Abstract
Serratia marcescens is a gram-negative environmental bacterium and opportunistic pathogen. S. marcescens expresses prodigiosin, a bright red and cell-associated pigment which has no known biological function for producing cells. We present here a kinetic model relating cell, ATP, and prodigiosin concentration changes for S. marcescens during cultivation in batch culture. Cells were grown in a variety of complex broth media at temperatures which either promoted or essentially prevented pigmentation. High growth rates were accompanied by large decreases in cellular prodigiosin concentration; low growth rates were associated with rapid pigmentation. Prodigiosin was induced most strongly during limited growth as the population transitioned to stationary phase, suggesting a negative effect of this pigment on biomass production. Mathematically, the combined rate of formation of biomass and bioenergy (as ATP) was shown to be equivalent to the rate of prodigiosin production. Studies with cyanide inhibition of both oxidative phosphorylation and pigment production indicated that rates of biomass and net ATP synthesis were actually higher in the presence of cyanide, further suggesting a negative regulatory role for prodigiosin in cell and energy production under aerobic growth conditions. Considered in the context of the literature, these results suggest that prodigiosin reduces ATP production by a process termed energy spilling. This process may protect the cell by limiting production of reactive oxygen compounds. Other possible functions for prodigiosin as a mediator of cell death at population stationary phase are discussed.
Serratia marcescens is a ubiquitous environmental bacterium which has been isolated from soil, water, and insects (6). This organism has emerged as an important nosocomial pathogen associated with respiratory infections, urinary tract infections, sepsis, wound infections, and conjunctivitis among wearers of contact lenses (13, 19, 27). Environmental isolates of S. marcescens express the red, cell-associated pigment prodigiosin, but identification of most clinical isolates must rely on other biochemical markers because the majority are not pigmented (13, 27).
Prodigiosin has been traditionally known as a secondary metabolite: it has no clearly defined function in cellular metabolism (4). Several physiological processes in S. marcescens, including prodigiosin pigmentation and biofilm formation, are activated at high cell density by quorum-sensing mechanisms (26, 28, 30). Low phosphate concentrations and temperatures below 37°C favor strong pigmentation (29). At least 12 pigmentation (pig) genes (A through J; M and N), arranged as part of a larger pig operon, encode enzymes for prodigiosin biosynthesis. In addition, a suite of regulatory proteins provides fine genetic control at the level of transcription. Quorum-sensing regulation of pig operon expression is mediated by SmaR and SmaI (secondary metabolite activator) (24, 25). SmaR is a repressor with DNA binding activity (8); transcriptional repression is mitigated by the N-acyl homoserine lactone (AHL) inducer synthesized by the enzyme SmaI (25). A distinct quorum-sensing system, mediated by autoinducer 2, is active in at least one strain of S. marcescens (5). Several genetic loci, including pigQ, pigR, and rap, function as activators of pigmentation (reviewed in reference 30). Expression of the pig operon is repressed by pigT when S. marcescens is grown in the presence of gluconate (7). Recent work has also revealed a role for pigX as a transcriptional repressor of the pig operon (9).
In order to address a putative physiological role for prodigiosin in pigmented cells, we undertook a kinetic analysis of pigment production by S. marcescens Nima in batch culture. Our data reveal an inverse association between cellular levels of prodigiosin and ATP. Experiments in a variety of growth media suggest that prodigiosin is synthesized as a negative regulator in response to ATP synthesis, particularly in energy-rich media. In view of prodigiosin's established role as a proton transporter, we postulate that prodigiosin may function as a mediator of energy spilling.
MATERIALS AND METHODS
Bacteria and media.
S. marcescens Nima is a pigmented strain which was used as a wild type by the Williams laboratory for early studies of prodigiosin biosynthesis (15). This strain was purchased from the American Type Culture Collection as culture number 29632. Strain KMR2 is a poorly pigmented, spontaneous mutant of Nima selected for kanamycin resistance on tryptic soy agar with 25 μg/ml kanamycin sulfate (Sigma). Escherichia coli K-12 was obtained from the E. coli Genetic Stock Center.
Bacteria were maintained as −80°C frozen stocks in 20% (wt/vol) glycerol-2% (wt/vol) peptone. Routine bacterial cultivation was performed on tryptic soy agar slants incubated at 26°C to 30°C. Base complex medium was essentially dilute nutrient broth no. 2 without sodium chloride (1). It contained the following components at the indicated weight/volume concentrations: beef extract at 0.3% and peptone at 0.5%. Complete complex media additionally contained one of the following carbohydrates at 0.5%: inositol, glycerol, glucose, maltose monohydrate, or sodium citrate. Noble agar (Difco) was added at 1.5% as needed for slant or plate growth. S. marcescens Nima was able to grow in M9 minimal salts medium (16) supplemented with either peptone, citrate, inositol, glycerol, glucose, maltose monohydrate, or galactose at 0.5% as a sole source of energy and carbon (data not shown).
Bacterial growth and prodigiosin assay.
Bacterial growth curves were performed for 50-ml cultures grown in 250-ml Erlenmeyer flasks. Cultures were aerated by rotation at 250 rpm in a Barnstead International MaxQ 7000 water bath shaker. One-milliliter or smaller culture aliquots were removed for both cell and prodigiosin measurements at 0.25-h intervals over 8 to 12 h for growth experiments.
Inocula for low-density and low-prodigiosin (37°C) growth experiments were prepared in several ways. Overnight broth culture pregrowths were centrifuged at 8,000 × g at room temperature. Cell pellets were either washed twice in 10 ml of growth medium prior to inoculation or simply suspended in 5 ml of growth medium for direct inoculation. Both washed and resuspended inocula were found to lag for longer periods. More commonly, slant pregrowth cultures were incubated for 1 to 3 days. Pregrowth was washed from a slant with the corresponding broth medium and mixed vigorously prior to direct inoculation of growth medium to an initial optical density at 750 nm (OD750) of 0.05 to 0.2. The slant pregrowth method provided both high initial cell density and high cellular pigmentation at 26°C.
Inocula for high-density growth experiments were also prepared by the slant method. Here, the slant wash was diluted 1:100 in the corresponding broth, and inoculation was made to an extremely low initial optical density (e.g., OD750 of 10−5). In the context of a known low-density growth rate, such inocula produced late low-density phase cultures when grown with aeration overnight (12 to 15 h) in preparation for high-density growth experiments.
The relatively long wavelength of 750 nm was chosen for cell optical density measurements because it was not affected by the presence of prodigiosin pigment. Cell-associated prodigiosin absorbs light over a broad wavelength range and peaks at 543 nm (data not shown). The Beckman DU 520 spectrophotometer employed for optical density and absorbance measurements exhibited a linear OD750 range of 0.050 to 0.500. Samples from densely grown cultures were diluted to fall within this range for accurate optical density measurements.
Following OD750 measurement of cell concentration during growth experiments, the cuvette contents were poured into 1.5-ml microcentrifuge tubes for prodigiosin extraction and assay as described by Slater et al. (24). The cells were sedimented at 12,000 × g for 1 minute in a microcentrifuge. The supernatant was discarded, and the cell pellet was extracted with 1,000 μl of 4% (vol/vol) 1 M HCl in 95% ethanol. Following vigorous mixing, white cellular debris was removed by a second centrifugation. Prodigiosin per ml of original culture was determined as the absorbance at 536 nm.
ATP assay.
Undiluted 100-μl growth culture aliquots were mixed with equal volumes of BacTiter-Glo ATP measurement reagent (Promega Inc., Madison, WI). ATP detection was accomplished in a chemiluminescence reaction which utilized ATP as a rate-limiting reactant in the oxidation of beetle luciferin by a recombinant luciferase. The lyophilized luciferin-luciferase protein reagent was reconstituted in a proprietary cell lysis buffer. Optimal ATP release from S. marcescens Nima in lysis solution was empirically found to occur after 7.5 min of incubation. The 3-s integral of the chemiluminescence response in relative light units (RLU) was determined using a Turner Biosystems 2020n analog luminometer. Both cell lysis and chemiluminescence measurements of ATP concentration were performed at 24°C. ATP concentration was expressed as RLU per 100 μl of culture.
Two ATP assay blanks consistently produced negligible luminescence (less than 0.1% of the lowest experimental reading) over the course of growth experiments. The reagent blank consisted of 100 μl sterile medium plus the same volume of BacTiter-Glo reagent. The culture blank was 100 μl of growing culture diluted with an equal volume of medium. The specificity of the BacTiter-Glo reagent for detection of ATP has been affirmed by a personal communication from the manufacturer: CTP is the only alternative substrate known, and its Km for luciferase is approximately 1,000-fold greater than that for ATP.
Reconstituted BacTiter-Glo reagent was found to have a minimum half-life of 8.3 h at 20°C; reagent decay did not limit ATP detection during these experiments.
As a check against reagent decay during storage prior to reconstitution and use, ATP assays were performed for E. coli K-12 growth curves in base complex broth. For this bacterium, ATP levels were observed to increase exponentially at two to three times the cellular growth rate (data not shown). The optimal cell lysis time for this organism was empirically determined to be 3.5 min.
AHL assay.
The colorimetric assay of Yang et al. (31) is reported to specifically detect lactones, including quorum-sensing inducer molecules, irrespective of acyl chain length. Lactones react with assay reagents to produce a soluble, dark-brown compound that absorbs light at 520 nm. Since the lactones produced by S. marcescens Nima have not yet been chemically identified, we quantified total lactones relative to the assay A520 values produced by N-butanoyl homoserine lactone (C4-AHL).
A C4-AHL assay standard curve was constructed with commercially prepared compound (Sigma). The empirically determined extinction coefficient used to quantify C4-AHL in methanol was 2.92 × 10−4 μM−1 cm−1 at 218 nm. A lactone assay using serial twofold dilutions of C4-AHL produced a linear A520 range of approximately 0.040 to 0.413 (n = 5; r2 = 1.00). For assay, 400-μl samples in methanol were mixed with 500 μl each of reagents 1 and 2. Absorbance at 520 nm was read against a blank containing methanol solvent alone plus reagents 1 and 2. Reagent 1 was a 1:1 (vol/vol) mixture of 2.5 M hydroxylamine and 3.5 M sodium hydroxide. Reagent 2 was a 1:1 (vol/vol) mixture of 10% ferric chloride in 4 M HCl and 95% ethanol. Reagents 1 and 2 were stable for at least 1 month at 4°C.
The lactone assay of S. marcescens Nima culture samples was performed as follows. One-milliliter samples were centrifuged at 12,000 × g for 1 minute in a microcentrifuge. Two 400-μl aliquots of supernatant were transferred to sterile microcentrifuge tubes for assay as undiluted and three serially twofold diluted samples in growth medium. Serially decreasing A520 values falling in the strain Nima linear range of 0.042 to 0.153 were converted to C4-AHL equivalents using the following relationship: equivalent C4-AHL micromolar concentration = (A520 − 0.029)/(2.11 × 10−4). This relationship produced an effective S. marcescens Nima lactone detection range of 62 to 588 μM C4-AHL equivalents. Equivalent concentration values were corrected for assay dilution and were standardized by the Grubbs outlier test using a critical z score of 1.15 (http://www.graphpad.com/quickcalcs/GrubbsHowTo.cfm). Following a single round of outlier removal, assay averages were calculated from two to three measurements. The spectrophotometric blank used for culture supernatant assays substituted sterile medium for methanol solvent. Cell pellets were assayed similarly, except that they were first rinsed with sterile medium and then dissolved by extraction with 1 ml methanol. Results were read using the methanol spectrophotometric blank. Undetectable lactone levels were converted to zero for median calculations.
Inhibition of oxidative phosphorylation.
Potassium cyanide was made to 4% (wt/vol) in base complex broth. This stock was diluted 100-fold to a working concentration of 0.04% (18) by addition either to sterile medium or to actively growing cultures. Control spikes of growing cultures with base complex medium did not noticeably affect growth or ATP or pigmentation kinetics (data not shown). ATP chemiluminescence assays with commercially prepared ATP (Sigma) diluted into base complex medium with cyanide showed that this inhibitor did not directly affect the performance of the ATP assay (data not shown).
Kinetic and statistical analyses.
All physiological processes described here were adequately modeled by first-order kinetics. That is, the rate of change of a quantity was directly proportional to the first power of its concentration. Rate equation proportionality constants, k, were given subscript designations to identify the following rates of change as population averages: cell concentration (OD750; kcell), prodigiosin concentration (A536/ml; kpig), prodigiosin per cell (A536/ml/OD750; kpig/cell), ATP concentration (RLU/100 μl; kATP), and ATP per cell (RLU/100 μl/OD750; kATP/cell). Plots of the natural logarithm of a quantity as a function of time in hours were analyzed by linear regression to yield rate constants as curve slopes. Unless otherwise noted, rate constants were calculated from 7 to 25 growth curve points which returned correlation squared values of at least 0.92. Rate constants have units of hour−1. Values for proportionality constant c were calculated as linear regression slopes from plots of prodigiosin per cell (y) versus (1/{(kcell)·(kpig/cell)·[cell]}) (x). Statistical calculations were performed using Excel 2003 (Microsoft, Inc.) and SPSS version 13.0 for Windows (Thomson Wadsworth, Inc., 2004). Tests of significant difference between sample means used a significance level of P = 0.05 and employed either two-tailed t tests that assumed unequal variance or single-factor analysis of variance.
RESULTS
ATP assay validation.
Our modification of the BacTiter-Glo ATP assay is facile and has high throughput, but it does require several minutes for complete cell lysis and optimal ATP release (see Materials and Methods). Recent work by Buckstein et al. (3) employed a rapid cell lysis technique in concentrated formic acid to preserve nucleotides extracted from logarithmically multiplying E. coli cells. Since our ATP results could potentially be altered during the cell lysis period, we validated our assay by comparison with results from the rapid cell lysis assay.
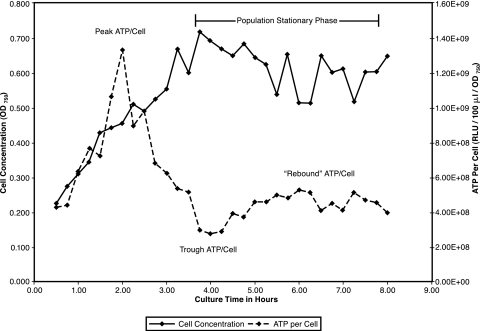
Figure 1 shows the kinetics of growth and ATP per cell of E. coli K-12 in base complex broth at 37°C. Similar to the findings of others (3), we observed that cellular ATP levels declined in late logarithmic phase. In addition, the phenomenon of a severalfold drop in peak cellular ATP to a trough level, followed by a partial recovery or “rebound,” was also observed. Results from three experiments (mean ± standard deviation [SD]) are expressed here as fractions of peak ATP per cell values. Trough values were 26% ± 5%, and the recovery or “rebound” values were 46% ± 11%. We conclude that the BacTiter-Glo ATP assay is accurate and generally applicable for this work.
FIG. 1.
Growth and ATP per cell of E. coli in base complex broth at 37°C. E. coli K-12 was pregrown in base complex broth with aeration at 37°C and diluted into fresh broth to an initial OD750 of 0.10 for continued growth. Culture aliquots were removed at 0.25-h intervals for assay of ATP concentration and cell concentration.
Strain Nima pigmentation and population phases.
In keeping with prodigiosin production by other strains of S. marcescens (24, 25, 26, 28, 30), pigmentation of strain Nima was most strongly induced by growth at relatively high cell density (see Fig. 6A). This suggests regulation of pigment production by a quorum-sensing mechanism. Even though they are not chemically defined, complex media with or without a single carbohydrate were chosen to provide relatively high rates of change for the kinetic parameters under investigation.
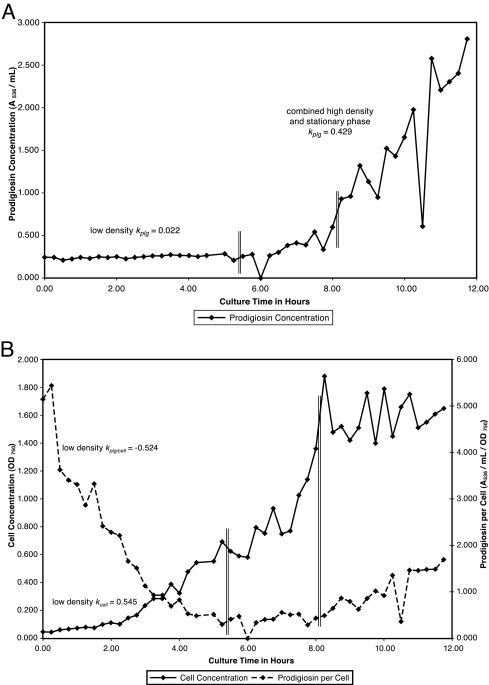
FIG. 6.
(A) Prodigiosin production throughout the population cycle in inositol complex broth at 26°C. S. marcescens Nima was pregrown in inositol complex broth and inoculated to an initial OD750 of 0.05. Growth was continued with aeration at 26°C. Samples were removed at 0.25-h intervals for assay of cell concentration and prodigiosin concentration. Vertical double lines separate low-density (0.25 to 5.25 h), high-density (5.50 to 8.00 h), and stationary (8.25 to 11.75 h) phases. (B) Cell growth and prodigiosin per cell throughout the population cycle in inositol complex broth at 26°C. Data for cell concentration and prodigiosin per cell from the experiment described for panel A are presented.
We detected the production of potential quorum-sensing inducer lactones using a colorimetric assay which is effective for AHLs of essentially all acyl chain lengths (31). Logarithmic growth of strain Nima in base complex broth at 26°C exhibited transient lactone production, from undetectable levels to approximately 1,200 μM C4-AHL equivalents, and a median of 318 μM. Inositol is a carbohydrate which promotes strong prodigiosin production (see Table 1). Growth at 26°C in M9 minimal medium with 0.5% inositol produced approximately 1,000 to 4,000 C4-AHL equivalents at stationary phase. Experiments are under way to precisely identify the AHLs produced by S. marcescens Nima.
TABLE 1.
Correlation of ATP per cell and prodigiosin per cell during low-density growth
| Complex medium | Avg correlation for n = 3 or 4 | Avg no. of data pairs per correlation | [ATP]/[prodigiosin] normalized to base medium |
|---|---|---|---|
| Inositol | 0.96 | 20 | 0.19 |
| Glycerol | 0.96 | 13 | 0.49 |
| Base | 0.94 | 15 | 1.0 |
| Maltose | 0.93 | 14 | 1.6 |
| Glucose | 0.98 | 16 | 1.7 |
We defined three major population metabolic phases based upon the kinetics of cell, ATP, and pigmentation changes. The low-density phase for initially pigmented cells was characterized by logarithmic growth at 26°C through the time point corresponding to a prodigiosin-per-cell minimum (see Fig. 3B and 4B). Subsequent experiments in which ATP was assayed refined this definition to include the peak ATP-per-cell value as the beginning of low density (see Fig. 3B). As another defining property, ATP-per-cell values were found to strongly correlate with prodigiosin-per-cell values throughout low density (see Table 1). The OD750 range corresponding to growth at low density in complex media was approximately 0.05 to 0.95 (5.2 × 107 to 9.9 × 108 CFU/ml).
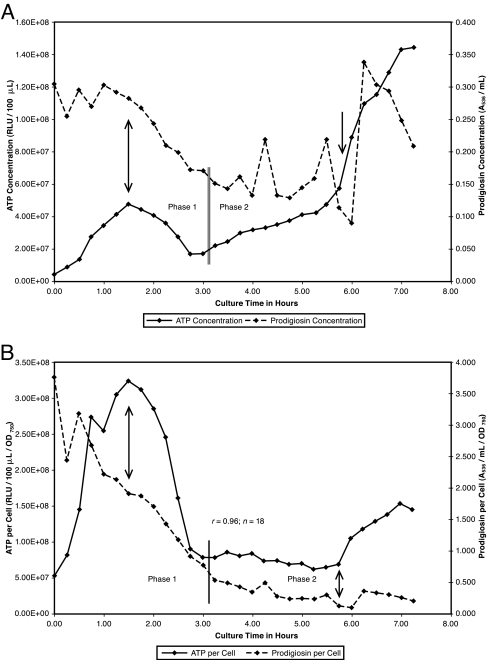
FIG. 3.
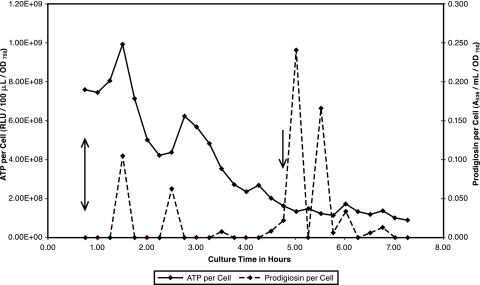
(A) ATP and prodigiosin during low-density growth in base complex broth. S. marcescens Nima was pregrown on a base complex medium slant at 26°C and washed into base complex broth to an initial OD750 of 0.12. Growth was continued with aeration. Samples were removed at 0.25-h intervals for assay of cell, ATP, and prodigiosin concentrations. Vertical arrows identify low density as 1.50 through 5.75 h. The vertical double line separates low-density phase 1 (1.50 to 3.00 h) from phase 2 (3.25 to 5.75 h). (B) ATP per cell and prodigiosin per cell during low-density growth in base complex broth. Data for ATP per cell and prodigiosin per cell from the experiment in panel A are presented.
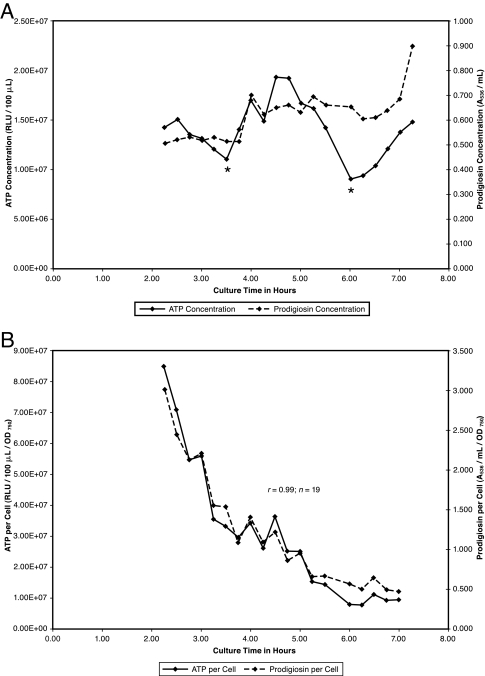
FIG. 4.
(A) ATP and prodigiosin during low-density growth in inositol complex broth. S. marcescens Nima pregrown for 1 day on an inositol complex medium slant was washed into inositol complex broth to an initial OD750 of 0.15. Growth was continued with aeration at 26°C. Samples were removed at 0.25-h intervals for assay of cell, prodigiosin, and ATP concentrations. Asterisks denote ATP minima preceding ATP synthesis. (B) ATP per cell and prodigiosin per cell during low-density growth in inositol complex broth. Data for ATP per cell and prodigiosin per cell from the experiment in panel A are presented.
The high-density phase was more simply defined as the culture time interval at 26°C during which prodigiosin production increased exponentially (see Fig. 6A). During high density, population growth occurred at a greatly reduced rate (see Table 3), and there was no correlation between ATP per cell and prodigiosin per cell. The OD750 range corresponding to high-density growth in complex media was approximately 1.0 to 2.0.
TABLE 3.
Cell growth, ATP and pigmentation rate constants for S. marcescens Nima grown in complex medium brothsa
| Growth medium | Temp (°C) | Phaseb | kcell | kATP | kpig | kcell + kATP − kpig |
|---|---|---|---|---|---|---|
| Maltose | 26 | LD | 0.837 | 0.459 | 0 | 1.30 |
| Base | 37 | LP | 0.857 | 0.398 | 0 | 1.26 |
| Base | 26 | LD-2 | 0.478 | 0.258 | 0 | 0.736 |
| Glucose | 26 | LD | 0.667 | 0 | 0 | 0.667 |
| Glycerol | 26 | LD | 0.652 | −0.214 | 0 | 0.438 |
| Inositol | 26 | LD | 0.437 | 0 | 0 | 0.437 |
| Inositol | 37 | LP | 0.739 | −0.582 | 0 | 0.156 |
| Glucose | 37 | LP | 0.444 | −0.462 | 0 | 0 |
| Base | 26 | LD-1 | 0.478 | −0.735 | −0.272 | 0 |
| Base | 26 | HD | 0.199 | 0.275 | 0.744 | −0.271 |
| Maltose | 26 | HD | 0.071 | 0 | 0.479 | −0.409 |
| Inositol | 26 | HD | 0.128 | 0 | 0.732 | −0.490 |
| Glucose | 26 | HD | 0.068 | 0 | 0.704 | −0.635 |
| Glycerol | 26 | HD | 0.172 | 0 | 1.43 | −1.25 |
Data are averages of three to five measurements. Values reported as “0” lie within the range ±0.050. Most rate constants with values in the range ±0.200 returned correlation-squared values of less than 0.92.
LD, low density (entire); LD-1, low-density phase 1; LD-2, low-density phase 2; HD, high density; LP, low-prodigiosin phase.
The low-prodigiosin phase was defined as the culture time interval during which the cell concentration increased logarithmically at 37°C. Pigmentation was essentially zero when cells were grown at 37°C; measured A536 values were low and transient under these conditions (see Fig. 5).
FIG. 5.
ATP per cell and prodigiosin per cell during low-prodigiosin growth in base complex broth. S. marcescens Nima pregrown for 1 day on a base complex medium slant at 37°C was washed into base complex broth to an initial OD750 of 0.04. Growth was continued with aeration at 37°C. Samples were removed at 0.25-h intervals for assay of cell, prodigiosin, and ATP concentrations. Vertical arrows denote the logarithmic growth phase.
Rates of growth and prodigiosin per cell.
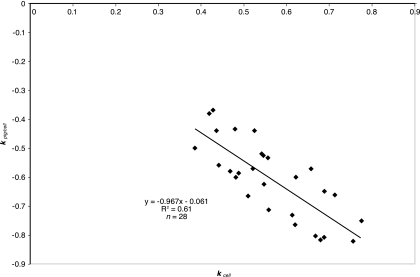
A rate constant for the quotient of two simultaneous, first-order kinetic processes may be described mathematically as the difference between the rate constants of the individual processes. We may therefore write kpig/cell = kpig − kcell. In the course of our studies with pigmented cells grown through low density, we consistently observed that kpig was a small fraction of the rate of change of the other two parameters. The preceding equation therefore reduces to −(kpig/cell) ≈ kcell. The latter expression suggests that the rate of decrease of prodigiosin per cell should be approximately equal to the growth rate. To verify this, we plotted 28 points for kpig/cell (y) versus kcell (x), representing a twofold range of cellular growth rates in six different complex media. The plot produced significant scatter but overall identity between these kinetic parameters (Fig. 2). Statistical tests for significance against a y intercept of zero and a slope of −1 revealed insignificant deviation from these expected null values (data not shown). The observed point scatter (Fig. 2) suggests the presence of another variable(s) influencing growth rate and cellular prodigiosin concentration. Work described below presents a more accurate equation that expresses prodigiosin per cell in terms of the cell concentration and two additional parameters which are constants within a single growth experiment.
FIG. 2.
Prodigiosin-per-cell rate of change as a function of low-density growth rate. S. marcescens Nima was pregrown at 26°C in solid or liquid base complex medium with a single carbohydrate (citrate, inositol, glycerol, glucose, or maltose). Pregrowth was diluted into the corresponding broth to an initial OD750 of 0.05 to 0.2 for continued growth at low density. Samples were removed at 0.25-h intervals for assay of cell and prodigiosin concentrations.
The relationship between growth rate and the rate of decrease of pigment per cell suggested two important ideas. First, the growth rate determines the rate of cellular pigmentation decrease. This was the first indication that prodigiosin is antagonistic to the production of biomass. Second, energy storage compounds could be involved as intermediates linking growth rate and pigmentation. In this context, we began to investigate the rate of change of ATP concentration along with cell growth and pigmentation.
Growth, ATP, and prodigiosin without carbohydrate.
Low-density growth of pigmented S. marcescens cells in base complex broth occurred at a uniform rate of kcell = 0.460 for the experiment depicted in Fig. 3A. However, this growth may be divided into two distinct phases on the basis of ATP and prodigiosin kinetics. Following net ATP synthesis during lag phase, both ATP and prodigiosin were consumed during phase 1 low-density growth. Phase 2 was defined on the basis of net ATP synthesis. During phase 2, prodigiosin synthesis and degradation were balanced to maintain a relatively low and gradually decreasing cellular pigment concentration as the cells continued to multiply at the phase 1 rate (Fig. 3B). Figure 3B shows similar low-density decay kinetics for ATP per cell and prodigiosin per cell as well as an excellent correlation between these parameters.
Growth, ATP, and prodigiosin with inositol.
Inositol complex medium has been observed to promote visibly strong pigmentation. Figure 4A depicts ATP and prodigiosin concentrations during low-density growth of pigmented cells in inositol complex broth. ATP synthesis and consumption alternated, while prodigiosin was not consumed but rather induced in a stepwise manner at a very low overall rate (kpig = 0.058). Figure 4B shows that ATP per cell and prodigiosin per cell exhibited proportional decreases during low-density growth in a manner similar to that seen without carbohydrate. In the context of ongoing cell multiplication at a rate greatly exceeding minimal pigment synthesis (kcell = 0.456), cellular levels of prodigiosin were diluted.
Relationship of ATP per cell and prodigiosin per cell.
The correlation between cellular levels of ATP and prodigiosin during the same growth experiment was found to hold for all media tested (Table 1). The average ratio of ATP to prodigiosin for low-density growth of pigmented cells in any medium may be calculated as the slope of the curve plotting ATP concentration (y) versus prodigiosin concentration (x). Thus, carbohydrate-supplemented media may be ranked according to energy production relative to base medium (Table 1). Supplementation with either inositol or glycerol favored prodigiosin production, while addition of glucose or maltose favored energy production.
The top two rows of Table 2 list the kinetic parameters calculated for the strain Nima experiments depicted in Fig. 3A through 4B. This analysis is particularly informative because the low-density growth rates in these two media were nearly identical, removing growth rate as a variable. Table 2 shows that ATP and prodigiosin were inversely related: ATP levels were low when prodigiosin levels were high and vice versa. Similar data for the low-density growth of strain KMR2, a Nima derivative which exhibited greatly reduced pigmentation, are also presented in Table 2. KMR2 showed higher ATP levels than strain Nima did in base complex broth, suggesting that prodigiosin lessens ATP production. This mutant also showed a reduced growth rate, indicating a complex interrelationship among growth, ATP, and prodigiosin. Cultivation of KMR2 on glucose-containing medium enabled low-frequency reversion to a fully pigmented, larger-colony phenotype (data not shown). In view of the results presented in Tables 1 and 2, we suggest that prodigiosin is a negative regulator of ATP production.
TABLE 2.
Growth, ATP, and prodigiosin at low density in complex media with or without inositola
| Strain | Complex broth | kcell | Median ATP/cell (RLU/100 μl/OD750) | Median prodigiosin/cell (A536/ml/OD750) |
|---|---|---|---|---|
| Nima | Base | 0.460 | 7.95 × 107 | 0.491 |
| Inositol | 0.456 | 2.63 × 107 | 1.08 | |
| KMR2b | Base | 0.210 | 3.54 × 108 | 0.052 |
Data were calculated from 18 to 20 measurements.
Values are averages of duplicate assays.
ATP during low prodigiosin growth.
Growth of S. marcescens at 37°C is a physiological condition which promotes minimal pigmentation (reviewed in reference 29). Growth in base complex broth at 37°C was relatively rapid and was characterized by an overall decrease in cellular ATP levels (Fig. 5). ATP consumption was punctuated by periodic synthesis. In keeping with the notion that prodigiosin production is antagonistic to biomass and bioenergy production, we observed very little pigment induction during this period of rapid growth and net ATP decrease. Furthermore, sporadic pigmentation roughly corresponded to periods of ATP synthesis and the transition to population stationary phase (Fig. 5). Also interesting are the maximum median value of 4.8 × 108 for ATP per cell and the minimum median value of 0.000 for prodigiosin per cell measured during growth under these conditions. These extreme values accompany the greatest observed average rate of biomass production (see Table 3).
Prodigiosin throughout the population cycle.
Figure 6A shows prodigiosin production by pigmented cells through low-density growth, high-density growth, and early population stationary phase in inositol complex broth. Similarly to other strains of S. marcescens (24, 25, 26, 28, 30), prodigiosin production was induced at high cell density. It is therefore possible that prodigiosin may function to reduce ATP levels as exponentially growing cells approach stationary phase. Figure 6A also shows virtually no change in prodigiosin levels as pigmented cells grew through low density, affirming the relationship between growth rate and the rate of decrease of prodigiosin per cell derived from Fig. 2.
Figure 6B depicts growth and prodigiosin-per-cell kinetics for the experiment shown in Fig. 6A. Rate constants for cell concentration and prodigiosin per cell at low density were of equal magnitude but opposite signs. Therefore, in the context of no net change in prodigiosin concentration (Fig. 6A), prodigiosin appeared to be diluted by cell multiplication. These processes may be modeled mathematically as an inverse relationship between the rate of decrease of prodigiosin per cell and the growth rate: −ratepig/cell α (1/ratecell). Substitution of a proportionality constant, c, and the corresponding first-order rate expressions for pigment per cell and cell growth produces the following equation: −(kpig/cell)·(pig/cell) = c/{(kcell)·[cell]}. A simple rearrangement yields an expression for calculated values of prodigiosin per cell: prodigiosin per cell = −c/{(kcell)·(kpig/cell)·[cell]}.
Calculated low-density prodigiosin-per-cell values were compared with at least 35 measured values from growth in each of five complex media containing a carbohydrate: citrate, inositol, glycerol, glucose, or maltose. Plots of calculated (y) versus observed (x) values yielded linear regression slopes statistically identical to the expected value of unity in four of five cases (data not shown). For maltose complex medium, the slope of 0.658 suggested a ca. 50% increase in prodigiosin levels during low-density growth in this energy-rich medium, although at a kinetically near-zero rate (see Table 3). These quantitative data for low-density growth of pigmented cells extend the results of Fig. 6A and B to a variety of carbohydrate-containing complex media.
Relationship among rate constants.
Results to this point have suggested an inverse relationship between cellular prodigiosin levels and the production of biomass (as growth rate) (Fig. 2). Our findings have also indicated an inverse relationship between cellular levels of prodigiosin and ATP (Table 2). Combination and generalization of these relationships are possible with the data of Table 3, which show a comparison of the rate constants for cell concentration, ATP concentration, and pigmentation across all population phases.
Table 3 shows that growth is associated with a wide range of net ATP changes, from consumption to no change to accumulation. In addition, rapid growth is not associated with pigment production. Rather, the strongest consistently measurable pigment production occurred at high density in the context of reduced growth and no ATP accumulation (Table 3). These results suggest that prodigiosin production is antagonistic to the combined production of biomass and bioenergy.
The instance of low-density phase 1 growth in base complex medium (Table 3; also Fig. 3A) enables the derivation of a quantitative relationship among rate constants for cell, ATP, and prodigiosin concentrations. Importantly, the rates of change of all three measured quantities are significantly different from zero. The processes of growth, ATP consumption, and pigment degradation appear to have been at equilibrium such that kcell + kATP = kpig or kcell + kATP − kpig = 0. The left side of the second equation may be applied to all growth experiments (Table 3). Positive values of this expression are associated with rapid growth at low density, zero values are associated with intermediate rates of growth at low density, and negative values are associated with slow to zero growth at high density preceding stationary phase. A simpler form of this equilibrium is observed with glucose complex medium at 37°C, where kpig = 0 and −(kATP) = kcell. In this case, ATP consumption appeared to directly fuel population growth.
Table 3 also permits direct comparison of rate constants between pigmented (26°C) and nonpigmented (37°C) growth in three types of complex media: base, inositol, and glucose. We consider phase 2 low-density growth (Fig. 3A and B) representative of steady-state growth at 26°C in base complex medium. Among these rate constants, by far the highest combined rate of biomass and bioenergy production was observed with nonpigmented cells grown without carbohydrate.
Finally, we note that glycerol complex medium promoted the highest rate of prodigiosin production under all conditions tested (Table 3). Many have observed that glycerol promotes strong culture pigmentation (29). Considered together, these kinetic data support the notion that prodigiosin promotes energy consumption without the concomitant production of biomass and/or bioenergy as ATP.
Prodigiosin and the inhibition of oxidative phosphorylation.
The early literature regarding prodigiosin production established an oxygen requirement for prodigiosin synthesis by S. marcescens (12, 18), a facultative anaerobe (11). Since our media and growth conditions at 26°C have consistently promoted pigmentation when growth was monitored to high density, we may reasonably assume that the cells have multiplied under aerobic conditions and have synthesized at least some ATP by oxidative phosphorylation. Therefore, studies regarding the inhibition of oxidative phosphorylation could potentially reveal a direct link between ATP production and prodigiosin synthesis.
Potassium cyanide, an inhibitor of oxidative phosphorylation which interferes with the reduction of oxygen to water, has been reported to inhibit prodigiosin production but not growth of S. marcescens colonies (18). Preliminary experiments measuring pigment levels in the presence of cyanide indicated that existing pigment levels exhibited no net change during logarithmic growth in base complex medium (kpig ≈ 0; data not shown). Table 4 shows a comparison of kinetic parameters from S. marcescens base complex broth cultures grown with and those grown without 0.04% potassium cyanide at 26°C. As expected, culture lag time with cyanide was increased relative to the no-cyanide culture, presumably due to a reduced rate of ATP production. Consistent with a negative regulatory role for prodigiosin in ATP production by oxygen-dependent mechanisms, the rates of biomass and bioenergy production were significantly increased when oxidative phosphorylation was inhibited.
TABLE 4.
Cyanide inhibition of oxidative phosphorylation in S. marcescens base complex broth culture at low densitya
| Culture | Lag-phase kATP | Lag time (h) | kcell | kATP | kcell + kATP − kpigb |
|---|---|---|---|---|---|
| Base | 1.51 ± 0.06 | 2.50 ± 0.00 | 0.425 ± 0.049c | 0.443 ± 0.013c | 0.868 |
| Base + cyanide | 0.410 ± 0.060 | 7.00 ± 0.00 | 0.638 ± 0.008 | 0.648 ± 0.025 | 1.29 |
Pregrowth from base complex slants at 30°C was pooled for inoculation of four shake flasks to an OD750 of 0.12. Growth was continued with aeration at 26°C. Values are means ± SDs for duplicate assays.
kpig was shown to be zero in separate experiments.
Values are reported for low-density phase 2 only.
A more definitive test of the association between prodigiosin and oxidative phosphorylation was performed by spiking late low-density base complex medium cultures with potassium cyanide just prior to significant pigment induction. Table 5 shows that cyanide treatment inhibited both pigmentation and residual growth at high density. On the other hand, it did not inhibit the ATP synthesis characteristic of the high-density phase in this medium (also Table 3). Interestingly, there was no statistical difference among the rates of ATP synthesis measured during low-density phase 2, high density, and high density with cyanide in base complex broth (n = 4 or 5; P = 0.84) (Table 3). It is therefore possible that net ATP accumulation during aerobic growth of S. marcescens is due primarily to substrate-level phosphorylation.
TABLE 5.
Cyanide inhibition of oxidative phosphorylation in S. marcescens base complex broth culture at high densitya
| Culture | kcell | kATP | kpig | kcell + kATP − kpig |
|---|---|---|---|---|
| Baseb | 0.199 ± 0.073 | 0.275 ± 0.041 | 0.744 ± 0.251 | −0.271 |
| Base + cyanidec | 0d | 0.280 ± 0.024 | 0d | 0.280 |
Values are averages of four or five measurements ± SDs.
Data were taken from Table 3.
Cyanide was added by spike addition to cultures grown overnight to the beginning of high-density phase. Values were recorded following a ca.-2-h lag of no net change.
Values were in the range ±0.050.
Considered together, our results provide strong evidence that prodigiosin is associated with negative regulation of ATP production by the oxidative pathway. As such, the pigment appears to be central to the energy-dependent mechanisms controlling aerobic cell growth of S. marcescens.
DISCUSSION
Prodigiosin is a red, hydrophobic pigment associated with the membrane fraction of Serratia marcescens cells. Even though some potentially significant nonphysiological functions for the molecule have been described, prodigiosin's role in the producing cell has remained elusive (2, 29, 30). This molecule has been shown to possess immunosuppressive and antitumor activities (17); cytotoxicity may be mediated by cytoplasmic acidification with a subsequent activation of caspase enzymes and apoptosis. Prodigiosin has been reported to possess antibiotic activity against bacteria, protozoa, and fungi (reviewed in reference 10). Significantly, none of these reported functions addresses a physiological role within the producing bacterial cell. Given the pigment's intracellular location, an intracellular function is extremely likely.
Prodigiosin induction by a quorum-sensing mechanism at relatively high cell density is well documented. However, this work is the first to show low-level, incremental induction at lower cell densities in carbohydrate-containing media, such as inositol complex broth (Fig. 4A). Prodigiosin was induced to a slightly higher and stable level during low-density growth just following the onset of ATP synthesis; these increased levels were then diluted by cell multiplication. The cycle of prodigiosin induction and dilution repeated as the cells depleted ATP and initiated its synthesis. More investigation will be needed to determine whether the degree of stepwise prodigiosin induction is matched by a similar increase in AHL quorum-sensing inducers.
Our kinetic data for cell growth rate, ATP production, and pigmentation combine to produce a mathematical relationship in which prodigiosin negatively impacts the total production of biomass and bioenergy (as ATP) under aerobic growth conditions. Since oxidative phosphorylation is known to complete the extraction of biologically useful energy from nutrients catabolized by heterotrophic bacteria, we were surprised to discover significantly higher rates of biomass and bioenergy production with cyanide-treated cultures (Table 4). An explanation for this phenomenon may be found in the coupling of oxidative phosphorylation to an energy dissipation process. Table 5 shows that an inhibitor of oxidative phosphorylation also mitigated prodigiosin biosynthesis, affirming earlier work with bacterial colonies (18). These data as well as the reduced growth rate of a poorly pigmented mutant (Table 2) highlight the critical influence of prodigiosin on aerobic growth rate.
A negative role for prodigiosin in ATP synthesis is reminiscent of a well-documented but not widely appreciated phenomenon called energy spilling (reviewed in references 21 and 22). Streptococcus bovis synthesizes ATP only by substrate-level phosphorylation. When cells whose growth is limited by nitrogen are pulsed with glucose, creating an intracellular ATP excess, the membrane F1Fo ATPase “spills” extra ATP by coupling ATP hydrolysis to an export of protons. Membrane permeability to protons is thought to transiently increase by an uncharacterized mechanism during periods of ATP hydrolysis, allowing proton reentry and completing a futile cycle of proton transport across the cytoplasmic membrane. Interestingly, a phenomenon resembling energy spilling for E. coli is demonstrated in Fig. 1: the cellular ATP concentration decreased over the 1.75-h interval between peak cellular ATP and growth cessation at population stationary phase.
In view of our results, we propose that prodigiosin functions in an energy-spilling process as a tightly regulated uncoupler of proton transport and ATP synthesis by oxidative phosphorylation. Central to this proposal is the demonstration of an H+/Cl− symport activity for prodigiosin in artificial liposomes (23). Additional support for this idea can be found in the earlier work of Kobayashi and Ichikawa (14). These investigators showed that membrane fractions from nonpigmented mutants grown on certain carbon sources consumed more oxygen than did membranes from pigmented strains during in vitro respiration assays. This finding suggests that prodigiosin indirectly limits oxygen consumption (and ATP synthesis) during aerobic respiration by relocating substrate protons. Taken together, results to date suggest a model in which the transmembrane potential difference is partially short circuited by prodigiosin prior to ATP generation by oxidative phosphorylation, thereby controlling oxygen consumption, ATP production, and ultimately growth rate under aerobic conditions. ATP production by oxidative phosphorylation is known to be accompanied by the production of reactive oxygen by-products; perhaps prodigiosin limits intracellular oxidative stress under aerobic growth conditions. This model makes the testable prediction that nonpigmented S. marcescens strains would experience greater oxidative stress during aerobic growth than would pigmented organisms.
A comprehensive physiological description of prodigiosin function must also address the high rate of prodigiosin production at high density preceding population stationary phase. Exhaustion of a growth-limiting nutrient may suspend oxidative phosphorylation, allowing diversion of pyruvate into prodigiosin biosynthesis at a rate determined by levels of AHL quorum-sensing activators present in the culture. Interestingly, Rice and Bayles have recently postulated for Staphylococcus aureus that diversion of pyruvate from the production of acetyl coenzyme A promotes cell death by membrane depolarization mechanisms which activate murein hydrolase enzymes (20). Further research into possible functional roles for prodigiosin should more clearly define the importance of this hydrophobic molecule to the physiology of S. marcescens and also contribute to a broader understanding of bacterial cell death in stationary phase.
Acknowledgments
This research was supported with funding from the Auburn University Montgomery Research and Equipment Grant-in-Aid programs as well as the Auburn University Montgomery School of Sciences.
We acknowledge the technical assistance of Abby Mann, Sarah A. Edgell-Hardinger, and Pius Nwobi. We thank Benedict C. Okeke and Stuart B. Price for critical reviews of the manuscript.
This work is dedicated to Gerald L. Seebach and Terry F. Werner.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Atlas, R. M. 1993. Handbook of microbiological media, p.673. CRC Press, Boca Raton, FL.
- 2.Bennett, J. W., and R. Bentley. 2000. Seeing red: the story of prodigiosin. Adv. Appl. Microbiol. 471-32. [DOI] [PubMed] [Google Scholar]
- 3.Buckstein, M. H., J. He, and H. Rubin. 2008. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 190718-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bu'Lock, J. D. 1961. Intermediary metabolism and antibiotic synthesis. Adv. Appl. Microbiol. 3293-342. [DOI] [PubMed] [Google Scholar]
- 5.Coulthurst, S. J., C. L. Kurz, and G. P. Salmond. 2004. luxS mutants of Serratia defective in autoinducer-2 dependent quorum sensing show strain-dependent impacts on virulence and production of carbapenem and prodigiosin. Microbiology 1501901-1910. [DOI] [PubMed] [Google Scholar]
- 6.Daschner, F. D. 1980. The epidemiology of Serratia marcescens, p. 187-196. In A. von Graevenitz and S. J. Rubin (ed.), The genus Serratia. CRC Press, Boca Raton, FL.
- 7.Fineran, P. C., L. Everson, H. Slater, and G. P. C. Salmond. 2005. A GntR family transcriptional regulator (PigT) controls gluconate-mediated repression and defines a new, independent pathway for regulation of the tripyrrole antibiotic, prodigiosin, in Serratia. Microbiology 1513833-3845. [DOI] [PubMed] [Google Scholar]
- 8.Fineran, P. C., H. Slater, L. Everson, K. Hughes, and G. P. C. Salmond. 2005. Biosynthesis of tripyrrole and β-lactam secondary metabolites in Serratia: integration of quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production. Mol. Microbiol. 561495-1517. [DOI] [PubMed] [Google Scholar]
- 9.Fineran, P. C., N. R. Williamson, K. S. Lilley, and G. P. C. Salmond. 2007. Virulence and prodigiosin antibiotic biosynthesis in Serratia are regulated pleiotropically by the GGDEF/EAL domain protein, PigX. J. Bacteriol. 1897653-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber, N. N. 1975. Prodigiosin-like pigments. Crit. Rev. Microbiol. 3469-485. [DOI] [PubMed] [Google Scholar]
- 11.Grimont, P. A. D., and F. Grimont. 1984. Family I. Enterobacteriaceae. Genus VIII. Serratia Bizio 1823, 288AL, p. 477-484. In N. R. Krieg et al. (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 12.Heinemann, B., A. J. Howard, and H. J. Palocz. 1970. Influence of dissolved oxygen levels on production of l-asparagine and prodigiosin by Serratia marcescens. Appl. Microbiol. 19800-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hejazi, A., and F. R. Falkiner. 1997. Serratia marcescens. J. Med. Microbiol. 46903-912. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, N., and Y. Ichikawa. 1985. Decrease in respiration activity related to prodigiosin synthesis in Serratia marcescens. Microbiol. Immunol. 29301-308. [DOI] [PubMed] [Google Scholar]
- 15.Lim, D. V., S. M. Qadri, C. Nichols, and R. P. Williams. 1977. Biosynthesis of prodigiosin by nonproliferating wild-type Serratia marcescens and mutants deficient in catabolism of alanine, histidine, and proline. J. Bacteriol. 129124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, J. H. 1972. Experiments in molecular genetics, p. 431. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Montaner, B., and R. Pérez-Tomás. 2003. The prodigiosins: a new family of anticancer drugs. Curr. Cancer Drug Targets 357-65. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima, M. 1965. The mechanism of prodigiosin biosynthesis. 1. External factors for prodigiosin biosynthesis. Bull. Osaka Med. Sch. 1139-55. [PubMed] [Google Scholar]
- 19.Parment, P. A. 1997. The role of Serratia marcescens in soft contact lens associated ocular infections. A review. Acta Ophthalmol. Scand. 7567-71. [DOI] [PubMed] [Google Scholar]
- 20.Rice, K. C., and K. W. Bayles. 2008. Molecular control of bacterial death and lysis. Microbiol. Rev. 7285-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell, J. B. 2007. The energy spilling reactions of bacteria and other organisms. J. Mol. Microbiol. Biotechnol. 131-11. [DOI] [PubMed] [Google Scholar]
- 22.Russell, J. B., and G. M. Cook. 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 5948-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato, T., H. Konno, Y. Tanaka, T. Kataoka, K. Nagai, H. Wasserman, and S. Ohkuma. 1998. Prodigiosins as a new group of H+/Cl− symporters that uncouple proton translocators. J. Biol. Chem. 27321455-21462. [DOI] [PubMed] [Google Scholar]
- 24.Slater, H., M. Crow, L. Everson, and G. P. C. Salmond. 2003. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing dependent and -independent pathways. Mol. Microbiol. 47303-320. [DOI] [PubMed] [Google Scholar]
- 25.Thomson, N. R., M. A. Crow, S. J. McGowan, A. Cox, and G. P. C. Salmond. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36539-566. [DOI] [PubMed] [Google Scholar]
- 26.van Houdt, R., M. Givskov, and C. W. Michiels. 2007. Quorum sensing in Serratia. FEMS Microbiol. Rev. 31407-424. [DOI] [PubMed] [Google Scholar]
- 27.von Graevenitz, A. 1980. Infection and colonization with Serratia, p. 167-185. In A. von Graevenitz and S. J. Rubin (ed.), The genus Serratia. CRC Press, Boca Raton, FL.
- 28.Wei, J., and H. Lai. 2006. N-acylhomoserine lactone-dependent cell-to-cell communication and social behavior in the genus Serratia. Int. J. Med. Microbiol. 296117-124. [DOI] [PubMed] [Google Scholar]
- 29.Williams, R. P., and M. H. Qadri. 1980. The pigment of Serratia, p. 31-75. In A. von Graevenitz and S. J. Rubin (ed.), The genus Serratia. CRC Press, Boca Raton, FL.
- 30.Williamson, N. R., P. C. Fineran, F. J. Leeper, and G. P. C. Salmond. 2006. The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 4887-899. [DOI] [PubMed] [Google Scholar]
- 31.Yang, Y.-H., T.-H. Lee, J. H. Kim, E. J. Kim, H.-S. Joo, C.-S. Lee, and B.-G. Kim. 2006. High-throughput detection method of quorum-sensing molecules by colorimetry and its applications. Anal. Biochem. 356297-299. [DOI] [PubMed] [Google Scholar]