Abstract
Impact-induced ejections of rocks from planetary surfaces are frequent events in the early history of the terrestrial planets and have been considered as a possible first step in the potential interplanetary transfer of microorganisms. Spores of Bacillus subtilis were used as a model system to study the effects of a simulated impact-caused ejection on rock-colonizing microorganisms using a high-explosive plane wave setup. Embedded in different types of rock material, spores were subjected to extremely high shock pressures (5 to 50 GPa) lasting for fractions of microseconds to seconds. Nearly exponential pressure response curves were obtained for spore survival and linear dependency for the induction of sporulation-defective mutants. Spores of strains defective in major small, acid-soluble spore proteins (SASP) (α/β-type SASP) that largely protect the spore DNA and spores of strains deficient in nonhomologous-end-joining DNA repair were significantly more sensitive to the applied shock pressure than were wild-type spores. These results indicate that DNA may be the sensitive target of spores exposed to ultrahigh shock pressures. To assess the nature of the critical physical parameter responsible for spore inactivation by ultrahigh shock pressures, the resulting peak temperature was varied by lowering the preshock temperature, changing the rock composition and porosity, or increasing the water content of the samples. Increased peak temperatures led to increased spore inactivation and reduced mutation rates. The data suggested that besides the potential mechanical stress exerted by the shock pressure, the accompanying high peak temperatures were a critical stress parameter that spores had to cope with.
Endospores of Bacillus subtilis are highly resistant to inactivation by environmental stresses such as biocidal agents and toxic chemicals, desiccation, pressure and temperature extremes, and high doses of UV and ionizing radiation (reviewed in references 36, 37, and 57). The reason for the high resistance of bacterial spores to environmental extremes lies in the structure of the spore. Spores possess thick layers of highly cross-linked coat proteins (11), a modified peptidoglycan spore cortex, abundant intracellular constituents such as the calcium chelate of dipicolinic acid, and small, acid-soluble spore proteins (SASP) (α/β-type SASP) as protectants of spore DNA (50, 51). During germination of spores, accumulated DNA damage is efficiently repaired, e.g., UV-induced spore photoproducts by spore photoproduct lyase, DNA double-strand breaks (DSB) by nonhomologous-end joining (NHEJ), and oxidative stress-induced apurinic/apyrimidinic sites by apurinic/apyrimidinic endonucleases (23, 29, 32, 33, 35, 45, 56).
Spores of B. subtilis have been used as biological dosimeters for terrestrial and extraterrestrial UV radiation (18, 43) and in outer space for astrobiological studies of the likelihood of the interplanetary transfer of life (15-17, 19, 20, 36). The latter scenario, first formulated as “panspermia” by Arrhenius (1), has recently been revisited after the discovery of 44 lunar meteorites (44) and about 40 Martian meteorites (14, 27) on Earth, which have provided evidence that rocks may naturally be transferred between the terrestrial planets. Those lunar and Martian meteorites have been ejected from their parent bodies following impacts of asteroids or comets (26, 28). Petrographic studies of shock metamorphism of Martian meteorites and numerical simulations of the impact-induced ejection of Martian rocks beyond the escape velocity of Mars have demonstrated a launch window for Martian meteorites of between about 5 to 10 GPa and about 55 GPa (2, 14). The recognition of high numbers of microorganisms inhabiting the Earth's crust (3, 13, 40) has provided additional support to the impact-driven scenario of panspermia, now termed “lithopanspermia” (21).
The scenario of lithopanspermia involves three basic hypothetical steps with which microorganisms have to cope: (i) escape from the planet of origin, (ii) wandering through the solar system, and (iii) capture by another planet and atmospheric entry. Several studies have dealt with the survival of bacterial spores in outer space and during hypervelocity atmospheric entry (12, 15, 16, 57; reviewed in reference 36). In shock recovery experiments based on the so-called reverberation technique (53), a systematic simulation of the impact ejection scenario was achieved (21, 54). For spores of B. subtilis sandwiched between two layers of gabbro rock, which is highly similar to the rock composition of the Martian shergottite meteorites, a vital launch window ranging from 5 to about 40 GPa has been determined.
However, so far, little is known about the influence of the host rock material and the initial humidity on spore survival and also about the mechanisms involved in the resistance of B. subtilis spores to the simulated hypervelocity impact. In addition, the preshock temperature determines the magnitude of the peak shock temperature and postshock temperature, which may affect spore survival.
In this article, we report a realistic and high-precision simulation of a hypervelocity impact ejection from the surface of a planet using spores of B. subtilis in different arrangements with their host rock: (i) sandwiched between thin layers of nonporous igneous rock (gabbro or dunite), (ii) embedded in a porous rock (i.e., sandstone), or (iii) mixed with rock and soil powder (halite or artificial Martian regolith). A low preshock temperature of about −80°C was applied for simulating Martian surface temperature (27). To obtain insight into mechanisms of spore resistance to hypervelocity impacts, strains deficient in DNA-protecting α/β-type SASP or in NHEJ DNA repair were studied. Besides survival as the colony-forming ability, the mutagenicity of the treatment was studied with regard to the occurrence of asporogenous mutants (Spo−) in the surviving fraction.
MATERIALS AND METHODS
Bacillus subtilis spores, sporulation, purification, and sample preparation.
Endospores of three different Bacillus subtilis 168-derived strains were used in this work: RM01, a trpC2 strain with resistance to rifampin (carrying a point mutation causing a change in amino acids Q469L in rpoB) (R. Moeller, unpublished data) in the following called wild-type strain; PS3722, deficient in DNA damage repair capacity by NHEJ (ykoU ykoV) (56); and PS356, lacking two major DNA-protective α/β-type SASP (encoded by sspA and sspB) (32). Strains PS356 and PS3722 are prototrophic. They were a generous gift from Peter Setlow, University of Connecticut Health Center. Spores were obtained by cultivation under vigorous aeration in double-strength liquid Schaeffer sporulation medium (SSM) (46) under identical conditions for each strain, and the spores were purified and stored as described previously (30-33). Spore preparations were free (>99%) of growing cells, germinated spores, and cell debris, as determined by phase-contrast microscopy.
Spore exposure to ultrahigh shock pressure.
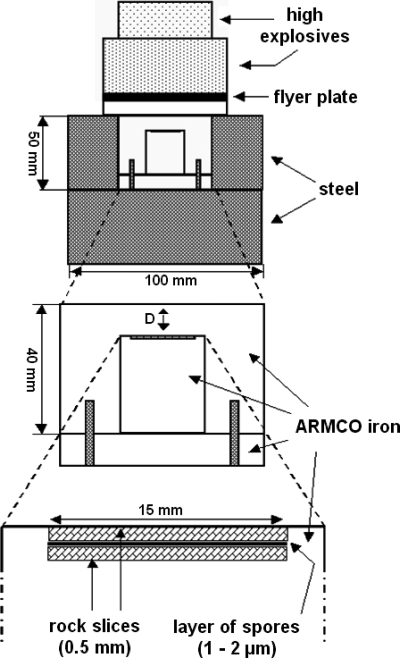
Test samples totaling 2 × 107 spores each were prepared in three alternative ways: (i) a spore suspension was spotted onto a 0.5-mm-thick disk of gabbro or dunite that was 15 mm in diameter, air dried, and covered by a spore-free layer of the same rock material as described in detail previously by Horneck et al. (20, 21); (ii) a spore suspension was added to a powder composed of artificial Martian regolith (MRS07) or rock salt (NaCl), air dried, and ground; and (iii) a spore suspension was soaked in porous sandstone and air dried. The mineralogical composition of the host materials used in this study is shown in Table 1. In ultrahigh shock pressure experiments using a high-explosive plane wave setup (Fig. 1), shock pressures of about 10, 20, 30, 40, or 50 GPa were systematically applied to the test samples as described in detail previously by Horneck et al. (20, 21) and Stöffler et al. (54). The selection of explosive materials used to generate the respective final ultrahigh shock pressures as well as further details on the setup of the individual parameters of the high-explosive device were previously reported (21). Experiments were conducted at room temperature (20°C) or at −78.5°C by using frozen carbon dioxide for sample cooling. The nominal shock pressures were calculated on the basis of the shock reverberation technique (53) and checked by refractive index measurements of particle grains from the shocked and recovered rock material (55). In the reverberation technique used in these experiments, the maximum shock pressure is achieved by a sequence of increasingly stronger shock waves reflected at the steel-rock interfaces in pulse durations of 1 to 2 μs (21). For each experiment, untreated control samples (mounted on the same host rock material) experienced the same sample preparation and processing as the test samples except for the shock pressure treatment. The shock pressure experiments were performed at the Fraunhofer Institute for High-Speed Dynamics, Ernst Mach Institute, Freiburg, Germany, under the aegis of DFG (German Research Foundation) research project RA 1049/1-2 (“Experimental Study of the Survival of Endolithic Microorganisms During Impact and Ejection of Martian Meteorites: First Phase of the Transfer of Life between Mars and Earth”) in the years 2005 to 2007. Further details on the experiment design and setup, reverberation technique, pressure calculation, and sample preparation were described previously by Stöffler and Langenhorst (53) and Horneck et al. (21).
TABLE 1.
Survival and sporulation deficiency rates of B. subtilis spores treated with ultrahigh shock pressure at 20°C when embedded in nonporous igneous and porous rock material or soil powderg
| Host rock material | Avg pressure (GPa) ± SD | Survival rate (avg ± SD) | Avg sporulation deficiency (%)f ± SD |
|---|---|---|---|
| Gabbro (solid rock)a | 32.0 ± 3.0 | (9.0 ± 4.7) × 10−4 | 7.7 ± 1.5 |
| Dry sandstone (solid rock)b | 27.0 ± 1.0 | (4.5 ± 1.6) × 10−3* | 4.1 ± 0.8 |
| Moisturized sandstone (solid rock)b | 29.0 ± 0.3 | (4.3 ± 2.3) × 10−4 | 2.9 ± 0.4 |
| Artificial Martian regolith MRS07 (powder)c | 32.5 ± 0.5 | (5.2 ± 1.6) × 10−4 | 8.2 ± 1.4 |
| Artificial rock salt (NaCl powder)d | 30.0 ± 0.5 | (7.3 ± 0.8) × 10−4 | 5.4 ± 1.2 |
| Gabbro (solid rock) | 42.0 ± 3.0 | (2.1 ± 0.9) × 10−4 | 9.3 ± 1.6 |
| Dunite (solid rock)e | 42.0 ± 7.0 | (6.5 ± 1.3) × 10−4* | 10.1 ± 2.9 |
Gabbro is an analogue for the relatively coarse-grained basaltic Martian shergottites meteorite (69% plagioclase, 27% ortho- and clinopyroxene, and traces of quartz) obtained from the Bushveld compley (eucritic basalts); the sample origin was South Africa.
Metamorphic sandstone was composed of 71% quartz and trace of plagioclase, with a porosity of 11% and pore size of 0.05 to 1.00 mm and complete recrystallization; the sample origin was Hesse, Germany. For the moisturization of sandstone, 100 μl of sterile distilled H2O was applied to the air-dried spore-sandstone assembly prior to exposure to ultrahigh shock pressure.
Powder composition of the artificial Martian regolith (MRS07) was 47.7% Na-montmorillonite, 9.9% kaolinite, 21.3% hematite (19.2% Fe2O3 with 1.3% SiO2), 13.0% anhydrite, 7.1% MgSO4, 1.0% NaCl, 2.5% Na2O, 3.4% MgO, 14.1% Al2O3, 34.6% SiO2, 5.1% SO3, 0.2% Cl−, 0.2% K2O, 6.1% CaO, 0.1% TiO, and 18.5% FeO, artificially composed according to the spectral data and information from the NASA Mars Exploration Rover Spirit and Opportunity (52) and OMEGA/Mars Express observations (4, 5).
Halite is composed of 99.5% NaCl (obtained from Merck KGaA, Darmstadt, Germany).
Dunite corresponds to the Martian chassignite meteorites; intrusive, 84% olivine, 11% othropyroxene, and minor phases of chromite, magnetite, ilmenite, and pyrrhotite. The sample origin was Aheim, Norway.
Sporulation deficiency was determined by visual inspection of the colony morphology (Fig. 4).
Asterisks indicate values that are significantly different (P ≤ 0.05) from those of the respective gabbro shock pressure experiments, as determined by multigroup pairwise combinations (Student's t test). Data are expressed as averages ± standard deviations.
FIG. 1.
Sketch of the high-explosive device used for shock recovery experiments in studies of the survival of rock-embedded B. subtilis spores after a simulated impact ejection. (Modified from reference 54 with permission from Elsevier.)
Survival assay.
To recover the spores of B. subtilis from the host rock slides after pressurization, spore monolayers were covered by a 10% aqueous polyvinyl alcohol (PVA) solution, and after drying the spore, the PVA layer was removed as described previously (32, 33) and was resuspended in 1 ml of sterile distilled water, resulting in >95% recovery of the spores. The PVA recovery method was repeated three times. This procedure has no geno- or cytotoxic effect on the spore viability (20). Spores mixed with powdered materials were directly resuspended in 10 ml of sterile distilled water, vortexed, and sonicated to ensure spore separation as described in detail previously by Brown et al. (8). Sandstone samples mixed with spores were ground until a homogenous powder was produced and then further treated as samples from powdered material. Spore survival was determined from appropriate dilutions in distilled water as the colony-forming ability after incubation overnight at 37°C on nutrient broth (NB) agar plates (Difco, Detroit, MI) as described previously (30-33). In order to control contamination, serial dilutions were plated onto NB agar plates containing rifampin (50-μg/ml final concentration) for RM01, chloramphenicol (3-μg/ml final concentration) for PS356, and lincomycin and erythromycin (25- and 1-μg/ml final concentrations, respectively) for PS3722.
Detection of sporulation deficiency.
To verify mutation induction caused by ultahigh shock pressure exposure, 200 B. subtilis colonies arising from each surviving spore fraction were picked onto SSM agar plates, supplied with the respective antibiotics, and incubated at 37°C for 7 days. Sporulation deficiency was determined by changes in the colony morphology and pigmentation. Sporulating colonies show definite brownish pigmentation after 7 days of incubation (22), whereas a decrease in pigmentation and appearance of translucence are characteristic for Spo− B. subtilis mutants as described previously by Piggot and Coote (41) and Fajardo-Cavazos et al. (12). Sporulation deficiency was expressed as the ratio of the number of translucent colonies (Spo−) to the total number of colonies (brownish-pigmented Spo+ plus translucent) that arose on SSM agar plates after 7 days of incubation. To verify the Spo− mutation, after growth for 3 to 4 days on NB agar plates, 10 Spo− colonies of each sample set (20°C and −78.5°C at 20, 30, and 40 GPa) were individually transferred into 5 ml of fresh SSM and incubated for 24 h at 37°C. Sporulation was then induced by diluting the culture grown overnight 1:100 into 5 ml of SSM medium, which lacked the sporulation inhibitor glucose. To determine the number of spores formed, the cultures were diluted after 24 h of cultivation and plated before and after heat shocking the culture (80°C for 10 min), as described in detail previously by Maughan et al. (25). Each analysis of sporulation deficiency was repeated at least three times.
Numerical and statistical analysis.
The surviving fraction of B. subtilis spores was determined from the quotient N/N0, where N is the number of CFU of the sample exposed to ultrahigh shock pressure and N0 is that of the nontreated controls. The frequency of induced Spo− mutations was determined by the quotient M/N − mS, where M is the total number of mutants of the pressure-exposed sample and mS is the rate of spontaneous mutations. The sporulation frequency of the induced asporogenous mutants was determined by dividing the titer after heat shock (spores) by the titer before heat shock (viable cells and spores). The data shown are expressed as averages ± standard deviations. The results were compared statistically using Student's t test. Values were analyzed in multigroup pairwise combinations, and differences with P values of ≤0.05 were considered to be statistically significant (21, 29-33).
RESULTS
To assess the survival of B. subtilis spores after short-term ultrahigh shock pressurization, air-dried spore monolayers at 20°C or −78.5°C were subjected to high shock pressures from 5 to 50 GPa, the range of shock pressures observed in Martian meteorites (14), by using a high-explosive plane wave setup (53). Inspection of the spores treated by shock pressure by phase-contrast microscopy showed that >99% of the spores were phase bright; i.e., germination was not induced by the treatment. This observation was further confirmed by testing the heat resistance of the treated sample: heat shocking (80°C for 10 min) did not change the CFU value of the sample. Spore resistance to extreme shock pressure was determined by plating pressure-treated and untreated spores. Genetic marker tests proved that all colonies were of the respective B. subtilis strain, which demonstrates that the samples exposed to ultrahigh shock pressure were not contaminated (20, 21).
Influence of preshock temperature on spore survival after exposure to ultrahigh shock pressures.
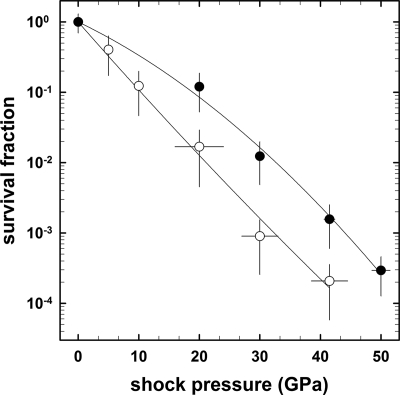
The absolute peak shock temperature depends on the initial preshock temperature of the samples (for details, see Fig. 18 in reference 21). To determine this temperature-dependent effect, shock pressure survival curves of spores of B. subtilis were obtained at preshock temperatures of 20°C (21) and at −78.5°C (Fig. 2). For both temperatures, the survival rate decreased nearly exponentially with increasing shock pressure. The survival curves were calculated using SigmaPlot 8.0 (Systat Software Inc., San Jose, CA). The regression factor (Pearson correlation coefficient) of a second-order regression amounted to an r value of 0.989 for 20°C and an r value of 0.992 for −78.5°C. It is interesting that at lower preshock temperatures, the survival rate was always higher than that at room temperature (20°C). For spores exposed to 50 GPa, the highest shock pressure applied, the survival rate dropped below the detection threshold of the initial spore burden of 2 × 107 spores if the preshock temperature was 20°C; in contrast, at a preshock temperature of −78.5°C, a survival rate of 3 × 10−4 was observed at the same shock pressure (Fig. 2).
FIG. 2.
Shock pressure survival curves of wild-type B. subtilis spores sandwiched between gabbro disks. Shown are data for preshock temperatures of −78.5°C (filled circles) and 20°C (open circles). Data are expressed as averages and standard deviations. Survival data for spores at a preshock temperature of 20°C were taken from data described previously by Horneck et al. (21). The variation of the error bars of 20, 30, and 40 GPa represents the variation of the shock pressure measured on the basis of the refractive index of plagioclase grains. For 5 and 10 GPa, the refractive indices were not measured. Otherwise, error bars for pressure data not shown were smaller than the symbol.
Calculations of the peak shock temperature have also shown that the temperature increases drastically with an increasing water content of the biological layer (21). Therefore, spores were embedded in sandstone and exposed to a shock pressure of about 30 GPa (20°C preshock temperature) either in a dry or in a moist state. The spores of the dry samples showed an approximately 10-times-higher survival rate than did the wet spores (Table 1).
Influence of host rock material on spore survival.
To study the influence of different host rock and soil material on the survival of spores of B. subtilis exposed to shock pressure, the spores were either sandwiched between thin layers of nonporous igneous rocks (i.e., gabbro or dunite), embedded in porous rock material (i.e., sandstone), or mixed with rock and soil powder (i.e., rock salt [halite or artificial Martian regolith]). All experiments were performed at a preshock temperature of 20°C. Gabbro as the host rock was used as analogue material for Martian shergottite meteorites, as previously reported by Horneck et al. (21). The survival rates of these gabbro samples were used as a basis for comparison with data obtained using other host material (Table 1). In some cases, host materials other than gabbro resulted in higher survival rates: spores sandwiched between thin layers of dunite, which was used as analogue material for the Martian chassignite meteorites, exhibited a 3.1-fold-higher survival rate after an exposure to about 40 GPa than did similarly treated spores sandwiched between gabbro. Air-dried spores in porous sandstone exposed to about 30 GPa showed a 5.0-fold-higher survival than did similarly treated gabbro samples. For other host materials, such as salt powder and artificial Martian regolith, the survival rates were not significantly different from those of similarly treated gabbro samples. The dependence of the survival rates on the host rock material is caused by the geological, physicochemical, and mineralogical properties of the host material, as will be elaborated in Discussion.
Role of NHEJ and α/β-type SASP in spore resistance to ultrahigh shock pressures.
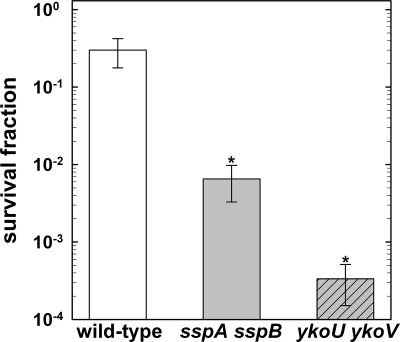
To assess whether the major DNA-protecting SASP and NHEJ, one of the main DNA repair mechanisms in B. subtilis spores (32, 33, 56), play a role in spore resistance to high shock pressures, wild-type and α/β-type SASP- and NHEJ-deficient spores, sandwiched between gabbro, were subjected to 10 GPa at a preshock temperature of 20°C. Whereas the wild type demonstrated a relatively high survival rate of 31% ± 4%, both mutant strains were more sensitive than the wild-type spores by orders of magnitude (Fig. 3). The sensitivity of the spores increased in the following order (from the most resistant to the most sensitive one): wild type > sspA sspB > ykoU ykoV. The ykoU ykoV mutant was about 3 orders of magnitude more sensitive than the wild-type spores. The survival fraction of the wild-type spores (Fig. 3) after exposure to 10 GPa is in quite good agreement with data from previous reports by Stöffler et al. (54) and Horneck et al. (21), where a survival rate of 12% ± 3% was reported. The results of the current experiments provide support to the assumption (i) that α/β-type SASP encoded by sspA and sspB are important protectants of DNA, also from damage induced by shock pressures and/or the accompanying high shock and postshock temperatures, and (ii) that the DSB repair pathway encoded by the ykoWVU operon is very important in the resistance of spores to shock pressures, in addition to its role in resistance to other DSB-inducible treatments, such as exposure to ionizing radiation and extremely low pressures (32, 33).
FIG. 3.
Survival fraction of B. subtilis spores exposed to 10 GPa (20°C). Strains are 168 (wild type) (white bar) and sspA sspB (gray bar) and ykoU ykoV (gray shaded bar) mutants. Data are expressed as averages and standard deviations. Asterisks indicate significant differences from the wild type (P < 0.05).
Sporulation deficiency induced by ultrahigh shock pressure.
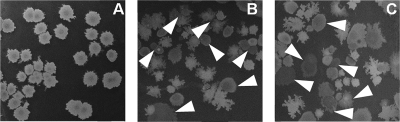
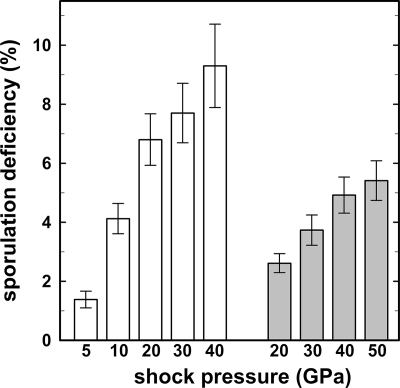
After exposure to ultrahigh shock pressures in the range of 5 to 50 GPa, it was noted by visual inspection of the colony morphology that a fraction of colonies arising from spores treated with high shock-pressure treated were different from the untreated laboratory controls (Fig. 4). To determine the phenotype of the spores, a total of 200 colonies of the wild-type strain (RM01) each from the sample treated with extreme shock pressure and the untreated laboratory sample were picked onto SSM agar plates with rifampin (50 μg/ml) as the antibiotic and on chemically defined supplemented minimal medium (9); all were Rifr and Trp−, lending further support to the notion that all colonies were genetically identical to the used wild-type strain RM01 and not environmental contaminants. After incubation for 7 days, colonies arising from untreated laboratory control appeared homogeneous and indistinguishable from those of wild-type strain RM01 (Fig. 4A). They were opaque and brownish and showed uniform colony morphology (13, 22). In contrast, spore survivors from 30-GPa and 40-GPa treatments, respectively, and a preshock temperature of 20°C formed colonies that resembled those of the RM01 controls, but a certain fraction of them exhibited decreased pigmentation and appeared translucent (Fig. 4B and C), which is characteristic of Spo− B. subtilis mutants (41). It was noted that the Spo− colonies showed fractal and diffuse colony morphology compared to the uniform colony growth of the untreated controls, which is a further attribute of mutated B. subtilis cells (7). Further examination of colonies from all samples treated with ultrahigh shock pressure revealed a high frequency of induced sporulation deficiency, ranging from 1.4% to 10.1%, compared to <0.02%, i.e., none, for the colonies of the untreated laboratory samples (Fig. 5 and Table 1). The induction of the sporulation deficiency increased linearly with increasing shock pressure (Fig. 5), indicating that the mutations leading to the Spo− phenotype were induced by the ultrahigh shock pressure treatment. Furthermore, the induction of Spo− mutants depended on the preshock temperature (Fig. 5). Spores that were shock pressure treated at a preshock temperature of 20°C exhibited an approximately twofold-higher Spo− mutation rate than spores treated with the same shock pressure but at a preshock temperature of −78.5°C. In addition to the visual screening of Spo− mutants by their level of pigmentation, the sporulation frequencies of Spo− colonies grown overnight were determined after heat shock treatment. After transfer in sporulation medium and incubation for 24 h at 37°C, less than 0.013% ± 0.002% of the descendants of the Spo− mutants induced by shock pressure survived a heat shock treatment, thereby confirming the stability of the Spo− mutation. Equally treated control samples (Spo+) showed sporulation frequencies of 10.8% ± 1.3% after overnight incubation.
FIG. 4.
Colony morphology of B. subtilis spores exposed to shock pressure. Colonies arising on sporulation medium were control colonies (A) and those exposed to 30 GPa (20°C) (B) and 40 GPa (20°C) (C). Arrowheads indicate Spo− mutants.
FIG. 5.
Sporulation deficiency of spores exposed to ultrahigh shock pressure at 20°C (white bar) and −78.5°C (gray bar). Data are expressed as averages and standard deviations.
DISCUSSION
Through the simulated hypervelocity impact process, spores of B. subtilis were subjected to a complex matrix of physical stress parameters including (i) defined extreme shock pressures of 5 to 50 GPa, (ii) peak shock temperature increases up to about 1,000°C lasting for nanoseconds, (iii) shock temperature increases up to 200°C lasting for fractions of microseconds, (iv) postshock temperature increases up to 300°C lasting for several seconds to few minutes, and, finally, (v) mechanical stress by friction and/or crushing. The magnitude of the temperature depends not only on the preshock temperature but also on the mineralogical composition, porosity, and water content of the host rock of the sample (21; C. Meyer, D. Stöffler, S. Ott, U. Hornemann, C. S. Cockell, R. Moeller, J. P. de Vera, J. Fritz, S. Schade, and N. A. Artemieva, unpublished observations).
From the shock pressure survival curves (Fig. 2), which decreased nearly exponentially with increasing shock pressure, one may deduce that the shock pressure may be the decisive physical stress parameter. Cell wall rupture and delaminating by shock pressures have been reported for cells of Escherichia coli (58) as well as cells of Chroococcidiopsis sp. strain 029 (21) at much lower pressure values than those used in this study. However, dormant spores are known to be highly resistant to shear forces and mechanical stress, and no disruption of the spore envelop was observed (24). These observations make it unlikely that the pressure alone is the critical physical parameter for killing spores in shock recovery experiments. However, it remains to be investigated whether and to what extent mechanical stress causes direct or indirect effects on the spores during ultrahigh shock pressurization.
The second critical physical parameter in shock recovery experiments is temperature, reaching extremely high values for very short periods (fractions of microseconds to seconds). Directly after the dramatic increase of the temperature in a very short thermal peak (lasting for fractions of microseconds), the temperature falls immediately to the postshock temperature, lasting for a few minutes, followed by quenching to room temperature (as shown in Fig. 18 in reference 21). The temperature reached is dependent on the applied shock pressure but also on the initial preshock temperature, the water content of the sample, and the type of host rock. Our data clearly show that for the same shock pressure but at a lower preshock temperature (−78.5°C), the survival of the spores was about 1 order of magnitude higher than at a preshock temperature of 20°C (Fig. 2). At 50 GPa, the highest pressure value used, a significant amount (3 × 10−4 spores) of −78.5°C-tempered spores survived, whereas no survivors were found at a preshock temperature of 20°C.
Computer code calculations of the shock temperature increases within a several-micrometer-thick water layer (substitute for a microbial endolithic layer) have shown that they reach values that are distinctly higher (during a very short time) than those achieved in the host rock (21). Our studies showed that spores in dry sandstone survived the shock treatment better than moist samples by an order of magnitude (Table 1). These data further support the assumption that the high temperature peaks, which reach higher values in wet than in dry samples, may be essential for the inactivation of spores in shock recovery experiments.
Several mineralogical attributes of the host rock such as composition and porosity are prone to have an influence on the temperature-time profile, especially on the postshock temperature. In our study, spores were either sandwiched between slides of ultramafic rock (i.e., gabbro or dunite) or embedded in porous sandstone, halite, or Martian regolith-analogous material: only small differences in spore survival (after exposure to about 30 GPa) were observed between the different samples (Table 1). These results indicate a minor role of the host rock material and hence the host rock-dependent postshock temperature on the survival of spores treated with shock pressure.
Mutagenesis in stressed microorganisms generally means damage to the DNA induced by the stressor. We have observed an increase of the Spo− mutation rate as a function of the applied shock pressure, and this effect was higher at the 20°C preshock temperature than at −78.5°C (Fig. 5). Similar observations of induced Spo− mutations were reported by Fajardo-Cavazos et al. (12) using B. subtilis spores in artificial meteorite material recovered after a hypervelocity atmospheric (re)entry. From those observations, it was suggested that the DNA of the spores had been affected. However, whether the shock pressure and/or the high temperatures caused Spo− mutagenesis in our experiments is still an open question. Dry heat (120°C for 45 min) is known to raise the mutation frequency of B. subtilis spores, e.g., to nalidixic acid resistance in the gyrA gene by 2 orders of magnitude (10). But it should be noted that in our experiments, the high peak shock temperatures lasted for fractions of a microsecond only and the postshock temperatures lasted from seconds to a few minutes.
In B. subtilis, sporulation genes comprise almost 5% of the genome (25). Therefore, increases in the mutation rate of Spo− phenotypes after exposure to the potentially mutagenic treatments are more likely than the induction of single point mutations, such as revertants or resistances to antibiotics. In preliminary studies of the resistance to kanamycin and spectinomycin, we did not find any significant increase in the mutation rates after shock pressure treatment (Moeller, unpublished). We monitored a response to wet heat exposure in the induced Spo− mutants that was 4 orders of magnitude lower than that in Spo+ spores, indicating that the vast majority of the Spo− mutants were at least blocked at stage IV of sporulation, whereas spore heat resistance was developed (39; reviewed in reference 36). While the loss of sporulation is a dramatic change in the life cycle of B. subtilis cells, further study of the type and nature of the induced Spo− mutation(s) is needed.
Further support for the notion that spore DNA appears to be the sensitive target of shock pressure treatment comes from the increased sensitivity of SASP-deficient spores as well as NHEJ-deficient spores compared to wild-type spores (Fig. 3). The SASP bind to DNA, thereby changing the DNA from the B-like to the A-like helix conformation (34, 38, 48, 49). As a consequence, DNA properties and reactivity to a variety of physicochemical treatments are dramatically changed. Spores deficient in their α/β-type SASP formation are much more sensitive to UV radiation, heat, oxidizing agents, and freeze-drying than wild-type spores. Our results show that α/β-type SASP are also major factors in spore resistance to ultrahigh short-term pressurization.
The extremely high sensitivity of NHEJ-deficient spores, they are 900 times more sensitive to 10 GPa than are wild-type spores, provides the third and strongest evidence for DNA being the shock-pressure-sensitive target. NHEJ is an efficient repair pathway for DSB induced in spore DNA (6, 32, 33, 42, 56). The first step in the NHEJ repair mechanism involves the binding of the Ku complex (encoded by YkoV) to the two DSB ends. The next proteins to be recruited to the complex are those that lead to the resection of the ends of a DSB. The final set of steps in NHEJ leads to the direct joining of the two ends by a specific DNA ligase (encoded by YkoU) that restores the integrity of the DNA. The ligation of DNA strands is an energy-dependent process, and ATP is required for the catalysis of the formation of a phosphodiester at the site of a single-strand break in a duplex DNA (reviewed in reference 42). It is worthwhile that dormant spores do not contain detectable amounts of endogenous ATP; rather, high-energy phosphate is stored in the spore as a large depot of 3-phosphoglyceric acid. In the first minutes of spore germination, 3-phosphoglyceric acid is rapidly converted to ATP by the lower branch of glycolysis (47). Thus, the activity of the NHEJ pathway is dependent upon the reactivation of spore metabolism during germination. After exposure to dry heat, polychromatic UV radiation at >280 nm, ionizing radiation from X rays, high-energy charged-particle bombardment, or ultrahigh vacuum-induced extreme desiccation (10−7 Pa), procedures that are known to cause breaks in spore DNA, ykoV and ykoVU mutant spores were found to be significantly more sensitive to those treatments than wild-type spores (32, 33, 56).
Our results strongly support the supposition that DNA is the sensitive target of B. subtilis spores subjected to hypervelocity impacts. This is indicated by the pressure-dependent survival and Spo− mutation induction curves as well as by the important role of the DNA-protecting SASP and the NHEJ repair pathways in the resistance of spores to this treatment. Besides the potential mechanical stress exerted by the shock pressure itself, the elevated temperatures, although lasting for fractions of seconds or microseconds only, may play a decisive role in the inactivation and mutagenesis of spores exposed to ultrahigh shock pressures. This assumption is supported by our findings that (i) lowering the preshock temperature and, hence, lowering the peak temperatures led to a higher survival of the spores and a reduced mutation rate and (ii) increasing the water content of the sample and, hence, increasing the peak temperature dramatically reduced the survival of the spores.
It can be concluded from our experiments that a substantial fraction of spores inside rocks may be capable of surviving impact ejections of solid rock fragments to velocities of more than 5 km/s, which are, e.g., required for escaping from planet Mars. Assuming a mean spore density of 5 × 102 spores/g in granite rock, as reported previously by Fajardo-Cavazos and Nicholson (13), a 1-kg rock would accommodate approximately 5 × 105 spores, up to 50 to 100 spores of which would survive a shock pressure of 50 GPa when embedded in basaltic rock and tempered at −78.5°C, to consider a worst-case scenario. However, one has to bear in mind that in dormant bacterial spores, DNA damage will accumulate and will be preserved until the spores germinate and may repair their damage to ensure survival and genome integrity. Furthermore, impact ejection is only the first step of the lithopanspermia scenario, and more damage may occur in spores during the interplanetary journey, a further obstacle to lithopanspermia.
Acknowledgments
We are grateful to Hendryk Schneider, Hans-Rudolf Knöfler, Simon Jokisch, and Hartmut Friedrich for skillful technical assistance during the construction and recovery of the experiment hardware and Heiko Will for assisting with the high-explosive experiments. We express gratitude to Peter Setlow for his generous donation of the B. subtilis strains.
This study was supported by the German Research Foundation (DFG) (HO 1508/3-1 and RA 1049/1-2).
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Arrhenius, S. 1903. Die Verbreitung des Lebens im Weltenraum. Umschau 7:481-485. [Google Scholar]
- 2.Artemieva, N. A., and B. A. Ivanov. 2004. Launch of Martian meteorites in oblique impacts. Icarus 171:183-196. [Google Scholar]
- 3.Benardini, J. N., J. Sawyer, K. Venkateswaran, and W. L. Nicholson. 2003. Spore UV and acceleration resistance of endolithic Bacillus pumilus and Bacillus subtilis isolates obtained from Sonoran desert basalt: implications for lithopanspermia. Astrobiology 3:709-717. [DOI] [PubMed] [Google Scholar]
- 4.Bibring, J. P., Y. Langevin, A. Gendrin, B. Gondet, F. Poulet, M. Berthé, A. Soufflot, R. E. Arvidson, N. Mangold, J. Mustard, P. Drossart, and the OMEGA Team. 2005. Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 307:1576-1581. [DOI] [PubMed] [Google Scholar]
- 5.Bibring, J. P., Y. Langevin, J. F. Mustard, F. Poulet, R. Arvidson, A. Gendrin, B. Gondet, N. Mangold, P. Pinet, F. Forget, the OMEGA Team, M. Berthé, J. P. Bibring, A. Gendrin, C. Gomez, B. Gondet, D. Jouglet, F. Poulet, A. Soufflot, M. Vincendon, M. Combes, P. Drossart, T. Encrenaz. T. Fouchet, R. Merchiorri, G. Belluci, F. Altieri, V. Formisano, F. Capaccioni, P. Cerroni, A. Coradini, S. Fonti, O. Korablev, V. Kottsov, N. Ignatiev, V. Moroz, D. Titov, L. Zasova, D. Loiseau, N. Mangold, P. Pinet, S. Douté, B. Schmitt, C. Sotin, E. Hauber, H. Hoffmann, R. Jaumann, U. Keller, R. Arvidson, J. F. Mustard, T. Duxbury, F. Forget, and G. Neukum. 2006. Global mineralogical and aqueous mars history derived from OMEGA/Mars Express data. Science 312:400-404. [DOI] [PubMed] [Google Scholar]
- 6.Bowater, R., and A. J. Doherty. 2006. Making ends meet: repairing breaks in bacterial DNA by non-homologous end-joining. PLoS Genet. 2:e8—e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branda, S. S., J. E. González-Pastor, E. Dervyn, S. D. Ehrlich, R. Losick, and R. Kolter. 2004. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J. Bacteriol. 186:3970-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, G. S., R. G. Betty, J. E. Brockmann, D. A. Lucero, C. A. Souza, K. S. Walsh, R. M. Boucher, M. Tezak, M. C. Wilson, and T. Rudolph. 2007. Evaluation of a wipe surface sample method for collection of Bacillus spores from nonporous surfaces. Appl. Environ. Microbiol. 73:706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawes, I. W., and J. Mandelstam. 1970. Sporulation of Bacillus subtilis in continuous culture. J. Bacteriol. 103:529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.del Carmen Huesca Espitia, L., C. Caley, I. Bagyan, and P. Setlow. 2002. Base-change mutations induced by various treatments of Bacillus subtilis spores with and without DNA protective small, acid-soluble spore proteins. Mutat. Res. 503:77-84. [DOI] [PubMed] [Google Scholar]
- 11.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajardo-Cavazos, P., L. Link, H. J. Melosh, and W. L. Nicholson. 2005. Bacillus subtilis spores on artificial meteorites survive hypervelocity atmospheric entry: implications for lithopanspermia. Astrobiology 5:726-736. [DOI] [PubMed] [Google Scholar]
- 13.Fajardo-Cavazos, P., and W. L. Nicholson. 2006. Bacillus endospores isolated from granite: close molecular relationships to globally distributed Bacillus spp. from endolithic and extreme environments. Appl. Environ. Microbiol. 72:2856-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz, J., N. A. Artemieva, and A. Greshake. 2005. Ejection of Martian meteorites. Meteorit. Planet. Sci. 9/10:1393-1412. [Google Scholar]
- 15.Horneck, G., H. Bücker, G. Reitz, H. Requardt, K. Dose, K. D. Martens, H. D. Mennigmann, and P. Weber. 1984. Microorganisms in the space environment. Science 225:226-228. [DOI] [PubMed] [Google Scholar]
- 16.Horneck, G., H. Buecker, and G. Reitz. 1994. Long-term survival of bacterial spores in space. Adv. Space Res. 10:41-45. [DOI] [PubMed] [Google Scholar]
- 17.Horneck, G., C. Miliekowsky, H. J. Melosh, J. W. Wilson, F. A. Cucinotta, and B. Gladman. 2002. Viable transfer of microorganisms in the solar system and beyond, p. 55-76. In G. Horneck and C. Baumstark-Khan (ed.), Astrobiology: the quest for the conditions of life. Springer, Berlin, Germany.
- 18.Horneck, G., P. Rettberg, E. Rabbow, W. Strauch, G. Seckmeyer, R. Facius, G. Reitz, K. Strauch, and J. U. Schott. 1996. Biological dosimetry of solar radiation for different simulated ozone column thickness. J. Photochem. Photobiol. B Biol. 32:189-196. [DOI] [PubMed] [Google Scholar]
- 19.Horneck, G., P. Rettberg, G. Reitz, J. Wehner, U. Eschweiler, K. Strauch, C. Panitz, V. Starke, and C. Baumstark-Khan. 2001. Protection of bacterial spores in space, a contribution to the discussion on panspermia. Orig. Life Evol. Biosph. 31:527-547. [DOI] [PubMed] [Google Scholar]
- 20.Horneck, G., D. Stöffler, U. Eschweiler, and U. Hornemann. 2001. Bacterial spores survive simulated meteorite impact. Icarus 149:285-293. [Google Scholar]
- 21.Horneck, G., D. Stöffler, S. Ott, U. Hornemann, C. S. Cockell, R. Moeller, C. Meyer, J. P. de Vera, J. Fritz, S. Schade, and N. A. Artemieva. 2008. Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 8:17-44. [DOI] [PubMed] [Google Scholar]
- 22.Hullo, M. F., I. Moszer, A. Danchin, and I. Martin-Verstraete. 2001. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 183:5426-5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibarra, J. R., A. D. Orozco, J. A. Rojas, K. López, P. Setlow, R. E. Yasbin, and M. Pedraza-Reyes. 2008. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in repair of DNA damage during outgrowth of Bacillus subtilis spores. J. Bacteriol. 190:2031-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones, C. A., N. L. Padula, and P. Setlow. 2005. Effect of mechanical abrasion on the viability, disruption and germination of spores of Bacillus subtilis. J. Appl. Microbiol. 99:1484-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maughan, H., J. Masel, C. W. Birky, Jr., and W. L. Nicholson. 2007. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 177:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melosh, H. J. 1984. Impact ejection, spallation and the origin of meteorites. Icarus 59:234-260. [Google Scholar]
- 27.Meyer, C. 2006. The Mars Meteorite Compendium. http://curator.jsc.nasa.gov/antmet/mmc/index.cfm.
- 28.Mileikowsky, C., F. A. Cucinotta, J. W. Wilson, B. Gladman, G. Horneck, L. Lindegren, J. Melosh, H. Rickman, M. Valtonen, and J. Q. Zheng. 2000. Natural transfer of viable microbes in space. 1. From Mars to Earth and Earth to Mars. Icarus 145:391-427. [DOI] [PubMed] [Google Scholar]
- 29.Moeller, R., T. Douki, J. Cadet, E. Stackebrandt, W. L. Nicholson, P. Rettberg, G. Reitz, and G. Horneck. 2007. UV radiation induced formation of DNA bipyrimidine photoproducts in Bacillus subtilis endospores and their repair during germination. Int. Microbiol. 10:39-46. [PubMed] [Google Scholar]
- 30.Moeller, R., G. Horneck, R. Facius, and E. Stackebrandt. 2005. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol. Ecol. 51:231-236. [DOI] [PubMed] [Google Scholar]
- 31.Moeller, R., G. Horneck, P. Rettberg, H.-J. Mollenkopf, E. Stackebrandt, and W. L. Nicholson. 2006. A method for extracting RNA from dormant and germinating Bacillus subtilis strain 168 endospores. Curr. Microbiol. 53:227-231. [DOI] [PubMed] [Google Scholar]
- 32.Moeller, R., P. Setlow, G. Horneck, T. Berger, G. Reitz, P. Rettberg, A. J. Doherty, R. Okayasu, and W. L. Nicholson. 2008. Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy charged-particle bombardment. J. Bacteriol. 190:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moeller, R., E. Stackebrandt, G. Reitz, T. Berger, P. Rettberg, A. J. Doherty, G. Horneck, and W. L. Nicholson. 2007. Role of DNA repair by nonhomologous-end joining (NHEJ) in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J. Bacteriol. 189:3306-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohr, S. C., N. V. H. A. Sokolov, C. He, and P. Setlow. 1991. Binding of small, acid-soluble proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc. Natl. Acad. Sci. USA 88:77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munakata, N., and C. S. Rupert. 1972. Genetically controlled removal of “spore photoproduct” from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J. Bacteriol. 111:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of bacterial endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson, W. L., A. C. Schuerger, and P. Setlow. 2005. The solar UV environment and bacterial spore UV resistance: considerations for Earth-to-Mars transport by natural processes and human spaceflight. Mutat. Res. 571:249-264. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson, W. L., B. Setlow, and P. Setlow. 1990. Binding of DNA in vitro by a small, acid-soluble spore protein from Bacillus subtilis and the effect of this binding on DNA topology. J. Bacteriol. 172:6900-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Sussex, England.
- 40.Onstott, T. C. 2002. Biogeochemical and geological significance of subsurface microbiology, p. 1453-1468. In G. Britton (ed.), Encyclopedia of environmental microbiology. John Wiley Press, New York, NY.
- 41.Piggot, P. J., and J. G. Coote. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitcher, R. S., N. C. Brissett, and A. J. Doherty. 2007. Nonhomologous end-joining in bacteria: a microbial perspective. Annu. Rev. Microbiol. 61:259-282. [DOI] [PubMed] [Google Scholar]
- 43.Puskeppeleit, M., L. E. Quintern, S. el Naggar, J. U. Schott, U. Eschweiler, G. Horneck, and H. Buecker. 1992. Long-term dosimetry of solar UV radiation in Antarctica with spores of Bacillus subtilis. Appl. Environ. Microbiol. 58:2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Righter, K. 2007. The Lunar meteorite compendium. http://curator.jsc.nasa.gov/antmet/lmc/index.cfm.
- 45.Salas-Pacheco, J. M., B. Setlow, P. Setlow, and M. Pedraza-Reyes. 2005. Role of the Nfo (YqfS) and ExoA apurinic/apyrimidinic endonucleases in protecting Bacillus subtilis spores from DNA damage. J. Bacteriol. 187:7374-7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaeffer, P., J. Millet, and J.-P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 45:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Setlow, B., and P. Setlow. 1995. Small, acid-soluble spore proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl. Environ. Mircobiol. 61:2787-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Setlow, P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49:29-54. [DOI] [PubMed] [Google Scholar]
- 51.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 52.Squyres, S. W., J. P. Grotzinger, R. E. Arvidson, J. F. Bell, W. Calvin, P. R. Christensen, B. C. Clark, J. A. Crisp, W. H. Farrand, K. E. Herkenhoff, J. R. Johnson, G. Klingelhofer, A. H. Knoll, S. M. McLennan, H. Y. McSween, R. V. Morris, J. W. Rice, R. Rieder, and L. A. Soderblom. 2004. In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306:1709-1714. [DOI] [PubMed] [Google Scholar]
- 53.Stöffler, D., and F. Langenhorst. 1994. Shock metamorphism of quartz in nature and experiment. I. Basic observations and theory. Meteoritics 29:155-188. [Google Scholar]
- 54.Stöffler, D., G. Horneck, S. Ott, U. Hornemann, C. S. Cockell, R. Moeller, C. Meyer, J. P. de Vera, J. Fritz, and N. A. Artemieva. 2007. Experimental evidence for the potential impact ejection of viable microorganisms from Mars and Mars-like planets. Icarus 186:585-588. [Google Scholar]
- 55.Stöffler, D., R. Ostertag, C. Jammes, G. Pfannenschmitt, P. R. Sen Gupta, S. B. Simon, J. J. Papike, and R. H. Beauchamp. 1986. Shock metamorphism and petrography of the Shergotty achondrite. Geochim. Cosmochim. Acta 50:889-903. [Google Scholar]
- 56.Wang, S. T., B. Setlow, E. M. Conlon, J. L. Lyon, D. Imamura, T. Sato, P. Setlow, R. Losick, and P. Eichenberger. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 358:16-37. [DOI] [PubMed] [Google Scholar]
- 57.Weber, P., and J. M. Greenberg. 1985. Can spores survive in interstellar space? Nature 316:403-407. [Google Scholar]
- 58.Willis, M. J., T. J. Ahrens, L. E. Bertani, and C. Z. Nash. 2006. Bugbuster—survivability of living bacteria upon shock compression. Earth Planet. Sci. 247:185-196. [Google Scholar]