Abstract
We describe a novel, rapid, and safe method for extracting RNA and DNA from refractory microbes, which avoids the use of phenol or chloroform. It has been used successfully to isolate high-quality nucleic acids from pure cultures and environmental populations known to resist widely used extraction protocols.
Critical to the success of culture-independent analyses of natural communities is the extraction of high-quality nucleic acids (2, 16). Although often ignored, the method used for RNA/DNA extraction can be an important source of bias, as only organisms susceptible to the method selected are detectable (16). In some instances, the choice of inappropriate methods may result in even the dominant microbial populations being missed in subsequent analyses (1, 12, 17, 18). To overcome this problem, laborious and hazardous protocols requiring phenol and chloroform combined with physical disruption such as bead beating have been used (4, 7, 11). Here we describe a rapid and safe method for the coextraction of RNA and DNA that avoids the use of enzymes, chloroform, or phenol.
Our method employs the strongest known chaotrophic agent, sodium trichloroacetate (NaTCA) (8). NaTCA was used by Summerton et al. (15) to isolate plasmid DNA but to our knowledge has never been used to extract nucleic acids from natural microbial communities. We reasoned that NaTCA in combination with physical disruption may provide high-quality DNA and RNA from difficult samples without the need for phenol or chloroform.
Our optimized method is as follows. A stock solution of 4.5 M NaTCA is prepared according to the method of Summerton et al. (15). Briefly, sufficient sterile distilled water is added to 367.63 g TCA to allow its magnetic stirring on ice. Ten molar NaOH is added slowly, dropwise (ensuring that the solution remains below 50°C), until the pH is ∼7. The final volume is adjusted to 500 ml (4.5 M NaTCA) with water and stored at −70°C, as NaTCA degrades at 4°C. Cell biomass (10 to 15 mg) is collected by centrifugation (4,000 × g for 10 min at 4°C) in standard 2-ml microcentrifuge tubes, and the supernatant discarded. The pelleted biomass is resuspended in 1.5 ml TCA lysis buffer (3 M NaTCA, 50 mM Tris-HCl, pH 8.0, 15 mM EDTA, pH 8.0, 1% [wt/vol] N-lauroylsarcosine, 1% [wt/vol] polyvinylpyrrolidone, 10 mM dithiothreitol), and 0.6 g Ballotini beads (0.1 mm) are added. Samples are homogenized with a bead beater (Biospec, Bartlesville, OK) at maximum speed for 1 to 3 min. The optimal beating time is chosen based on the yield and integrity of nucleic acids as assessed by their appearance on agarose electrophoresis gels (Fig. 1). A time of 3 min was selected here for activated sludge samples based on successful isolation and PCR of DNA from cluster II Defluviicoccus organisms. Samples are centrifuged (14,000 × g for 5 min at 4°C) to remove cell debris, and supernatants carefully transferred to new 1.5-ml microcentrifuge tubes containing 0.6 volume of 2-propanol. After being mixed, the samples are incubated on ice (20 min) to precipitate nucleic acids. The tubes are centrifuged for 20 min at 14,000 × g, the supernatants removed, and the pellets washed two times with 70% ethanol, air dried for 10 min, and resuspended in 40 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0).
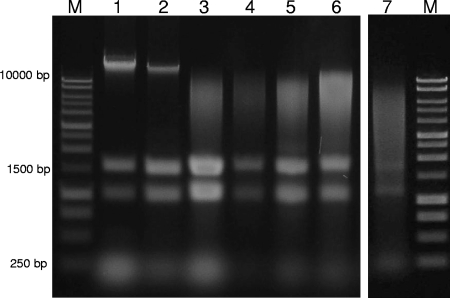
FIG. 1.
Gel electrophoresis (1% agarose, 1× TAE) of the total nucleic acid extracted from different organisms using the NaTCA method (2.5-μl load). Lanes: M, 1-kb DNA ladder (catalog no. G5711; Promega); 1, E. coli (no bead beating); 2, P. aeruginosa (no bead beating); 3, M. luteus (1 min of bead beating); 4, B. subtilis (1.5 of min bead beating); 5, M. phlei (1 min of bead beating); 6, M. smegmatis (1 min of bead beating); 7, aerobic laboratory scale reactor (3 min of bead beating).
This method isolated DNA and RNA simultaneously from a range of gram-negative and -positive bacteria, including Escherichia coli, Pseudomonas aeruginosa, Micrococcus luteus, Bacillus subtilis, Mycobacterium phlei, and Mycobacterium smegmatis, the latter two being recalcitrant acid-fast organisms. Bead beating was essential for gram-positive strains but proved to be optional for gram-negative strains (Fig. 1). Some DNA pellets were gelatinous, resulting possibly from high levels of polymeric material present in some biomasses (13). Our attempts to avoid this by modifying the precipitation protocol proved unsuccessful. However, gelatinous material could be removed by resuspending the nucleic acids (20 min at 37°C or 4°C overnight), centrifuging for 30 s, and transferring the supernatants to fresh tubes.
This method was also tested on complex samples from four phosphorus-removing activated-sludge plants (two laboratory scale and two full scale). Initial difficulties were encountered in disrupting the large flocs present due to foaming during the bead beating step. This was overcome by incorporating a silicone-based antifoam (1.67% [vol/vol]) (product no. 1520-US; Dow Corning) in the lysis buffer. Nucleic acids isolated by this method proved sufficiently free of inhibitory substances for successful performance of PCR, reverse transcription-PCR, real-time PCR, and restriction digestion (data not shown). When samples were incubated for 2 h at 37°C in 1× EcoRI restriction enzyme buffer (Roche, Basel, Switzerland), no DNase activity was detected (data not shown). Furthermore, a comparison of the results of our method with those of eight different published methods and kits developed for difficult environmental samples showed equal or better DNA and RNA yields (S. McIlroy, K. Porter, S. Schroeder, R. J. Seviour, and D. Tillett, unpublished data).
Real-time PCR data (McIlroy et al., unpublished) confirmed that this protocol extracted DNA from “Candidatus Accumulibacter phosphatis” and Defluviicoccus-related organisms, both of which can form heavily capsulated clustered cells. Their RNA has been difficult to recover in 16S rRNA gene clone libraries (1, 12). Semiquantitative fluorescence in situ hybridization analyses of these populations (6) gave data that corresponded well with data obtained by quantitative PCR, suggesting that our method does not introduce significant extraction bias. Real-time PCR results for Defluviicoccus-related organisms were 11.4% ± 2.8% (mean ± standard error) of total 16S rRNA genes and for “Candidatus Accumulibacter phosphatis” were 66.3% ± 14.4%. The universal primers used were 1492R and 1369F (10); the primers for Defluviicoccus were 518F (14) and DEF1020R (12); and the primers for “Candidatus Accumulibacter” were PAO462F and PAO651R (5). Semiquantitative fluorescence in situ hybridization results for Defluviicoccus-related organisms showed that 4.1% ± 0.6% of the total biovolume hybridizing with the EUBmix probes (5a) also hybridized with the DF1020 probe (12) and that 67.7% ± 3.0% hybridized with the PAOmix probes designed to target “Candidatus Accumulibacter phosphatis” (5, 19). Furthermore, comparisons with a range of phenol-based extraction methods (3, 4, 7, 11) with activated-sludge samples showed that our method was equal to or better than these as assessed by PCR targeting the same problematic populations (McIlroy et al., unpublished). Increased safety and a reduced number of pipetting steps make the method suitable for high-throughput analysis. Consequently, it should prove valuable in molecular microbial ecology where current extraction protocols can fail to recover sequences from recalcitrant populations in clone libraries (1, 9, 12, 16).
Acknowledgments
This research was funded by Australian Research Council Discovery (DP0557646DS) and Victorian State Government Smart Water grants. S. McIlroy was supported by an Australian Postgraduate Award.
We thank Johwan Ahn for his technical assistance.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Ahn, J., S. Schroeder, M. Beer, S. McIlroy, R. C. Bayly, J. W. May, G. Vasiliadis, and R. J. Seviour. 2007. Ecology of the microbial community removing phosphate from wastewater under continuously aerobic conditions in a sequencing batch reactor. Appl. Environ. Microbiol. 73:2257-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corgié, S. C., T. Beguiristain, and C. Leyval. 2006. Profiling 16S bacterial DNA and RNA: difference between community structure and transcriptional activity in phenanthrene polluted sand in the vicinity of plant roots. Soil Biol. Biochem. 38:1545-1553. [Google Scholar]
- 4.Costa, R., N. C. M. Gomes, A. Milling, and K. Smalla. 2004. An optimized protocol for simultaneous extraction of DNA and RNA from soils. Braz. J. Microbiol. 35:230-234. [Google Scholar]
- 5.Crocetti, G. R., P. Hugenholtz, P. L. Bond, A. Schuler, J. Keller, D. Jenkins, and L. L. Blackall. 2000. Identification of polyphosphate-accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl. Environ. Microbiol. 66:1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Daims, H., A. Brühl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 6.Daims, H., K. Stoecker, and M. Wagner. 2005. Fluorescence in situ hybridization for the detection of prokaryotes, p. 213-239. In A. M. Osborn and C. J. Smith (ed.), Molecular microbial ecology. Taylor & Francis, New York, NY.
- 7.Griffiths, R., A. Whiteley, A. O'Donnell, and M. Bailey. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamaguchi, K., and E. Geiduschek. 1962. The effect of electrolytes on the stability of the deoxyribonucleate helix. J. Am. Chem. Soc. 84:1329-1338. [Google Scholar]
- 9.Juretschko, S., A. Loy, A. Lehner, and M. Wagner. 2002. The microbial community composition of a nitrifying-denitrifying activated sludge from an industrial sewage treatment plant analyzed by the full-cycle rRNA approach. Syst. Appl. Microbiol. 25:84-99. [DOI] [PubMed] [Google Scholar]
- 10.Lane, D. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Modern microbial methods: nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 11.McVeigh, H., J. Munro, and T. Embley. 1996. Molecular evidence for the presence of novel actinomycete lineages in a temperate forest soil. J. Ind. Microbiol. Biotechnol. 17:197-204. [Google Scholar]
- 12.Meyer, R. L., A. M. Saunders, and L. L. Blackall. 2006. Putative glycogen-accumulating organisms belonging to the Alphaproteobacteria identified through rRNA-based stable isotope probing. Microbiology 152:419-429. [DOI] [PubMed] [Google Scholar]
- 13.Moore, E., A. Arnscheidt, A. Krüger, C. Strömpl, and M. Mau. 2004. Simplified protocols for the preparation of genomic DNA from bacterial cultures, p. 1.01. In G. Kowalchuk, F. J. De Bruijn, I. Head, A. D. L. Akkermans, and J. D. van Elsas (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, London, England.
- 14.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summerton, J., T. Atkins, and R. Bestwick. 1983. A rapid method for preparation of bacterial plasmids. Anal. Biochem. 133:79-84. [DOI] [PubMed] [Google Scholar]
- 16.von Wintzingerode, F., U. B. Göbel, and E. Stackebrandt. 1997. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 21:213-229. [DOI] [PubMed] [Google Scholar]
- 17.Wallner, G., B. Fuchs, S. Spring, W. Beisker, and R. Amann. 1997. Flow sorting of microorganisms for molecular analysis. Appl. Environ. Microbiol. 63:4223-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong, M.-T., F. M. Tan, W. J. Ng, and W.-T. Liu. 2004. Identification and occurrence of tetrad-forming Alphaproteobacteria in anaerobic-aerobic activated sludge processes. Microbiology 150:3741-3748. [DOI] [PubMed] [Google Scholar]
- 19.Zilles, J., J. Peccia, M. Kim, C. Hung, and D. Noguera. 2002. Involvement of Rhodocyclus-related organisms in phosphorus removal in full-scale wastewater treatment plants. Appl. Environ. Microbiol. 68:2763-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]