Abstract
We report pyruvate formation in Escherichia coli strain ALS929 containing mutations in the aceEF, pfl, poxB, pps, and ldhA genes which encode, respectively, the pyruvate dehydrogenase complex, pyruvate formate lyase, pyruvate oxidase, phosphoenolpyruvate synthase, and lactate dehydrogenase. The glycolytic rate and pyruvate productivity were compared using glucose-, acetate-, nitrogen-, or phosphorus-limited chemostats at a growth rate of 0.15 h−1. Of these four nutrient limitation conditions, growth under acetate limitation resulted in the highest glycolytic flux (1.60 g/g · h), pyruvate formation rate (1.11 g/g · h), and pyruvate yield (0.70 g/g). Additional mutations in atpFH and arcA (strain ALS1059) further elevated the steady-state glycolytic flux to 2.38 g/g · h in an acetate-limited chemostat, with heterologous NADH oxidase expression causing only modest additional improvement. A fed-batch process with strain ALS1059 using defined medium with 5 mM betaine as osmoprotectant and an exponential feeding rate of 0.15 h−1 achieved 90 g/liter pyruvate, with an overall productivity of 2.1 g/liter · h and yield of 0.68 g/g.
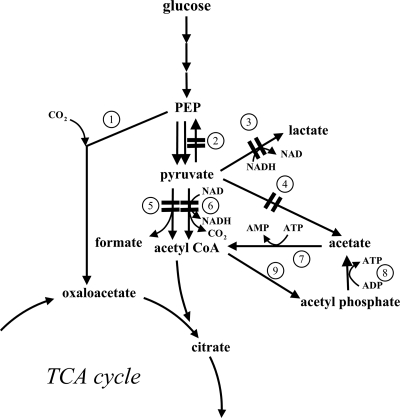
Pyruvic acid (pyruvate) is widely used in food, chemicals, and pharmaceuticals. The chemical is a precursor for the enzymatic production of l-tryptophan, l-tyrosine, d-/l-alanine, and l-dihydroxyphenylalanine (22), and it also serves in several health-related roles, including weight loss (25, 33, 34), exercise endurance (32), cholesterol reduction (35), and acne treatment (10). Recently, pyruvate has been used as the key metabolic precursor to the second-generation biofuels isobutanol and 3-methyl-1-butanol (2). By applying metabolic engineering strategies to them, microorganisms such as Escherichia coli and yeasts can be used to produce significant quantities of pyruvate from glucose and other renewable resources (22). In general, such approaches must delete or repress pathways which metabolize pyruvate. For example, pyruvate accumulates readily in E. coli strains having mutations in aceEF, encoding components of the pyruvate dehydrogenase complex (37). Additional mutations, of the ldhA, poxB, pfl, and pps genes, further improve pyruvate formation (48). Figure 1 shows the principal metabolic pathways and enzymes involved in the formation of pyruvate.
FIG. 1.
Key enzymatic reactions in the production of pyruvate by Escherichia coli strains. Enzymes: 1, PEP carboxylase, 2, PEP synthase, 3, lactate dehydrogenase, 4, pyruvate oxidase, 5, pyruvate formate lyase, 6, pyruvate dehydrogenase complex, 7, acetyl-CoA synthetase, 8, acetate kinase, 9, phosphotransacetylase.
Because pyruvate resides biochemically at the end of glycolysis, pyruvate production is directly related to the glycolytic flux. Metabolic engineering strategies to form pyruvate therefore also aim to enhance glycolysis (8, 45, 46). Glycolysis is not transcriptionally limited, and control principally resides outside the pathway in cellular demand for global cofactors, such as ATP and NADH (19, 23, 40). Glycolytic flux is substantially increased by disrupting oxidative phosphorylation or by increasing ATP hydrolysis (8, 19). Increased glycolytic flux assists pyruvate accumulation: for example, an F1-ATPase-defective mutant (E. coli lipA2 bgl+ atpA401) generated pyruvate more quickly than its parent (45, 46). Similarly, E. coli atpFH (strain TC44), deficient in oxidative phosphorylation but having hydrolytic F1-ATPase activity, showed an elevated glycolytic flux and generated 64.9 g/liter pyruvate, with a yield of 0.75 g/g and a volumetric productivity of 1.2 g/liter · h (8). In addition to reducing oxidative phosphorylation, reducing NADH availability directly also increases glycolytic flux. For example, glycolytic flux was increased 70% by introducing water-forming NADH oxidase from Streptococcus pneumoniae into E. coli and into an arcA mutant (40).
Another goal in a pyruvate production process is of course high yield. Since the principal by-product of pyruvate formation is biomass, high glycolytic flux relative to low biomass generation is sought. Glycolytic flux will achieve a maximum from supplying glucose in excess and limiting cell growth by the availability of another substrate, during a chemostat or fed-batch culture, for example. With mutations in the pathways leading to acetyl-coenzyme A (CoA) from pyruvate, E. coli YYC202 ldhA requires both glucose and acetate as carbon sources for cell growth (48), making this strain useful to examine the effects of nutrient limitation using acetate or noncarbon compounds. In a previous study, 62 g/liter pyruvate was generated, with a yield from glucose of 0.56 g/g at a rate of 1.75 g/liter · h (48), but glycolytic flux was not maximized and cell growth was not limited. A fed-batch process limited by the availability of a substrate other than glucose would limit cell growth and could simultaneously elevate glycolytic flux, pyruvate yield, and productivity. The objective of the present study was to use chemostat and fed-batch experiments to study the effects of growth-limiting substrate availability and genetic perturbations on glycolytic flux and pyruvate generation. Specifically, using E. coli YYC202 ldhA (strain ALS929), we compared the effects of glucose, acetate, nitrogen, and phosphorus limitation on growth, and using the best conditions, compared the effects of mutations in atpFH and arcA and the result of introducing heterologous NADH oxidase activity.
MATERIALS AND METHODS
Strains.
Table 1 lists the strains used in this study. Derivatives of YYC202 that contained ldhA::Kan, ΔarcA726::(FRT)Kan, or ΔatpFH::Cam were constructed using P1 transduction. KanR was deleted from ΔarcA726::(FRT)Kan transductants using the curable pCP20 plasmid which overproduces FLP recombinase (9). The pTrc99A-nox plasmid which overproduces Streptococcus pneumoniae NADH oxidase has been previously described (40). All manipulations that involved YYC202 were performed using TYA medium (described below).
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| YYC202 | Hfr zbi::Tn10 poxB1 Δ(aceEF) rpsL pps-4 pfl-1 | John E. Cronan, Jr., University of Illinois |
| ALS929 | YYC202 ldhA::Kan | This study |
| ALS1054 | YYC202 ldhA::Kan arcA726::FRT | This study |
| ALS1058 | YYC202 ldhA::Kan atpFH::Cam | This study |
| ALS1059 | YYC202 ldhA::Kan arcA726::FRT atpFH::Cam | This study |
| CGSC11117 (JW4364-1) | Δ(araD-araB)567 ΔlacZ4787(::rrnB-3) Δ(rhaD-rhaB)568 hsdR514 rph-1 λ− ΔarcA726::(FRT)Kan | 3 |
| DY330 | F−mcrA mcrB IN(rrnD-rrnE)1 Δ(lac)U169 gal490 λ[cI857 Δ(cro-bioA)] | 47 |
| NZN111 | F+ λ−rpoS396(Am) rph-1 ldhA::Kan Δ(pflAB::Cam) | 6 |
The F1F0 proton-translocating ATPase complex of E. coli, which catalyzes the synthesis of ATP from inorganic phosphate, is encoded by the atpIBEFHAGDC operon. Because the atpFH genes are transcriptionally coupled, a deletion that encompassed both atpF and atpH could easily be constructed using the lambda Red recombination system (11, 47). Primers were designed which could amplify the chloramphenicol acetyltransferase gene and promoter from pACYC184, bracketed by the first 50 bases of the atpF gene and the last 50 bases of the atpH gene. The forward primer 5′ GTGAATCTTAACGCAACAATCCTCGGCCAGGCCATCGCGTTTGTCCTGTTTTGAGAAGCACACGGTCACA3′ contains the first 50 bases of the atpF coding sequence followed by bases 3601 to 3620 of pACYC184, while the reverse primer 5′ TTAAGACTGCAAGACGTCTGCAAGGCGCTCAAGACGACCGCGTACGCTGCTACCTGTGACGGAAGATCAC3′ contains the last 50 bases of the atpH coding sequence followed by bases 400 to 419 of pACYC184. The bases homologous to pACYC184 are underlined in the primers. The two primers were used to amplify a 1,162-bp fragment from pACYC184 DNA using PCR and Pfu polymerase. The resulting DNA was gel isolated and electroporated into DY330 electrocompetent cells, and CamR colonies were then selected. The presence of the ΔatpFH::Cam knockout was confirmed by performing PCR with the following two primer pairs which could amplify the atpFH genes. The forward primer 5′CTTAACGCAACAATCCTCGG3′ contains bases 7 to 26 of the atpF gene, while the reverse primer 5′TAAGACTGCAAGACGTCTGC 3′ contains bases 514 to 533 of the atpH gene. PCR amplification with these two primers yields a 1,012-bp fragment from the wild-type atpFH gene and a 1,155-bp fragment from the ΔatpFH::Cam knockout.
Growth conditions.
For all experiments, cells were first grown in a 250-ml shake flask containing 30 ml TYA medium for about 8 h before 5 ml was transferred to 50 ml of SF medium (described below) in a 250-ml shake flask. After 12 h of growth, the contents of this shake flask were used to inoculate a bioreactor containing GAM or CGAM medium (described below). Flasks were incubated at 37°C and 250 rpm (19 mm pitch). TYA medium contained (per liter): 10.0 g tryptone, 5.0 g NaCl, 1.0 g yeast extract, 1.36 g Na(CH3COO)·3H2O. SF medium contained (per liter) 10.0 g glucose, 2.3 g Na(CH3COO)·3H2O, 5.66 g Na2HPO4·7H2O, 1.5 g KH2PO4, 0.25 g NaCl, 0.5 g NH4Cl, 0.1 g MgSO4·7H2O, 0.013 g CaCl2·2H2O, 0.02 g thiamine·HCl, 0.5 g l-isoleucine. GAM medium contained (per liter) 30.0 g glucose, 2.75 g Na(CH3COO)·3H2O, 1.5 g NaH2PO4·H2O, 3.25 g KH2PO4, 3.275 g K2HPO4·3H2O, 0.2 g NH4Cl, 2.0 g (NH4)2SO4, 1.024 g MgSO4·7H2O, 0.01 g CaCl2·2H2O, 0.5 mg ZnSO4·7H2O, 0.25 mg CuCl2·2H2O, 2.5 mg MnSO4·H2O, 1.75 mg CoCl2·6H2O, 0.12 mg H3BO3, 1.772 mg Al2(SO4)3, 0.5 mg Na2MoO4·2H2O, 18.29 mg FeSO4·7H2O, 0.02 g thiamine·HCl, 0.75 g l-isoleucine. CGAM medium contained (per liter) 35.0 g glucose, 9.22 g Na(CH3COO)·3H2O, 2.51 g K2HPO4, 1.44 g KH2PO4, 0.4 g NH4Cl, 4.0 g (NH4)2SO4, 0.15 g MgSO4·7H2O, 0.01 g CaCl2·2H2O, 0.05 g Na2EDTA·2H2O, 0.25 mg ZnSO4·7H2O, 0.125 mg CuCl2·2H2O, 1.25 mg MnSO4·H2O, 0.875 mg CoCl2·6H2O, 0.06 mg H3BO3, 0.8859 mg Al2(SO4)3, 0.25 mg Na2MoO4·2H2O, 5.50 mg FeSO4·7H2O, 0.02 g thiamine·HCl, 0.2 g l-isoleucine.
Chemostat.
Continuous fermentations of 1.0-liter volumes operated as chemostats and were initiated in batch mode in a 2.5-liter bioreactor (Bioflow 2000; New Brunswick Scientific Co., Inc., Edison, NJ). The pH was maintained at 7.0 with 20% (wt/vol) NaOH, the temperature at 37°C, and the agitation at 400 rpm. An airflow rate of 1.0 liter/min ensured that the dissolved oxygen concentration remained above 40% saturation (Unit Instruments mass flow controllers; Orange, CA). Using constant medium feed and effluent culture rates of 0.15 liter/min, a steady-state condition was assumed after five residence times, at which time the oxygen and CO2 concentrations in the effluent gas remained unchanged. Several limited-nutrient conditions were examined by reducing the concentration of one nutrient in CGAM medium while leaving others unchanged. Specifically, for glucose limitation, the feed glucose concentration was 5.0 g/liter, for acetate limitation the feed contained 2.30 g/liter Na(CH3COO)·3H2O, for nitrogen limitation the feed contained 0.1 g/liter NH4Cl and 1.0 g/liter (NH4)2SO4, and for a phosphate-limited chemostat the feed contained 0.08 g/liter K2HPO4 and 0.04 g/liter KH2PO4. Nutrient limitation was verified by measurement of (the absence of) each nutrient in the respective chemostat effluent. For example, for the nitrogen-limited chemostat, no nitrogen was detected in the cell-free medium exiting the bioreactor, while glucose, acetate, and phosphorus were each detected in this effluent. For chemostat experiments, the dry cell mass concentration was calculated by drying a centrifuged and washed 20-ml sample at 60°C for 24 h.
Fed-batch processes.
Fed-batch processes were carried out in a 2.5-liter bioreactor (Bioflow 2000; New Brunswick Scientific Co., Edison, NJ) initially containing 1.0 liter GAM medium. Cells grew first in batch mode at their maximum specific growth rate until the initial acetate was nearly exhausted (optical density at 600 nm [OD600] of about 3.0). Then, fed-batch mode was commenced by exponentially feeding a solution containing 600 g/liter glucose and 30 g/liter acetate so that cell growth was controlled at a constant specific rate of about 0.15 h−1. The pH was controlled at 7.0 by using 5% (wt/vol) NH4OH-25% KOH; the temperature was controlled at 37°C; and the agitation maintained at 400 rpm. Air and O2 were mixed as necessary at 1.0 liter/min total flow rate to maintain a dissolved oxygen concentration of above 40% saturation. To calculate specific rates for fed-batch experiments, the OD600 (UV-650 spectrophotometer; Beckman Instruments, San Jose, CA) of a 10 times or 50 times diluted sample was correlated to the dry cell mass concentration by using the formula 1 OD600 (undiluted) = 0.40 g/liter.
Analyses.
The presence of numerous soluble organic compounds (e.g., pyruvate, acetate, glucose, malate, α-ketoglutarate, citrate, glucuronate, isocitrate, tartrate, malonate, glyoxylate, glycerate, sorbitol, succinate, glycolate, methylglyoxal, fumarate, lactate, formate, glycerol, dihydroxyacetone, acetoacetate, diacetyl, 1,2-propanediol, 1,3-propanediol, propionate, acetoin, acetaldehyde, ethanol, and 2,3-butanediol) was determined above a quantification limit of about 0.05 g/liter by liquid chromatography using a Coregel 64H ion exclusion column (Interaction Chromatography, San Jose, CA) with refractive index detection (14). The concentrations of oxygen and CO2 in the off-gas were measured (Ultramat 23 gas analyzer, Siemens, Germany).
RESULTS
Selection of limiting substrate.
Escherichia coli cells containing gene mutations corresponding to major pathways involved in pyruvate metabolism (i.e., aceEF ldhA pfl poxB pps) accumulate that product under aerobic conditions in a medium containing both glucose and acetate (48). The highest productivity and pyruvate yield from glucose should occur when cell growth is limited but under conditions of excess glucose to maximize glycolytic flux. To test this hypothesis, we conducted chemostat experiments to compare the results of limiting four different nutrients: glucose, acetate, nitrogen (ammonium), and phosphorus (phosphate). Table 2 summarizes key parameters determined from these steady-state experiments at the dilution rate of 0.15 h−1.
TABLE 2.
Comparison of results of limiting nutrients during chemostats of E. coli ALS929a using a dilution rate of 0.15 h−1
| Growth-limiting nutrient | qg (g/g · h) | qA (g/g · h) | qPyr (g/g · h) | qO2 (mmol/g · h) | qCO2 (mmol/g · h) | YX/G (g/g) | YPyr/G (g/g) | YPyr/X (g/g) |
|---|---|---|---|---|---|---|---|---|
| Glucose | 0.36 | 0.28 | 0.085 | 10.97 | 6.27 | 0.43 | 0.24 | 0.56 |
| Nitrogen | 0.85 | 0.20 | 0.57 | 12.35 | 4.30 | 0.18 | 0.67 | 3.72 |
| Phosphorus | 0.77 | 0.36 | 0.20 | 13.05 | 8.06 | 0.20 | 0.26 | 1.32 |
| Acetate | 1.60 | 0.065 | 1.11 | 8.65 | 1.10 | 0.10 | 0.70 | 7.15 |
qg, specific glucose consumption rate; qA, specific acetate consumption rate; qPyr, specific pyruvate production rate; qO2, specific oxygen consumption rate; qCO2, specific carbon dioxide evolution rate; YX/G, mass yield coefficient of biomass/glucose; YPyr/G, mass yield coefficient of pyruvate/glucose; YPyr/X, mass yield coefficient of pyruvate/biomass.
The greatest specific glucose consumption rate was observed when the availability of acetate limited growth. This maximum of 1.60 g/g · h was about twice as great as the value observed when the availability of either nitrogen or phosphorus limited growth and over four times greater than the level attained when the availability of glucose limited growth. The value of the specific glucose consumption rate was indeed related to pyruvate formation, with acetate-limited growth also achieving the greatest rate of pyruvate formation, at 1.10 g/g · h. Acetate-limited growth thereby resulted in a pyruvate mass yield based on glucose consumed of 0.70 g/g. Nitrogen-limited conditions generated pyruvate at about 50% of this maximal rate and achieved a similar mass yield of 0.67 g/g. However, phosphorus-limited conditions accumulated pyruvate at a much-lower rate: the rate of pyruvate formation was only 0.20 g/g · h, with a yield of 0.26 g/g. The pyruvate yield was similar for glucose- and phosphorus-limited conditions.
Since acetate is required for the growth of strain ALS929, the molar ratio of glucose-to-acetate consumed should be an indicator of the excess glucose which is used by the cell but does not generate biomass. The values for this molar glucose/acetate ratio were 0.41, 1.4, 0.70, and 8.1, respectively, for glucose-, nitrogen-, phosphorus-, and acetate-limited growth. Thus, under acetate-limited conditions, the cells consumed 8 glucose molecules for every acetate molecule, while under glucose-limited conditions, the cells consumed almost 2.5 acetate molecules for every glucose molecule.
The specific nutrients which limited growth also affected respiration and carbon dioxide evolution. Phosphorus-limited cells consumed 58% more oxygen and generated over seven times more CO2 than acetate-limited cells. The very low carbon dioxide evolution rate when acetate limited growth (the respiratory quotient was 0.127) provides further evidence that glucose was very effectively converted to pyruvate with minimal “loss” of carbon, and therefore, for subsequent studies we used acetate as the limiting substrate.
Genetic approaches to elevate glycolytic flux.
With mutations in major metabolic pathways from pyruvate, strain ALS929 also has a limited ability to generate other soluble by-products from glucose. The acetate-limited chemostat achieved a molar carbon recovery of 82%, though no other extracellular product was detected by chromatography (see Materials and Methods). In order to increase the yield further, the yield of biomass itself would have to be reduced. We therefore explored several genetic modifications to increase glycolytic flux and/or reduce the formation of biomass. Three genetic modifications were examined: knockouts in arcA and atpFH and the overexpression of heterologous water-forming NADH oxidase. We first conducted acetate-limited chemostats at the same dilution rate of 0.15 h−1 using strains with one or more of these genetic modifications.
Previous reports suggest that the ArcA regulator system is controlled by the redox environment or, specifically, a high NADH/NAD+ ratio, and NADH accumulates in cells which have high glycolytic flux (15, 17, 40). The expression levels of several respiratory genes repressed by ArcA are increased over sevenfold in an arcA knockout (40). In order to test whether glycolytic flux and pyruvate generation could be increased by relieving this respiratory repression, we knocked out arcA in ALS929 to construct strain ALS1054 and characterized this strain in an acetate-limited chemostat (Table 3). ALS1054 had biomass and pyruvate yields on glucose similar to the biomass and pyruvate yields of ALS929, and the specific glucose consumption rate and specific pyruvate production rate were slightly lower, suggesting that respiratory processes and other enzymes under the control of the ArcA system did not limit glycolysis in ALS929 under these conditions.
TABLE 3.
Comparison of E. coli strains during acetate-limited chemostatsa using a dilution rate of 0.15 h−1
| Strain | qg (g/g · h) | qA (g/g · h) | qPyr (g/g · h) | qO2 (mmol/g · h) | qCO2 (mmol/g · h) | YX/G (g/g) | YPyr/G (g/g) | YPyr/X (g/g) |
|---|---|---|---|---|---|---|---|---|
| ALS929 | 1.60 | 0.065 | 1.11 | 8.65 | 1.10 | 0.10 | 0.70 | 7.15 |
| ALS1054 | 1.46 | 0.054 | 1.04 | 7.30 | 1.29 | 0.11 | 0.72 | 6.72 |
| ALS1058 | 2.18 | 0.085 | 1.65 | 10.81 | 1.89 | 0.07 | 0.76 | 10.67 |
| ALS1059 | 2.38 | 0.091 | 1.86 | 10.60 | 3.00 | 0.07 | 0.78 | 11.97 |
| ALS929/pTrc99A-nox | 1.42 | 0.061 | 0.97 | 6.55 | 1.27 | 0.11 | 0.68 | 6.28 |
| ALS1054/pTrc99A-nox | 1.17 | 0.086 | 0.85 | 7.90 | 1.46 | 0.13 | 0.73 | 5.51 |
| ALS1059/pTrc99A-nox | 2.67 | 0.117 | 2.01 | 9.76 | 1.71 | 0.06 | 0.75 | 12.94 |
qg, specific glucose consumption rate; qA, specific acetate consumption rate; qPyr, specific pyruvate production rate; qO2, specific oxygen consumption rate; qCO2, specific carbon dioxide evolution rate; YX/G, mass yield coefficient of biomass/glucose; YPyr/G, mass yield coefficient of pyruvate/glucose; YPyr/X, mass yield coefficient of pyruvate/biomass.
Membrane-bound (F1F0) H+-ATP synthase (EC 3.6.1.34) is responsible for oxidative phosphorylation (31) and catalyzes ATP synthesis by capturing the energy of the transmembrane electrochemical potential (24). In previous studies, knockouts in subunits b and c of F0 encoded by atpFH reduced cell yield and growth rates slightly but increased by twofold the glycolytic flux compared to the results for a wild-type strain (8). In our study, we similarly eliminated ATP synthesis via oxidative phosphorylation by constructing the atpFH knockout strain ALS1058. With ALS1058 under steady-state conditions, the specific glucose consumption rate was increased 36% and the specific pyruvate productivity was increased 49% compared to those of the parent strain ALS929 (Table 3). An atpFH deletion strain also reduced cell yield on glucose by 30% and increased pyruvate yield to 0.76 g/g. These factors resulted in a 49% increase in the yield of pyruvate on biomass: 10.7 g pyruvate was generated for every 1 g of ALS1058 biomass generated. Under acetate-limited steady-state conditions, ALS1058 consumed 9.2 glucose molecules for every acetate molecule consumed. The vast majority of glucose has been diverted to the product, pyruvate, instead of to biomass.
We next examined the combination of an atpFH knockout and the arcA knockout by constructing strain ALS1059. Whereas the arcA mutation alone did not improve glucose consumption and pyruvate formation, the arcA knockout in the atpFH strain did improve these parameters beyond the levels observed with the atpFH knockout alone (Table 3). ALS1059 showed a specific glucose consumption rate of 2.38 g/g · h, 49% greater than that of the parent ALS929, and a pyruvate yield of 0.78 g/g. Interestingly, ALS1059 generated almost three times as much CO2 as ALS929 and 59% more than ALS1058, containing only the atpFH knockout. Despite this increase in CO2 generation, CO2 remains a very small fraction of the total carbon products. Only 3.7% of the total carbon consumed by ALS1059 leads to CO2 formation, compared to 2.0% of the total carbon consumed by ALS929 in an acetate-limited chemostat.
During elevated glucose consumption, respiration cannot keep pace with glycolysis and cells accumulate NADH (40). The introduction of water-forming NADH oxidase can provide cells with another means to reoxidize NADH to NAD+ without energy generation. To determine whether providing cells with another outlet for NADH would further increase pyruvate production, we transformed strains ALS929, ALS1054, and ALS1059 with pTrc99A-nox expressing the water-forming NADH oxidase from S. pneumoniae. The introduction of NADH oxidase activity into ALS929 and ALS1054 resulted in slight decreases in specific glucose consumption and pyruvate formation rates (Table 2). However, for ALS1059/pTrc99A-nox, these rates were increased by about 10% compared to those for ALS1059. Furthermore, 12.9 g of pyruvate were generated for every 1 g of strain ALS1059/pTrc99A-nox.
Fed-batch processes.
Although chemostat experiments provide steady-state information and high volumetric rates, such an operational mode does not permit the product, pyruvate, to accumulate to a significant final concentration. We therefore next examined a fed-batch process using an exponential feed in order to maintain a constant specific growth rate of 0.15 h−1. Similar to the chemostat experiments, the growth was limited by acetate (except for a brief initial batch portion of the process).
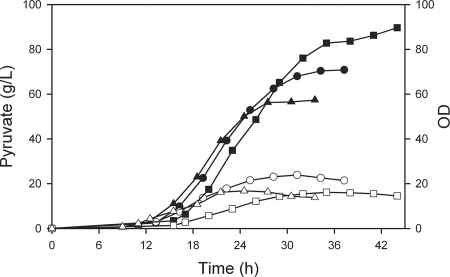
One disadvantage of fed-batch processes is that numerous substances accumulate, not only the desired product. Because the accumulation of pyruvate in our current study would simultaneously require the accumulation of K+ (or another counterion) to maintain the pH, we conducted an experiment to determine whether the addition of the known osmoprotectant betaine (38, 50) would improve pyruvate accumulation. Without betaine present in the medium under acetate-limited conditions, strain ALS929 was able to accumulate over 56 g/liter pyruvate in about 28 h (Fig. 2). At this time, cell growth ceased at an OD600 of 17, even though acetate was still being consumed. In the presence of 5 mM betaine, ALS929 accumulated over 70 g/liter pyruvate in 34 h, with the OD600 achieving 24. Because growth was limited by the feed rate, the presence of betaine did not affect the growth rate or the rate of pyruvate formation. Similar to the chemostat results (Table 3), the maximum specific rate of pyruvate formation was about 1.2 g/g · h. The average productivity over the course of the process was 2.1 g/liter · h, although the maximum rate was about 5.8 g/liter · h. Betaine did not improve the pyruvate yield but merely allowed the process to continue to a 25%-higher final pyruvate concentration. We therefore used 5 mM betaine in subsequent fed-batch experiments.
FIG. 2.
Results of fed-batch process using E. coli strains with and without 5 mM betaine at a growth rate of 0.15 h−1. OD (at 600 nm): ○, ALS929 with betaine; ▵, ALS929 without betaine; □, ALS1059 with betaine. Pyruvate: •, ALS929 with betaine; ▴, ALS929 without betaine; ▪, ALS1059 with betaine. L, liter.
We next examined pyruvate formation in a fed-batch process with ALS1059, the strain which attained a 67%-higher steady-state rate of pyruvate formation than ALS929 (Table 3). In the presence of 5 mM betaine, ALS1059 accumulated about 90 g/liter pyruvate in 44 h, with a yield of 0.68 g/g (Fig. 2). As expected, the biomass generation with ALS1059 was lower than observed for ALS929. Interestingly, the higher specific rate was compensated by lower biomass concentration, resulting in an average volumetric productivity of about 2.1 g/liter · h, identical to that of ALS929. With a higher final pyruvate concentration and lower biomass concentration, 14.4 g pyruvate was generated for each 1 g of biomass.
DISCUSSION
E. coli generates pyruvate aerobically primarily through the Embden-Meyerhof-Parnas pathway and the phosphoenolpyruvate:carbohydrate phosphotransferase system (PEP:PTS). Pyruvate is metabolized by numerous pathways, including pyruvate dehydrogenase, pyruvate formate lyase, pyruvate oxidase, PEP synthase, and lactate dehydrogenase. A strain with knockouts in these pathways accumulates pyruvate as the main product (48). In order to increase pyruvate yield from glucose, cell growth must be limited and excess glucose supplied. An exponential fed-batch process is ideal to limit growth at a constant rate, reducing the aeration demand and offering controlled growth conditions (4, 21). We selected the unoptimized growth rate of 0.15 h−1 to focus our study of the effects of nutrient limitation and genetic perturbations on glycolytic flux and pyruvate yield.
In the present study, we found that limiting acetate resulted in the highest rate of pyruvate accumulation among four limiting nutrients examined. Glucose limitation would be expected to limit glycolysis directly, and in our study, this operational mode resulted in the lowest rate of pyruvate formation. Acetate-limited growth also led to a nearly twofold-greater rate of glucose consumption and pyruvate accumulation than phosphorus or nitrogen limitation. Strain ALS929 is distinct from wild-type E. coli because it is unable to synthesize the essential biomass precursor acetyl-CoA from pyruvate, and therefore, the strains used in this study require acetate in the medium for the synthesis of acetyl-CoA via acetyl-CoA synthase (Fig. 1). When the availability of glucose, nitrogen, or phosphorus limits growth, acetate and, presumably, acetyl-CoA are therefore always in excess. These two compounds are known to affect E. coli in a variety of ways, including the inhibition of phosphoglucomutase (13, 29), both NAD+- and NADP+-dependent malic enzymes (27, 28, 36), malonyl-CoA-ACP transacylase (18), and aldose-1-epimerase (41). Acetate also induces the RpoS-mediated stress response (1, 30) and notably downregulates the expression of ptsG, encoding the key protein in glucose uptake (1). Malic enzyme particularly may serve an important role in strain ALS929 as one remaining link between the TCA cycle and glycolysis (51). These numerous factors which would likely occur during glucose-, nitrogen-, or phosphorus-limited conditions provide explanations for the comparatively elevated rate of glucose consumption under acetate limitation. Typically in wild-type E. coli, excess glucose leads to excess acetyl-CoA and acetate “overflow” (16, 43). Because acetate formation is uniquely decoupled from glycolysis in this strain, ALS929 in acetate-limited conditions may be useful for future studies of glucose utilization under excess-glucose conditions in the absence of overflow metabolism.
We speculate that the reduced pyruvate yield under phosphorus-limited conditions (compared to that under nitrogen-limited conditions) may also be a result of very strong uncoupling of anabolism and catabolism in cultures with excess carbon which limits glycolysis in favor of the less energy-efficient pentose phosphate pathway (12). For Bacillus subtilis cells, the oxidative pentose phosphate pathway flux exceeded 50% of the total glucose flux in a slow-growing (0.10 h−1) phosphorus-limited chemostat (12). In E. coli cells, the gnd gene encoding the pentose phosphate pathway enzyme phosphogluconate dehydrogenase, which releases CO2, was upregulated fivefold under phosphorus-limited conditions (39). During the conversion of one mole glucose to pyruvate, the Embden-Meyerhof-Parnas pathway alone would generate two ATP (and therefore require two Pi), while the exclusive flux of carbon through the pentose phosphate pathway would generate 1.67 ATP and require 1.67 Pi. In the current study, the high CO2 evolution rate and low pyruvate yield observed during phosphorus-limited conditions is consistent with a higher carbon flux through the pentose phosphate pathway. Our results therefore similarly support the conclusion that E. coli responds to phosphate limitation by favoring the pentose phosphate pathway, a path which consumes 17% less phosphate than glycolysis. Of course, phosphate limitation could globally reduce the concentrations of phosphorylated intermediates, as well as ATP and ADP (12).
Given acetate-limited conditions, genetic modifications also increased glycolytic flux while minimizing biomass formation. The general conclusion from the results of previous studies is that glycolytic flux control primarily resides outside the glycolytic pathway. For example, glycolytic flux is enhanced by decreasing the cell's energy level through overexpressing the F1 subunit or disrupting the F0 subunit of (F1F0) H+-ATP synthase complex (8, 19, 23). Moreover, overexpression of enzyme IIGlc of the PTS did not increase the flux in E. coli (26). The (F1F0) H+-ATP synthase complex consists of two subunits, the membrane-bound F0, which forms a proton channel, and the cytoplasmic F1, which contains the catalytic site for ATP formation (19). The whole enzyme complex catalyzes ATP synthesis by utilizing the energy of the transmembrane electrochemical potential of a proton, while the soluble F1 subunit can hydrolyze ATP in vitro independently of the F0 subunit (24). The deletion of subunits b and c of F0 (encoded by atpFH) inactivated F0 and resulted in the separation of the F1 sector from the membrane (7, 31). Therefore, ATP synthesis by oxidative phosphorylation through F0 was disrupted while the hydrolytic activity of the F1 subunit in the cytoplasm was preserved. The deletion of atpFH increases the availability of ADP and AMP (8), which respectively activate phosphofructokinase-I and pyruvate kinase-II in E. coli (5, 20). Consistent with the results of previous studies (8, 19), our results show that the (F1F0) H+-ATP synthase complex mutation (ΔatpFH) significantly increased the steady-state specific glucose consumption and pyruvate production rates while reducing the cell yield (Table 3).
NADH is another global cofactor which affects the physiological state. Acetate overflow metabolism commences when the NADH/NAD+ ratio reaches a threshold value, and overflow can be reduced by increasing the NADH oxidation rate (40). A high NADH/NAD+ ratio limits the availability of NAD+ required for glycolysis, since the total NAD pool is normally constant (42), and NADH inhibits several enzymes in glycolysis allosterically (8, 40). Our chemostat results show that the introduction of NADH oxidase with or without the arcA deletion had little effect on the specific glucose consumption rate and pyruvate productivity in strain ALS929, suggesting that respiratory processes under the control of the ArcA system and NADH/NAD+ ratio (40) did not limit glycolysis in ALS929. However, when the glycolytic rate was substantially increased as a result of the atpFH arcA double mutation (strain ALS1059), the introduction of NADH oxidase did further improve glycolytic flux. The higher rate of NADH generation in ALS1059 may cause NAD+ availability to limit glycolytic flux. Although the CO2 generated always represented less than 4% of the carbon consumed, it remains unclear why the atpFH arcA mutant ALS1059 generated CO2 more quickly than either strain with only one or the other of these mutations (ALS1054 or ALS1058) or more quickly than strain ALS1059/pTrc99A-nox.
Although the fed-batch process using ALS929 at a low constant growth rate achieved a high specific pyruvate generation rate, cell growth ceased when pyruvate reached about 40 g/liter, with pyruvate ultimately reaching 56.3 g/liter. Previous studies proposed that cell growth and pyruvate formation were inhibited by a high extracellular pyruvate concentration (48, 49). Another explanation for the observed termination of cell growth and pyruvate formation is the high osmotic stress caused by the K+ ion required to maintain the pH. Organic osmolytes, such as betaine, often accumulate naturally to protect cells from high osmotic stress (44), and the production of ethanol (38) and lactate (50) has been improved by the addition of betaine to the medium. Our fed-batch results similarly demonstrated that 5 mM betaine prolonged cell growth and thereby increased the final pyruvate concentration by at least 25%. The 1 M pyruvate concentration ultimately attained in this study implies that in previous studies, pyruvate generation was not limited by pyruvate.
In summary, limiting cell growth by limiting acetate provides a convenient means to sustain a high rate of glycolysis in a metabolically engineered E. coli strain blocked in pyruvate metabolism. The specific glucose consumption rate was further elevated by the combination of atpFH and arcA knockouts, with heterologous NADH oxidase providing only a modest further increase in the glycolytic rate in the double-knockout strain. We report the highest pyruvate concentration achieved using E. coli in defined medium, 90 g/liter, with an overall productivity of 2.1 g/liter · h and yield of 0.68 g pyruvate/g glucose.
Acknowledgments
We acknowledge the financial support of the U.S. Department of Agriculture NRI program (2003-35504-13666) and the Georgia Experiment Station.
We thank John E. Cronan, Jr., for E. coli strain YYC202 and Sarah Lee for technical assistance.
Footnotes
Published ahead of print on 19 September 2008.
REFERENCES
- 1.Arnold, C. N., J. McElhanon, A. Lee, R. Leonhart, and D. A. Siegele. 2001. Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J. Bacteriol. 183:2178-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atsumi, S., T. Hanai, and J. C. Liao. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86-90. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura, M. Baba, K. A. Datsenko, M. Tomita, B. L. Wanner, and H. Mori. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babaeipour, V., S. A. Shojaosadati, R. Khalilzadeh, N. Maghsoudi, and F. Tabandeh. 2008. A proposed feeding strategy for the overproduction of recombinant proteins in Escherichia coli. Biotechnol. Appl. Biochem. 49:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Babul, J. 1978. Phosphofructokinases from Escherichia coli. J. Biol. Chem. 253:4350-4355. [PubMed] [Google Scholar]
- 6.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 142:187-195. [DOI] [PubMed] [Google Scholar]
- 7.Causey, T. B., S. Zhou, K. T. Shanmugam, and L. O. Ingram. 2003. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc. Natl. Acad. Sci. USA 100:825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Causey, T. B., K. T. Shanmugam, L. P. Yomano, and L. O. Ingram. 2004. Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc. Natl. Acad. Sci. USA 101:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 10.Cotellessa, C., T. Manunta, I. Ghersetich, B. Brazzini, and K. Perist. 2004. The use of pyruvic acid in the treatment of acne. J. Eur. Acad. Dermatol. Venereol. 18:275-278. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauner, M., T. Storni, and U. Sauer. 2001. Bacillus subtilis metabolism and energetics in carbon-limited and excess-carbon chemostat culture. J. Bacteriol. 183:7308-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duckworth, H. W., B. H. Barber, and B. D. Sanwal. 1973. The interaction of phosphoglucomutase with nucleotide inhibitors. J. Biol. Chem. 248:1431-1435. [PubMed] [Google Scholar]
- 14.Eiteman, M. A., and M. J. Chastain. 1997. Optimization of the ion-exchange analysis of organic acids from fermentation. Anal. Chim. Acta 338:69-75. [Google Scholar]
- 15.Georgellis, D., O. Kwon, and E. C. C. Lin. 2001. Quinones as the redox signal for the Arc two-component system of bacteria. Science 292:2314-2316. [DOI] [PubMed] [Google Scholar]
- 16.Holms, H. 1996. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol. Rev. 19:85-116. [DOI] [PubMed] [Google Scholar]
- 17.Iuchi, S., A. Aistarkhov, J. M. Dong, J. S. Taylor, and E. C. C. Lin. 1994. Effects of nitrate respiration on expression of the Arc-controlled operons encoding succinate dehydrogenase and flavin-linked l-lactate dehydrogenase. J. Bacteriol. 176:1695-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi, V. C., and S. J. Wakil. 1971. Studies on the mechanism of fatty acid synthesis. XXVI. Purification and properties of malonyl-coenzyme A-acyl carrier protein transacetylase of Escherichia coli. Arch. Biochem. Biophys. 143:493-505. [DOI] [PubMed] [Google Scholar]
- 19.Koebmann, B. J., H. V. Westerhoff, J. L. Snoep, D. Nilsson, and P. R. Jensen. 2002. The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J. Bacteriol. 184:3909-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotlarz, D., Garreau, H., and H. Buc. 1975. Regulation of the amount and of the activity of phosphofructokinases and pyruvate kinases in Escherichia coli. Biochim. Biophys. Acta 381:257-268. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J., S. Y. Lee, S. Park, and A. P. Middelberg. 1999. Control of fed-batch fermentations. Biotechnol. Adv. 17:29-48. [DOI] [PubMed] [Google Scholar]
- 22.Li, Y., J. Chen, and S. Y. Lun. 2001. Biotechnological production of pyruvic acid. Appl. Microbiol. Biotechnol. 57:451-459. [DOI] [PubMed] [Google Scholar]
- 23.Oliver, S. 2002. Metabolism: demand management in cells. Nature 418:33-34. [DOI] [PubMed] [Google Scholar]
- 24.Ono, S., N. Sone, M. Yoshida, and T. Suzuki. 2004. ATP synthase that lacks F0a-subunit: isolation, properties, and indication of F0b2-subunits as an anchor rail of a rotating c-ring. J. Biol. Chem. 279:33409-33412. [DOI] [PubMed] [Google Scholar]
- 25.Roufs, J. B. 1996. Pyruvate: does it amp endurance and burn more fat? Muscle Fitness 57:195-197. [Google Scholar]
- 26.Ruyter, G. J. G., P. W. Postma, and K. Dam. 1991. Control of glucose metabolism by enzyme IIGlc of the phosphoenolpyruvate-dependent phosphotransferase system in Escherichia coli. J. Bacteriol. 173:6184-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanwal, B. D., J. A. Wright, and R. Smando. 1968. Allosteric control of the activity of malic enzyme in Escherichia coli. Biochem. Biophys. Commun. 31:623-627. [DOI] [PubMed] [Google Scholar]
- 28.Sanwal, B. D. 1970. Regulatory characteristics of the diphosphopyridine nucleotide-specific malic enzyme of Escherichia coli. J. Biol. Chem. 245:1212-1216. [PubMed] [Google Scholar]
- 29.Sanwal, B. D., H. W. Duckworth, and M. L. Hollier. 1972. Regulation of phosphoglucomutase. Proc. Biochem. Soc. 128:26P-27P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schellhorn, H. E., and V. L. Stones. 1992. Regulation of katF and katE in Escherichia coli K-12 by weak acids. J. Bacteriol. 174:4769-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorgen, P. L., T. L. Caviston, R. C. Perry, and B. D. Cain. 1998. Deletions in the second stalk of F1F0-ATP synthase in Escherichia coli. J. Biol. Chem. 273:27873-27878. [DOI] [PubMed] [Google Scholar]
- 32.Stanko, R. T., R. J. Robertson, R. W. Galbreath, J. J. Reilly, K. D. Greenawalt, and F. L. Goss. 1990. Enhanced leg exercise endurance with a high-carbohydrate diet and dihydroxyacetone and pyruvate. J. Appl. Physiol. 69:1651-1656. [DOI] [PubMed] [Google Scholar]
- 33.Stanko, R. T., D. L. Tietze, and J. E. Arch. 1992. body-composition, energy-utilization, and nitrogen-metabolism with a 4.25-Mj/D low-energy diet supplemented with pyruvate. Am. J. Clin. Nutr. 56:630-635. [DOI] [PubMed] [Google Scholar]
- 34.Stanko, R. T., D. L. Tietze, and J. E. Arch. 1992. Body-composition, energy-utilization, and nitrogen-metabolism with a severely restricted diet supplemented with dihydroxyacetone and pyruvate. Am. J. Clin. Nutr. 55:771-776. [DOI] [PubMed] [Google Scholar]
- 35.Stanko, R. T., H. R. Reynolds, R. Hoyson, J. E. Janosky, and R. Wolf. 1994. Pyruvate supplementation of a low-cholesterol, low-fat diet: effects on plasma-lipid concentrations and body-composition in hyperlipidemic patients. Am. J. Clin. Nutr. 59:423-427. [DOI] [PubMed] [Google Scholar]
- 36.Takeo, K., T. Murai, J. Nagai, and H. Katsuki. 1967. Allosteric activation of DPN-linked malic enzyme from Escherichia coli by aspartate. Biochem. Biophys. Res. Commun. 29:717-722. [DOI] [PubMed] [Google Scholar]
- 37.Tomar, A., M. A. Eiteman, and E. Altman. 2003. The effect of acetate pathway mutations on the production of pyruvate in Escherichia coli. Appl. Microbiol. Biotechnol. 62:76-82. [DOI] [PubMed] [Google Scholar]
- 38.Underwood, S. A., M. L. Buszko, K. T. Shanmugam, and L. O. Ingram. 2004. Lack of protective osmolytes limits final cell density and volumetric productivity of ethanologenic Escherichia coli KO11 during xylose fermentation. Appl. Environ. Microbiol. 70:2734-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanBogelen, R. A., E. R. Olson, B. L. Wanner, and F. C. Neidhardt. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 178:4344-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vemuri, G. N., E. Altman, D. P. Sangurdekar, A. B. Khodursky, and M. A. Eiteman. 2006. Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl. Environ. Microbiol. 72:3653-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallenfels, K., F. Hucho, and K. Herrmann. 1965. Die enzymatisch katalysierte Mutarotation der Aldosen: untersuchungen an der Aldose-1-epimerase aus E. coli. Biochem. Z. 343:307-325. [PubMed] [Google Scholar]
- 42.Wimpenny, J. W., and A. Firth. 1972. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J. Bacteriol. 111:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yancey, P. H. 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 208:2819-2830. [DOI] [PubMed] [Google Scholar]
- 45.Yokota, A., H. Shimizu, Y. Terasawa, N. Takaoka, and F. Tomita. 1994. Pyruvic acid production by a lipoic acid auxotroph of Escherichia coli W1485. Appl. Microbiol. Biotechnol. 41:638-643. [DOI] [PubMed] [Google Scholar]
- 46.Yokota, A., Y. Terasawa, N. Takaoka, H. Shimizu, and F. Tomita. 1994. Pyruvic-acid production by an F-1-ATPase-defective mutant of Escherichia coli W1485lip2. Biosci. Biotechnol. Biochem. 58:2164-2167. [DOI] [PubMed] [Google Scholar]
- 47.Yu, D., H. M. Ellis, E.-C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelic, B., T. Gerharz, M. Bott, D. Vasič-Racki, C. Wandrey, and R. Takors. 2003. Fed-batch process for pyruvate production by recombinant Escherichia coli YYC202 strain. Eng. Life Sci. 3:299-305. [Google Scholar]
- 49.Zelic, B., S. Gostovic, K. Vuorilehto, B. Vasic-Racki, and R. Takors. 2004. Process strategies to enhance pyruvate production with recombinant Escherichia coli: from repetitive fed-batch to in situ product recovery with fully integrated electrodialysis. Biotechnol. Bioeng. 85:638-646. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, S., T. B. Grabar, K. T. Shanmugam, and L. O. Ingram. 2006. Betaine tripled the volumetric productivity of D(−)-lactate by Escherichia coli strain SZ132 in mineral salts medium. Biotechnol. Lett. 28:671-676. [DOI] [PubMed] [Google Scholar]
- 51.Zhu, Y., M. A. Eiteman, K. DeWitt, and E. Altman. 2007. Homolactate fermentation by metabolically engineered Escherichia coli strains. Appl. Environ. Microbiol. 73:456-464. [DOI] [PMC free article] [PubMed] [Google Scholar]