Abstract
MgrA is a global regulator in Staphylococcus aureus. Differences in the effects of MgrA on norA expression have been reported for different strains, which varied in rsbU, a gene that affects the expression of sigB, which encodes an alternative σ factor involved in stress responses. We hypothesized that MgrA was modified by sigB-dependent factors that affected its ability to control the expression of the norA efflux pump. Heterologously expressed MgrA purified from Escherichia coli was incubated with crude extracts (CE) from strains RN6390 (rsbU) and SH1000 (rsbU+) and tested for binding to the norA promoter. Purified MgrA exhibited greater binding to norA promoter DNA after being incubated with SH1000 CE than MgrA incubated with the RN6390 CE. Phosphorylation of MgrA occurring in cell extracts caused it to lose the ability to bind norA promoter DNA. Overexpression of pknB, encoding a candidate serine/threonine kinase, produced increased phospho-MgrA and led to a fivefold increase in the transcript level of norA for both RN6390 and SH1000, as well as a fourfold increase in the MICs of norfloxacin and ciprofloxacin for these two strains. The levels of expression of pknB in RN6390 and SH1000, however, indicated that additional factors related to rsbU or sigB contribute to the differential regulatory effects of MgrA on norA expression.
Staphylococcus aureus is an organism of great medical importance as a cause of serious infections in the hospital and community. This pathogen is responsible for a broad range of infections, ranging from skin and soft tissue infections to more serious illnesses such as bacteremia and endocarditis. Resistance to antimicrobial compounds in S. aureus can result from drug target modifications, drug inactivation, or extrusion of drugs by efflux pumps, some of which have broad substrate profiles and can cause multidrug resistance. S. aureus NorA is a well-studied multidrug efflux pump that includes certain fluoroquinolones in its substrate profile. Its expression is controlled in part by the global transcriptional regulator MgrA (4, 5). MgrA is a homolog of MarR and SarA and is involved in the regulation of expression of virulence genes (α-hemolysin, protein A, lipase, protease, and coagulase), autolysins, and capsular polysaccharide (11, 12, 17). In addition to controlling norA expression, MgrA is also involved in modulating the expression of other efflux pumps such as NorB, NorC, Tet38, and AbcA (23, 25). Depending on the nature of the target genes, MgrA functions as a repressor or an activator in a direct or indirect manner (11, 19). Recently, NorG, a GntR-like transcriptional regulator, was discovered due to its ability to bind the norA promoter. The role of NorG in this control is unclear, since no change in the levels of norA transcripts was found with alterations in the levels of norG expression (24). Other regulators, such as the arlRS two-component regulatory system, also influence the expression of norA, but many details remain to be elucidated (4, 26).
Recent studies of the role of MgrA in the regulation of the expression of various genes suggested that the effect of MgrA on norA expression may be affected by the alternative σ factor SigB (13, 16). In gram-positive bacteria such as Bacillus subtilis and S. aureus, the sigB regulon has been studied extensively and was shown to be involved in the expression of various genes, including genes of the general stress response and genes associated with virulence (3, 9, 15). In S. aureus, the sigB regulon is associated with increased expression of at least 27 genes and decreased expression of at least 10 genes (6). It also has been shown to play a role in mediating antibiotic resistance (28) but has not been previously implicated in the regulation of expression of efflux transporters. Three genes in the sigB regulon, rsbU, rsbV, and rsbW, were found to exist in both B. subtilis and S. aureus and had similar functions in these two organisms. RsbU belongs to the serine/threonine phosphatase family, and RsbW is a protein kinase (8). In S. aureus, the activation of the sigB regulon was found to be controlled by RsbU via a series of dephosphorylation and phosphorylation by several phosphatases and kinases (22).
In our previous study, we determined that MgrA acted as a positive regulator of norA expression (26). In later studies by other investigators, norA expression measured as β-galactosidase activity generated by the norA-lac gene fusion reporter suggested, in contrast, that MgrA functioned as a negative regulator of norA expression (13), and a transcriptional profile of strain Newman and its mgrA mutant showed the negative effect of MgrA on norA expression (16). One common difference in strain backgrounds for these two studies, in comparison to our original findings, was the absence and presence of mutations in the sigB regulon. Thus, we hypothesized that SigB or sigB-dependent factors modulate the effect of MgrA on norA expression. To be able to compare rsbU-mediated effects, we selected two strains, RN6390 and SH1000, both derived from strain 8325-4, as well as the parental strain 8325-4, which came from strain NCTC 8325 harboring an 11-bp deletion in the rsbU locus, which encodes a positive regulator essential for the expression of SigB (15). Strain SH1000 was constructed from strain 8325-4 with the restoration of the rsbU deletion (7, 9).
In this report, we determined the role of rsbU in the MgrA-dependent regulation of norA expression and investigated the modification of purified MgrA by cell extracts of strains differing in rsbU. We also demonstrated the effect of these modifications on the ability of MgrA to bind norA promoter DNA and identified a putative kinase, PknB, which affected phosphorylation of MgrA and annulled its ability to bind the norA promoter.
MATERIALS AND METHODS
Bacterial strains, plasmids, growth media, and other materials.
Bacterial strains and plasmids used in this study are listed in Table 1. The presence of an 11-bp deletion in rsbU was confirmed by DNA sequencing in strains RN6390 and 8325-4, and an intact rsbU locus was confirmed in strain SH1000. The cna gene was absent from all three strains. S. aureus strains were cultivated in brain heart infusion broth (Difco, Sparks, MD) at 37°C, unless otherwise stated. Escherichia coli strains were grown in Luria-Bertani medium. Lysostaphin, kanamycin, anhydrotetracycline, chloramphenicol, and norfloxacin were obtained from Sigma Chemical Co., St. Louis, MO. Ciprofloxacin and moxifloxacin were obtained from Bayer Corp., West Haven, CT. Sparfloxacin was obtained from Parke-Davis Pharmaceutical Research Division, Ann Arbor, MI. All primers used in this study were synthesized at the Tufts University Core Facility, Boston, MA, and are listed in Table 2.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotypes or relevant characteristic(s)a | Reference or source |
|---|---|---|
| S. aureus | ||
| 8325-4 | 8325 strain cured of three prophages | 10 |
| RN4220 | Restriction-deficient transformation recipient | 14 |
| RN6390 | Laboratory strain related to 8325-4 | 18 |
| SH1000 | Functional rsbU+ derivative of 8325-4 | 9 |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 phoA hsdR17(rK− mK−) supE44 λ− thi-1 gyrA96 relA1 | Gibco-BRL |
| BL21 | E. coli B F−dcm ompT hsdSB(rB− mB−) gal λ | Stratagene |
| Plasmids | ||
| pSK950 | 10.5-kb plasmid carrying the attP site of phage L54a, replicon of pE194; Tcr Emr (S. aureus) | 20 |
| pQT5 | pTrcHisA-mgrA | 26 |
| pQT16 | pknB gene cloned into pSK950 | This study |
| pWN2018 | 10.5-kb S. aureus transcriptional promoter probe vector; Cmr | 25, 27 |
| pBF8-30 | 315-bp sequence containing the entire promoter of norA cloned upstream of the blaZ gene of pWN2018 | 25 |
| pLZ113 | E. coli-S. aureus shuttle vector pRB373 with xyl/tet promoter cloned into the MCS | 29 |
| pLZ-mgrA | pLZ113-mgrA | This study |
MCS, multiple cloning site.
TABLE 2.
Primers used in this study
| Gene | Primer
|
||
|---|---|---|---|
| Pair | Nucleotide sequence (5′→3′) | DNA length (bp) | |
| Based on RT-PCR and real-time RT-PCR | |||
| norA(1) | Sense | ATGAATAAACAGATTTTTGT | 250 |
| Antisense | TGATGTTATCGAGAGTGATT | ||
| norA(2) | Sense | ATCGGTTTAGTAATACCAGTCTTGC | 100 |
| Antisense | GCGATATAATCATTTGAGATAACGC | ||
| mgrA | Sense | ATGTCTGATCAACATAATTT | 300 |
| Antisense | TATTTATTCACTTGACTGAC | ||
| gmk | Sense | TATCAGGACCATCTGGAGTAGG | 100 |
| Antisense | CATCAACTTCACCTTCACGC | ||
| pknB | Sense | CGCATGATATGCGTATTGTA | 150 |
| Antisense | TTGTTCTGGCGAAAAGTACT | ||
| Based on DNA-protein gel mobility shift binding assays | |||
| norA promoter | Sense | TGCAATTTCATATGATCAATCCC | 150 |
| Antisense | AGATTGCAATTCATGCTAAATATT | ||
β-Lactamase assays.
We introduced plasmid pBF8-30, which harbors the entire promoter of norA cloned upstream of the blaZ gene of the promoter-probe plasmid pWN2018 (5, 27), into strains RN6390, SH1000, RN6390 (mgrA), and SH1000 (mgrA) and measured the β-lactamase activity of the transformants. Cells were grown in trypticase soy broth at 37°C to an optical density at 600 nm (OD600) of 0.9. The culture was assayed for β-lactamase activity by using nitrocephin as a substrate (1, 4, 5, 27). β-Lactamase activity was expressed in micromoles of nitrocephin hydrolyzed per milligram of cell protein per minute. Protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA). The promoter-probe plasmid pWN2018 was included in this assay as a control for the background level of β-lactamase expression.
RT-PCR assays.
Total S. aureus RNA was prepared by extraction from lysostaphin-treated cells grown to the exponential phase at 37°C or 30°C, using an RNeasy mini-kit (Qiagen, Valencia, CA). The concentration of RNA was determined spectrophotometrically at A260. The reverse transcription-PCR (RT-PCR) analyses were performed using SuperScript One-Step RT-PCR (Invitrogen Inc., Carlsbad, CA) with 8 ng of total RNA as a template. The sense and antisense pair of primers for norA(1) listed in Table 2 generated a 250-bp amplicon for the norA gene. The amplification conditions were 1 cycle for 30 min at 40°C, 1 cycle for 2 min at 94°C, 30 cycles for 45 s at 94°C, 45 s at 40°C, 30 s at 72°C, and 1 cycle for 10 min at 72°C.
Quantitative real-time RT-PCR assays were carried out using a QPCR SYBR green kit (ABgene; Thermoscientific, Surrey, United Kingdom) and a Chromo4 system for real-time PCR detection (Bio-Rad). Gene-specific primers were designed to yield ∼100 bp of specific products (Table 2), and the gmk housekeeping gene was used as an internal control. All samples were analyzed in triplicate and normalized against gmk gene expression.
Construction of RN6390 (mgrA negative) and SH1000 (mgrA negative) mutants.
We constructed mgrA mutants from strain RN6390 (rsbU) and strain SH1000 (rsbU+) by phage Φ85 transduction of mgrA::cat, as described previously (23). Colonies of interest were selected on trypticase soy agar plates containing sodium citrate (10 μg/ml) and chloramphenicol (5 μg/ml). DNA sequencing was performed to verify the presence of mutations.
We also subcloned the mgrA gene into plasmid pLZ113 to create the plasmid pLZ-mgrA in which mgrA expression is under the control of the xyl/tet promoter and could be induced by anhydrotetracycline (5 μg/ml).
Purification of MgrA protein.
The mgrA gene was subcloned into plasmid pTrcHisA (Invitrogen, Carlsbad, CA) and then introduced into E. coli BL21, as described previously (23). For purification of histidine-tagged MgrA, E. coli BL21 harboring the expression plasmid was grown to mid-log phase in Luria-Bertani medium, at which time isopropyl-β-d-thiogalactopyranoside (1 mM) was added to the culture. After 3 h, the cells were harvested by centrifugation and then resuspended in 20 mM sodium phosphate buffer (pH 7.4). The cells were lysed with lysozyme (0.02%) and then centrifuged (100,000 × g) for 90 min. The supernatant was applied to a nickel affinity column (iminodiacetic acid-Sepharose-Ni; Amersham Pharmacia Biotech, Uppsala, Sweden) and then washed with buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5% glycerol) supplemented with concentrations of imidazole, increasing from 10 to 60 mM. MgrA protein was eluted with 100 mM imidazole. The homogeneity of the eluted protein was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
Phosphorylated and unphosphorylated forms of MgrA were separated by using the phosphoprotein purification kit (Qiagen, MD) following the manufacturer's instructions.
DNA mobility shift analysis.
Primers designed to amplify the promoter region of norA are listed in Table 2. One member of a primer pair was biotinylated by Tufts University Core Facility (Boston, MA). Bacterial cells collected from 200 ml of S. aureus culture at late exponential phase (OD600 of 0.9) were used to prepare the crude cell extracts as previously described (26). The gel mobility shift assay was carried out using a LightShift chemiluminescent electrophoretic mobility shift assay kit (Pierce, Rockford, IL), as recommended by the manufacturer. After a 30-min incubation with various S. aureus cell extracts, the purified protein (MgrA) was repurified by Ni affinity chromatography and mixed with biotin-labeled DNA in 20 μl of binding buffer (10 mM HEPES [pH 8], 60 mM KCl, 4 mM MgCl2, 0.1 mM EDTA, 0.1 mg/ml of bovine serum albumin, 0.25 mM dithiothreitol) containing 1 μg of poly(dI-dC), 200 ng of sheared herring sperm DNA, and 10% glycerol. After incubation, the binding mixture was analyzed by 5% nondenaturing PAGE (4).
RESULTS
Effects of mgrA on norA expression in strains RN6390 and SH1000.
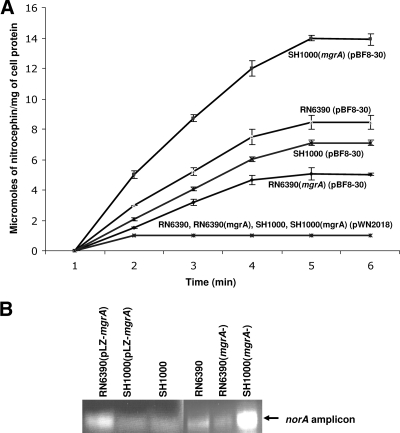
We measured the influence of mgrA on norA transcription levels, using β-lactamase assays for the norA-blaZ reporter gene fusion and RT-PCRs. S. aureus strains used for these experiments were RN6390, RN6390 (mgrA negative), SH1000, and SH1000 (mgrA negative) (Table 1). For the β-lactamase assays, we introduced into these strains the pBF8-30 plasmid, which carried the norA promoter upstream of the β-lactamase gene (5, 27). We measured the β-lactamase activity as micromoles of nitrocephin hydrolyzed per milligram of cell protein per minute in strains carrying plasmid pBF8-30. This activity was consistently higher in strain SH1000 (mgrA negative) at 3 μmol of nitrocephin/mg/min than in SH1000 (1.5 μmol of nitrocephin/mg/min). In contrast, the activity level in strain RN6390 (mgrA negative) was lower at 1 μmol of nitrocephin/mg/min than in RN6390 (2 μmol of nitrocephin/mg/min) (Fig. 1A). Plasmid pWN2018, which lacks the norA promoter, was similarly introduced and used as a negative control.
FIG. 1.
Expression of β-lactamase from the norA promoter in a transcriptional fusion in S. aureus RN6390, SH1000, SH1000 (mgrA), and RN6390 (mgrA). (A) The β-lactamase activities were normalized to that of the strains carrying the vector alone (pWN2018). The β-lactamase activities are expressed in micromoles of nitrocephin hydrolyzed per milligram of cell protein per minute, and each assay was done in triplicate (values represent means and the error bars the standard deviations). In pBF8-30, the norA promoter was cloned upstream of the β-lactamase gene of plasmid pWN2018. (B) RT-PCRs using RNA extracted at the exponential phase. Each reaction used 10 picograms of total RNA as template and primers specific to an internal region of norA.
Similar differences were observed for the norA transcript levels, as determined by RT-PCR assays. norA transcripts increased threefold in SH1000 (mgrA negative) in comparison to those in SH1000 and decreased slightly in RN6390 (mgrA negative) in comparison to those in RN6390 (Fig. 1B). In addition, to assess the effect of overexpression of mgrA on norA transcript levels, plasmid pLZ-mgrA was introduced into RN6390 and SH1000. Levels of norA transcripts increased threefold when the plasmid was present in the RN6390 strain and decreased slightly in SH1000 (Fig. 1B). We also confirmed the findings for RN6390 by using strain 8325-4 (rsbU negative), the direct parental strain of SH1000, in which plasmid pLZ-mgrA resulted in a 2.5-fold increase in norA transcript levels, by qRT-PCR. We also constructed an mgrA knockout mutant from 8325-4 and checked the norA transcript level of this mutant. The presence of the mgrA mutation in 8325-4 resulted in a 1.5-fold decrease in norA transcript levels, as seen with RN6390 and ISP794 backgrounds. Thus, mgrA affects expression of norA differently in strains differing in rsbU, with mgrA acting as a positive regulator in the rsbU-negative strains and a negative regulator in the rsbU+ strains.
Posttranslational modifications of MgrA.
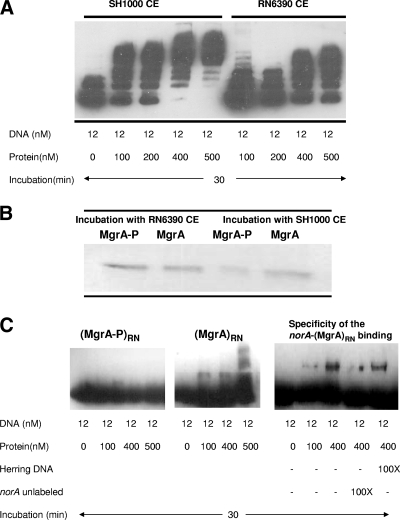
We postulated that these differential effects might be due to posttranslational modification of MgrA. To detect posttranslational modification of MgrA, we incubated heterologously expressed and purified histidine-tagged MgrA with crude cell extracts prepared from RN6390 and SH1000. After 30 min of incubation, we repurified the MgrA protein by nickel affinity chromatography. After the column was washed with Tris-HCl buffer containing 50 and 100 mM imidazole, MgrA was eluted with buffer containing 200 mM imidazole. The eluted protein was dialyzed overnight in water to eliminate the imidazole before protein-DNA gel mobility shift assays using the norA promoter as target DNA was performed. We used increasing amounts of protein and kept the DNA concentration constant at 12 nM for the norA promoter DNA. MgrASH (after incubation with SH1000 crude extract) generated a more rapid and extensive shift of the norA promoter DNA than did MgrARN (after incubation with RN6390 crude extract) and reached total binding at a concentration 500 nM of protein. This assay suggested that MgrA was modified differently by incubation with extracts of RN6390 and SH1000 in a manner that affected its binding to the norA promoter DNA (Fig. 2A).
FIG. 2.
A. Gel mobility shift analyses of the interactions of the purified MgrA protein with the biotinylated norA promoter DNA. (A) Protein and DNA were in contact for 30 min at room temperature, followed by electrophoresis through a 5% acrylamide gel. CE, crude extract. (B) SDS-PAGE analysis of histidine-tagged MgrA purified by Ni affinity chromatography after incubation with crude cell extracts. The column was washed with buffers containing 10 and 60 mM imidazole, and the purified protein was eluted with buffer containing 300 mM imidazole. The phosphorylated protein was then purified using a phosphocolumn (Qiagen) following the manufacturer's protocol. The presence of phospho-MgrA was confirmed by Western blotting using AntiSerine antibody (Qiagen) (data not shown). CE, crude extract. (C) Gel mobility shift analyses of the interactions of the purified MgrA and phospho-MgrA proteins with the biotinylated promoter of norA. The two forms of MgrA were purified after incubation with RN6390 crude extract. Unlabeled norA fragment and herring sperm DNA were used to assess the specificity of binding. Protein and DNA concentrations and ratios of unlabeled to labeled DNA used in this assay are indicated in the table below the figures. RN, RN6390.
We hypothesized that the modification of MgrA by phosphorylation could be responsible for differences in binding to norA promoter DNA. We repeated the purification, the incubation with crude cell extracts, and the repurification of MgrA and then separated the phosphorylated form of MgrA, using a phospho-protein purification kit. Starting from the same amounts of MgrA (5 μg), we observed a ratio for MgrA versus phospho-MgrA of 1:1 and 2:1 after incubation with RN6390 and SH1000 crude extracts, respectively (Fig. 2B). We then performed the gel mobility shift assays, using both forms of MgrA with equivalent concentrations of protein. While unphosphorylated MgrA generated a binding pattern similar to that of heterologously expressed and purified MgrA, phospho-MgrA showed no binding to norA promoter DNA (Fig. 2C). MgrARN binding was demonstrated to be specific by competition with unlabeled norA promoter DNA and nonspecific herring sperm DNA (Fig. 2C). Western blot analysis using anti-Xpress antibody showed that the MgrA protein remained intact after incubations with cell extracts (data not shown). Thus, phospho-MgrA and unphosphorylated MgrA differ in their ability to bind norA promoter DNA, and relative proportions of these two forms of MgrA differ with incubations with RN6390 and SH1000 cell extracts, suggesting that rsbU modulates the relative levels of the two forms of MgrA.
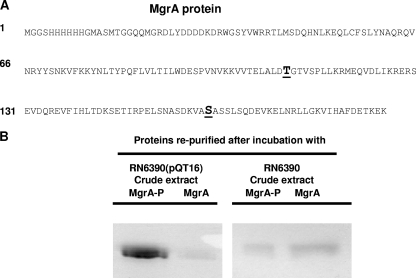
We also performed mass spectrometry to assess posttranslational modification of MgrA protein incubated with RN6390 or SH1000 crude extracts. Phosphorylation of the threonine at position 109 and the serine at position 161 were unequivocally identified (Tufts Core Facility) for MgrA incubated with RN6390 extracts (Fig. 3A). These residues were also phosphorylated in MgrA incubated with the SH1000 extracts, but a control analysis using the histidine-tagged MgrA without incubation with cell extracts revealed no phosphorylation at either residue (data not shown).
FIG. 3.
(A) Mass spectrometry data indicating two phosphorylated residues, threonine 109 and serine 161, of the MgrA amino acid sequence, are shown in boldface and underlined. (B) SDS-PAGE analysis of histidine-tagged MgrA purified with a Ni affinity column after incubation with crude extracts prepared from RN6390 and RN6390(pQT16), which overexpressed pknB.
Effects of the kinases PknB and RsbW on phosphorylation of MgrA.
We searched the published genome sequence of S. aureus N315 for protein kinase candidates that might be responsible for phosphorylation of MgrA. One open reading frame (SA1063) encoded a 664-amino-acid protein that showed 37% identity and 56% similarity with the serine/threonine kinase PrkC protein of Bacillus subtilis and 99% similarity with the protein kinase PknB of S. aureus RF122. We called this open reading frame pknB based on these similarities. Further searches indicated that pknB is present in all published S. aureus genomes (N315, Mu50, COL, MW2, MRS252, MSSA476, USA300, JH9, JH1, and Newman). We performed PCR, RT-PCR, and Southern blotting to confirm the presence of pknB in RN6390 and SH1000. The pknB gene amplified from the genome of S. aureus RN6390 was 1,995 bp in length and was identified as pknB by DNA sequencing and BLAST searches. To determine the effect of pknB expression on phosphorylation on MgrA, we cloned pknB into plasmid pSK950 to generate plasmid pQT16 and incubated MgrA with cell extracts prepared from RN6390(pQT16), which overexpressed pknB. After phosphoseparation using a Qiagen kit, we observed an increase of threefold in the amount of phospho-MgrA compared to that of RN6390(pSK950) by SDS-PAGE electrophoresis (Fig. 3B).
For comparison, we evaluated the effect of overexpression of rsbW, which encodes a histidine kinase-like ATPase and is found in the rsbU-rsbV-rsbW-sigB regulon. We amplified the 480-bp rsbW gene from the genome of RN6390 and generated plasmid pQT17, which overexpressed rsbW. Incubation of MgrA with crude extracts prepared from rsbW-overexpressor RN6390(pQT17) failed to generate a significant increase in the amount of phospho-MgrA (data not shown).
PknB affects the expression of norA.
We introduced pQT16 into S. aureus RN6390 and SH1000 after passage through S. aureus RN4220. Real-time RT-PCRs were then performed to measure the levels of transcription of pknB, mgrA, and norA. We observed an increase of fivefold for the norA transcript levels in the two pknB overexpressors RN6390(pQT16) and SH1000(pQT16) relative to those for strains that carried the vector plasmid pSK950 (Table 3). MICs were determined to assess changes in the quinolone resistance phenotype of strains with overexpression of pknB. We observed a fourfold increase in the MICs of norfloxacin and ciprofloxacin, which are known NorA substrates, for both strains RN6390 and SH1000 in the presence of plasmid pQT16. No change in MICs was found, however, for sparfloxacin and moxifloxacin, which are not substrates of NorA (Table 4). Thus, pknB overexpression results in increased phosphorylation of MgrA and is associated with increased expression of norA and a quinolone resistance profile consistent with that of NorA substrates. These results suggest that MgrA in its unphosphorylated form is a repressor of norA expression and that this repression can be removed by MgrA phosphorylation.
TABLE 3.
Effect of pknB expression on levels of expression of norA and mgrA
| S. aureus strain(plasmid)a | Genotype expression levelb | Relative transcript levelc
|
||
|---|---|---|---|---|
| norA | mgrA | pknB | ||
| RN6390 | rsbU− | 1 | 1 | 1 |
| SH1000 | rsbU+ | 1.3 | 1 | 1.7 |
| RN6390(pSK950) | rsbU− | 1 | 1 | 1 |
| SH1000(pSK950) | rsbU+ | 1.3 | 1 | 1.7 |
| RN6390(pQT16) | rsbU−; pknB+++ | 5 | 1 | 8 |
| SH1000(pQT16) | rsbU+; pknB+++ | 5 | 1 | 8 |
Strains harboring plasmid pSK950 or pQT16 were grown in the presence of tetracycline (5 μg/ml).
−, negative; +, positive; +++, overexpression.
Values represent transcript (n-fold) changes relative to that of RN6390.
TABLE 4.
Effects of the expression of pknB on quinolone susceptibility
| Strain (plasmid)a | Genotype expression levelb | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| Nor- floxacin | Cipro- floxacin | Moxi- floxacin | Spar- floxacin | ||
| RN6390 | rsbU − | 0.5 | 0.25 | 0.03 | 0.06 |
| SH1000 | rsbU+ | 0.5 | 0.5 | 0.03 | 0.06 |
| RN6390 (pSK950) | rsbU− | 0.5 | 0.25 | 0.03 | 0.06 |
| SH1000 (pSK950) | rsbU+ | 0.5 | 0.5 | 0.03 | 0.06 |
| RN6390 (pQT16) | rsbU−; pknB+++ | 2 | 1 | 0.03 | 0.06 |
| SH1000 (pQT16) | rsbU+; pknB+++ | 2 | 2 | 0.03 | 0.06 |
Strains harboring plasmid pSK950 or pQT16 were grown in the presence of tetracycline (5 μg/ml).
−, negative; +, positive; +++, overexpression.
Notably, the level of expression of chromosomal pknB was slightly higher (1.7-fold) in SH1000 than in RN6390, suggesting that PknB itself cannot account for the differences in the extent of phosphorylation of MgrA by incubation with the extracts of these two strains. These findings further imply that other factors regulated by rsbU and possibly sigB contribute to the extent of phosphorylation of MgrA and its differential regulation of norA expression.
DISCUSSION
After MgrA was discovered and shown to be a global regulator affecting expression of many genes, disparities were identified among studies regarding the role of MgrA in the regulation of the expression of several genes, including those encoding NorA, NorB, and AbcA (13, 16, 17, 26). In the case of norA, studies of strain ISP794 from the 8325-4 lineage generated findings consistent with MgrA acting as a positive regulator of norA expression (26). In contrast, in a later study, transcriptional profiling of the mgrA regulon in strain Newman showed a 2.9-fold increase in the norA transcript level in an mgrA mutant in the late-log phase of growth, suggesting that MgrA functioned as a negative regulator of norA (16). In addition, in a study using strain SH1000, mgrA overexpression resulted in decreased norA expression as measured by a norA::lacZ transcriptional fusion reporter (13). The last two studies used different strains, both of which differed from the original 8325 strain at the rsbU locus. To assess the role of rsbU in mgrA regulation of norA expression, we compared two related widely used 8325-4 laboratory strains, RN6390 and SH1000, which differed at the rsbU locus.
We have now shown by genetic studies that mgrA functions as a positive regulator of norA expression in the rsbU mutant background in strains RN6390 and 8325-4, whereas it functions as a negative regulator in the rsbU+ reconstituted derivative of 8325-4, SH1000 (9). In S. aureus, rsbU is required for the activation of the sigB regulon, which controls the expression of multiple genes in response to stress (2). We hypothesized that the rsbU- or sigB-dependent factor(s) might modify MgrA in such a way as to affect its function and binding to the norA promoter.
mgrA cloned and heterologously expressed in E. coli may generate a form of MgrA that lacks possible posttranslational modifications that occur specifically in the S. aureus cytoplasm. Thus, we sought to determine if MgrA, repurified after incubation with cell extracts of RN6390 and SH1000, differed in its ability to bind to the norA promoter DNA. Remarkably, the rate and extent of binding of MgrA to the norA promoter differed depending on incubation with cell extracts from RN6390 or SH1000, suggesting that MgrA is modified differentially by the two extracts in a manner that alters its binding and possibly regulatory properties. Analyses of the repurified MgrA, using mass spectrometry, revealed phosphorylation of at least two amino acid residues (serine 161 and threonine 109). Less MgrA became phosphorylated after incubation with crude extract prepared from SH1000 than after incubation with RN6390 crude extract. Notably, phospho-MgrA lost its ability to bind to the norA promoter. These findings are consistent with prior DNA mobility shift data showing that heterologously expressed MgrA purified from E. coli that lacks phosphorylation was able to bind norA promoter DNA (26) and explained the phenomenon observed with MgrASH, for which more unphosphorylated MgrA was available for DNA binding.
We also identified a putative serine/threonine kinase, PknB, based on its relationship to a similar kinase of B. subtilis. The kinase activity of PknB was suggested by the observed threefold increase in the quantity of phospho-MgrA after incubation of MgrA with extracts of cells expressing cloned pknB. Plasmid-expressed pknB also resulted in a fivefold increase in the norA transcript levels in both RN6390 and SH1000, as well as increases in the MICs of the substrates norfloxacin and ciprofloxacin for NorA. Thus, increased phospho-MgrA is associated with increased expression of norA and increased quinolone resistance.
Although we cannot as yet distinguish whether PknB itself or other phosphorelay proteins triggered by pknB expression resulted in the increased phosphorylation of MgrA, our data taken together suggest that MgrA can be posttranslationally phosphorylated by PknB or another kinase and that phosphorylation modifies the ability of MgrA to bind to the norA promoter and affects the expression of the norA efflux pump. The direct link between the rsbU-rsbV-rsbW-sigB regulon and the differences in the regulatory role of MgrA for norA in the rsbU+ and rsbU strains, however, remains elusive, since pknB is not overexpressed in RN6390 relative to that in SH1000. Although overexpression of MgrA has been shown to result in a twofold increase in the expression of the rsbW-encoded kinase (17), overexpression of rsbW appeared not to result in increased phosphorylation of MgrA, possibly because RsbW is a histidine kinase and the physiologically relevant modifications of MgrA involve phosphorylation of serine(s) and threonine(s).
Thus, our findings suggest that the differing roles of MgrA in regulation of norA are under the control of some other factor(s) regulated by rsbU or sigB. In B. subtilis, the sigB regulon controls the environmental stress response by a cascade of signal transduction, initiated by a kinase (RsbT) which activates RsbU after forming a complex with this phosphatase (8). We hypothesize that the regulation of norA expression by MgrA depends on a cascade of regulators linked to the sigB regulon that control one another by a series of phosphorylations and dephosphorylations and that MgrA is a substrate in this phosphorelay system.
We propose a preliminary model to explain our findings in the presence of intact rsbU. With rsbU intact, lower proportions of phospho-MgrA and more binding of MgrA to the norA promoter are associated with findings from genetic studies indicating that mgrA behaves as a negative regulator of norA expression in the rsbU+ background. Furthermore, exceptionally high levels of phospho-MgrA resulting from the overexpression of pknB result in increased expression of norA. These associations would suggest that unphosphorylated MgrA functions as a norA repressor and that increased phosphorylation relieves repression. In this model, for a strain with disruption of functional rsbU, a higher proportion of phospho-MgrA would be expected to result in less binding of MgrA to norA promoter DNA and lower levels of repression, thereby causing higher levels of norA expression. Two findings, however, suggest that additional factors must be invoked to explain the role of mgrA as a positive regulator of norA expression in genetic studies in several strains with the rsbU-negative background (ISP794, RN6390, and 8325-4). First, baseline levels of norA transcripts are similar in the rsbU+ and rsbU-negative strain backgrounds, rather than the higher levels that would be predicted in the rsbU-negative background with a higher proportion of phospho-MgrA. Second, the increased expression of norA seen when mgrA is overexpressed in the rsbU-negative background would not be predicted to occur from increased levels of either unphosphorylated MgrA or phospho-MgrA if unphosphorylated MgrA is a repressor of norA, and phospho-MgrA loses its effect by lack of binding to the norA promoter. Thus, we predict that the additional regulators that are also affected by rsbU may be interacting with the norA promoter alone or together with MgrA, phospho-MgrA, or both to modulate norA expression. The interactions of potential additional regulators of norA with MgrA are the subject of ongoing work.
Finally our findings imply that efflux pump expression will likely vary in response to specific stresses to which the sigB regulon is known to respond, including heat shock, addition of MnCl2 or NaCl, and alkaline shock (21), responses that are consistent with the native roles of a centrally regulated complement of multiple efflux pumps functioning to protect the cell in response to environmental stresses.
Acknowledgments
This work was supported in part by grant R37 AI023988 from the U.S. Public Health Service, National Institutes of Health (to D.C.H.).
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Ahn, B. Z., K. U. Baik, G. R. Kweon, K. Lim, and B. D. Hwang. 1995. Acylshikonin analogues: synthesis and inhibition of DNA topoisomerase-I. J. Med. Chem. 381044-1047. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff, M., P. Dunman, J. Kormanec, D. Macapagal, E. Murphy, W. Mounts, B. Berger-Bachi, and S. Projan. 2004. Microarray-based analysis of the Staphylococcus aureus σB regulon. J. Bacteriol. 1864085-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 1835171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier, B., R. Aras, and D. C. Hooper. 2000. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J. Bacteriol. 182664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fournier, B., Q. C. Truong-Bolduc, X. Zhang, and D. C. Hooper. 2001. A mutation in the 5′ untranslated region increases stability of norA mRNA, encoding a multidrug resistance transporter of Staphylococcus aureus. J. Bacteriol. 1832367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gertz, S., S. Engelmann, R. Schmid, A. K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 1826983-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 1831843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardwick, S. W., J. Pane-Farre, O. Delumeau, J. Marles-Wright, J. W. Murray, M. Hecker, and R. J. Lewis. 2007. Structural and functional characterization of partner switching regulating the environmental stress response in Bacillus subtilis. J. Biol. Chem. 28211562-11572. [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 1845457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iandolo, J. J., V. Worrell, K. H. Groicher, Y. D. Qian, R. Y. Tian, S. Kenton, A. Dorman, H. G. Ji, S. P. Lin, P. Loh, S. L. Qi, H. Zhu, and B. A. Roe. 2002. Comparative analysis of the genomes of the temperate bacteriophages phi11, phi12 and phi13 of Staphylococcus aureus 8325. Gene 289109-118. [DOI] [PubMed] [Google Scholar]
- 11.Ingavale, S., W. van Wamel, T. T. Luong, C. Y. Lee, and A. L. Cheung. 2005. Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus. Infect. Immun. 731423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingavale, S. S., W. Van Wamel, and A. L. Cheung. 2003. Characterization of RAT, an autolysis regulator in Staphylococcus aureus. Mol. Microbiol. 481451-1466. [DOI] [PubMed] [Google Scholar]
- 13.Kaatz, G. W., R. V. Thyagarajan, and S. M. Seo. 2005. Effect of promoter region mutations and mgrA overexpression on transcription of norA, which encodes a Staphylococcus aureus multidrug efflux transporter. Antimicrob. Agents Chemother. 49161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreiswirth, B. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305709-712. [DOI] [PubMed] [Google Scholar]
- 15.Kullik, I., and P. Giachino. 1997. The alternative sigma factor σB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167151-159. [DOI] [PubMed] [Google Scholar]
- 16.Luong, T. T., P. M. Dunman, E. Murphy, S. J. Projan, and C. Y. Lee. 2006. Transcription profiling of the mgrA regulon in Staphylococcus aureus. J. Bacteriol. 1881899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luong, T. T., S. W. Newell, and C. Y. Lee. 2003. mgr, a novel global regulator in Staphylococcus aureus. J. Bacteriol. 1853703-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manna, A., and A. L. Cheung. 2001. Characterization of sarR, a modulator of sar expression in Staphylococcus aureus. Infect. Immun. 69885-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manna, A. C., S. S. Ingavale, M. Maloney, W. van Wamel, and A. L. Cheung. 2004. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J. Bacteriol. 1865267-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemeyer, D. M., M. J. Pucci, J. A. Thanassi, V. K. Sharma, and G. L. Archer. 1996. Role of mecA transcriptional regulation in the phenotypic expression of methicillin resistance in Staphylococcus aureus. J. Bacteriol. 1785464-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pane-Farre, J., B. Jonas, K. Forstner, S. Engelmann, and M. Hecker. 2006. The σB regulon in Staphylococcus aureus and its regulation. Int. J. Med. Microbiol. 296237-258. [DOI] [PubMed] [Google Scholar]
- 22.Shaw, L. N., J. Aish, J. E. Davenport, M. C. Brown, J. K. Lithgow, K. Simmonite, H. Crossley, J. Travis, J. Potempa, and S. J. Foster. 2006. Investigations into σB-modulated regulatory pathways governing extracellular virulence determinant production in Staphylococcus aureus. J. Bacteriol. 1886070-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truong-Bolduc, Q. C., P. M. Dunman, J. Strahilevitz, S. J. Projan, and D. C. Hooper. 2005. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 1872395-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truong-Bolduc, Q. C., and D. C. Hooper. 2007. Transcriptional regulators NorG and MgrA modulate resistance to both quinolones and β-lactams in Staphylococcus aureus. J. Bacteriol. 1892996-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truong-Bolduc, Q. C., J. Strahilevitz, and D. C. Hooper. 2006. NorC, a new efflux pump regulated by MgrA of Staphylococcus aureus. Antimicrob. Agents Chemother. 501104-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truong-Bolduc, Q. C., X. Zhang, and D. C. Hooper. 2003. Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus. J. Bacteriol. 1853127-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, P. Z., S. J. Projan, K. R. Leason, and R. P. Novick. 1987. Translational fusion with a secretory enzyme as an indicator. J. Bacteriol. 1693082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 1786036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, L., F. Fan, L. M. Palmer, M. A. Lonetto, C. Petit, L. L. Voelker, A. St. John, B. Bankosky, M. Rosenberg, and D. McDevitt. 2000. Regulated gene expression in Staphylococcus aureus for identifying conditional lethal phenotypes and antibiotic mode of action. Gene 255297-305. [DOI] [PubMed] [Google Scholar]