PN1. J. Chem. Eng. Jpn. 361178-1184. [Google Scholar]
- 45.Takeo, M., M. Nishimura, M. Shirai, H. Takahashi, and S. Negoro. 2007. Purification and characterization of catechol 2,3-dioxygenase from the aniline degradation pathway of Acinetobacter sp. YAA and its mutant enzyme, which resists substrate inhibition. Biosci. Biotechnol. Biochem. 711668-1675. [DOI] [PubMed] [Google Scholar]
- 46.Xun, L., and E. R. Sandvik. 2000. Characterization of 4-hydroxyphenylacetate 3-hydroxylase (HpaB) of Escherichia coli as a reduced flavin adenine dinucleotide-utilizing monooxygenase. Appl. Environ. Microbiol. 66481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeyer, J., and H. P. Kocher. 1988. Purification and characterization of a bacterial nitrophenol oxygenase which converts ortho-nitrophenol to catechol and nitrite. J. Bacteriol. 1701789-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zylstra, G. J., S.-W. Bang, L. M. Newman, and L. L. Perry. 2000. Microbial degradation of mononitrophenols and mononitrobenzoates, p. 145-160. In J. C. Spain, J. B. Hughes, and H.-J. Knackmuss (ed.), Biodegradation of nitroaromatic compounds and explosives. Lewis Publishers, Boca Raton, FL.