Abstract
Pseudomonas aeruginosa Pa5196 produces type IV pilins modified with unusual α1,5-linked d-arabinofuranose (α1,5-d-Araf) glycans, identical to those in the lipoarabinomannan and arabinogalactan cell wall polymers from Mycobacterium spp. In this work, we identify a second strain of P. aeruginosa, PA7, capable of expressing arabinosylated pilins and use a combination of site-directed mutagenesis, electrospray ionization mass spectrometry (MS), and electron transfer dissociation MS to identify the exact sites and extent of pilin modification in strain Pa5196. Unlike previously characterized type IV pilins that are glycosylated at a single position, those from strain Pa5196 were modified at multiple sites, with modifications of αβ-loop residues Thr64 and Thr66 being important for normal pilus assembly. Trisaccharides of α1,5-d-Araf were the principal modifications at Thr64 and Thr66, with additional mono- and disaccharides identified on Ser residues within the antiparallel beta sheet region of the pilin. TfpW was hypothesized to encode the pilin glycosyltransferase based on its genetic linkage to the pilin, weak similarity to membrane-bound GT-C family glycosyltransferases (which include the Mycobacterium arabinosyltransferases EmbA/B/C), and the presence of characteristic motifs. Loss of TfpW or mutation of key residues within the signature GT-C glycosyltransferase motif completely abrogated pilin glycosylation, confirming its involvement in this process. A Pa5196 pilA mutant complemented with other Pseudomonas pilins containing potential sites of modification expressed nonglycosylated pilins, showing that TfpW's pilin substrate specificity is restricted. TfpW is the prototype of a new type IV pilin posttranslational modification system and the first reported gram-negative member of the GT-C glycosyltransferase family.
Pseudomonas aeruginosa is a gram-negative bacterium that inhabits diverse environments and is an important opportunistic pathogen of immunocompromised individuals and cystic fibrosis patients (14, 34, 39, 43, 47, 48). The ubiquity of these bacteria relies in part on their ability to adhere to a wide variety of surfaces via the key virulence factor type IV pili (T4P) (12, 21, 25, 26, 44). T4P are strong and flexible proteinaceous surface filaments expressed by a wide range of bacteria and are involved in twitching motility, adherence, competence, and bacteriophage adsorption (8, 26, 42, 60). The T4P of P. aeruginosa are composed primarily of monomeric subunits of pilin encoded by the pilA gene (19, 38, 55). We previously identified within the P. aeruginosa species five PilA variants (designated groups I through V) which differ in amino acid sequence and length, in their association with specific accessory genes, and in the presence or absence of posttranslational modifications (PTMs) (34).
Glycosylation is now recognized as a common PTM of surface-associated proteins of mucosal pathogens (57). Recent studies have demonstrated roles for this modification in adhesion, protection from proteolytic cleavage, solubility, antigenic variation, and protective immunity (21, 33, 36, 40, 41, 58). Among the best-studied examples of glycosylated bacterial proteins are the T4P of Neisseria gonorrhoeae, Neisseria meningitidis, and P. aeruginosa (reviewed in references 5, 45, and 57). Pilins of N. meningitidis are O glycosylated at Ser63 with Gal (β1-4) Gal (α1-3) 2,4-diacetamido-2,4,6-trideoxyhexose (DATDH) (53), and genetic variation in the pilin glycosylation locus can result in modification with 2-acetamido-4-glyceramido-2,4,6-trideoxyhexose (10). Recent data indicate that N. gonorrhoeae pilins can be modified at Ser63 with a related glycan, HexNAcDATDH (2, 27). The pilin glycan does not appear to influence pilus-mediated adhesion, but in the case of N. meningitidis, plays a role in production of truncated soluble S-pilins (40, 41).
To date, two different pilin glycosylation systems have been identified in P. aeruginosa. The first is the well-described system of strain 1244 (group I T4P), in which the oligosaccharide transferase TfpO (also called PilO) attaches an O-antigen subunit of the host lipopolysaccharide to the C-terminal Ser148 of PilA from strain 1244 (PilA1244). This site of modification is immediately adjacent to the C-terminal disulfide-bonded loop implicated as the adhesintope (15, 62). tfpO mutants produce functional, nonglycosylated pili with increased hydrophobicity compared to those of the wild type (49). The mutants exhibit reduced twitching motility and are less virulent than the wild-type strain in competitive infection assays, suggesting that the glycan may provide a colonization advantage. The glycan may contribute to T4P adhesiveness or may serve a protective role, shielding the disulfide-bonded loop from proteolytic damage, thus enhancing colonization and virulence over the nonglycosylated mutant strains (15, 49). The pilin glycan may also provide additional antigenic diversity, allowing glycosylated strains to evade immune responses that target nonmodified T4P or to alter their interaction with the immune system.
We recently identified a second pilin glycosylation system in P. aeruginosa strain Pa5196 (group IV) (59). Initial studies of strain Pa5196 showed that its pilins were modified at multiple positions with homopolymers of α-1,5-linked d-arabinofuranose (α-1,5-d-Araf) (59); this rare sugar configuration is typically found in the lipoarabinomannan (LAM) and arabinogalactan components of the cell walls of mycobacteria. Initial analysis of the glycosylation pattern of PilA5196 indicated that the sites of modification were likely to be on predicted loops in the antiparallel beta sheet region of the subunits, more similar to pilins of Neisseria than to those of P. aeruginosa 1244.
In P. aeruginosa strain 1244 and other group I strains, the pilin glycosyltransferase TfpO is encoded immediately downstream of pilA (9, 20). Strain Pa5196 contains two novel open reading frames, tfpW and tfpX, in the corresponding locus (34). Based on the location of tfpW in the pilin operon and the predicted membrane association and topology of TfpW, which resembles that of TfpO despite the lack of sequence identity between the two proteins, we hypothesized that it may be the arabinosyltransferase responsible for attaching the d-Araf to the pilin subunits.
The present study used a multifaceted approach to specifically map the glycosylation sites within PilA5196, using alanine replacement mutagenesis of candidate residues, as well as electron transfer dissociation mass spectrometry (ETD-MS) of tryptic glycopeptides. ETD-MS involves a novel method of peptide fragmentation that cleaves the bond between the amide nitrogen and the alpha carbon of the peptide, while delicate posttranslational modifications, such as O-linked phosphorylation and glycosylation, remain unaltered (16, 29, 56, 63). To our knowledge, this is the first report of this technique being used in the characterization of bacterial glycoproteins and demonstrates that it is an excellent tool for bottom-up studies of complex modified proteins. Finally, we present evidence that TfpW is a glycosyltransferase C family member involved in posttranslational glycosylation of the Pa5196 pilin and propose a model for this novel bacterial protein modification system.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this work are summarized in Table 1. Escherichia coli and P. aeruginosa strains were maintained as glycerol stocks at −80°C and grown on Luria-Bertani (LB) agar (Difco) under standard conditions. For E. coli strains in LB, 100 μg/ml ampicillin and 15 μg/ml gentamicin (Gm) were used; for P. aeruginosa strains in LB, 50 μg/ml gentamicin, 200 μg/ml carbenicillin (Carb), and 5% sucrose were used. P. aeruginosa strains complemented with genes cloned into the pBADGr vector were grown on LB agar supplemented with gentamicin and 0.01% to 0.2% l-arabinose (BioShop). Mycobacterium smegmatis strain mc2155 was grown, and lysates were prepared as described previously (59).
TABLE 1.
Bacterial strains and constructs used in this work
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli | ||
| XL10-Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔM15 Tn10 (Tetr) Amy Camr] | Stratagene |
| DH5α | F− φ80dlacZM15 (lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 λ− | Lab stocks |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| M. smegmatis mc2155 | Avirulent lab strain | J. Liu |
| P. aeruginosa | ||
| Pa5196 | Group IV T4P; rectal isolate | 34 |
| PA7 | Group IV T4P; multidrug-resistant clinical isolate | P. Roy |
| 5196pilA+GentFRT | pilA::aacC1FRT | This study |
| Pa5196tfpW | EZ::Tn FRT insertion in tfpW | This study |
| PAO1 | Group II T4P; lab strain | |
| PAK | Group II T4P | J. Boyd |
| 1244 | Group I T4P; clinical isolate | P. Castric |
| PA14 | Group III T4P; clinical isolate | |
| Pa281457 | Group V T4P; clinical isolate | 34 |
| Plasmids | ||
| pEX18AP | Apr; oriT+sacB+ | 28 |
| pEX18AP+5196BAW | 3′ end of pilB, pilA, 5′ end of tfpW | This study |
| pEX18Ap+5196BAW-SphI | SDM of base pairs C359-T and T360-G to create SphI site in pilA | This study |
| pEX18AP+5196BAW-GentFRT | Gent-FRT insertion in SphI site of pilA | This study |
| pMOD EZ::TN 2 | Transposon construction plasmid | Epicentre |
| pMOD EZ::TN 2_Gent-FRT | Insertion of Gent-FRT into SacI site of EZ::TN transposon | This study |
| pEX18AP+5196pilAtfpW::EZ::TN Gent-FRT-48 | EZ::TN Gent-FRT insertion in tfpW | This study |
| pBADGr | ori araC-PBAD Gmrmob+ | H. Harvey et al., unpublished data |
| pBADGr+5196pilA | pilA5196 | This study |
| pBADGr+5196pilA-tfpW | pilA5196 and tfpW | This study |
| pBADGr+5196pilA-tfpW-tfpX | pilA5196, tfpW, and tfpX | This study |
| pBADGr+5196pilA-tfpX | pilA5196 and tfpX | This study |
| pBADGr+MPAO1pilA | pilAMPAO1 | This study |
| pBADGr+PAKpilA | pilAPAK | This study |
| pBADGr+1244pilA | pilA1244 | This study |
| pBADGr+1244pilA-pilO | pilA1244 and pilO/tfpO | This study |
| pBADGr+PA14pilA | pilAPA14 | This study |
| pBADGr+1457pilA | pilA1457 | This study |
| pBADGr+5196pilA T64A | PilA Thr64Ala | This study |
| pBADGr+5196pilA T66A | PilA Thr66Ala | This study |
| pBADGr+5196pilA T64A and T66A | PilA Thr64Ala, Thr66Ala | This study |
| pBADGr+5196pilA T64S and T66S | PilA Thr64Ser, Thr66Ser | This study |
| pBADGr+5196pilA T75A | PilA Thr75Ala | This study |
| pBADGr+5196pilA T86A | PilA Thr86Ala | This study |
| pBADGr+5196pilA T97A | PilA Thr97Ala | This study |
| pBADGr+5196pilA T99A | PilA Thr99Ala | This study |
| pBADGr+5196pilA T101A | PilA Thr101Ala | This study |
| pBADGr+5196pilA T97A, T99A, and T101A | PilA Thr97Ala, Thr99Ala, and Thr101Ala | This study |
| pBADGr+5196pilA T86A, T97A, T99A, and T101A | PilA Thr86Ala, Thr97Ala, Thr99Ala, and Thr101Ala | This study |
| pBADGr+5196pilA T64A, T66A, T97A, T99A, and T101A | PilA Thr64Ala, Thr66Ala, Thr86Ala, Thr97Ala, Thr99Ala, and Thr101Ala | This study |
Sequence and structural analyses of PilA and TfpW.
DNA sequence information was obtained through direct sequencing, from the J. Craig Venter Institute (www.tigr.org) and NCBI (www.ncbi.nlm.nih.gov). Homology searches were performed with BLASTP and PSI-BLAST (4). Alignments of amino acid sequences were performed by ClustalW (11). Secondary structure predictions for PilA were performed by PHYRE version 2.0 (http://www.sbg.bio.ic.ac.uk/phyre/). TfpW transmembrane topology and structural predictions were performed using Transmembrane Hidden Markov Model (TMHMM v.2.0; http://www.cbs.dtu.dk/services/TMHMM-2.0/), and TMRPres2D (51). Domain searches of TfpW were done using Pfam 22.0 (http://pfam.sanger.ac.uk/) (41). TfpW was classified within glycosyltransferase families using the CAZy database (carbohydrate-active enzymes) (http://www.cazy.org/fam/acc_GT.html) (17).
Generation of pilA and tfpW mutants.
For creation of the pilA mutant, a 2,052-bp PCR product, encoding pilA and flanking DNA, was amplified from P. aeruginosa Pa5196 genomic DNA using the primers 5196B-int-rev3-XbaI and 5196W-int-rev3-HindIII (Table 2) and gel purified (Qiagen). The PCR product was cloned into the XbaI and HindIII sites of the P. aeruginosa suicide vector pEX18AP (28), creating pEX18AP+5196BAW, and transformed into E. coli DH5α. A SphI restriction site was introduced into pilA of pEX18AP+5196BAW via site-directed mutagenesis (below) using primers 5196A-SDM-SphI_F and 5196A-SDM-SphI_R, creating pEX18AP+5196BAW-SphI. A gentamicin resistance cassette flanked by FLP recombination target (FRT) sites was released from pPS856 (28) by digestion with SphI and cloned into the SphI site of pEX18AP+5196BAW-SphI, creating pEX18AP+5196BAW-GmFRT. This construct was electroporated into strain Pa5196 (13), and recombinant strains were selected on LB containing 50 μg/ml Gm. The resulting gentamicin-resistant pilA knockout was transformed with pFLP2 (28) to excise the gentamicin marker and plated on LB containing 200 μg/ml Carb. The resulting carbenicillin-resistant, gentamicin-sensitive transformants were cured of pFLP2 by plating on LB plus 5% sucrose, resulting in the unmarked pilA mutant 5196NP.
TABLE 2.
Primers used in this work
| Primer | Oligonucleotide sequencea |
|---|---|
| 5196B-int-rev3-XbaI | 5′-AATCTAGATGGTGGTTGGCGGGGTCGGAG |
| 5196W-int-rev3-HindIII | 5′-AAAAGCTTGAGAACCACCATCTGCCCAGC |
| 5196A_SDM-SphI_F | 5′-AGAAGCTGACCTTCAGCATGCAAGACGGGGGTTCGTCTTGGGCGTG |
| 5196A_SDM-SphI_R | 5′-ACGAACCCCCGTCTTGCATGCTGAAGGTCAGCTTCTTACCAGAAGC |
| 5196pilAup(noSD)-EcoRI | 5′-AAGAATTCCAATGAAAGCGCAAAAAGGC |
| 5196tfpWdown (HindIII) | 5′-CAGAAGCTTATACTGGAAAAAAGAAGATG |
| 5196tfpWup (EZTN) (for tfpW) | 5′-GTTCGTCTTGGGCGTGTGGT |
| Gm_up | 5′-AAGCCTGTTCGGTTCGTAA |
| Gm_down | 5′-TTCTTCCCGTATGCCCAA |
| 5196pilAup-EcoRI | 5′-AAGAATTCATGAAAGCGCAAAAAGGC |
| 5196pilAdown-HindIII | 5′-AGAAGCTTAAAAAGAGACAAGCCCCGCA |
| tfpWdown-HindIII | 5′-AGAAGCTTATACTGGAAAAAAGAAGATG |
| tRNAThrdown-HindIII | 5′-AAAAAGCTTCGAATGAGCTGCTCTACCGACAGAGCT |
| tfpXbeg+pilAend_down | 5′-TCACGTAACTCACCACACCTATTTTGAGCGCTATTACGGC |
| pilAend+tfpWbeg_up | 5′-TAATAGCGCTCAAAATAGGTGTGGTGAGTTACGTGAGC |
| pilAII, III, V up EcoRI | 5′-AAGAATTCATGAAAGCTCAAAAAGGC |
| pilAIII down HindIII | 5′-AGAAGCTTTGCCATCCTCCTGCTATTC |
| pilAV down HindIII | 5′-AGAAGCTTCTCACAACTTTCCGTCTTTT |
| pilAI up EcoRI | 5′-AAGAATTCATGAAAGCTCAGAAGGGT |
| pilAI down HindIII | 5′-AGAAGCTTCAAAACAACTCAAAAAACC |
| 5196pilA SDM T64A fwd | 5′-GACTCTGGTTGCCCCAgCTGCTACTCCTGGGGCTGGTCAATTGAATGC |
| 5196pilA SDM T64A rev | 5′-GCCCCAGGAGTAGCAGcTGGGGCAACCAGAGTCGGCCAAGCG |
| 5196pilA SDM T66A fwd | 5′-GTTGCCCCAACTGCTgCTCCTGGGGCTGGTCAATTGAATGC |
| 5196pilA SDM T66A rev | 5′-GACCAGCCCCAGGAGcAGCAGTTGGGGCAACCAGAGTCGGCC |
| 5196pilA SDM T64A, T66A fwd | 5′-GACTCTGGTTGCCCCAgCTGCTgCTCCTGGGGCTGGTCAATTGAATGC |
| 5196pilA SDM T64A, T66A rev | 5′-GACCAGCCCCAGGAGcAGCAGcTGGGGCAACCAGAGTCGGCCAAGCG |
| 5196pilA SDM T64/66S fwd | 5′-GACTCTGGTTGCCCCAtCTGCTtCTCCTGGGGCTGGTCAATTGAATGC |
| 5196pilA SDM T64/66S rev | 5′-GACCAGCCCCAGGAGaAGCAGaTGGGGCAACCAGAGTCGGCCAAGCG |
| 5196PilA T75A_fwd | 5′-GGTCAATTGAATGCAgCTCTGGTTGGCAAGTATTCGAGC |
| 5196PilA T75A_rev | 5′-GCCAACCAGAGcTGCATTCAATTGACCAGCCCCAGG |
| 5196pilA SDM T86A fwd | 5′-CGAGCGTAGATAGCgCCATTGCCAGCGGCTATCCTAACG |
| 5196pilA SDM T86A rev | 5′-GATAGCCGCTGGCAATGGcGCTATCTACGCTCGAATACTTGCC |
| 5196pilA SDM T97A fwd | 5′-CCTAACGGTCAGATCgCTGTCACCATGACTCAAGGTAAGGC |
| 5196pilA SDM T97A rev | 5′-GAGTCATGGTGACAGcGATCTGACCGTTAGGATAGCCGCTGGC |
| 5196pilA SDM T99A fwd | 5′-GGTCAGATCACTGTCgCCATGACTCAAGGTAAGGCTTCTGG |
| 5196pilA SDM T99A rev | 5′-CTTACCTTGAGTCATGGcGACAGTGATCTGACCGTTAGGATAGC |
| 5196pilA SDM T101A fwd | 5′-GATCACTGTCACCATGgCTCAAGGTAAGGCTTCTGGTAAGAAGC |
| 5196pilA SDM T101A rev | 5′-GAAGCCTTACCTTGAGcCATGGTGACAGTGATCTGACCG |
| 5196pilA SDM T97A, T99A, T101A fwd | 5′-CCTAACGGTCAGATCgCTGTCgCCATGgCTCAAGGTAAGGCTTCTGGTAAGAAGC |
| 5196pilA SDM T97A, T99A, T101A rev | 5′-GAAGCCTTACCTTGAGcCATGGcGACAGcGATCTGACCGTTAGGATAGCCGC |
Nucleic acids in a restriction site (underlined), nucleic acids in a start site (italicized), nucleic acid residues altered in the SDM (lowercase), and codons (boldface) are indicated.
For creation of a tfpW mutant, a 2,541-bp PCR product encompassing pilA and tfpW was amplified from chromosomal DNA from P. aeruginosa Pa5196 using the primers 5196pilAup-EcoRI and tfpwdown-HindIII and subsequently cloned into the EcoRI and HindIII sites of pEX18AP, creating pEX18AP-5196AW. A gentamicin-marked transposon was generated in the EZ::Tn5 transposon construction vector pMOD-2 (Epicentre). The gentamicin resistance gene flanked by FRT sites was released from pPS856 (28) using SmaI and cloned into the SacI site within the multiple cloning site of pMOD-2, resulting in pMODEZ::Tn5_GmFRT. The transposon was released from the vector via digestion with PvuII and gel purified. In vitro mutagenesis of pEX18AP-5196AW with the transposon was performed per the manufacturer's directions. Gentamicin-resistant clones were screened via PCR with the primers 5196tfpWupEZTn and 5196tfpWdown-HindIII, as well as Gm_up and Gm_down, to determine the location and orientation of the transposon within tfpW. Clone 48, in which the transposon had inserted in the forward orientation and within the first third of the gene, was selected for use as the knockout construct. This construct was electroporated into strain Pa5196, and recombinant strains were selected on LB containing 50 μg/ml Gm. Insertion of the transposon into tfpW was verified by PCR of the gentamicin-resistant clones. The gentamicin resistance cassette was removed through the same procedure as described above, resulting in the unmarked tfpW insertion mutant tfpW::FRT.
Complementation of mutants.
Mutants were complemented in trans by expression of genes cloned into a modified pMLBAD vector (35), pBADGr in which a gentamicin resistance cassette (aacC1) had been inserted into the trimethoprim resistance gene dhfrII. Pilin variants representing each group, with and without their unique downstream genes, were amplified using the primers listed in Table 2 and cloned into the EcoRI and HindIII sites of the multiple cloning site of pBADGr. The strains used were MPAO1 (group II), 1244 (group I), PA14 (group III), Pa5196 (group IV), and Pa281457 (group V). To create the pilA-tfpX insert, gene splicing by overlap extension PCR was used to fuse these genes (30). Briefly, in two separate reaction mixtures using P. aeruginosa Pa5196 genomic DNA as a template, pilA was amplified with a tfpX extension (using primers 5196pilAup-EcoRI and tfpXbeg+pilAend_down), and tfpX was amplified with a upstream pilA extension (using primers pilAend+tfpXbeg_up and tRNAThr-down-HindIII). These PCR products were combined in a third PCR with no primers for three cycles in order for the overlapping regions to ligate, and then the flanking primers (5196pilAup-EcoRI and tRNAThr-down-HindIII) were added for an additional 27 cycles to amplify the fused product. The resulting PCR product was cloned as described above and verified by DNA sequence analysis.
Site-directed mutagenesis.
QuikChange mutagenesis (Stratagene, Cedar Creek, TX) was used for introducing site-directed mutations via PCR, with modifications described by Wang and Malcolm (61). The site-directed mutants (SDMs) generated to map the sites of glycosylation of PilA were created in the pBADGr+5196pilA plasmid. The primers used to generate SDMs are listed in Table 2. To determine whether the Asp43-Asp44 residues of TfpW were required for arabinosylation of pilins, PCR using mutageneic primers containing the desired changes (Table 2) was used to amplify two separate fragments (pilA-tfpW and tfpW-tfpX), overlapping at the site of mutation. These fragments were then used as overlapping templates in a third PCR to generate a pilA-tfpW-tfpX construct with the desired changes, verified by DNA sequencing. The product was cloned into pCR2.1-TOPO and subcloned into pBADGr using XbaI and ScaI, and the resulting construct (tfpWD43AD44A) was introduced into the tfpW mutant by electroporation.
Twitching motility assays.
Twitching assays were performed as previously described (23). Briefly, single colonies were stab inoculated with a sterile 200-μl pipette tip to the bottom of a 1% LB agar plate containing appropriate amounts of antibiotic and l-arabinose where indicated and incubated for 24 h at 37°C and then for a further 24 h at room temperature. The agar was carefully removed, and the adherent twitching zones were visualized by staining with 1% crystal violet for 10 min and then rinsing with tap water to remove unbound dye.
Surface pilin isolation, SDS-PAGE, and Western blots.
Pili were harvested and analyzed on gels by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue staining as described previously (59), and where indicated, transferred to nitrocellulose for Western blot analysis by using standard methods. For Western blots, polyclonal rabbit antibodies against the P. aeruginosa Pa5196 pilin were generated by Cedarlane Laboratories. Polyclonal antibodies against Mycobacterium tuberculosis LAM were obtained through the NIH, NIAID contract no. HHSN266200400091C, entitled “Tuberculosis Vaccine Testing and Research Materials,” awarded to Colorado State University.
MS analysis of intact pilin.
Pilins for mass spectrometry (MS) analysis were isolated as described previously (59) by shearing surface-expressed pili from bacteria and dissociating these structures into their component subunits. The purified pilin protein tended to form a precipitate that was impossible to resolubilize in the water-based solutions normally used for electrospray ionization MS (ESI-MS). Therefore, a nonaqueous method was developed to overcome this problem. Pilin solutions were evaporated to complete dryness on a Savant centrifugal evaporator and resuspended in concentrated formic acid (10 μl). The proteins were solubilized by the addition of hexafluroisopropanol (90 μl). All mass spectra were acquired on a Q-TOF2 hybrid quadrupole time-of-flight mass spectrometer (Waters). The nonaqueous pilin solutions were infused into the nanoelectrospray interface at 1 μl/minute, and spectra were recorded in the m/z range from 800 to 2,400 (one acquisition per second). Protein molecular weight profiles were derived from the spectra using MaxEnt (Waters).
Enzymatic digestion and liquid chromatography MS (LC-MS) analysis of the PilA protein from strain 5196NP plus PilA.
The PilA protein from a pilin-less background strain of P. aeruginosa Pa5196 (5196NP) (NP for no pili) was reduced, alkylated, and tryptically digested according to standard protocols. In summary, approximately 200 μl of 1 μg/μl of PilA was dissolved in 1 M Tris HCl and 6 M guanidine HCl (pH 7.5) containing 2 mM dithiothreitol and incubated for 1 h at 50°C. The reduced cysteines were converted to carboxyamidomethyl derivatives using 10-fold excess of iodoacetamide over dithiothreitol. The protein solution was desalted by concentrating twice on a 3,000 MWL Centricon centrifugal filter. The protein was then diluted to approximately 1 μg/μl with 50 mM ammonium bicarbonate after each concentration step. Trypsin (10 μg) was added to the solution, which was then incubated overnight at 37°C.
The PilA digestion products were analyzed and fractionated by LC-MS using a series 1100 high-performance liquid chromatography (HPLC) system (Agilent) interfaced with the Q-TOF2 mass spectrometer. Approximately 50 μg of the digestion products was injected onto a Jupiter C18 column (4.6 by 250 mm; 5 μm; 300 Å) (Phenomenex, Torrance, CA). The peptides and glycopeptides were separated using the following gradient: 5% to 60% acetonitrile, 0.2% formic acid in 45 min, increasing to 95% after 50 min. The HPLC eluate (1 ml/min) was split so that 60 μl/min was directed to the electrospray interface of the Q-TOF2 mass spectrometer, while the remainder was collected in 1-min fractions. The Q-TOF2 mass spectrometer was set to acquire one spectrum per second (m/z, 400 to 2,000). Glycopeptides were identified by a characteristic pattern of ions separated by 132 Da, the residue mass of a single d-arabinose sugar.
ETD-MS.
Glycopeptide-containing HPLC fractions were infused at 0.5 μl/minute into the ESI source of an LTQ XL linear ion trap (Thermo Fischer Scientific) equipped to perform ETD. Glycopeptide ions were first interrogated by collision-activated dissociation to confirm their identity. The sites of glycosylation were identified by ETD using fluoranthene as the anionic reagent and with supplementary activation enabled. The optimal ETD reaction time for these glycopeptides was 500 ms.
RESULTS
Identification of a second strain of P. aeruginosa expressing arabinosylated pilins and generation of antipilin antibodies cross-reacting with mycobacterial cell wall material.
The genome of the multidrug-resistant P. aeruginosa strain PA7 was recently sequenced (GenBank accession number NC_009656), and examination of its pilin operon revealed a group IV pilA gene, similar to that of P. aeruginosa Pa5196 (proteins 81% identical over 156 residues; Fig. 1), as well as two open reading frames downstream whose predicted products are 56% identical over 640 residues and 53% identical over 129 residues, respectively, with TfpW5196 and TfpX5196. The PA7 strain produces fewer recoverable surface pili than strain Pa5196 does, as determined by twitching motility assays and SDS-PAGE of sheared pili (Fig. 2). However, like the pilins from strain Pa5196, sheared pilins from strain PA7 reacted with anti-LAM antibody, indicating that they are also posttranslationally modified with d-Araf (Fig. 2). Many residues identified as potential sites of modification in the Pa5196 pilin are conserved in the PA7 pilin (Fig. 1).
FIG. 1.
ClustalW amino acid alignment of mature pilins and predicted secondary structure. Pilin sequences from P. aeruginosa strains representing each pilin group and N. gonorrhoeae MS11 PilE are shown. Strains Pa5196 and PA7 represent pilin group IV, strain PA14 represents pilin group III, strain Pa281457 represents pilin group V, strain PAO1 represents pilin group II, and strain 1244 represents pilin group I. The T3 tryptic peptide of Pa5196 PilA is highlighted in pink, the T4 peptide in yellow and the T5 tryptic peptide in cyan. Candidate sites of glycosylation in PilA5196 are shown in boldface type; residues underlined were determined to be modified through SDM and/or ETD-MS. The boldface residues of the PA7 pilin are putative sites of glycosylation based on similarity to the Pa5196 pilin. The Ser63 residue of strain MS11 is glycosylated (22), as is the Ser148 residue of strain1244 (29); both are highlighted in green. The gray-shaded residues are Ser/Thr residues of pilin alleles I, II, III, and V corresponding to those in regions that are modified in PilA5196. The top line represents the predicted secondary (2°) structure of Pa5196 PilA based on the analysis by PHYRE, compared to the PAK PilA structure. The boldface E represents beta-strands, C represents coiled regions, and H represents α-helices. Gaps introduced to maximize alignment of sequences are indicated by dashes. Amino acids that are identical in all strains are indicated by asterisks below the sequence.
FIG. 2.
Pilins from strains Pa5196 and PA7 are modified with D-Araf and elicit antibodies that cross-react with mycobacterial lipoarabinomannan. (A) Strain PA7 produces functional pili when tested for twitching motility by agar subsurface assay, but it is less motile than strain PAO1 or Pa5196; for scale, the dish is 10 cm in diameter. See strain 5196NP in Fig. 3 for an example of a nonmotile strain. (B) SDS-PAGE and Western blot analyses of sheared surface pili demonstrate that Pa5196 and PA7 produce pilins with a larger mass than (nonglycosylated) PAO1, though PA7 produces fewer pili than Pa5196, corresponding with the reduced motility seen in panel A. Both Pa5196 and PA7 pilins react with anti-LAM and anti-PilA5196 antibodies, while the PAO1 pilins do not, confirming that PA7 pilins are also arabinosylated. The positions (in kilodaltons) of molecular mass markers (lane M) are shown to the left of the gel. (C) Immunization of rabbits with a sheared surface protein preparation from Pa5196 containing pilin and flagellin elicits antibodies that recognize cell wall material from Mycobacterium smegmatis (M.sm) of the same size range as anti-LAM sera. The flagellins of Pa5196 are not arabinosylated and do not react with anti-LAM sera. The positions of 15-kDa molecular mass markers (lane M) and of flagellin (F) and pilin (P) are shown to the left of the gel.
We demonstrated previously that a polyclonal M. tuberculosis anti-LAM antibody specifically recognized the d-Araf glycosylated pilins of P. aeruginosa Pa5196, but not other P. aeruginosa pilins (59). Polyclonal antisera from rabbits immunized with sheared surface proteins (pilins and flagellins) from strain Pa5196 reacted with the pilins from strains Pa5196 and PA7 and with M. smegmatis cell wall extract at a mass range similar to material that reacts with anti-LAM antibodies, demonstrating recognition of both protein and sugar moieties (Fig. 2). These results confirm that antibodies to wild-type Pa5196 pilins are cross-reactive with Mycobacterium cell wall material and that PA7 is the second strain of P. aeruginosa that is capable of arabinosylating its pilins.
Modification sites on Pa5196 PilA.
We previously showed that the pilin from P. aeruginosa Pa5196 was posttranslationally modified via an O linkage with d-Araf at multiple sites within two tryptic peptides of the protein (59) (Fig. 1). Initial analysis of the intact mass of the wild-type Pa5196 pilin indicated that glycosylation was somewhat heterogeneous, with up to 16 pentoses per subunit (59). Within the T4 tryptic peptide, potential sites for O-linked glycosylation were determined to be Thr64 and Thr66, as the other residues were eliminated as possible modification sites via ESI-MS analysis (59). However, it was not clear whether both residues were modified in the same protein or whether the observed profile resulted from a mixed population of proteins modified at one site or at the other site. The T5 tryptic peptide was also determined to be glycosylated, but we were unable to identify which of its five Ser residues and four Thr residues (Fig. 1) serve as sites of modification using standard ESI-MS.
Residues Thr97, Thr99, and Thr101 are predicted to occur in a loop region that in N. meningitidis pilins is modified at Ser93 with a α-glycerophosphate (52). We therefore focused our attention first on five candidate residues, Thr64 and Thr66 in the T4 peptide and Thr97, Thr99, and Thr101 in the T5 peptide. Each of these residues, individually and in combination, was changed to Ala via site-directed mutagenesis, and the mutant pilins were expressed from the pBADGr vector in a pilin-less background strain of P. aeruginosa Pa5196 (5196NP). To determine whether the pili were being expressed and were functional, twitching motility assays were performed for each SDM (Fig. 3). Twitching motility was moderately reduced in the Thr66Ala and Thr64/66Ala mutants, and the twitching zones appeared to contain more adherent bacteria, as indicated by more intense staining with crystal violet. A Thr64/66Ser mutant had a wild-type twitching phenotype, suggesting that Thr and Ser are interchangeable at these positions. Twitching motility was complemented to wild-type levels for all other SDMs tested, with the exception of the compound mutant Thr64/66/97/99/101Ala, where no twitching zone was observed (Fig. 3).
FIG. 3.
Twitching motility of PilA5196 site-directed mutants. All mutant pilins were able to complement twitching motility with the exception of the compound mutant T-5-A. The Thr66Ala and Thr64/Thr66Ala mutants produced slightly smaller, more intensely stained twitching zones, indicating increased adherence of cells to the plastic dish. The wild-type phenotype was restored when select Thr residues were replaced with Ser residues (Thr64/Thr66Ser). Each of the mutant pilins was expressed in the 5196NP (no pili) background from the pBADGr plasmid. T-3-A, Thr97/Thr99/Thr101Ala; T-4-A, Thr86/Thr97/Thr99/Thr101Ala; T-5-A, Thr64/Thr66/Thr97/Thr99/Thr101Ala.
Sheared surface pili of the SDMs were analyzed by SDS-PAGE and Western blotting to assess the amount of pilin being produced, the mass of the protein, and the presence of d-Araf. Equal amounts of sample were loaded in each lane, as determined by the similar intensities of the flagellin band, which is not affected by pilin mutations. SDS-PAGE analysis demonstrated that pili were being produced by all SDMs with the exception of the Thr64/66/97/99/101Ala mutant, suggesting that the quintuple mutant protein was not stable (Fig. 4). More significant was the reduction in both the mass and amount of the pilin isolated from the Thr64Ala, Thr66Ala, and Thr64/66Ala SDMs. There was a decrease in mass of the single mutants compared to the wild-type pilin, and a further decrease in mass was evident in the Thr64/66Ala double mutant, suggesting that both Thr64 and Thr66 were glycosylated in the same molecule. These data were further supported by the weak reaction of the Thr64/66Ala double mutant pilin with the anti-LAM antibody compared with the single mutants and the wild-type protein. In addition to the reduction in anti-LAM reactivity, there was a reduction in the amount of surface pilin isolated from these strains as determined by the reduced intensity of the pilin band on the Coomassie blue-stained gel as well as the Western blot with the anti-PilA5196 antibody, particularly in variants with the Thr66Ala mutation. The reduced levels of surface pilins were not due to protein instability, as there was no difference in the amount of pilin as determined by anti-PilA Western blotting of whole-cell lysates of the mutants (not shown). The SDMs of the Thr97, Thr99, and Thr101 residues within the T5 tryptic peptide showed no alterations in mass, glycosylation (as determined by Western blotting with anti-LAM antisera), or abundance compared to the wild-type protein. Therefore, the sites that are glycosylated on the second modified peptide were likely to be Ser, not Thr.
FIG. 4.
SDS-PAGE and Western blots of surface T4P from site-directed mutants. (A) Coomassie blue-stained SDS-PAGE of sheared surface pilins expressed in the 5196NP (no pili) background. The levels of pili recovered from the Thr66Ala and Thr64/Thr66Ala mutants were reduced compared to those of other mutants. No pili could be recovered from the Thr64/Thr66/Thr97/Thr99/Thr101Ala mutant. WT, wild-type Pa5196 strain; NP, 5196NP (pilA mutant). The positions of molecular mass markers (lanes M) (in kilodaltons) and of flagellin (F) and pilin (P) are indicated to the left and right, respectively, of the gels. (B) Western blot with anti-PilA5196 antibody. Pilins from Thr64Ala, Thr66Ala, and Thr64/Thr66Ala mutants had reduced masses compared with the strain complemented with the wild-type pilA5196 gene. Thr66Ala and Thr64 Thr66Ala mutants also produced less surface pili than other mutants; to better visualize the pilin band, the samples are more concentrated compared with the others (as shown by the increased intensity of the flagellin band). (C) Western blot with anti-LAM antibody. All pilins reacted with the anti-LAM sera, although the Thr64/Thr66Ala mutant reacted weakly. Antibody reactivity is restored to the wild-type reactivity in the Thr64/Thr66Ser mutant.
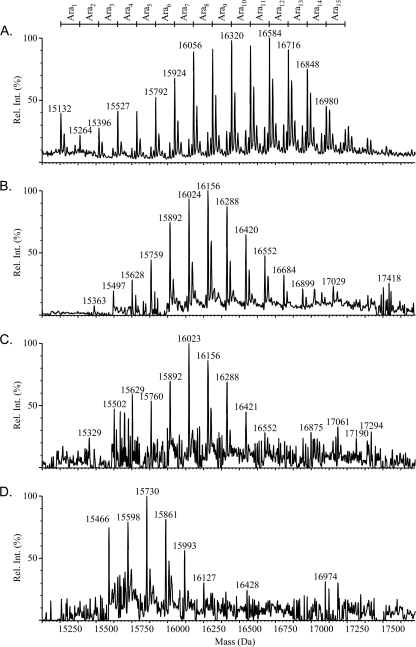
MS was used to obtain the molecular weight profile for the Pa5196 pilin expressed in trans in the 5196NP background (positive control for the SDMs), as well as for the Thr64Ala, Thr66Ala, and Thr64/66Ala variants (Fig. 5). The molecular weight profile of the recombinant PilA (Fig. 5A) indicates that it was not as extensively modified with arabinose as we reported previously for the pilin isolated from the wild-type strain (59) and that there is a slightly broader distribution of glycoforms. This modest difference in PTM was most likely due to the change in stoichiometry between the pilin protein, expressed in this case from a multicopy plasmid, and the pilin modification enzyme, expressed from a single copy on the chromosome (see below). The mass profiles of the Thr64Ala and Thr66Ala mutants (Fig. 5B and C, respectively) confirmed the presence of Ala residues in each case and showed that glycosylation was reduced by approximately three or four arabinose residues compared to the wild-type PilA5196 (Fig. 5A). The molecular weight profile obtained for the double mutant (Fig. 5D) clearly demonstrated loss of six to eight arabinose residues compared with the intact pilin protein, additional evidence that both of these Thr residues are glycosylated in the same protein subunit. The continued presence of arabinose residues on the Thr64/66Ala double mutant protein also confirmed that additional residues in the protein, presumably within the region corresponding to the T5 tryptic peptide, can act as sites of modification.
FIG. 5.
Mass spectrometry of sheared intact pilins. Pili were isolated and subjected to ESI-MS as described in Materials and Methods to determine the extent of pilin glycosylation. (A) A characteristic pattern of evenly spaced peaks, each separated by 132 Da (the mass of a single d-Araf unit), is observed in the reconstructed molecular mass profile for the wild-type pilin expressed in the nonpiliated 5196NP background. The y axis shows the percent relative intensity [Rel. Int. (%)]. (B to D) Mutation of potential acceptor residue Thr64 (B) or Thr66 (C) to Ala causes the loss of approximately three or four d-Araf units each, while mutation of both sites (D) causes a cumulative loss of roughly six to eight d-Araf units. In addition, the glycoform distribution is narrower in the mutants (B to D) relative to the wild type (A), indicating a degree of glycan heterogeneity at these sites in the wild type.
ETD-MS analysis of Pa5196 pilins.
To precisely determine the distribution of arabinose sugars on Thr64 and Thr66 and to test our hypothesis that one or more Ser residues are the acceptor residues on the T5 tryptic glycopeptide, we performed ETD-MS on glycopeptides obtained by tryptic digestion of the wild-type pilin expressed in the 5196NP background. This technique fragments the peptides without loss of delicate O-linked glycans, allowing for detailed mapping of the sites of modification. The pilin tryptic digest was fractionated by reverse-phase HPLC-MS, and glycopeptides were observed in two fractions (Fig. 6). The T3-4 glycopeptide dominated the 26- to 27-min fraction (Fig. 6A), while the T5 glycopeptide was eluted in the 21- to 22-min fraction (Fig. 6B). ETD analysis of the principal glycoform of the T3-4 tryptic peptide, 45SPLAEYGAD(K/N)AWPTLVAPTATPGAGQLNATLVGK79, revealed that both Thr64 and Thr66 are modified, with three d-Araf units each (Fig. 7A).
FIG. 6.
LC-MS analysis of the tryptic digestion products of pilin protein isolated from P. aeruginosa 5196NP plus PilA5196. (A) Mass spectrum of the 26- to 27-min HPLC fraction containing the T3-4 glycopeptide. The triply protonated ions of this glycopeptide are indicated and demonstrate the heterogeneity of the glycosylation associated with this peptide. The y axis shows the percent relative intensity [Rel. Int. (%)]. (B) Mass spectrum of the 21- to 22-min fraction containing the T5 glycopeptide. The doubly protonated glycopeptide ions are indicated in this instance.
FIG. 7.
ETD-MS analysis of glycosylated peptides from strain 5196NP plus pilA5196. (A) ETD-MS spectrum of the triply protonated ion at m/z 1424.7 corresponding to the T3-4 peptide modified with six d-Araf residues. The major c and z fragment ions are indicated in the spectrum and identify Thr64 and Thr66 as the sites of O glycosylation. Furthermore, each site is modified with three d-Araf residues. (B) ETD-MS spectrum of the doubly protonated ion at m/z 1634.2 corresponding to the T5 peptide modified with five d-Araf residues. This fragment ion spectrum indicates that all the serine residues in this peptide are partially modified with one or two d-Araf residues. Thr97, Thr99, and Thr101 are not glycosylated, and it is also very likely that Thr86 is not modified.
The ETD tandem MS (MS-MS) spectrum for the T5 peptide 80YSSVDSTIASGYPNGQITVTMTQGKASGK108, modified with five arabinose residues is presented in Fig. 7B. Though more complex to interpret, the fragmentation pattern clearly demonstrated that the sites of the d-Araf modification are the four Ser residues, not Thr, as was predicted from the SDM results. The degree of modification at each of the Ser residues is more modest than the Thr glycosylation sites on the T4 glycopeptide, with only one arabinose unit, and sometimes two arabinose units, per Ser residue.
Glycosylation of other pilins in the 5196NP background.
There are five known type IVa pilin alleles within P. aeruginosa (34) (Fig. 1), but only groups I and IV pilins have been experimentally confirmed to be glycosylated. The group I oligosaccharide transferase TfpO is flexible in terms of the glycan that it can transfer to the pilin and in its protein substrate specificity (15, 22, 31). To investigate the protein substrate specificity of the glycosylation system of strain Pa5196, each of the P. aeruginosa pilin variants was expressed in the 5196NP strain. Each of the pilin variants could complement twitching motility in the 5196NP strain; however, the amounts of purifiable surface pili were very low compared to those obtained upon complementation with the cognate pilin. A combination of Western blot analysis with the anti-LAM antibody and intact protein ESI-MS showed that none of the other pilins tested were modified with d-arabinose when expressed in the 5196NP background (not shown), even though all have potential acceptor residues in regions that correspond to those modified in the Pa5196 pilin (Fig. 1). Therefore, additional features of the Pa5196 pilin must be involved in its specific recognition by the group IV glycosylation system.
Characterization of the putative arabinosyltransferase, TfpW.
Directly downstream of pilA in both P. aeruginosa Pa5196 and PA7 is a novel open reading frame we named tfpW, predicted to encode a 646-residue protein with a cytoplasmic N terminus, followed by 11 transmembrane segments and a large periplasmic C-terminal region (Fig. 8). BLASTP and PSI-BLAST searches revealed only a few weak matches to the predicted product of tfpW (Table 3). Most are hypothetical proteins; however, some similarities of interest include hypothetical tetratricopeptide repeat-containing proteins and putative O-GlcNAc transferases.
FIG. 8.
Topology model of TfpW. TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0) and TMRPres2D (http://biophysics.biol.uoa.gr/TMRPres2D) were used to predict the putative transmembrane domains and topology of TfpW (646 residues). It has a short N-terminal cytoplasmic sequence, followed by 11 predicted membrane-spanning domains (numbered gray boxes) and a large (232-residue) C-terminal periplasmic domain containing predicted tetratricopeptide domains. The small black numbers at the top and bottom of the gray boxes correspond to residue numbers. The putative GT-C DDS motif is located in the first large periplasmic loop between transmembrane domains 1 and 2 and is followed by a characteristic hydrophobic region 45ILFL48.
TABLE 3.
Selected BLASTP results for TfpW5196
| Organism | GenBank accession no. | Homologya | Similarity |
|---|---|---|---|
| Geobacter bemidjiensis Bem | ZP_01775296 | Putative TPR 2 protein | 31% identical, 48% positive over 190 residues |
| Kuenenia stuttgartiensis | CAJ71278 | Putative TPR protein | 24% identical over 385 residues |
| Desulfatibacillum alkenivorans | ZP_02130537 | Putative TPR protein | 30% identical over 206 residues |
| Desulfococcus oleovorans | ZP_01674191 | Putative O-GlcNAc transferase | 25% identical over 466 residues |
| Rhodopirellula baltica SH 1 | NP_870507 | Putative O-GlcNAc transferase | 26% identical over 183 residues |
TPR, tetratricopeptide.
To examine the potential role of TfpW in pilin glycosylation, a tfpW knockout mutant of Pa5196 was generated as outlined in Materials and Methods. The tfpW mutant strain retained twitching motility, although it was reduced compared to the wild type (Fig. 9). Twitching motility was restored to wild-type levels by complementation with a plasmid expressing pilA-tfpW or pilA-tfpW-tfpX, but not pilA alone or pilA-tfpX, demonstrating that the reduction in twitching motility was specifically due to the loss of tfpW (Fig. 9). Twitching motility was not restored to wild-type levels with the addition of a plasmid containing tfpO, demonstrating that tfpO cannot complement the tfpW mutation (Fig. 9).
FIG. 9.
TfpW influences twitching motility. A tfpW mutant displays an altered twitching phenotype compared to that of wild-type strain Pa5196; it produces a smaller, more densely stained twitching zone, suggesting that the mutant is more adherent to plastic than the wild type is. The wild-type twitching phenotype was restored when tfpW was expressed in trans from either pBADGr+5196pilA-tfpW or pBADGr+5196pilA-tfpW-tfpX, but not with constructs lacking tfpW. The P. aeruginosa 1244 oligosaccharide transferase PilO/TfpO was not able to complement the tfpW mutant. All recombinant strains are on the Pa5196 tfpW mutant background, with the exception of the wild-type control, Pa5196.
SDS-PAGE analysis of sheared pilins from the wild-type Pa5196 strain, the tfpW mutant strain, and complemented strains showed that despite only a modest decrease in motility, the tfpW mutant strain produced substantially fewer recoverable pili with a reduced mass compared with those of the wild-type strain. The identity of the lower-molecular-mass protein isolated from the tfpW mutant strain was confirmed by MS-MS analysis to be the unmodified Pa5196 pilin protein (not shown). Addition of tfpW in trans restored the wild-type mass and levels of recoverable surface pili in the complemented strain (Fig. 10). A Western blot of sheared T4P with anti-LAM sera showed that pilins from the tfpW mutant strain were not recognized by the antibody; reactivity was restored in tfpW mutant strains complemented with tfpW in trans (Fig. 10A). Together, these data demonstrate that tfpW encodes a protein that is necessary for the addition of d-Araf to the pilins of Pa5196.
FIG. 10.
TfpW is a putative GT-C family glycosyltransferase. (A) Coomassie blue-stained SDS-polyacrylamide gel of sheared surface pili from P. aeruginosa Pa5196 and mutant strains. The tfpW mutant strain produces reduced amounts of surface pili as well as pilins of reduced mass. These lower-molecular-mass bands were confirmed to be PilA5196 by MS-MS (not shown). The pilins were restored to wild-type mass with the addition of tfpW in trans, but not by other genes in the pilin operon. The positions of molecular mass markers (lane M) (in kilodaltons) and of flagellin (F) and pilin (P) are shown to the left and right of the gel, respectively. (B) Western blot using anti-LAM antibody shows that the pilins from the tfpW mutant strain fail to react, while those strains complemented with tfpW in trans regain anti-LAM reactivity. (C) Mutation of the putative GT-C motif within TfpW results in loss of pilin glycosylation.
TfpW could promote pilin glycosylation by synthesizing/polymerizing the d-Araf residues, by transporting them across the inner membrane to the periplasm (flippase), by acting as a pilin glycosyltransferase, or through a combination of these activities. Because all detectable glycosylation was abolished in the tfpW mutant, including the addition of single sugars, TfpW is likely to be a glycosyltransferase involved in transfer of one or more residues of d-Araf to PilA. Pfam analysis showed that residues 16 to 249 of TfpW have limited similarity to the dolichyl-phosphate-mannose-protein mannosyltransferase family of O-linked glycosyltransferases, a member of the Carbohydrate-Active Enzymes (CAZy) family GT39 (17). Notably, this family belongs to the same clan (CAZy family GT22) as the Mycobacterium arabinosyltransferases EmbA, -B, and -C (CAZy family GT53). All are members of the small glycosyltransferase GT-C superfamily, defined as integral membrane proteins with 8 to 13 membrane-spanning helices, a proposed active site within the loop between helices 1 and 2, and inverting enzyme activity (37). Although a number of GT-C glycosyltransferases are found in eukaryotes, they are not found in members of the domain Archaea and are extremely rare in prokaryotes (6, 32, 37). The proteins contain a flexible binding or catalytic DXD motif (variants include EXD, DXE, DDX, and DEX) in the N-terminal portion of the first or second extracytoplasmic loop, followed by an essential short series of hydrophobic residues (37). In the few GT-C proteins that have been characterized, mutation of either of the conserved acidic residues causes loss of glycosyltransferase activity (37). The first predicted periplasmic loop of TfpW5196 (between transmembrane helices 1 and 2; Fig. 8) contains a putative GT-C motif (42DDS44) followed by hydrophobic residues (45ILFL48); these features are conserved in the PA7 TfpW orthologue. Figure 10B shows that an Asp42/43Ala double mutant of TfpW5196 was unable to complement pilin glycosylation in the tfpW mutant background, supporting our hypothesis that it is a glycosyltransferase of the GT-C family.
Although TfpW is necessary for pilin glycosylation, it is not sufficient, as transfer of the construct expressing pilA5196-tfpW-tfpX (pilA5196 is the pilA gene from strain Pa5196) to a pilA mutant of the PAO1 laboratory strain of P. aeruginosa resulted in the expression of only small amounts of nonglycosylated pilins (not shown). Therefore, additional gene products specific to P. aeruginosa Pa5196 and related strains, such as PA7, are likely involved in biosynthesis of the glycan.
DISCUSSION
Although the basic architecture of type IV pilins is highly conserved, considerable variation exists with respect to amino acid sequence and posttranslational modifications, even within species (9, 18, 34, 50, 54, 57). We previously demonstrated that P. aeruginosa strain Pa5196 produces pili that are modified with O-linked homopolymers of α-1,5-d-arabinofuranose and postulated that the novel open reading frame downstream of pilA5196, tfpW, encoded the protein involved in this modification (59).
The P. aeruginosa arabinans are chemically and immunologically similar to the oligosaccharides within the LAM and arabinogalactan polymers of the cell walls of mycobacteria and other members of the suborder Corynebacterineae as determined by nuclear magnetic resonance analysis and reaction of LAM-specific antibodies with glycosylated PilA from strain Pa5196 (PilA5196) (59). Antibodies raised against the glycosylated PilA5196 reacted with cell wall material from M. smegmatis and with the pili from strain PA7 (Fig. 2), which has an P. aeruginosa Pa5196-like group IV pilin gene as well as tfpW and tfpX orthologues. We will use the publicly available genome sequence of strain PA7 to identify additional genes that may be involved in the synthesis of d-Araf precursors and any other glycosyltransferase(s) potentially involved in the progressive assembly of the glycan, since transfer of the pilA-tfpW-tfpX gene cassette from Pa5196 to PAO1 did not result in pilin glycosylation. Preliminary inspection of the PA7 genome did not reveal open reading frames with similarity to Corynebacterineae arabinosyltransferase genes, suggesting a novel pathway.
Based on our previous work, PilA5196 was predicted to have up to 16 arabinose units per pilin subunit (59). Two tryptic peptides encompassing the predicted αβ-loop region of the protein (T4 peptide) and the β-strands 1 and 2 of the antiparallel beta-sheet domain (T5 peptide) were modified, though the exact sites could not be identified. In this work, SDS-PAGE and Western blot analyses of SDMs of candidate sites Thr64 and Thr66 in the T4 peptide indicated that both residues were modified in each PilA molecule, while ETD-MS of the principal glycoform of the wild-type pilin showed that each residue is modified with a trisaccharide of d-Araf. Notably, the Thr64Ala Thr66Ala double mutant reacted only weakly with the anti-LAM antibody, despite the MS data showing that the T5 peptide is modified with up to eight sugar residues. In N. gonorrhoeae, pilin PTMs were shown to be interdependent; when the modification of Ser68 with phosphoethanolamine and/or phosphocholine was prevented, there was a corresponding decrease in glycosylation at Ser63 (2). In this case, however, it is more likely that the affinity of the anti-LAM antibody for mono- or disaccharides of d-Araf is weaker than for the trisaccharides, leading to reduced reactivity.
On the T5 tryptic peptide, Thr97, Thr99, and Thr101 were considered the most likely sites for modification based on their position in an accessible loop region and approximate correspondence to the Ser93 site of phosphorylation in Neisseria pilins (52). To our surprise, mutagenesis and ETD-MS analyses demonstrated that instead, Ser81, Ser82, Ser85, and Ser89 were modified with mono- and disaccharides of d-Araf. Ser81 and Ser82 map to the predicted loop region and the adjacent β-strand region, respectively (Fig. 1), upstream of the first β-strand, while Ser85 is located within the first predicted β-strand, followed by Ser89, which is in the loop between the first and second β-strands. These residues were not uniformly modified, and different glycoforms, likely to represent a natural heterogeneous population of glycosylated pilins, were detected. We previously reported that sheared pilins from strain Pa5196 typically run as a smear on SDS-polyacrylamide gels (34, 59), indicating a range of masses that is likely due to various levels of modification. Further studies will be necessary to understand the mechanisms that control both selection of pilin glycosylation sites and the numbers of sugars added at each position.
The Thr64Ala and Thr66Ala single and double mutants, as well as the nonglycosylated tfpW mutant, had fewer surface pili and reduced motility compared with the wild type, indicating that glycosylation affects pilus assembly or extension/retraction dynamics. This was an unexpected finding, since loss of pilin glycosylation in P. aeruginosa 1244 or Neisseria does not reduce the amount of recoverable surface pili; in N. meningitidis, mutation of Ser63 to Ala actually increased piliation (9, 40). Since the Pa5196 pilins are not degraded in the absence of glycosylation, the d-Araf modifications may influence the pilin's structure or properties, resulting in optimal surface expression, function, or dynamics of T4P. The low levels of recoverable surface pili from the 5196NP strain expressing other P. aeruginosa pilin variants compared with levels recovered from their native backgrounds may be similarly due to the observed lack of glycosylation of the heterologous proteins in strain Pa5196.
Our hypothesis that TfpW is the enzyme involved in the modification of PilA with d-Araf (59) was supported by data showing that a tfpW mutant produced pilins with reduced mass that no longer reacted with anti-LAM antibodies. In addition to the GT-C motif described above, TfpW is predicted to have a large C-terminal periplasmic region containing tetratricopeptide repeats, a motif typically involved in protein-protein interactions (7). This region of TfpW may be involved in interactions with the pilin subunits and/or components of the pilus assembly complex. The possibility that TfpW could be involved in the biosynthesis or translocation of d-Araf to the periplasm, in addition to acting as a glycosyltransferase, cannot be ruled out at this point. However, the inability of recombinant strain PAO1 carrying the pilA5196-tfpW-tfpX genes to express glycosylated pilins argues against the sole involvement of TfpW in arabinose biosynthesis. TfpX does not mediate pilin glycosylation, as complementation of the tfpW mutant with pilA5196-tfpX did not restore modification. TfpX has approximately 50% sequence similarity with the pilin accessory proteins TfpY and TfpZ from group III and V strains of P. aeruginosa (34), which modulate pilus retraction dynamics (4a).
The current model for type IV pilin glycosylation (Fig. 11) involves progressive assembly of the glycan on an undecaprenol carrier on the cytoplasmic face of the inner membrane through the action of a series of glycosyltransferases (1, 46). Once the mature glycan is assembled, a flippase translocates the undecaprenol-linked glycan to the periplasmic face of the membrane where the oligosaccharide is transferred to the mature pilin subunit (1, 46). Glycosylated pilin subunits are then shuttled to the pilus assembly complex where they are polymerized to form the fiber. In Neisseria, these steps are performed by a dedicated series of Pgl enzymes, while in P. aeruginosa 1244, a subset of the O-antigen units translocated by Wzx of the lipopolysaccharide machinery are transferred to PilA1244 by TfpO (5, 15). In both cases, the glycans are defined hetero-oligosaccharides and are attached to a single acceptor site on the pilin substrate.
FIG. 11.
Models of type IV pilin glycosylation systems. Three systems involved in the glycosylation of type IV pilins have been identified: the Pgl system of Neisseria spp., the TfpO system of group I strains of P. aeruginosa, and the TfpW system of group IV strains of P. aeruginosa. The first two systems attach short hetero-oligosaccharides to a single site on the pilin, while the TfpW system attaches multiple single sugars or short homo-oligosaccharides (two to four units) to seven different acceptor sites on the protein. The Pgl system transfers a heterosaccharide synthesized by dedicated glycosyltransferases, while the TfpO system transfers a lipopolysaccharide O unit corresponding to the serotype of the host strain. The TfpW system transfers d-Araf which is synthesized by an as-yet unknown pathway. P, phosphate.
In contrast, P. aeruginosa Pa5196 expresses the prototype of a new pilin glycosylation system (Fig. 11) where the pilin glycan comprises single d-Araf residues or short homopolymers thereof, attached at multiple sites on each subunit. Gram-negative bacteria typically use cytoplasmic nucleotide sugar substrates to synthesize both hetero- and homopolysaccharides that are attached to either protein or nonprotein acceptors, while studies of the Corynebacterineae showed that decaprenol-linked d-ribose precursors are epimerized to d-Araf and incorporated directly into cell wall polymers without a nucleotide sugar intermediate in a process reminiscent of peptidoglycan biosynthesis.
Since no mechanism for the translocation of d-Araf in strain Pa5196 has been identified, it is possible that TfpW could act as a flippase as well as a glycosyltransferase or oligosaccharyltransferase (Fig. 11). Its predicted structure containing multiple transmembrane domains (Fig. 8) is consistent with that of known glycan flippases including Wzx homologues from various species, which have been shown to be capable of translocating one or more isoprenoid-linked sugars. Wzx proteins lack characteristic signature motifs and are in general poorly conserved at the primary sequence level (3). Investigation of whether d-Araf residues are exposed at the periplasmic face of the inner membrane in a tfpW mutant will help to resolve its potential role in glycan translocation.
In summary, strains Pa5196 and PA7 constitute a unique subset of P. aeruginosa strains that are of biological and evolutionary interest. TfpW is the gram-negative prototype of an unusual member of the GT-C family that includes the M. tuberculosis Emb arabinosyltransferases. Future studies will reveal the specific mechanism by which TfpW glycosylates Pil and the pathway for biosynthesis of its unusual glycan substrate.
Acknowledgments
We thank Ylan Nguyen and Tony Zhang for excellent technical assistance.
This work was supported by grants to L.L.B. from the Canadian Institutes of Health Research (MOP49577) and from the Advanced Food and Materials Network of Centres of Excellence (AFMnet). L.L.B. holds a CIHR New Investigator Award. J.V.K. was a University of Toronto Faculty of Dentistry Harron Scholar and held a studentship with the CIHR Strategic Training Program in Cell Signaling in Mucosal Inflammation and Pain (STP-53877).
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Aas, F. E., A. Vik, J. Vedde, M. Koomey, and W. Egge-Jacobsen. 2007. Neisseria gonorrhoeae O-linked pilin glycosylation: functional analyses define both the biosynthetic pathway and glycan structure. Mol. Microbiol. 65607-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aas, F. E., W. Egge-Jacobsen, H. C. Winther-Larsen, C. Lovold, P. G. Hitchen, A. Dell, and M. Koomey. 2006. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchical modifications with phosphoethanolamine and phosphocholine requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J. Biol. Chem. 28127712-27723. [DOI] [PubMed] [Google Scholar]
- 3.Alaimo, C., I. Catrein, L. Morf, C. L. Marolda, N. Callewaert, M. A. Valvano, M. F. Feldman, and M. Aebi. 2006. Two distinct but interchangeable mechanisms for flipping of lipid-linked oligosaccharides. EMBO J. 25967-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 4a.Asikyan, M. L., J. V. Kus, and L. L. Burrows. 2008. Novel proteins that modulate type IV pilus retraction dynamics in Pseudomonas aeruginosa. J. Bacteriol. 1907022-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45267-276. [DOI] [PubMed] [Google Scholar]
- 6.Berg, S., D. Kaur, M. Jackson, and P. J. Brennan. 2007. The glycosyltransferases of Mycobacterium tuberculosis—roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology 1735R-56R. [DOI] [PubMed] [Google Scholar]
- 7.Blatch, G. L., and M. Lassle. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21932-939. [DOI] [PubMed] [Google Scholar]
- 8.Burrows, L. L. 2005. Weapons of mass retraction. Mol. Microbiol. 57878-888. [DOI] [PubMed] [Google Scholar]
- 9.Castric, P. 1995. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology 1411247-1254. [DOI] [PubMed] [Google Scholar]
- 10.Chamot-Rooke, J., B. Rousseau, F. Lanternier, G. Mikaty, E. Mairey, C. Malosse, G. Bouchoux, V. Pelicic, L. Camoin, X. Nassif, and G. Dumenil. 2007. Alternative Neisseria spp. type IV pilin glycosylation with a glyceramido acetamido trideoxyhexose residue. Proc. Natl. Acad. Sci. USA 10414783-14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang, P., and L. L. Burrows. 2003. Biofilm formation by hyperpiliated mutants of Pseudomonas aeruginosa. J. Bacteriol. 1852374-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi, K. H., A. Kumar, and H. P. Schweizer. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64391-397. [DOI] [PubMed] [Google Scholar]
- 14.Coleman, F. T., S. Mueschenborn, G. Meluleni, C. Ray, V. J. Carey, S. O. Vargas, C. L. Cannon, F. M. Ausubel, and G. B. Pier. 2003. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc. Natl. Acad. Sci. USA 1001949-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comer, J. E., M. A. Marshall, V. J. Blanch, C. D. Deal, and P. Castric. 2002. Identification of the Pseudomonas aeruginosa 1244 pilin glycosylation site. Infect. Immun. 702837-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coon, J. J., B. Ueberheide, J. E. Syka, D. D. Dryhurst, J. Ausio, J. Shabanowitz, and D. F. Hunt. 2005. Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proc. Natl. Acad. Sci. USA 1029463-9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coutinho, P. M., E. Deleury, G. J. Davies, and B. Henrissat. 2003. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 328307-317. [DOI] [PubMed] [Google Scholar]
- 18.Craig, L., M. E. Pique, and J. A. Tainer. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2363-378. [DOI] [PubMed] [Google Scholar]
- 19.Craig, L., R. K. Taylor, M. E. Pique, B. D. Adair, A. S. Arvai, M. Singh, S. J. Lloyd, D. S. Shin, E. D. Getzoff, M. Yeager, K. T. Forest, and J. A. Tainer. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol. Cell 111139-1150. [DOI] [PubMed] [Google Scholar]
- 20.DiGiandomenico, A., M. J. Matewish, A. Bisaillon, J. R. Stehle, J. S. Lam, and P. Castric. 2002. Glycosylation of Pseudomonas aeruginosa 1244 pilin: glycan substrate specificity. Mol. Microbiol. 46519-530. [DOI] [PubMed] [Google Scholar]
- 21.Doig, P., T. Todd, P. A. Sastry, K. K. Lee, R. S. Hodges, W. Paranchych, and R. T. Irvin. 1988. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect. Immun. 561641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faridmoayer, A., M. A. Fentabil, D. C. Mills, J. S. Klassen, and M. F. Feldman. 2007. Functional characterization of bacterial oligosaccharyltransferases involved in O-linked protein glycosylation. J. Bacteriol. 1898088-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallant, C. V., C. Daniels, J. M. Leung, A. S. Ghosh, K. D. Young, L. P. Kotra, and L. L. Burrows. 2005. Common beta-lactamases inhibit bacterial biofilm formation. Mol. Microbiol. 581012-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Giltner, C. L., E. J. van Schaik, G. F. Audette, D. Kao, R. S. Hodges, D. J. Hassett, and R. T. Irvin. 2006. The Pseudomonas aeruginosa type IV pilin receptor binding domain functions as an adhesin for both biotic and abiotic surfaces. Mol. Microbiol. 591083-1096. [DOI] [PubMed] [Google Scholar]
- 26.Hahn, H. P. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 19299-108. [DOI] [PubMed] [Google Scholar]
- 27.Hegge, F. T., P. G. Hitchen, F. E. Aas, H. Kristiansen, C. Lovold, W. Egge-Jacobsen, M. Panico, W. Y. Leong, V. Bull, M. Virji, H. R. Morris, A. Dell, and M. Koomey. 2004. Unique modifications with phosphocholine and phosphoethanolamine define alternate antigenic forms of Neisseria gonorrhoeae type IV pili. Proc. Natl. Acad. Sci. USA 10110798-10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 21277-86. [DOI] [PubMed] [Google Scholar]
- 29.Hogan, J. M., S. J. Pitteri, P. A. Chrisman, and S. A. McLuckey. 2005. Complementary structural information from a tryptic N-linked glycopeptide via electron transfer ion/ion reactions and collision-induced dissociation. J. Proteome Res. 4628-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8528-535. [PubMed] [Google Scholar]
- 31.Horzempa, J., J. E. Comer, S. A. Davis, and P. Castric. 2006. Glycosylation substrate specificity of Pseudomonas aeruginosa 1244 pilin. J. Biol. Chem. 2811128-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi, N., Y. D. Kwon, M. Gotoh, and H. Narimatsu. 2003. Comparison of glycosyltransferase families using the profile hidden Markov model. Biochem. Biophys. Res. Commun. 310574-579. [DOI] [PubMed] [Google Scholar]
- 33.Kuo, C., N. Takahashi, A. F. Swanson, Y. Ozeki, and S. Hakomori. 1996. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Investig. 982813-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kus, J. V., E. Tullis, D. G. Cvitkovitch, and L. L. Burrows. 2004. Significant differences in type IV pilin allele distribution among Pseudomonas aeruginosa isolates from cystic fibrosis (CF) versus non-CF patients. Microbiology 1501315-1326. [DOI] [PubMed] [Google Scholar]
- 35.Lefebre, M. D., and M. A. Valvano. 2002. Construction and evaluation of plasmid vectors optimized for constitutive and regulated gene expression in Burkholderia cepacia complex isolates. Appl. Environ. Microbiol. 685956-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 674084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, J., and A. Mushegian. 2003. Three monophyletic superfamilies account for the majority of the known glycosyltransferases. Protein Sci. 121418-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu, H. M., S. T. Motley, and S. Lory. 1997. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol. Microbiol. 25247-259. [DOI] [PubMed] [Google Scholar]
- 39.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 21051-1060. [DOI] [PubMed] [Google Scholar]
- 40.Marceau, M., and X. Nassif. 1999. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J. Bacteriol. 181656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marceau, M., K. Forest, J. L. Beretti, J. Tainer, and X. Nassif. 1998. Consequences of the loss of O-linked glycosylation of meningococcal type IV pilin on piliation and pilus-mediated adhesion. Mol. Microbiol. 27705-715. [DOI] [PubMed] [Google Scholar]
- 42.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56289-314. [DOI] [PubMed] [Google Scholar]
- 43.Murray, T. S., M. Egan, and B. I. Kazmierczak. 2007. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr. 1983-88. [DOI] [PubMed] [Google Scholar]
- 44.O'Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30295-304. [DOI] [PubMed] [Google Scholar]
- 45.Power, P. M., and M. P. Jennings. 2003. The genetics of glycosylation in Gram-negative bacteria. FEMS Microbiol. Lett. 218211-222. [DOI] [PubMed] [Google Scholar]
- 46.Power, P. M., K. L. Seib, and M. P. Jennings. 2006. Pilin glycosylation in Neisseria meningitidis occurs by a similar pathway to wzy-dependent O-antigen biosynthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 347904-908. [DOI] [PubMed] [Google Scholar]
- 47.Romling, U., B. Fiedler, J. Bosshammer, D. Grothues, J. Greipel, H. von der Hardt, and B. Tummler. 1994. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis. 1701616-1621. [DOI] [PubMed] [Google Scholar]
- 48.Saiman, L., and A. Prince. 1993. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J. Clin. Investig. 921875-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smedley, J. G., III, E. Jewell, J. Roguskie, J. Horzempa, A. Syboldt, D. B. Stolz, and P. Castric. 2005. Influence of pilin glycosylation on Pseudomonas aeruginosa 1244 pilus function. Infect. Immun. 737922-7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spangenberg, C., R. Fislage, W. Sierralta, B. Tummler, and U. Romling. 1995. Comparison of type IV-pilin genes of Pseudomonas aeruginosa of various habitats has uncovered a novel unusual sequence. FEMS Microbiol. Lett. 125265-273. [DOI] [PubMed] [Google Scholar]
- 51.Spyropoulos, I. C., T. D. Liakopoulos, P. G. Bagos, and S. J. Hamodrakas. 2004. TMRPres2D: high quality visual representation of transmembrane protein models. Bioinformatics 203258-3260. [DOI] [PubMed] [Google Scholar]
- 52.Stimson, E., M. Virji, S. Barker, M. Panico, I. Blench, J. Saunders, G. Payne, E. R. Moxon, A. Dell, and H. R. Morris. 1996. Discovery of a novel protein modification: alpha-glycerophosphate is a substituent of meningococcal pilin. Biochem. J. 31629-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stimson, E., M. Virji, K. Makepeace, A. Dell, H. R. Morris, G. Payne, J. R. Saunders, M. P. Jennings, S. Barker, and M. Panico. 1995. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 171201-1214. [DOI] [PubMed] [Google Scholar]
- 54.Strom, M. S., and S. Lory. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47565-596. [DOI] [PubMed] [Google Scholar]
- 55.Strom, M. S., and S. Lory. 1986. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J. Bacteriol. 165367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syka, J. E., J. J. Coon, M. J. Schroeder, J. Shabanowitz, and D. F. Hunt. 2004. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. USA 1019528-9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szymanski, C. M., and B. W. Wren. 2005. Protein glycosylation in bacterial mucosal pathogens. Nat. Rev. Microbiol. 3225-237. [DOI] [PubMed] [Google Scholar]
- 58.Szymanski, C. M., D. H. Burr, and P. Guerry. 2002. Campylobacter protein glycosylation affects host cell interactions. Infect. Immun. 702242-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voisin, S., J. V. Kus, S. Houliston, F. St-Michael, D. Watson, D. G. Cvitkovitch, J. Kelly, J. R. Brisson, and L. L. Burrows. 2007. Glycosylation of Pseudomonas aeruginosa strain Pa5196 type IV pilins with mycobacterium-like α-1,5-linked d-Araf oligosaccharides. J. Bacteriol. 189151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wall, D., and D. Kaiser. 1999. Type IV pili and cell motility. Mol. Microbiol. 321-10. [DOI] [PubMed] [Google Scholar]
- 61.Wang, W., and B. A. Malcolm. 1999. Two-stage PCR protocol allowing introduction of multiple mutations, deletions and insertions using QuikChange site-directed mutagenesis. BioTechniques 26680-682. [DOI] [PubMed] [Google Scholar]
- 62.Wong, W. Y., A. P. Campbell, C. McInnes, B. D. Sykes, W. Paranchych, R. T. Irvin, and R. S. Hodges. 1995. Structure-function analysis of the adherence-binding domain on the pilin of Pseudomonas aeruginosa strains PAK and KB7. Biochemistry 3412963-12972. [DOI] [PubMed] [Google Scholar]
- 63.Wuhrer, M., J. C. Stam, F. E. van de Geijn, C. A. Koeleman, C. T. Verrips, R. J. Dolhain, C. H. Hokke, and A. M. Deelder. 2007. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics 74070-4081. [DOI] [PubMed] [Google Scholar]