Abstract
Elongation factor Tu (EF-Tu), encoded by tuf genes, carries aminoacyl-tRNA to the ribosome during protein synthesis. Duplicated tuf genes (tufA and tufB), which are commonly found in enterobacterial species, usually coevolve via gene conversion and are very similar to one another. However, sequence analysis of tuf genes in our laboratory has revealed highly divergent copies in 72 strains spanning the genus Yersinia (representing 12 Yersinia species). The levels of intragenomic divergence between tufA and tufB sequences ranged from 8.3 to 16.2% for the genus Yersinia, which is significantly greater than the 0.0 to 3.6% divergence observed for other enterobacterial genera. We further explored tuf gene evolution in Yersinia and other Enterobacteriaceae by performing directed sequencing and phylogenetic analyses. Phylogenetic trees constructed using concatenated tufA and tufB sequences revealed a monophyletic genus Yersinia in the family Enterobacteriaceae. Moreover, Yersinia strains form clades within the genus that mostly correlate with their phenotypic and genetic classifications. These genetic analyses revealed an unusual divergence between Yersinia tufA and tufB sequences, a feature unique among sequenced Enterobacteriaceae and indicative of a genus-wide loss of gene conversion. Furthermore, they provided valuable phylogenetic information for possible reclassification and identification of Yersinia species.
The genus Yersinia, a member of the family Enterobacteriaceae, is composed of 14 known species: Y. aldovae, Y. aleksiciae, Y. bercovieri, Y. enterocolitica, Y. frederiksenii, Y. intermedia, Y. kristensenii, Y. massiliensis, Y. mollaretii, Y. pestis, Y. pseudotuberculosis, Y. rohdei, Y. ruckeri, and Y. similis (45, 49-51). Three of these species are important human pathogens. Y. pestis is the etiologic agent of plague, while Y. enterocolitica and Y. pseudotuberculosis usually cause self-limiting food-borne illnesses.
Interspecies and intraspecies genetic studies of relationships of yersiniae based on DNA-DNA hybridization and sequencing of housekeeping genes have provided additional information not encompassed by the current classification, which is based mainly on biochemical phenotypes (14, 16, 17, 36). Thus, Y. enterocolitica, Y. frederiksenii, and Y. kristensenii show more genetic diversity than other species in the genus (17, 36, 46). Indeed, more divergent strains that form their own clades could represent novel Yersinia species. Neubauer and colleagues (46) characterized two subspecies of Y. enterocolitica, Y. enterocolitica subsp. enterocolitica and Y. enterocolitica subsp. palearctica, that have distinctive 16S rRNA sequences. Sprague and Neubauer (49) recently proposed a novel species, Y. aleksiciae, which was differentiated from Y. kristensenii based on a comparison of a region of the 16S rRNA gene and by the presence of lysine decarboxylase activity. On the other hand, based on DNA-DNA hybridization, Y. pestis and Y. pseudotuberculosis are a single genomospecies, even though they are classified as two distinct nomenspecies (6). Previous analyses revealed that Y. pestis emerged within the last 9,000 to 40,000 years from a Y. pseudotuberculosis predecessor (2, 48a). Although Y. pestis and Y. pseudotuberculosis are members of the same genomospecies, the medical implications for these organisms preclude establishment of a new nomenclature, which could endanger laboratory workers and general public health (30, 31, 58, 60). Finally, Y. ruckeri is the most distant Yersinia species, and its inclusion in the genus is still debated (28, 36, 51). Clearly, further genetic studies of the genus Yersinia are required in order to clarify the phylogeny and reinforce the taxonomy of this group.
Previous studies using 16S rRNA gene sequences exposed this method's limited ability to resolve the evolutionary history of Yersinia species (36). The high level of sequence conservation among 16S rRNA genes in some bacteria and the presence of multiple copies with sequence variations in many other bacteria limit the use of these genes for taxonomic resolution of closely related microorganisms (12, 13). Sequence analysis of the tuf gene has been proven to be valuable for accurate evaluation of genetic relationships among closely related microorganisms, such as members of the genus Enterococcus and the family Enterobacteriaceae (32, 47). Elongation factor Tu (EF-Tu), encoded by tuf genes, carries aminoacyl-tRNA to the programmed ribosome during protein synthesis. The synteny of gammaproteobacterial tuf gene regions is conserved. Most enterobacterial genomes possess two copies of the tuf gene, tufA and tufB, in distinct operons, designated str and tRNA-tufB, respectively (20, 39). The fusA gene, which encodes elongation factor G, is located upstream of tufA in the str operon of gammaproteobacteria (35, 39). Four tRNA structural genes are located upstream of tufB in the tRNA-tufB operon (20), and the secE and nusG genes are downstream of tufB in most Enterobacteriaceae (20, 35, 39). The tufA and tufB genes appear to evolve in concert through gene conversion events that maintain the sequence homology (1). A previous analysis of duplicated bacterial tuf genes revealed identical or very similar nucleic acid sequences that differ by less than 1.4% (39). In contrast, the data presented here show that the tufA and tufB gene copies in 12 species of the genus Yersinia have a remarkable level of variability (up to 16%). Chain and colleagues have observed a similar level of divergence in Y. pestis and Y. pseudotuberculosis (10). In the current work, we performed an in-depth study of tuf gene sequence variation in the genus Yersinia. The data obtained were juxtaposed with data for closely related members of the Enterobacteriaceae, and mechanisms for the remarkable diversity were examined. As described here, the divergence among the Yersinia intragenomic tuf gene sequences appears to be unique among sequenced Enterobacteriaceae, as demonstrated by phylogenetic analyses.
MATERIALS AND METHODS
Bacterial strains.
Sixty-six Yersinia strains (Table 1) and 14 Enterobacteriaceae strains (Enterobacter agglomerans ATCC 27989, Escherichia vulneris ATCC 33821, Hafnia alvei ATCC 13337, ATCC 25927, ATCC 51873, CCRI-10616, CCRI-11829, and CCRI-16651, Klebsiella pneumoniae ATCC 13883, Obesumbacterium proteus ATCC 12841, Plesiomonas shigelloides ATCC 14029, Serratia fonticola DSM 4576, Serratia marcescens ATCC 13880, and Yokenella regensburgei ATCC 35313) were obtained from the American Type Culture Collection (Manassas, VA) (ATCC), the Belgian Coordinated Collections of Microorganisms (Ghent, Belgium) (LMG 22254), the Collection du Centre de Recherche en Infectiologie (Québec, Canada) (CCRI) (http://wdcm.nig.ac.jp/CCINFO/CCINFO.xml?861), the Culture Collection of the University of Göteborg (Göteborg, Sweden) (CCUG), the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany) (DSM 4576), and the Public Health Agency of Canada (Winnipeg, Canada) (ER 5307, ER 4215, ER, 5271, and 88-0762). All strains were grown overnight at 30 or 37°C under aerobic conditions on Trypticase soy agar with 5% sheep blood.
TABLE 1.
Yersinia strains used in this studya
| Species or subspecies | Strain | Abbreviation | Isolation
|
Accession no.
|
|||
|---|---|---|---|---|---|---|---|
| Country | Source | Year | tufA | tufB | |||
| Y. aldovae | ATCC 35236T | Yad1 | Czechoslovakia | Drinking water | NA | EF113985 | EF114034 |
| ATCC 35237 | Yad2 | Norway | Fish | NA | EF113986 | EF114035 | |
| CCUG 26532 | Yad3 | Germany | Feces | 1983 | EF113987 | EF114036 | |
| CCUG 26915 | Yad4 | Germany | Human feces | NA | EF113988 | EF114037 | |
| Y. aleksiciae | LMG 22254T | Yak1 | Finland | Human feces | NA | EF113989 | EF114038 |
| Y. bercovieri | 88-0762 | Yb1 | NA | NA | NA | EF113990 | EF114039 |
| ATCC 43970T | Yb2 | France | Human feces | NA | EF113991 | EF114040 | |
| CCUG 26330 | Yb3 | France | Food | NA | EF113992 | EF114041 | |
| CCUG 26539 | Yb4 | Germany | Feces | 1986 | EF113993 | EF114042 | |
| Y. enterocolitica subsp. | 8081 | Ye1 | United States | Septicemia | NA | AM286415b | AM286415b |
| enterocolitica | ATCC 9610T | Ye2 | United States | Human tissue | NA | EF113994 | EF114043 |
| ATCC 23715 | Ye3 | United States | Human blood | 1968 | EF113995 | EF114044 | |
| ATCC 27729 | Ye4 | Belgium | Human blood | 1972 | EF113996 | EF114045 | |
| CCUG 8238 | Ye5 | United States | Human | NA | EF113998 | EF114047 | |
| Y. enterocolitica subsp. | CCRI-905 | Ye6 | Canada | Human blood | NA | EU566877 | EU566908 |
| palearctica | CCRI-952 | Ye7 | Canada | Human feces | NA | EU566878 | EU566909 |
| CCRI-1044 | Ye8 | Canada | Human blood | NA | EU566875 | EU566906 | |
| CCRI-1139 | Ye9 | Canada | Human blood | NA | EU566876 | EU566907 | |
| CCRI-9984 | Ye10 | Canada | Human feces | NA | EU566879 | EU566910 | |
| CCRI-10035 | Ye11 | Canada | Human feces | NA | EU566872 | EU566903 | |
| CCRI-10046 | Ye12 | Canada | Human feces | NA | EU566873 | EU566904 | |
| CCRI-10098 | Ye13 | Canada | Human feces | NA | EU566874 | EU566905 | |
| CCRI-10461 | Ye14 | Canada | Human feces | 1976 | EU566881 | EU566912 | |
| CCRI-10462 | Ye15 | Canada | Human feces | 1976 | EU566882 | EU566913 | |
| CCRI-10463 | Ye16 | Canada | Human feces | 1976 | EU566883 | EU566914 | |
| CCRI-10464 | Ye17 | Canada | Human feces | 1976 | EU566884 | EU566915 | |
| CCRI-10465 | Ye18 | Canada | Human feces | NA | EU566885 | EU566916 | |
| CCRI-10603 | Ye19 | Finland | Human feces | 1981 | EU566886 | EU566917 | |
| CCUG 4586 | Ye20 | Sweden | Human MLN | 1963 | EF113997 | EF114046 | |
| CCUG 18381 | Ye21 | Sweden | Human feces | 1986 | EF113999 | EF114048 | |
| CCUG 21476 | Ye22 | Sweden | Human blood | 1987 | EF114000 | EF114049 | |
| CCUG 31436 | Ye23 | France | Bovine stools | NA | EF114001 | EF114050 | |
| CCUG 33553 | Ye24 | Denmark | Chinchilla | 1960 | EF114002 | EF114051 | |
| CCUG 46041 | Ye25 | Sweden | Human blood | 2002 | EU566880 | EU566911 | |
| Y. frederiksenii | ATCC 29912 | Yf1 | Belgium | Human | NA | EF114003 | EF114052 |
| ATCC 33641T | Yf2 | Denmark | Sewage | NA | EF114004 | EF114053 | |
| CCUG 8246 | Yf3 | NA | Human | NA | EF114005 | EF114054 | |
| CCUG 26594 | Yf4 | Norway | Forced pork | 1983 | EF114006 | EF114055 | |
| CCUG 26949 | Yf5 | Sweden | Human feces | 1990 | EF114007 | EF114056 | |
| CCUG 30114 | Yf6 | Sweden | Human feces | 1992 | EF114008 | EF114057 | |
| ER 5307 | Yf7 | NA | NA | NA | EF114009 | EF114058 | |
| Y. intermedia | ATCC 29909T | Yi1 | NA | Human urine | NA | EF114010 | EF114059 |
| ATCC 33647 | Yi2 | NA | Human feces | NA | EF114011 | EF114060 | |
| ATCC 33648 | Yi3 | NA | Human urine | NA | EF114012 | EF114061 | |
| Y. kristensenii | ATCC 33638T | Yk1 | NA | Human urine | NA | EF114013 | EF114062 |
| CCUG 26582 | Yk2 | Germany | Sewage | 1980 | EF114014 | EF114063 | |
| CCUG 26589 | Yk3 | Norway | Forced meat | 1983 | EF114015 | EF114064 | |
| CCUG 46842 | Yk4 | Sweden | NA | 2002 | EF114016 | EF114065 | |
| Y. mollaretii | ATCC 43969T | Ym1 | United States | Soil | NA | EF114017 | EF114066 |
| CCUG 26536 | Ym2 | Germany | Human feces | 1984 | EF114018 | EF114067 | |
| ER 4215 | Ym3 | NA | NA | NA | EF114019 | EF114068 | |
| Y. pestis | 91001 | Ype1 | NA | NA | NA | AE017042b | AE017042b |
| Antiqua | Ype2 | NA | NA | NA | CP000308b | CP000308b | |
| CO92 | Ype3 | United States | Human | NA | AL590842b | AL590842b | |
| KIM | Ype4 | NA | Human | NA | AE009952b | AE009952b | |
| Y. pseudotuberculosis | ATCC 13979 | Yps1 | Sweden | Hare | 1952 | EF114020 | EF114069 |
| ATCC 27802 | Yps2 | Denmark | Mink | 1943 | EF114021 | EF114070 | |
| ATCC 29833T | Yps3 | Turkey | NA | 1952 | EF114022 | EF114071 | |
| CCUG 7803 | Yps4 | Sweden | Human blood | 1978 | EU566890 | EU566921 | |
| CCUG 17342 | Yps5 | Sweden | Hare | 1951 | EU566887 | EU566919 | |
| CCUG 17345 | Yps6 | Japan | Guinea pig | 1951 | EU566888 | EU566920 | |
| CCUG 37903 | Yps7 | NA | NA | 1997 | EU566888 | EU566918 | |
| ER 5271 | Yps8 | NA | NA | NA | EU566891 | EU566922 | |
| IP 32953 | Yps9 | NA | NA | NA | BX936398b | BX936398b | |
| Y. rohdei | ATCC 43380T | Yro1 | Germany | Dog feces | NA | EF114023 | EF114072 |
| ATCC 43871 | Yro2 | Germany | Dog feces | NA | EF114024 | EF114073 | |
| ATCC 43873 | Yro3 | United States | Human feces | NA | EF114025 | EF114074 | |
| CCUG 26544 | Yro4 | Germany | Human feces | 1978 | EF114026 | EF114075 | |
| CCUG 26545 | Yro5 | Germany | Human feces | 1989 | EF114027 | EF114076 | |
| Y. ruckeri | ATCC 29473T | Yru1 | United States | Rainbow trout | NA | EF114028 | EF114077 |
| CCRI-10643 | Yru2 | United States | Rainbow trout | NA | EF114029 | EF114078 | |
| CCUG 21537 | Yru3 | NA | NA | NA | EF114030 | EF114079 | |
T = type strain. Abbreviations are those used in Fig. 2. NA, not available; MLN, mesenteric lymph node.
Sequences retrieved from completed genome sequences.
DNA isolation.
Bacterial DNA was isolated from mid-log-phase cultures of selected strains by using a BioSprint 15 DNA blood kit (Qiagen, Mississauga, Ontario, Canada) automated with a KingFisher mL instrument (Thermo Fisher Scientific, Waltham, MA). Alternatively, a GNOME DNA kit (Qbiogene Inc., Carlsbad, CA) was used (32).
Primer design.
The organization of the genes surrounding the tufA and tufB genes was used to design primers for specific amplification of tufA and tufB. The bacterial fusA or gammaproteobacterial and Deinococcus-Thermus nusG gene sequences in public databases were assembled in a multiple alignment using the CLUSTAL W software (version 1.83) (11, 55). Conserved regions were identified at the 3′ end of the fusA gene and at the 5′ end of the nusG gene. The fusA primer (primer F1; 5′-GTICCIYTIKCIGARATGTTYGGITAYGC-3′; positions 1972 to 2000 in the Escherichia coli K-12 GenBank accession number U00096 sequence) was combined with a previously designed universal primer for the terminal region of tuf (primer T2; 5′-CCIACIGTICKICCRCCYTCRCG-3′; positions 1132 to 1154 in the E. coli K-12 sequence) (47) to amplify tufA sequences. The nusG primer (primer N1; 5′-AACGCCTGRACGACRTACCA-3′; positions 25 to 44 in the E. coli K-12 sequence) was used in combination with a second universal primer for the 5′ region of tuf (primer T1; 5′-AAYATGATIACIGGIGCIGCICARATGGA-3′; positions 271 to 299 in the E. coli K-12 sequence) (47) to amplify tufB sequences.
Sequencing of tufA and tufB genes.
A 1,369-bp fragment of the str operon (tufA) was amplified using the F1 and T2 primers. The N1 and T1 primers were used for specific amplification of a 1,594-bp fragment of the tufB region. The PCR mixtures contained primers F1 and T2 or primers T1 and N1 (each at a concentration of 1.0 μM), 200 μM deoxyribonucleoside triphosphates (Amersham Biosciences, Piscataway, NJ), 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2.5 mM MgCl2, 3.3 μg/μl bovine serum albumin (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada), 0.06 μg/μl methoxsalen (Sigma-Aldrich Canada Ltd.), and 0.025 U/μl Taq DNA polymerase (Promega, Madison, WI) combined with the TaqStart antibody (Clontech Takara Bio, Mountain View, CA) (33). Decontamination of the PCR mixtures prior to PCR was performed by using a Spectrolinker model XL-1000 UV cross-linker (Spectronics Corporation, Westbury, NY). Purified genomic DNA (3 ng) was added to each PCR mixture, which was subjected to thermal cycling with a PTC-200 DNA Engine thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA) as follows: 5 min at 94°C, followed by 40 cycles of 1 s at 95°C, 1 min at 60°C (for tufA) or 58°C (for tufB), and 1 min at 72°C and then a final extension for 7 min at 72°C. Amplicons were detected using 1.2% agarose gel electrophoresis with 0.25 μg/ml of ethidium bromide in Tris-borate-EDTA buffer (89 mM Tris, 89 mM boric acid, 2 mM EDTA). Amplicon sizes were verified with a 1-kb DNA ladder used as a molecular weight marker (Invitrogen, Carlsbad, CA). During all of these steps, precautions were taken to avoid carryover of amplified DNA, as previously described (42). Amplification products were purified using gel electrophoresis (1.2% agarose at 120 V for 1 h), followed by staining with methylene blue (Mallinckrodt Baker, Inc., Phillipsburg, NJ) and DNA purification with a QIAquick gel extraction kit (Qiagen), as previously described (32). Both strands of each amplicon were sequenced using an automated ABI sequencer (Applied Biosystems, Foster City, CA) with 5 μM sequencing primer (primer F1, T1, or T2 for tufA and primer T1 or T2 for tufB). Chromatogram assembly and analysis were performed using the Sequencher 3.1 software (Gene Codes Corp., Ann Arbor, MI).
Phylogenetic analyses.
In order to investigate tuf genetic variability within the genus Yersinia, 72 strains were chosen to represent 12 species; greater proportions of strains of known genetically diverse species were used (Table 1). Representative Enterobacteriaceae other than Yersinia were selected based on their close relationships at the phylogenetic level as determined by 16S rRNA gene sequence analysis (28, 47). Vibrio cholerae N16961 was used as a nonenterobacterial outgroup. The tuf gene sequences of Erwinia carotovora subsp. atroseptica SCRI1043 (accession number BX950851), E. coli K-12 (accession number U00096), Photorhabdus luminescens subsp. laumondii TTO1 (accession number BX470251), Salmonella enterica subsp. enterica serovar Typhimurium strain LT2 (accession number AE006468), Shigella flexneri 2a 2457T (accession number AE014073), V. cholerae O1 biovar El Tor strain N16961 (accession number AE003852), Y. enterocolitica subsp. enterocolitica 8081, Y. pestis strains 91001, Antiqua, CO92, and KIM, and Y. pseudotuberculosis strain IP 32953 (Table 1) were retrieved from complete genome sequences available from the GenBank database (2, 4, 9, 10, 18, 23, 40, 44, 55a, 59, 61). A multiple-sequence alignment of the newly generated tuf sequences and sequences from the GenBank database was constructed using the CLUSTAL W software. The alignment was verified by visual inspection using the SeqLab editor (GCG Wisconsin Package, version 10.3; Accelrys Software Inc., San Diego, CA). A 771-bp region of the tufA and tufB genes (positions 316 to 1086 in Y. pestis CO92) was analyzed. The nucleic acid sequences were translated into amino acid sequences (residues 105 to 361) using the Translate function of the GCG Wisconsin Package and then inspected with the SeqLab editor to identify variations in residues between Yersinia EF-TuA (encoded by tufA) and EF-TuB (encoded by tufB). Levels of identity between tufA and tufB nucleic acid sequences and levels of similarity between the gene products were calculated with the GAP function of the GCG Wisconsin Package (BLOSUM62 amino acid substitution matrix) (Tables 2 and 3) (24). Phylogenetic analyses of comparative (see Fig. 1A) and concatenated (see Fig. 1B) tufA and tufB nucleic acid sequences were performed with the MEGA4 software (52) in order to compute evolutionary distances using the maximum composite likelihood method (52, 54). The differences in composition bias among sequences were considered in evolutionary comparisons (53). One thousand bootstrap analyses were performed to estimate the robustness of the phylogenetic inference (19). A split network was computed using SplitsTree 4.8 for Unix, and genetic distances were calculated using the uncorrected P method (27). A split network was also constructed using the Neighbor-Net method (7) and EqualAngle split transformation settings.
TABLE 2.
tuf sequence variations in Yersiniaa
| Species | n | Intragenomic variation
|
Intraspecies variation
|
||||
|---|---|---|---|---|---|---|---|
| Nucleic acid identity (%)
|
Amino acid similarity (%)
|
||||||
| Nucleic acid identity (%) of tufA and tufB | Amino acid similarity (%) of EF-TuA and EF-TuB | tufA | tufB | EF-TuA | EF-TuB | ||
| Y. aldovae | 4 | 87.4-87.7 | 96.1 | 99.9-100.0 | 98.8-100.0 | 100.0 | 100.0 |
| Y. aleksiciae | 1 | 83.8 | 96.1 | NA | NA | NA | NA |
| Y. bercovieri | 4 | 83.9 | 95.7-96.1 | 99.6-100.0 | 99.9-100.0 | 100.0 | 99.6-100.0 |
| Y. enterocolitica | 25 | 90.5-91.7 | 96.1-98.1 | 93.9-100.0 | 97.7-100.0 | 97.7-100.0 | 100.0 |
| Y. frederiksenii | 7 | 86.0-87.8 | 95.3-95.7 | 92.5-100.0 | 92.0-100.0 | 96.9-100.0 | 99.6-100.0 |
| Y. intermedia | 3 | 84.7-85.2 | 94.9-95.3 | 99.7-99.9 | 98.7-99.6 | 100.0 | 99.6-100.0 |
| Y. kristensenii | 4 | 88.8-89.9 | 96.1 | 100.0 | 96.9-100.0 | 100.0 | 100.0 |
| Y. mollaretii | 3 | 84.7-85.1 | 94.9-95.7 | 96.9-99.7 | 99.9-100.0 | 98.8-100.0 | 100.0 |
| Y. pestis | 4 | 85.7-85.9 | 96.5 | 100.0 | 99.9-100.0 | 100.0 | 98.8-100.0 |
| Y. pseudotuberculosis | 9 | 85.9-86.0 | 96.5 | 99.7-100.0 | 97.5-100.0 | 100.0 | 99.6-100.0 |
| Y. rohdei | 5 | 84.7-85.1 | 96.1 | 99.7-100.0 | 99.2-100.0 | 100.0 | 100.0 |
| Y. ruckeri | 3 | 85.2 | 94.9 | 100.0 | 100.0 | 100.0 | 100.0 |
| Yersinia | 72 | 83.8-91.7 | 94.9-98.1 | 92.5-100.0 | 92.0-100.0 | 96.9-100.0 | 98.8-100.0 |
| H. alvei | 6 | 96.4-97.0 | 97.7-98.8 | 98.4-100.0 | 95.3-100.0 | 100.0 | 98.4-100.0 |
| O. proteus | 1 | 97.1 | 97.3 | NA | NA | NA | NA |
| Other Enterobacteriaceae | 12 | 98.8-100.0 | 99.6-100.0 | NA | NA | NA | NA |
| V. cholerae (outgroup) | 1 | 99.1 | 99.6 | NA | NA | NA | NA |
n, number of strains; NA, not applicable.
TABLE 3.
Interspecies variations of tuf sequences in Yersinia
| Species (no. of strains) | % tuf identity (% EF-Tu similarity)a
|
% tuf identity (% EF-Tu similarity)a
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y. aldovae | Y. aleksiciae | Y. bercovieri | Y. enterocolitica | Y. frederiksenii | Y. intermedia | Y. kristensenii | Y. mollaretii | Y. pestis | Y. pseudotuberculosis | Y. rohdei | Y. ruckeri | |
| Y. aldovae (4) | 95.7-95.9 (100.0) | 95.3-95.6 (100.0) | 91.8-93.9 (97.3-97.7) | 92.1-95.7 (96.9-100.0) | 96.1-96.4 (99.6) | 93.3-93.4 (97.7) | 93.5-96.0 (98.8-100.0) | 92.6-92.7 (97.7) | 92.6-92.7 (97.7) | 93.3-93.6 (97.7) | 93.3-93.4 (97.7) | |
| Y. aleksiciae (1) | 84.7-85.2 (97.3) | 99.4-99.5 (100.0) | 92.6-94.6 (97.3-97.7) | 93.8-97.8 (96.9-100.0) | 95.9-96.0 (99.6) | 94.0 (97.7) | 96.1-97.9 (98.8-100.0) | 92.6 (97.7) | 92.6 (97.7) | 92.6-92.9 (97.7) | 93.4 (97.7) | |
| Y. bercovieri (4) | 85.2-85.6 (96.9-97.3) | 94.8-94.9 (99.6-100.0) | 92.1-94.2 (97.3-97.7) | 93.3-97.8 (96.9-100.0) | 95.5-95.7 (99.6) | 93.5-93.6 (97.7) | 96.0-97.9 (98.8-100.0) | 92.1-92.2 (97.7) | 92.1-92.2 (97.7) | 92.1-92.5 (97.7) | 92.9-93.0 (97.7) | |
| Y. enterocolitica (25) | 92.3-93.4 (100.0) | 84.7-85.1 (97.3) | 85.5-85.9 (96.9-97.3) | 91.6-95.7 (97.3-99.2) | 91.6-93.7 (96.9-98.1) | 93.9-98.1 (97.7-100.0) | 92.1-95.1 (97.3-98.4) | 94.4-95.3 (98.1-99.6) | 94.2-95.3 (98.1-99.6) | 94.0-95.6 (98.1-99.6) | 94.0-94.9 (98.1-98.8) | |
| Y. frederiksenii (7) | 86.3-87.7 (97.3-97.7) | 86.1-87.8 (99.2-99.6) | 86.5-87.7 (98.8-99.6) | 87.9-88.8 (97.3-97.7) | 92.1-95.7 (96.5-99.6) | 92.5-94.2 (97.3-97.7) | 93.1-96.1 (96.9-100.0) | 91.1-96.4 (97.7-99.6) | 91.1-96.4 (97.7-99.6) | 91.6-97.4 (97.7-99.6) | 92.3-95.2 (97.7-98.8) | |
| Y. intermedia (3) | 85.2-86.3 (97.3-97.7) | 85.0-85.2 (99.2-99.6) | 83.9-84.4 (98.8-99.6) | 85.9-87.0 (97.3-97.7) | 89.1-90.8 (99.2-100.0) | 93.8-94.0 (98.1) | 94.0-96.2 (98.4-99.6) | 92.3-92.5 (97.3) | 92.3-92.5 (97.3) | 93.0-93.4 (97.3) | 93.9-94.0 (97.3) | |
| Y. kristensenii (4) | 90.8-92.5 (100.0) | 83.9-84.7 (97.3) | 83.7-84.6 (96.9-97.3) | 92.1-94.7 (100.0) | 85.9-87.5 (97.3-97.7) | 84.6-86.0 (97.3-97.7) | 93.6-94.4 (97.7-98.4) | 94.0 (98.1) | 93.8-94.0 (98.1) | 94.0-94.3 (98.1) | 94.3 (98.1) | |
| Y. mollaretii (3) | 87.4-87.9 (96.9) | 87.8 (98.4) | 87.7-87.8 (98.1-98.4) | 88.8-89.4 (98.8) | 85.0-86.6 (98.4-98.8) | 84.8-85.0 (98.4-98.8) | 86.8-87.9 (98.8) | 92.9-94.0 (97.7-98.4) | 92.9-94.0 (97.7-98.4) | 92.7-93.6 (97.7-98.4) | 93.8-94.7 (97.7-98.4) | |
| Y. pestis (5) | 83.7-84.2 (96.9) | 86.0-86.1 (98.8) | 85.5-85.7 (98.4-98.8) | 83.4-84.6 (96.9) | 86.6-86.9 (99.2-99.6) | 85.6-86.0 (98.8-99.2) | 83.7-85.0 (96.9) | 83.4-83.5 (98.1) | 99.7-100.0 (100.0) | 96.1-96.4 (100.0) | 95.7 (99.2) | |
| Y. pseudotuberculosis (4) | 83.9-84.8 (96.9) | 86.0-86.3 (98.4-98.8) | 84.8-85.9 (98.1-98.8) | 83.5-84.8 (96.9) | 86.5-87.7 (98.8-99.6) | 85.5-86.3 (98.4-99.2) | 83.7-85.2 (96.9) | 83.7-84.0 (98.1) | 97.5-99.9 (99.6-100.0) | 95.9-96.4 (100.0) | 95.5-95.7 (99.2) | |
| Y. rohdei (5) | 86.6-87.3 (97.3) | 86.6-87.2 (99.2) | 87.2-87.8 (98.8-99.2) | 86.5-87.2 (97.3) | 90.7-91.8 (99.6-100.0) | 88.6-89.1 (99.2-99.6) | 84.3-85.5 (97.3) | 85.1-85.5 (98.4) | 85.1-85.6 (99.6) | 85.3-85.9 (99.2-99.6) | 96.6-96.9 (99.2) | |
| Y. ruckeri (3) | 84.8-85.1 (97.3) | 83.5 (98.4) | 82.4-82.5 (98.4) | 83.9-84.2 (97.3) | 85.2-85.6 (98.4-98.8) | 83.9-84.8 (98.4-98.8) | 83.8-84.4 (97.3) | 81.3 (97.7) | 84.0-84.2 (98.4) | 84.2-84.6 (98.1-98.4) | 85.2-85.3 (98.4) | |
The data above the diagonal are for tufA and EF-TuA sequences, and the data below the diagonal are for tufB and EF-TuB sequences.
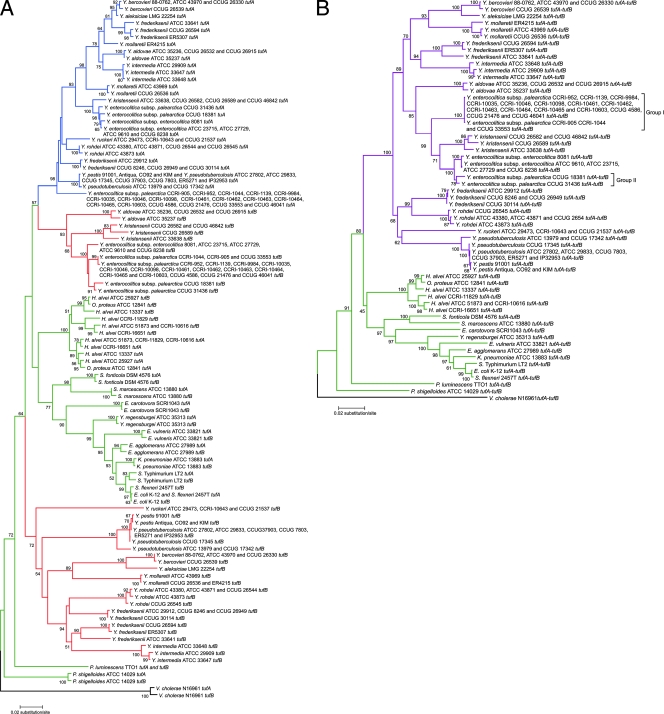
FIG. 1.
Phylogenetic trees for tufA and tufB nucleic acid sequences from Yersinia and non-Yersinia enterobacterial strains. (A) Phylogenetic tree based on a comparison of tufA and tufB nucleic acid sequences. Yersinia tufA and tufB tree branches are blue and red, respectively. (B) Phylogenetic tree based on concatenated tufA and tufB nucleic acid sequences. Yersinia concatenated tufA and tufB tree branches are purple. The tuf gene branches for other enterobacterial species and V. cholerae (outgroup) are green and black, respectively. Evolutionary distances were computed using the maximum composite likelihood method of the MEGA4 software. The topological accuracy of the tree was evaluated using 1,000 bootstrap replicates.
Nucleotide sequence accession numbers.
Partial tufA and tufB gene sequences have been deposited in the GenBank database under the following accession numbers: Yersinia strains, EF113985 to EF114030, EU566872 to EU566891, EF114034 to EF114079, and EU566903 to EU566922 (Table 1); E. agglomerans ATCC 27989, EU566892 and EU566923; E. vulneris ATCC 33821, EU566893 and EU566924; H. alvei ATCC 13337, ATCC 25927, ATCC 51873, CCRI-10616, CCRI-11829, and CCRI-16651, EF114031, EU566894 to EU566898, EF114080, and EU566925 to EU566929; K. pneumoniae ATCC 13883, EF114032 and EF114081; O. proteus ATCC 12841, EU566899 and EU566930; P. shigelloides ATCC 14029, EU566900 and EU566931; S. fonticola DSM 4576, EU566901 and EU566932; S. marcescens ATCC 13880, EF114033 and EF114082; and Y. regensburgei ATCC 35313, EU566902 and EU566933.
RESULTS
tufA and tufB gene similarities.
All 72 Yersinia strains analyzed in this study possessed two divergent copies of the tuf gene, tufA and tufB. The levels of intragenomic identity for tufA and tufB nucleic acid sequences varied from 83.8 to 91.7% for the 771-bp region studied (Table 2). In contrast, 12 Enterobacteriaceae strains (E. agglomerans ATCC 27989, E. carotovora SCRI1043, E. coli K-12, E. vulneris ATCC 33821, K. pneumoniae ATCC 13883, P. luminescens TTO1, P. shigelloides ATCC 14029, S. enterica subsp. enterica serovar Typhimurium LT2, S. fonticola DSM 4576, S. marcescens ATCC 13880, S. flexneri 2457T, and Y. regensburgei ATCC 35313), as well as a Vibrionaceae strain (V. cholerae N16961), had levels of intragenomic tufA and tufB identity ranging from 98.8 to 100.0% (Table 2). Interestingly, all six strains of H. alvei (ATCC 13337, ATCC 25927, ATCC 51873, CCRI-10616, CCRI-11829, and CCRI-16651) and O. proteus ATCC 12841 exhibited intermediate levels of identity for tufA and tufB, which ranged from 96.4 to 97.1%; this distinguished these strains from both Yersinia and other genera of the Enterobacteriaceae. The levels of intragenomic amino acid similarity for EF-TuA and EF-TuB exhibited a similar pattern and ranged from 94.9 to 98.1% for Yersinia strains, from 97.3 to 98.3% for H. alvei and O. proteus strains, and from 99.6 to 100% for other Enterobacteriaceae strains, as well as V. cholerae N16961 (Table 2). Amino acid sequence similarities for all EF-TuA and EF-TuB sequences from strains of Yersinia, strains of other Enterobacteriaceae, and Vibrionaceae strains were also examined (residues 105 to 361). Amino acid residues in functionally important sites are conserved in Yersinia (38). However, two amino acid positions that can distinguish all Yersinia tufA sequences from all tufB sequences were found (at position 167, Ile or Leu in EF-TuA and Thr in EF-TuB; at position 361, Thr in EF-TuA and Asn in EF-TuB). Therefore, these residues can be used as signature residues to discriminate between the tufA and tufB genes in Yersinia strains. Residue 167 is different (Ile, Leu, or Thr) in the other genera of the Enterobacteriaceae studied, and therefore the difference between EF-TuA and EF-TuB reported here is not specific to the genus Yersinia. In contrast, EF-Tu residue 361 is conserved among most of the Enterobacteriaceae strains (Thr) investigated in this study; the exceptions are the residues in EF-TuB in the genera Yersinia (Asn), Hafnia (Asn or Ser), and Obesumbacterium (Asn).
The levels of interspecies similarity for tufA and tufB genes and their products are shown in Table 3. Most notably, when the EF-TuA amino acid sequences of Y. rohdei were compared to those of Y. pestis and Y. pseudotuberculosis, the levels of interspecies similarity were 100%, even though the levels of tufA nucleotide sequence identity were 95.9 to 96.4% (Table 3). Also, the level of EF-TuB amino acid sequence similarity for Y. aldovae, Y. enterocolitica, and Y. kristensenii was 100.0%, while the levels of tufB nucleic acid sequence identity ranged from 90.8 to 94.7%. These examples suggest that there is selective pressure for both the EF-TuA and EF-TuB proteins to maintain amino acid sequence stability.
Phylogenetic tree.
A phylogenetic tree based on tufA and tufB nucleic acid sequences was constructed to study the evolution of orthologous and paralogous tuf genes in Yersinia compared to other genera in the family. Non-Yersinia enterobacterial reference strains were selected based on their relatively close relationships to the genus Yersinia at the phylogenetic level, based on 16S rRNA gene sequences. The paralogous genes of most of the non-Yersinia species are very similar and group together, forming an organismal tufA-tufB clade. On the other hand, the paralogous tuf genes of Yersinia strains showed the distinctive evolution of these strains (Fig. 1A). Based on the data obtained, it is clear that intragenomic tufA and tufB genes in Yersinia have diverged significantly. H. alvei and O. proteus are minor exceptions within the Enterobacteriaceae, because they form two other clades with separated tufA and tufB genes. The Yersinia, H. alvei, and O. proteus tufB interspecies distances (branch lengths) are approximately twice the interspecies distances for the tufA genes. The topology of the phylogenetic tree shows that Yersinia tufA gene sequences are monophyletic, while the tufB sequences are diphyletic. The Yersinia tufA clade branches with the Y. aldovae-Y. enterocolitica-Y. kristensenii tufB clade, which is separated from the tufB sequence cluster of other Yersinia species by other enterobacterial genera. However, this topology is not supported by the results of the bootstrap analysis.
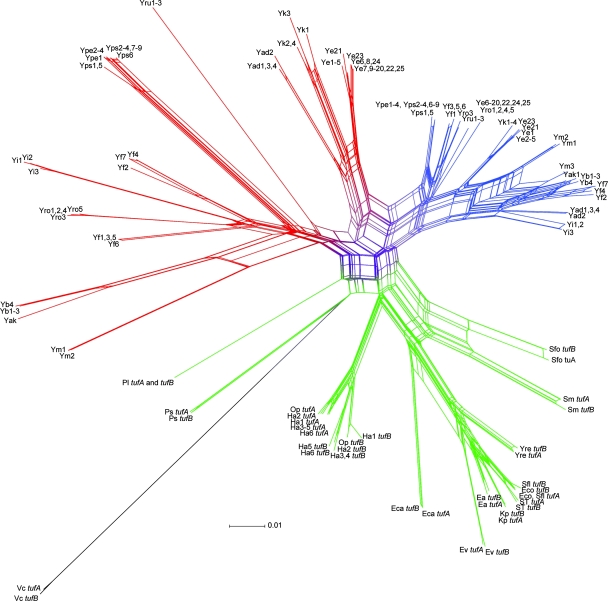
Phylogenetic network.
While phylogenetic trees describe evolutionary relationships based on mutational events, phylogenetic networks allow incorporation of more complex models of evolution, such as recombination and gene duplication (27). Because the evolution of tuf genes in Enterobacteriaceae was driven not only by mutation but also by gene conversion events, the Neighbor-Net method was used to visualize and analyze incompatible phylogenetic signals that are represented by edges (7, 34). Yersinia tufA and tufB sequences were analyzed by the Neighbor-Net method implemented in the SplitsTree software (Fig. 2). The Neighbor-Net analysis results support the clustering of Yersinia tufA and tufB sequences previously observed in the phylogenetic tree (Fig. 1A) and display the paralogous tufA and tufB genes in Yersinia as separate clusters linked by edges near the origin. In contrast, other paralogous enterobacterial tufA and tufB sequences are grouped and linked by edges at the extremities. This indicates that there was ancestral divergence of Yersinia tufA from tufB, while in other Enterobacteriaceae the tufA and tufB genes are still coevolving. The H. alvei-O. proteus group is an exception in which there are two separate tufA and tufB clusters, which have intermediate distances of the edges to the origin compared to Yersinia strains and the other Enterobacteriaceae strains. This suggests that there was more recent divergence compared to the genus Yersinia. There is no evidence that H. alvei and O. proteus tuf paralogs are still coevolving.
FIG. 2.
Phylogenetic network for tufA and tufB nucleic acid sequences from Yersinia and non-Yersinia enterobacterial strains. The Neighbor-Net graph was computed by using the SplitsTree 4.8 software. Yersinia tufA and tufB branches are blue and red, respectively. The tuf gene branches of other enterobacterial species and V. cholerae (outgroup) are green and black, respectively. Abbreviations for Yersinia strains are shown in Table 1. Abbreviations for non-Yersinia strains are as follows: Ea, E. agglomerans ATCC 27989; Eca, E. carotovora subsp. atroseptica SCRI1043; Eco, E. coli K-12; Ev, E. vulneris ATCC 33821; Ha1, H. alvei ATCC 13337; Ha2, H. alvei ATCC 25927; Ha3, H. alvei ATCC 51873; Ha4, H. alvei CCRI-10616; Ha5, H. alvei CCRI-11829; Ha6, H. alvei CCRI-16651; Kp, K. pneumoniae ATCC 13883; Op, O. proteus ATCC 12841; Pl, P. luminescens subsp. laumondii TTO1; Ps, P. shigelloides ATCC 14029; ST, S. enterica subsp. enterica serovar Typhimurium strain LT2; Sfo, S. fonticola DSM 4576; Sm, S. marcescens ATCC 13880; Sfl, S. flexneri 2a strain 2457T; Yre, Y. regensburgei ATCC 35313; and Vc, V. cholerae O1 biovar El Tor strain N16961.
Yersinia species.
The remarkable evolution of tuf genes in Yersinia has resulted in genetic variations that can be used to infer species clustering. Therefore, phylogenetic analyses of tuf genes in Yersinia provide information for reclassification and identification of Yersinia species. In order to enhance the number of sites analyzed simultaneously, we constructed a phylogenetic tree using concatenated tufA and tufB sequences (Fig. 1B). This tree showed the monophyletic nature of the genus Yersinia, its separation from other genera of the Enterobacteriaceae, and Yersinia species clustering, all of which were strongly supported by bootstrap analysis.
Based on the analysis of concatenated tufA and tufB sequences, Y. aldovae, Y. aleksiciae, Y. bercovieri, Y. intermedia, Y. kristensenii, Y. mollaretii, Y. rohdei, and Y. ruckeri strains form distinct clusters that correlate with the current species classification (Fig. 1B). Y. aleksiciae type strain LMG 22254 is clearly distinct from the Y. kristensenii strain cluster.
Nucleic acid sequences and tree topology showed the genotypic diversity of Y. enterocolitica (Fig. 1B). Y. enterocolitica concatenated tufA and tufB sequences form two distant clades supported by a high bootstrap value (99%). One clade includes 17 strains of Y. enterocolitica subsp. palearctica, which were designated group I (CCRI-905, CCRI-952, CCRI-1044, CCRI-1139, CCRI-9984, CCRI-10035, CCRI-10046, CCRI-10098, CCRI-10461, CCRI-10462, CCRI-10463, CCRI-10464, CCRI-10465, CCRI-10603, CCUG 4586, CCUG 21476, and CCUG 33553) and were isolated in Canada, Finland, Sweden, and Denmark (Table 1). The other clade contains two subgroups. One subgroup includes five Y. enterocolitica subsp. enterocolitica strains (8081, ATCC 9610, ATCC 23715, ATCC 27729, and CCUG 8238) isolated in the United States and Belgium, while the other subgroup contains two strains of Y. enterocolitica subsp. palearctica designated group II (CCUG 18381 and CCUG 31436) isolated in France and Sweden (46) (Table 1).
Y. frederiksenii, which also is known to be a genotypically heterogeneous species, consists of three concordant clades that correlate with three of the four previously characterized genomic groups (genomic groups 1a, 1b, and 3; no genomic group 2 reference strain was used in this study) (56). The intraspecies distances (branch length) of Y. frederiksenii ATCC 33641 (genomic group 1a type strain) from Y. frederiksenii CCUG 26594 (genomic group 1b) and Y. frederiksenii ER 5307 (unknown genomic group) are greater than those between strains of other Yersinia species. Y. frederiksenii strains ATCC 29912, CCUG 26949, and CCUG 30114 (all genomic group 3 strains) cluster with strain CCUG 8246 (unknown genomic group). The concatenated tuf gene tree clearly separates the genomic group 3 clade from genomic groups 1a and 1b. Y. pestis and Y. pseudotuberculosis strains cluster together based on the concatenated tuf gene tree and thus are presented as a unique genomospecies, as previously revealed by DNA-DNA hybridization analysis (6). Finally, although the taxonomy of Y. ruckeri is controversial, its tuf gene sequences cluster with those of other Yersinia species and support the conclusion that this species should be included in the genus (17, 28, 36, 51). In addition, the tufA and tufB genes of each Y. ruckeri strain exhibit a level of identity (85.2%) which is in the range observed for other Yersinia species (Table 2).
DISCUSSION
In this study, sequence analyses revealed that intragenomic tufA and tufB genes are divergent in 12 Yersinia species. In comparison, 12 non-Yersinia enterobacterial species investigated contained two intragenomic tuf genes which were very similar to one another. However, the intragenomic tuf sequences of the members of the H. alvei-O. proteus clade exhibited an intermediate level of divergence. The tufA and tufB genes have been described previously as genes evolving in concert through gene conversion events which maintain their remarkable level of nucleotide sequence identity (1, 3, 26, 39). Gene conversion driven by homologous recombination mechanisms explains the high levels of similarity usually observed for duplicated tuf genes of members of the Enterobacteriaceae, as shown in S. enterica serovar Typhimurium (1). Conversely, the remarkable divergence between the tufA and tufB genes in Yersinia strains may result from (i) the acquisition of an exogenous tuf gene by horizontal transfer, (ii) the gradual or spontaneous loss of effective conversion mechanisms (due either to defects in the mechanism or the level of dissimilarity of sequences), or (iii) either loss of function or neofunctionalization of one EF-Tu copy.
High levels of divergence (21 to 32%) of intragenomic tuf gene sequences have been observed previously for 11 enterococcal species, while six other enterococcal species contained only one tuf gene (32). Acquisition of an exogenous copy of the tuf gene by the ancestor of the 11 Enterococcus species having two tuf gene copies was the mechanism proposed to explain the presence of two different intragenomic tuf genes (32). In contrast, the organization of tuf genetic regions shows that in Enterobacteriaceae containing two tuf gene copies an ancestral duplication of tuf gene was conserved (35, 39). The organization of the tuf genetic regions (fusA-tufA and tufB-secE-nusG) in E. coli K-12 is conserved in Yersinia and all of the other Enterobacteriaceae genomes studied here except the P. luminescens subsp. laumondii strain TTO1 genome (27). The latter strain has an unusual chimeric gene order (fusA-tuf-secE-nusG), whereas the other copy of the tuf gene is located downstream from tRNA genes. This abnormal arrangement of the tuf regions in strain P. luminescens subsp. laumondii TTO1 can be explained by recent homologous recombination between the tufA and tufB genes, resulting in the observed chimeric configurations. The conserved synteny of the tuf gene neighborhood in the genus Yersinia, as well as in the other Enterobacteriaceae studied here, also shows that there was conservation of two ancestral duplicated copies.
The higher levels of divergence between tufA and tufB sequences in the 12 Yersinia species examined in this study suggest that gene conversion became inefficient or ceased to function in the ancestor that gave rise to the modern Yersinia species. This could have been due to loss of specific or general gene conversion mechanisms or simply to sequence divergence beyond the divergence that these mechanisms allowed. Gene conversion mechanisms require recombination between very similar sequences. It has been proposed that recombination events played a large role in the evolution and emergence of Y. pestis from Y. pseudotuberculosis and that active genome rearrangements in the form of inversions or translocations are responsible for a highly plastic genome with noticeable strain-to-strain variation (9, 15, 48). Moreover, multiple copies of the 16S and 23S rRNA genes are also influenced by gene conversion mechanisms (22, 43). The seven copies of the 16S and 23S rRNA genes in Y. enterocolitica subsp. enterocolitica 8081, Y. pestis strains 91001, Antiqua, CO92, and KIM, and Y. pseudotuberculosis IP 32953 have very similar nucleic acid sequences. Therefore, some gene conversion mechanisms are likely to still be operational in the genus Yersinia. More general models, in which the conversion frequency gradually declines as genes diverge via the accumulation of point mutations, have been studied previously (57). The tufA and tufB genes in the ancestor of the genus Yersinia could have mutated gradually, and thereby affected conversion mechanisms. The genes would then have evolved independently in parallel. This mutational evolution model could explain the high and relatively wide intragenomic tuf nucleic acid sequence divergence in Yersinia species, which ranges from 8.3 to 16.2%. To our knowledge, this is the first example of possible tuf gene conversion inefficiency. The intermediate level of sequence variation between intragenomic tuf genes in the H. alvei-O. proteus clade could be an attenuated result of the same phenomenon. The 16S rRNA gene sequence analysis performed by Ibrahim and colleagues (28) showed that H. alvei is the member of the Enterobacteriaceae most closely related to the genus Yersinia, but it was not included in this genus. However, the number of non-Yersinia Enterobacteriaceae included in this study was relatively small.
In the tuf mutational evolution model, it is possible that one of the two Yersinia EF-Tu copies ceased to function or evolved to perform new functions. The levels of similarity between EF-TuA and EF-TuB amino acid sequences are significantly lower in Yersinia (94.9 to 98.1%) than in other Enterobacteriaceae (99.6 to 100%). The H. alvei-O. proteus group exhibits intragenomic tuf nucleic acid sequence divergence lower than that observed for Yersinia. However, amino acid sequence divergence is similar for the two groups (97.3 to 98.3% similarity). Thus, the nucleic acid sequence divergence resulted in significant amino acid sequence changes. Although evolution of new functions for duplicate genes may be rare (41), EF-Tu proteins have been linked to functions other than elongation, including chaperone properties (8). EF-Tu residue 361 (Thr) is conserved among all of the Enterobacteriaceae strains investigated here except for EF-TuB in the genera Yersinia (Asn), Hafnia (Asn or Ser), and Obesumbacterium (Asn). However, this amino acid residue could not be linked to known functional activities of the protein (5, 38). Although tuf DNA sequences are divergent in Yersinia species, it appears that some EF-Tu proteins have identical or very similar sequences. The tufA nucleic acid sequences of Y. rohdei and the Y. pseudotuberculosis/Y. pestis genomospecies were clearly divergent, but the EF-TuA amino acid sequences of these organisms were identical. Also, the levels of tufB nucleic acid sequence identity for Y. aldovae, Y. enterocolitica, and Y. kristensenii ranged from 90.8 to 94.7%, while the levels of EF-TuB amino acid sequence similarity were 100.0%. These observations suggest that functional convergent evolution occurred in these species for EF-TuA and EF-TuB. The recently described genome sequence of Y. pestis Nepal516 revealed a 58-amino-acid C-terminal deletion in EF-TuB that might have led to a nonfunctional copy of this elongation factor (10). However, such a truncated tufB gene has been found only in this isolate and may be a strain-specific mutation that occurred since the original isolation. There is no evidence suggesting that a loss of EF-Tu function occurred in other Yersinia strains. Differential expression studies of tufA and tufB, as well as gene inactivation mutagenesis analysis, may help elucidate specific functions of EF-TuA and EF-TuB under different physiological conditions. Han and colleagues (21) recently compiled microarray data from numerous studies investigating the expression of Y. pestis strain 201 genes under 25 different stress conditions in vitro. Cold shock stimulation appears to downregulate tufB, while the presence of an antibacterial peptide (polymyxin B) apparently upregulates tufA expression.
This study of the genus Yersinia was performed with a wide diversity of strains representing 12 Yersinia species. The evolution of tuf genes in Yersinia has resulted in genetic variations that provide a high level of resolution within the genus and represent a powerful tool for the classification of the Yersinia genomospecies. Moreover, the greater divergence between the tufA and tufB genes in Yersinia species than in other Enterobacteriaceae helps distinguish the genus Yersinia from other genera. The concatenated tuf gene tree was used to infer species clustering (Fig. 1B). tuf-based genetic analyses of Y. aldovae, Y. aleksiciae, Y. bercovieri, Y. intermedia, Y. kristensenii, Y. rohdei, and Y. ruckeri showed that their distinctive clades correlate with the phenotypic classification.
Y. enterocolitica strains show great diversity genetically as well as phenotypically. Studies of Y. enterocolitica based on DNA-DNA hybridization, 16S rRNA gene sequences, and G+C content have subdivided Y. enterocolitica into two subspecies (Y. enterocolitica subsp. enterocolitica and Y. enterocolitica subsp. palearctica) (29, 46). A more recent study, based on DNA-DNA microarray hybridization with the genome of strain Y. enterocolitica subsp. enterocolitica 8081, separated Y. enterocolitica strains into three groups (high-pathogenicity, low-pathogenicity, and nonpathogenic clades) (25). Here, the tuf results also support the biodiversity of Y. enterocolitica strains. Great evolutionary distances separate Y. enterocolitica subsp. palearctica (group I) strains from Y. enterocolitica subsp. enterocolitica and Y. enterocolitica subsp. palearctica (group II) strains. Currently, it is not clear what caused the deviation in tree topology for these two clades. The divergence could not be completely explained by geographical biodiversity as both clusters contain strains isolated in North America and Europe. Based on tuf data, Y. enterocolitica subsp. palearctica strains were clearly separated into two groups (groups I and II). All 13 Y. enterocolitica strains isolated in Canada were members of Y. enterocolitica subsp. palearctica and did not cluster with strains of Y. enterocolitica subsp. enterocolitica isolated in the United States. Instead, the Canadian isolates clustered with other Y. enterocolitica subsp. palearctica strains isolated in Europe. Our analyses, based on tuf genes, showed that there are three groups of Y. enterocolitica, thus supporting previous findings (25).
Y. frederiksenii includes different genomic groups (genomic groups 1a, 1b, 2, 3, and 4) that are sufficiently different as determined by DNA-DNA hybridization, multilocus enzyme electrophoresis, and 16S rRNA gene and gyrB sequence analyses to belong to at least three different Yersinia genomospecies (14, 16, 17, 56). Our tufA and tufB phylogenetic analyses also showed that there are three distinct clades that could represent three distinct genomospecies and thus further support the need to reevaluate the classification of these organisms.
Finally, inclusion of Y. ruckeri in the genus Yersinia has been controversial ever since this organism was classified. The Y. ruckeri G+C content is more similar to those of Yersinia species even though DNA relatedness showed that Y. ruckeri strains were only 30% related to Yersinia and Serratia species (51). Analysis based on multilocus sequence typing identified Y. ruckeri as the most distant species of the genus (36). However, a previous study based on 16S rRNA gene sequence analysis supported inclusion of Y. ruckeri in the genus Yersinia (28). Phylogenetic trees (Fig. 1) and a network (Fig. 2) of tufA and tufB gene sequences, as well as the level of divergence, showed that Y. ruckeri should be included in the genus Yersinia and thus supported its current classification.
In summary, to our knowledge, this is the first report of significant divergence between tufA and tufB genes throughout a genus. The high level of divergence between tufA and tufB genes in Yersinia strains is a characteristic hallmark of this genus. Here, the evolution of tuf genes in Yersinia was used to investigate species clustering, and the results provided information useful for reclassification and identification. Our analyses suggest that further investigation using a wider diversity of strains, as well as other genetic analyses, is needed to clarify the taxonomic classification of Y. enterocolitica, Y. frederiksenii, and Y. ruckeri. tufA and tufB genes could also be genetic targets that are useful for identification of Yersinia species for diagnostic purposes. In this study, we provide the first evidence, to our knowledge, supporting the hypothesis that there may be gene conversion inefficiency for the tuf gene encoding EF-Tu. It is not known whether the genetic drift seen in duplicated tuf genes in Yersinia resulted in evolution of a new function or resulted in the nonfunctionalization of the product of one copy of the gene. Determining the reason for the natural selection of the high level of divergence between tufA and tufB sequences in Yersinia requires further investigation and could lead to a better understanding of multigene family evolution in bacteria.
Acknowledgments
We thank Martine Bastien, France Bégin, Ève Bérubé, Karel Boissinot, Xavier Bouhy, Gilles Chabot, Natalie Clairoux, Richard Giroux, Marie-Claude Hélie, Jean-Luc Simard, Viridiana Sistek, and Mario Vaillancourt for their technical support.
This research project was funded by the CBRN Research and Technology Initiative under project CRTI-0154RD, Genome Canada, Genome Québec, Infectio Diagnostic Inc., and the Canadian Institutes of Health Research (grant PA-15586). S.I. received scholarships from the Fondation Dr George Phénix (Montréal, Canada) and the Fonds de la Recherche en Santé du Québec (Montréal, Canada). P.S.G.C. received a scholarship from the Natural Sciences and Engineering Research Council of Canada (Ottawa, Canada).
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Abdulkarim, F., and D. Hughes. 1996. Homologous recombination between the tuf genes of Salmonella typhimurium. J. Mol. Biol. 260506-522. [DOI] [PubMed] [Google Scholar]
- 2.Achtman, M., K. Zurth, G. Morelli, G. Torrea, A. Guiyoule, and E. Carniel. 1999. Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 9614043-14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arwidsson, O., and D. Hughes. 2004. Evidence against reciprocal recombination as the basis for tuf gene conversion in Salmonella enterica serovar Typhimurium. J. Mol. Biol. 338463-467. [DOI] [PubMed] [Google Scholar]
- 4.Bell, K. S., M. Sebaihia, L. Pritchard, M. T. Holden, L. J. Hyman, M. C. Holeva, N. R. Thomson, S. D. Bentley, L. J. Churcher, K. Mungall, R. Atkin, N. Bason, K. Brooks, T. Chillingworth, K. Clark, J. Doggett, A. Fraser, Z. Hance, H. Hauser, K. Jagels, S. Moule, H. Norbertczak, D. Ormond, C. Price, M. A. Quail, M. Sanders, D. Walker, S. Whitehead, G. P. Salmond, P. R. Birch, J. Parkhill, and I. K. Toth. 2004. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. USA 10111105-11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berchtold, H., L. Reshetnikova, C. O. Reiser, N. K. Schirmer, M. Sprinzl, and R. Hilgenfeld. 1993. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature 365126-132. [DOI] [PubMed] [Google Scholar]
- 6.Bercovier, H., H. H. Mollaret, J. M. Alonso, J. Brault, G. R. Fanning, A. G. Steigerwalt, and D. J. Brenner. 1980. Intra- and interspecies relatedness of Yersinia pestis by DNA hybridization and its relationship to Yersinia pseudotuberculosis. Curr. Microbiol. 4225-229. [Google Scholar]
- 7.Bryant, D., and V. Moulton. 2004. Neighbor-net: an agglomerative method for the construction of phylogenetic networks. Mol. Biol. Evol. 21255-265. [DOI] [PubMed] [Google Scholar]
- 8.Caldas, T., A. El Yaagoubi, and G. Richarme. 1998. Chaperone properties of bacterial elongation factor EF-Tu. J. Biol. Chem. 27311478-11482. [DOI] [PubMed] [Google Scholar]
- 9.Chain, P. S., E. Carniel, F. W. Larimer, J. Lamerdin, P. O. Stoutland, W. M. Regala, A. M. Georgescu, L. M. Vergez, M. L. Land, V. L. Motin, R. R. Brubaker, J. Fowler, J. Hinnebusch, M. Marceau, C. Medigue, M. Simonet, V. Chenal-Francisque, B. Souza, D. Dacheux, J. M. Elliott, A. Derbise, L. J. Hauser, and E. Garcia. 2004. Insights into the evolution of Yersinia pestis through whole-genome comparison with Yersinia pseudotuberculosis. Proc. Natl. Acad. Sci. USA 10113826-13831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chain, P. S., P. Hu, S. A. Malfatti, L. Radnedge, F. Larimer, L. M. Vergez, P. Worsham, M. C. Chu, and G. L. Andersen. 2006. Complete genome sequence of Yersinia pestis strains Antiqua and Nepal516: evidence of gene reduction in an emerging pathogen. J. Bacteriol. 1884453-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cilia, V., B. Lafay, and R. Christen. 1996. Sequence heterogeneities among 16S ribosomal RNA sequences, and their effect on phylogenetic analyses at specific level. Mol. Biol. Evol. 13451-461. [DOI] [PubMed] [Google Scholar]
- 13.Coenye, T., and P. Vandamme. 2003. Intragenomic heterogeneity between multiple 16S ribosomal RNA operons in sequenced bacterial genomes. FEMS Microbiol. Lett. 22845-49. [DOI] [PubMed] [Google Scholar]
- 14.Demarta, A., S. De Respinis, M. Dolina, and R. Peduzzi. 2004. Molecular typing of Yersinia frederiksenii strains by means of 16S rDNA and gyrB genes sequence analyses. FEMS Microbiol. Lett. 238423-428. [DOI] [PubMed] [Google Scholar]
- 15.Deng, W., V. Burland, G. Plunkett III, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 1844601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolina, M., V. Gaia, and R. Peduzzi. 1995. Molecular typing of Yersinia frederiksenii strains by means of ribotyping and DNA-DNA hybridization. Contrib. Microbiol. Immunol. 13140-144. [PubMed] [Google Scholar]
- 17.Dolina, M., and R. Peduzzi. 1993. Population genetics of human, animal, and environmental Yersinia strains. Appl. Environ. Microbiol. 59442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 211307-1313. [DOI] [PubMed] [Google Scholar]
- 19.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39783-791. [DOI] [PubMed] [Google Scholar]
- 20.Grunberg-Manago, M. 1996. Regulation of the expression of aminoacyl-tRNA synthetases and translation factors, 2nd ed., vol. 2. ASM Press, Washington, DC.
- 21.Han, Y., J. Qiu, Z. Guo, H. Gao, Y. Song, D. Zhou, and R. Yang. 2007. Comparative transcriptomics in Yersinia pestis: a global view of environmental modulation of gene expression. BMC Microbiol. 796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto, J. G., B. S. Stevenson, and T. M. Schmidt. 2003. Rates and consequences of recombination between rRNA operons. J. Bacteriol. 185966-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, O. White, S. L. Salzberg, H. O. Smith, R. R. Colwell, J. J. Mekalanos, J. C. Venter, and C. M. Fraser. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henikoff, S., and J. G. Henikoff. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA 8910915-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard, S. L., M. W. Gaunt, J. Hinds, A. A. Witney, R. Stabler, and B. W. Wren. 2006. Application of comparative phylogenomics to study the evolution of Yersinia enterocolitica and to identify genetic differences relating to pathogenicity. J. Bacteriol. 1883645-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes, D. 2000. Co-evolution of the tuf genes links gene conversion with the generation of chromosomal inversions. J. Mol. Biol. 297355-364. [DOI] [PubMed] [Google Scholar]
- 27.Huson, D. H., and D. Bryant. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23254-267. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim, A., B. M. Goebel, W. Liesack, M. Griffiths, and E. Stackebrandt. 1993. The phylogeny of the genus Yersinia based on 16S rDNA sequences. FEMS Microbiol. Lett. 114173-177. [DOI] [PubMed] [Google Scholar]
- 29.International Journal of Systematic and Evolutionary Microbiology. 2000. IJSEM validation list no. 75. 501415-1417. [DOI] [PubMed] [Google Scholar]
- 30.International Journal of Systematic Bacteriology. 1981. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. List no. 7. Int. J. Syst. Bacteriol. 31382-383. [DOI] [PubMed] [Google Scholar]
- 31.Judicial Commission of the International Committee on Systematic Bacteriology. 1985. Opinion 60. Rejection of the name Yersinia pseudotuberculosis subsp. pestis (van Loghen) Bercovieri et al. 1981 and conversion of the name Yersinia pestis (Lehmann and Neumann) van Loghem 1944 for the plague bacillus. Int. J. Syst. Bacteriol. 35540. [Google Scholar]
- 32.Ke, D., M. Boissinot, A. Huletsky, F. J. Picard, J. Frenette, M. Ouellette, P. H. Roy, and M. G. Bergeron. 2000. Evidence for horizontal gene transfer in evolution of elongation factor Tu in enterococci. J. Bacteriol. 1826913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellogg, D. E., I. Rybalkin, S. Chen, N. Mukhamedova, T. Vlasik, P. D. Siebert, and A. Chenchik. 1994. TaqStart Antibody™: “hot start” PCR facilitated by a neutralizing monoclonal antibody directed against Taq DNA polymerase. BioTechniques 162888-2893. [PubMed] [Google Scholar]
- 34.Kloepper, T. H., and D. H. Huson. 2008. Drawing explicit phylogenetic networks and their integration into SplitsTree. BMC Evol. Biol. 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kondrashov, F. A., T. A. Gurbich, and P. K. Vlasov. 2007. Selection for functional uniformity of tuf duplicates in gamma-proteobacteria. Trends Genet. 23215-218. [DOI] [PubMed] [Google Scholar]
- 36.Kotetishvili, M., A. Kreger, G. Wauters, J. G. Morris, Jr., A. Sulakvelidze, and O. C. Stine. 2005. Multilocus sequence typing for studying genetic relationships among Yersinia species. J. Clin. Microbiol. 432674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Kushiro, M., M. Shimizu, and K. Tomita. 1987. Molecular cloning and sequence determination of the tuf gene coding for the elongation factor Tu of Thermus thermophilus HB8. Eur. J. Biochem. 17093-98. [DOI] [PubMed] [Google Scholar]
- 39.Lathe, W. C., III, and P. Bork. 2001. Evolution of tuf genes: ancient duplication, differential loss and gene conversion. FEBS Lett. 502113-116. [DOI] [PubMed] [Google Scholar]
- 40.Liao, X., T. Ying, H. Wang, J. Wang, Z. Shi, E. Feng, K. Wei, Y. Wang, X. Zhang, L. Huang, G. Su, and P. Huang. 2003. A two-dimensional proteome map of Shigella flexneri. Electrophoresis 242864-2882. [DOI] [PubMed] [Google Scholar]
- 41.Lynch, M., and J. S. Conery. 2000. The evolutionary fate and consequences of duplicate genes. Science 2901151-1155. [DOI] [PubMed] [Google Scholar]
- 42.Martineau, F., F. J. Picard, D. Ke, S. Paradis, P. H. Roy, M. Ouellette, and M. G. Bergeron. 2001. Development of a PCR assay for identification of staphylococci at genus and species levels. J. Clin. Microbiol. 392541-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattatall, N. R., and K. E. Sanderson. 1996. Salmonella typhimurium LT2 possesses three distinct 23S rRNA intervening sequences. J. Bacteriol. 1782272-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413852-856. [DOI] [PubMed] [Google Scholar]
- 45.Merhej, V., T. Adekambi, I. Pagnier, D. Raoult, and M. Drancourt. 2008. Yersinia massiliensis sp. nov., isolated from fresh water. Int. J. Syst. Evol. Microbiol. 58779-784. [DOI] [PubMed] [Google Scholar]
- 46.Neubauer, H., A. Stojanka, H. Andreas, F. Ernst-J.ürgen, and M. Hermann. 2000. Yersina enterocolitica 16S rRNA gene types belong to the same genospecies but form three homology groups. Int. J. Med. Microbiol. 29061-64. [DOI] [PubMed] [Google Scholar]
- 47.Paradis, S., M. Boissinot, N. Paquette, S. D. Bélanger, E. A. Martel, D. K. Boudreau, F. J. Picard, M. Ouelette, P. H. Roy, and M. G. Bergeron. 2005. Phylogeny of the Enterobacteriaceae based on genes encoding elongation factor TU and F-ATPase β-subunit. Int. J. Syst. Evol. Microbiol. 552013-2025. [DOI] [PubMed] [Google Scholar]
- 48.Parkhill, J., B. W. Wren, N. R. Thomson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Basham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413523-527. [DOI] [PubMed] [Google Scholar]
- 48a.Prentice, M. B., and L. Rahalison. 2007. Plague. Lancet 3691196-1207. [DOI] [PubMed] [Google Scholar]
- 49.Sprague, L. D., and H. Neubauer. 2005. Yersinia aleksiciae sp. nov. Int. J. Syst. Evol. Microbiol. 55831-835. [DOI] [PubMed] [Google Scholar]
- 50.Sprague, L. D., H. C. Scholz, S. Amann, H. J. Busse, and H. Neubauer. 2008. Yersinia similis sp. nov. Int. J. Syst. Evol. Microbiol. 58952-958. [DOI] [PubMed] [Google Scholar]
- 51.Sulakvelidze, A. 2000. Yersiniae other than Y. enterocolitica, Y. pseudotuberculosis, and Y. pestis: the ignored species. Microbes Infect. 2497-513. [DOI] [PubMed] [Google Scholar]
- 52.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 53.Tamura, K., and S. Kumar. 2002. Evolutionary distance estimation under heterogeneous substitution pattern among lineages. Mol. Biol. Evol. 191727-1736. [DOI] [PubMed] [Google Scholar]
- 54.Tamura, K., M. Nei, and S. Kumar. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 10111030-11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 224673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55a.Thomson, N. R., S. Howard, B. W. Wren, M. T. Holden, L. Crossman, G. L. Challis, C. Churcher, K. Mungall, K. Brooks, T. Chillingworth, T. Feltwell, Z. Abdellah, H. Hauser, K. Jagels, M. Maddison, S. Moule, M. Sanders, S. Whitehead, M. A. Quail, G. Dougan, J. Parkhill, and M. B. Prentice. 2006. The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet. 2e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ursing, J., and S. Aleksic. 1995. Yersinia frederiksenii, a genotypically heterogeneous species with few differential characteristics. Contrib. Microbiol. Immunol. 13112-116. [PubMed] [Google Scholar]
- 57.Walsh, J. B. 1987. Sequence-dependent gene conversion: can duplicated genes diverge fast enough to escape conversion? Genetics 117543-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wayne, L. G. 1986. Actions of the Judicial Commission of the International Committee on Systematic Bacteriology on requests for opinions published in 1983 and 1984. Int. J. Syst. Bacteriol. 36357-358. [Google Scholar]
- 59.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 9917020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams, J. E. 1984. Proposal to reject the new combination Yersina pseudotuberculosis subsp. pestis for violation of the first principle of the International Code of Nomenclature of Bacteria: request for an opinion. Int. J. Syst. Bacteriol. 34268-269. [Google Scholar]
- 61.Zhou, D., Z. Tong, Y. Song, Y. Han, D. Pei, X. Pang, J. Zhai, M. Li, B. Cui, Z. Qi, L. Jin, R. Dai, Z. Du, J. Wang, Z. Guo, J. Wang, P. Huang, and R. Yang. 2004. Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. J. Bacteriol. 1865147-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]