Abstract
Methanococcus maripaludis, an H2- and formate-utilizing methanogen, produced H2 at high rates from formate. The rates and kinetics of H2 production depended upon the growth conditions, and H2 availability during growth was a major factor. Specific activities of resting cells grown with formate or H2 were 0.4 to 1.4 U·mg−1 (dry weight). H2 production in formate-grown cells followed Michaelis-Menten kinetics, and the concentration of formate required for half-maximal activity (Kf) was 3.6 mM. In contrast, in H2-grown cells this process followed sigmoidal kinetics, and the Kf was 9 mM. A key enzyme for formate-dependent H2 production was formate dehydrogenase, Fdh. H2 production and growth were severely reduced in a mutant containing a deletion of the gene encoding the Fdh1 isozyme, indicating that it was the primary Fdh. In contrast, a mutant containing a deletion of the gene encoding the Fdh2 isozyme possessed near-wild-type activities, indicating that this isozyme did not play a major role. H2 production by a mutant containing a deletion of the coenzyme F420-reducing hydrogenase Fru was also severely reduced, suggesting that the major pathway of H2 production comprised Fdh1 and Fru. Because a Δfru-Δfrc mutant retained 10% of the wild-type activity, an additional pathway is present. Mutants possessing deletions of the gene encoding the F420-dependent methylene-H4MTP dehydrogenase (Mtd) or the H2-forming methylene-H4MTP dehydrogenase (Hmd) also possessed reduced activity, which suggested that this second pathway was comprised of Fdh1-Mtd-Hmd. In contrast to H2 production, the cellular rates of methanogenesis were unaffected in these mutants, which suggested that the observed H2 production was not a direct intermediate of methanogenesis. In conclusion, high rates of formate-dependent H2 production demonstrated the potential of M. maripaludis for the microbial production of H2 from formate.
Many hydrogenotrophic methanogens use H2 or formate for the reduction of CO2 to obtain energy for growth. Methanococcus maripaludis, the model microorganism in this study, is a hydrogenotrophic, formate-utilizing, mesophilic methanogen. It is common in salt marsh sediments, from which it was isolated (12). An extraordinarily active H2 consumer, M. maripaludis is exceptionally well equipped with enzymes responsible for H2 metabolism. M. maripaludis contains genes for seven different hydrogenases, whose expression depends upon the growth conditions (18). It possesses two membrane-bound, energy-converting [Ni-Fe] hydrogenases, designated Eha and Ehb, that are involved in the reduction of low-potential ferredoxins for anabolism (16, 26). There are also four cytoplasmic [Ni-Fe] hydrogenases, including two coenzyme F420-reducing (Fru and Frc) and two coenzyme F420-nonreducing (Vhu and Vhc) hydrogenases. One hydrogenase of each type (Fru and Vhu) contains a selenocysteinyl residue. The other hydrogenases (Frc and Vhc) contain cysteinyl residues at homologous positions (18). The selenocysteine-containing isozymes are abundant during cultivation in medium containing selenium, and the cysteine-containing isozymes (Frc and Vhc) are produced only upon selenium limitation (27). Lastly, the cells contain a cytoplasmic [Fe-S] cluster-free hydrogenase, the H2-forming methylenetetrahydromethanopterin (methylene-H4MPT) dehydrogenase (Hmd), which in other species has been shown to play an important role at high levels of H2 or under nickel limitation (1, 2, 29).
When formate is the substrate, it is oxidized for the reduction of CO2 to methane. The key enzyme for formate utilization is formate dehydrogenase, Fdh. The genome of M. maripaludis harbors two sets of genes encoding Fdh, fdhA1B1 and fdhA2B2 (33). Both Fdhs contain selenocysteinyl residues. While fdhA1B1 are found in an apparent operon with genes for a putative formate transporter and carbonic anhydrase, fdhA2B2 are not linked with other genes in formate utilization. In methanococci as well as in methanobacteria, the deazaflavin coenzyme F420 is the electron acceptor of the Fdhs (5, 20).
Formate-hydrogen lyase activity (reaction 1) is common in methanococci and other methanogens (5, 6, 11, 20, 34). Reaction 1: HCO2− + H2O → HCO3− + H2 (+1.3 kJ/reaction).
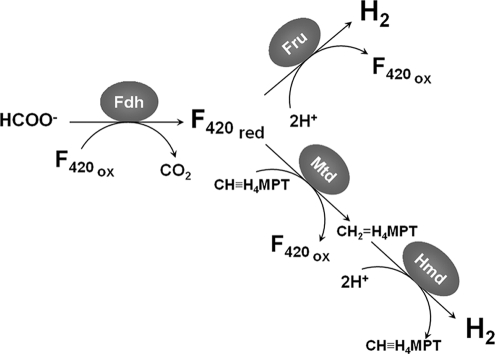
Two pathways are likely to contribute to this activity in whole cells of M. maripaludis. In the first pathway, reduced coenzyme F420 (F420H2) generated by Fdh is oxidized by the reversible F420-dependent hydrogenase Fru (Fig. 1). In the second pathway, F420H2 is oxidized by the F420-dependent methylene-H4MPT dehydrogenase (Mtd) and Hmd (1, 2, 29) (Fig. 1). While previous studies demonstrated H2 production from formate for some methanogens (7), the rates were very low and the pathways were not determined.
FIG. 1.
Potential pathways of H2 production in the hydrogenotrophic methanogens. The first pathway includes Fdh and Fru. In this pathway, Fdh reduces F420. In selenium-grown cells, F420H2 is oxidized by the [Ni-Fe] hydrogenase Fru. In the second pathway, F420H2 is oxidized by Mtd to reduce methenyl-H4MPT to methylene-H4MPT, which is then reoxidized by Hmd, a Ni-free hydrogenase, to produce H2. Abbreviations: Fdh, formate dehydrogenase; Fru, F420-reducing hydrogenase; F420 ox, oxidized coenzyme F420; F420 red, reduced coenzyme F420; Mtd, F420-dependent methylene-H4MPT dehydrogenase; Hmd, H2-forming methylene-H4MPT dehydrogenase.
Because of the low energy yield of CH4 production, these microorganisms possess extremely high specific activities for enzymes involved in methanogenesis, including the hydrogenases used for H2 consumption. If H2 utilization were a reversible process, high rates of H2 production would be possible. To test this hypothesis, the rates of H2 production from formate were tested in M. maripaludis, a representative of a diversified and important group of methanogens.
MATERIALS AND METHODS
Strains and growth conditions.
M. maripaludis wild-type strain S2 and the mutant strains are listed in Table 1. For growth experiments, the strains were grown in 28-ml Balch tubes (4) filled with 5 ml of either McNA (McN minimal medium [32] supplemented with 10 mM acetate) or McCV medium (McNA medium supplemented with 0.2% yeast extract and Casamino Acids as well as vitamin solution [32]). The tubes were pressurized to 276 kPa with H2-CO2 gas (80:20 [vol/vol]) and grown at 37°C. When sodium formate was used (100 mM), 100 mM Tris-HCl solution, pH 7, was added to reduce the increase in pH. The tubes were also pressurized to 138 kPa with N2-CO2 (80:20 [vol/vol]).
TABLE 1.
Strains used in this study
For obtaining cell mass, M. maripaludis cultures were grown in 1-liter Wheaton bottles (3) with 100 ml of McNA or McCV medium and 138 kPa of H2-CO2 (80:20 [vol/vol]) at 37°C. When sodium formate was the substrate (0.4 M), the bottles were flushed with N2-CO2 (80:20 [vol/vol]) and pressurized to 35 kPa.
Cells were collected by centrifugation in sealed plastic centrifuge bottles that had been equilibrated in an anaerobic glove box (Coy Laboratories, Ann Arbor, MI) for at least 24 h to remove O2 adsorbed to the plastic. The cells were centrifuged at 6,000 × g for 20 min at 4°C using a Beckman model J2-21 centrifuge (Beckman Coulter, Inc., Fullerton, CA) fitted with a Beckman JA-14 rotor. The cells were resuspended in 1/100 of the initial volume of an anaerobic buffer containing 50 mM piperazine-1,4-bis(2-ethanesulfonic acid) (PIPES), 400 mM NaCl, 20 mM KCl, 20 mM MgCl2, 1 mM CaCl2, and 5 mM dithiothreitol, pH 6.9.
H2 measurements.
The H2 measurements were performed at 37οC in a custom-made anaerobic, water-jacketed cuvette (2.8 ml) fitted with a rubber stopper and gassed with O2-free N2 (31). The concentration of the dissolved H2 in the anaerobic buffer was measured using a modified amperometric O2 Clark-type electrode (15, 31) (Yellow Springs Instrument, Yellow Springs, OH) connected to a picoammeter PA2000 (Unisense, Aarhus, Denmark). The connections for the electrode (6.3-mm TRS connector) and picoammeter (BNC connector) were incompatible, and so the appropriate connection was manufactured. Standard curves were prepared with H2-saturated distilled water. Cell suspensions of 0.1 mg (dry weight) were added via microsyringes to 1 ml of the same buffer used to suspend the cells. The assay was started by adding sodium formate. One unit was defined as 1 μmol of H2 produced per minute. The cell dry weight was calculated from the slope of a standard curve relating absorbance at 600 nm to dry weight. From this curve, a suspension with an A600 of 1 corresponded to 0.34 mg (dry weight)·ml−1.
CH4 detection.
Resting cells (0.1 mg [dry weight]) suspensions in 0.5 ml of buffer were transferred to 3.5-ml vials under an atmosphere of N2. The assay was initiated by adding formate to a final concentration of 20 mM or flushing the vials with H2-CO2 (80:20 [vol/vol]) for 1 min. The samples were incubated at 37°C for 10 min. CH4 was detected with an SRI 8610-C gas chromatograph (SRI Instruments, Torrance, CA) fitted with on-column injection, a Porpak Q teflon column at 90°C, and a flame ionization detector operating at 150°C. The carrier gas was N2. One unit was defined as 1 μmol of CH4 produced per minute.
Preparation of cell extracts.
All procedures were performed anaerobically. Cells were collected by centrifugation as described above and loaded into a chilled French pressure cell in the anaerobic glove box. Cell extracts were prepared by passing 10 ml of cell suspension (about 10 mg [dry weight] per ml) through the French pressure cell operated at 65 MPa equipped with a 22-gauge needle. Cell extracts were collected in the sealed tubes or serum bottles previously equilibrated in the anaerobic glove box. Subsequently, the extracts were centrifuged at 10,000 × g for 20 min at 4°C. Protein concentrations were determined using the Bradford protein kit (Bio-Rad, Hercules, CA).
Enzymatic assays.
The Fdh and F420-reducing hydrogenase activities were measured spectrophotometrically under anaerobic conditions. H2-dependent F420 reduction was assayed in 1 ml of anaerobic buffer containing 100 mM PIPES, pH 6.9, 20 mM NaCl, 10 mM KCl, 10 mM MgCl2, 1 mM CaCl2, and 5 mM dithiothreitol. The final concentration of F420 was 10 μM. The 1.6-ml glass cuvettes were sealed with rubber stoppers and flushed with O2-free H2 for 5 min before each assay. Changes in absorbance at a λ of 420 nm were measured using a Beckman DU-640B spectrophotometer. An extinction coefficient (ɛ420) of 40 mM·cm−1 was used for calculations. One unit was defined as 1 μmol of coenzyme F420 reduced per minute. F420 was purified from the M. maripaludis cell paste according to a modification of the method of Eirich et al. (9).
Fdh activity was assayed in 1 ml of the same buffer described above plus 2 mM methyl viologen (MV). The cuvettes were flushed with O2-free N2 for 5 min before each assay. The MV was reduced with a few microliters of 200 mM dithionite until the assay buffer turned slightly blue. The cell extract was added, and the reaction was initiated by adding formate to a final concentration of 10 mM. Changes in absorbance at a λ of 605 nm were recorded, and an extinction coefficient (ɛ605) of 13.9 mM·cm−1 was used for calculations. One unit was defined as 2 μmol of MV reduced per minute.
RESULTS AND DISCUSSION
Initial rates of H2 production.
Previous reports of formate-dependent H2 production measured formation of headspace H2 during growth (7, 19, 28). However, preliminary measurements of the rate of production of dissolved H2 with an H2 probe and using resting cells far exceeded these reports. Therefore, the earlier measurements underestimated the production rate, possibly because H2 uptake was occurring simultaneously or gas transfer to the headspace was rate limiting.
To examine these high rates in more detail, further experiments were performed to standardize the reaction conditions. Whole cells were grown either with H2 or formate, washed in buffer, and resuspended in a reaction cuvette. In both cases, formate-dependent H2 production proceeded linearly for the first minute (Fig. 2). In the subsequent 2 to 5 minutes, the apparent rate of H2 generation declined until a plateau was reached. With 20 mM formate, the maximal H2 concentrations ranged between 0.4 and 0.8 mM, depending upon the experiment. These concentrations approached 3 mM, the concentration expected at equilibrium. The observed rate would be lower than the true production rate if H2 utilization were occurring simultaneously. However, H2 utilization for methanogenesis was not likely to be a factor, because the initial concentration of CO2 was very low. In addition, the inhibition of methanogenesis by bromoethanesulfonate (1 mM) had little effect on the initial rates or maximum values of H2 production (data not shown). Therefore, the initial observed rate was not affected by simultaneous H2 consumption for methanogenesis.
FIG. 2.
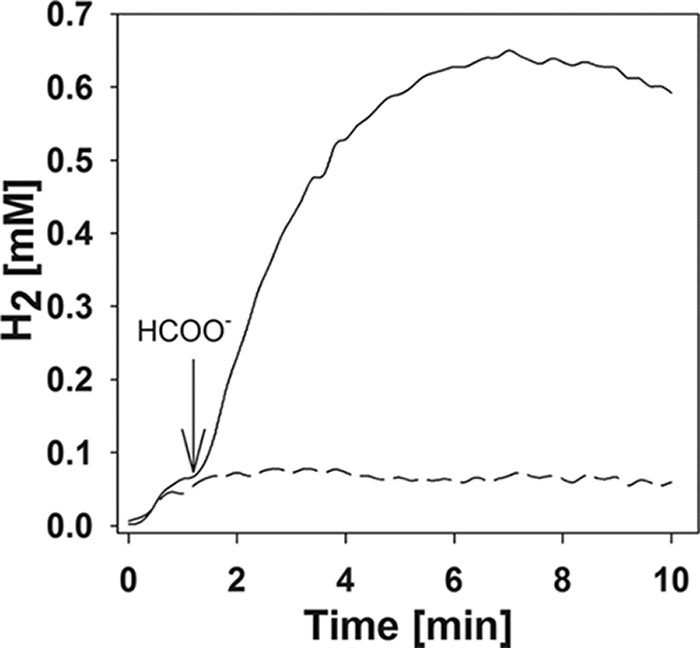
Initial rates of H2 production from formate (solid line) by resting cells of M. maripaludis. Cells were grown with formate, washed in buffer, and resuspended in the reaction cuvette. Assays were initiated by adding 20 mM sodium formate (arrow). Results from incubation of the cell suspension in the absence of formate is shown by the broken line.
Both H2- and formate-grown cells produced H2 from formate at comparable rates. Although the rates depended greatly upon the experiment (see below), the rates varied from about 0.4 to 1.4 U·mg−1 (dry weight) regardless of how the cells were grown (data not shown). These results suggested that the levels of Fdh and hydrogenase were high in both cell types. Since these enzymes are essential components of the methanogenesis system, the rates of methanogenesis were also compared. In one experiment, the rates of methanogenesis by H2-grown cells were 0.37 ± 0.02 U·mg−1 (dry weight) and 0.32 ± 0.04 U·mg−1 (dry weight) with H2 or formate, respectively (means ± standard deviations of triplicate measurements with two independently grown cultures). Similarly, the rates of methanogenesis by formate-grown cells were 0.25 ± 0.02 U·mg−1 (dry weight) and 0.43 ± 0.04 U·mg−1 (dry weight) with H2 or formate, respectively. Since four molecules of H2 or formate are consumed per molecule of CH4 formed, the capacity of H2 or formate consumption is comparable to the rate of formate-dependent H2 production regardless of the growth substrate.
Influence of growth conditions on cellular rates of H2 production.
The rate of formate-dependent H2 production varied greatly between experiments. To determine if some of this variability resulted from the growth phase, H2-grown cultures were monitored for formate-dependent H2 production (Fig. 3). In batch cultures of methanococci, exponential growth is only observed at low cell densities (25). Above an absorbance of ∼0.4, growth becomes linear as the rate of H2 transfer to the aqueous phase becomes rate limiting. Finally, the linear phase usually ends at absorbances of ∼0.8, as cells enter early stationary phase. When cultures were allowed to nearly exhaust H2 late in growth, the specific activity of H2 production increased 6- to 10-fold (Fig. 3). In addition to growth phase, the medium composition also affected these rates. At the end of growth, cells grown in the minimal medium possessed about 30% higher specific activities than cells grown in the rich medium (Fig. 3). Methanococci require higher levels of H2 for anabolism during growth on minimal medium (26). Taken together, these results were consistent with a role for H2 limitation in the regulation of H2 production.
FIG. 3.
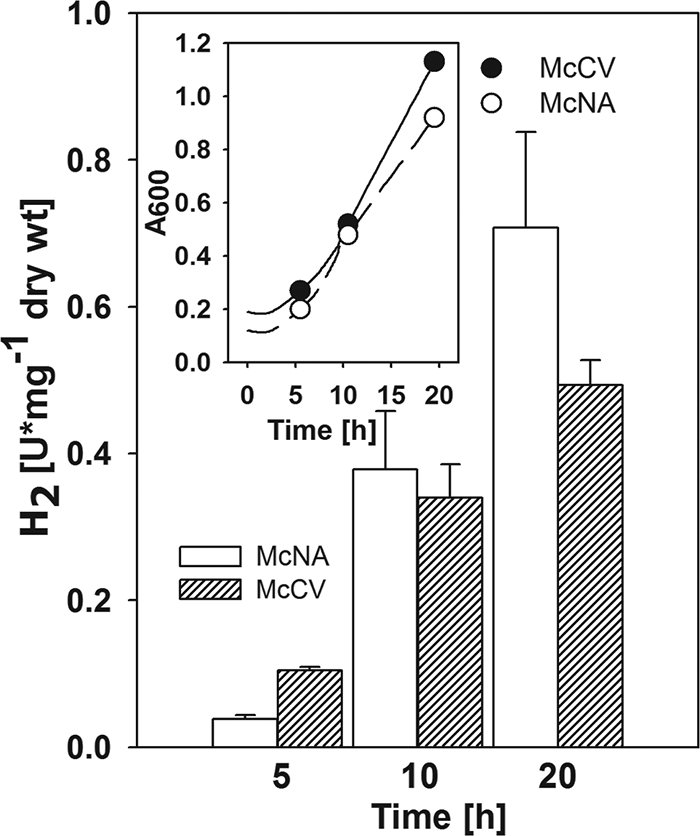
Changes in formate-dependent H2 production by resting cells following growth under H2 limitation. The wild-type S2 was grown in minimal or McNA (○) and rich or McCV (•) medium in 1-liter Wheaton bottles with 100 ml of broth. At the beginning of the experiment, the bottles were pressurized with 138 kPa of H2-CO2 (80:20 [vol/vol]). Cell samples (20 ml) were collected at different absorbances, as indicated by the points on the inset growth curves. After each sampling, the bottles were repressurized with N2-CO2 (80:20 [vol/vol]). This resulted in H2 limitation but maintained the concentration of CO2. Error bars represent 1 standard deviation from the four measurements.
To examine directly the role of H2 partial pressure on the specific activity of H2 production, cultures were grown in bottles with very large headspaces in order to minimize gas pressure fluctuations due to H2 consumption. Parallel cultures were grown with H2 partial pressures of 220 and 80 kPa to absorbances of 0.45 to 0.50. The specific activities of H2 production were 0.12 ± 0.03 and 0.41 ± 0.04 U·mg−1 (dry weight), respectively. Thus, the levels of H2 during growth had a direct effect on the expression of the enzymes involved in H2 production. These results are consistent with microarray observations, where the levels of mRNA for both the formate dehydrogenase (fdh1) and F420-reducing hydrogenase (fru) genes are significantly higher during H2 limitation (17).
Role of formate dehydrogenase.
To ascertain the importance of the two Fdh isozymes in H2 production, the mutant strains ΔfdhA1, ΔfdhA2, and ΔfdhA1-ΔfdhA2 were tested for their ability to grow with formate, MV-dependent Fdh activity, and for H2 production. The double mutant ΔfdhA1-ΔfdhA2, which was constructed by marker exchange of internal portions of both fdhA1 and fdhA2, was unable to utilize formate for either growth, which was followed for 140 h, or H2 production (Fig. 4, Table 2, and data not shown). In addition, in extracts of H2-grown cells, the MV-dependent Fdh activity was <0.02 U·mg−1 (dry weight). Thus, H2 production required Fdh. MV-dependent Fdh activities in cell extracts of the ΔfdhA2 mutant and the wild-type S2 strains were 1.0 ± 0.25 and 3.6 ± 1.2 U·mg−1 (dry weight), respectively. In contrast, growth on formate of strain ΔfdhA2 was comparable to that of the wild type, and H2 production was only slightly reduced. Therefore, the rate of formate oxidation did not appear to limit growth and H2 production in this mutant. Notably, the ΔfdhA1 mutant failed to grow with formate without an extended incubation of 70 h and produced H2 poorly (Fig. 4, Table 2, and data not shown). In addition, the MV-dependent Fdh activity was <0.02 U·mg−1 (dry weight). Subsequent transfers of formate-grown cultures of the ΔfdhA1 mutant to the fresh medium decreased the lag phase, and the third subculture grew with formate after a lag of about 48 h. Presumably, mutations at other sites on the genome were responsible for the adaptation of the ΔfdhA1 mutant to growth on formate. For instance, increased expression of Fdh2 could account for this phenotype. Thus, Fdh2 played only a small role in H2 production, and Fdh1 appeared to be the major isozyme in formate utilization under these conditions. In previous studies, the ΔfdhA1 mutant grew with formate at similar rates as the ΔfdhA2 strain (33). Presumably, this difference reflects the differences in the medium composition and the experimental design.
FIG. 4.
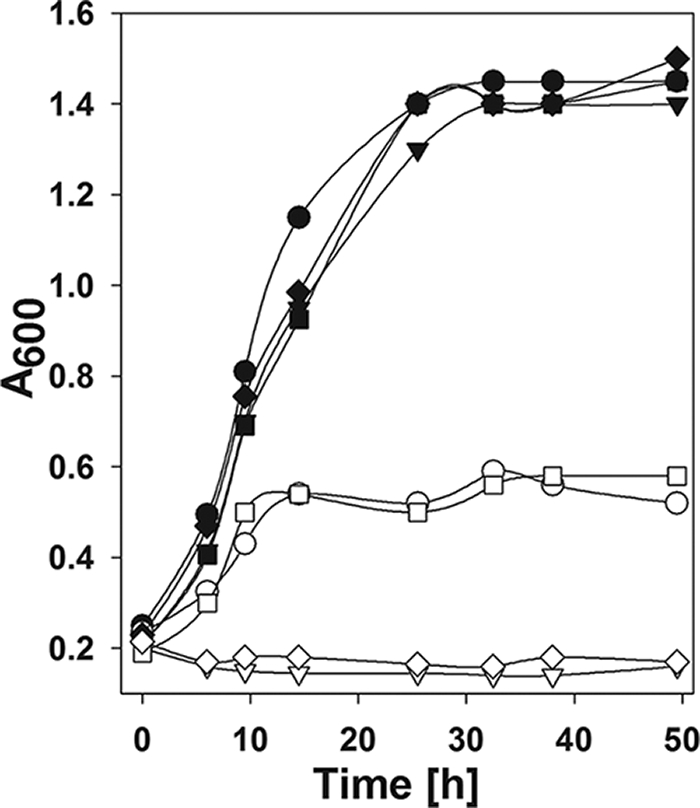
Growth of the wild-type and fdh mutant strains with H2 or formate. Solid symbols, growth with H2 (276 kPa; H2-CO2 80:20 [vol/vol]); open symbols, growth with formate (100 mM). Circles, S2; inverted triangles, ΔfdhA1; squares, ΔfdhA2; diamonds, ΔfdhA1-A2. Each point represents the mean value of two replicates. Similar curves were obtained in a replicate experiment.
TABLE 2.
Activities of formate-dependent H2 production by fdh mutant strainsa
| Genotype | Sp act (U·mg−1 [dry wt]) |
|---|---|
| Wild type | 0.32 ± 0.05 |
| ΔfdhA1 | 0.04 ± 0.01 |
| ΔfdhA2 | 0.29 ± 0.02 |
| ΔfdhA1-ΔfdhA2 | <0.02 |
Cultures were grown with H2 at 136 kPa of H2-CO2, (80:20 [vol/vol]) to an absorbance of about 0.5. The values are means ± standard deviations of triplicate measurements with two independently grown cultures.
Kinetics of formate-dependent H2 production by whole cells.
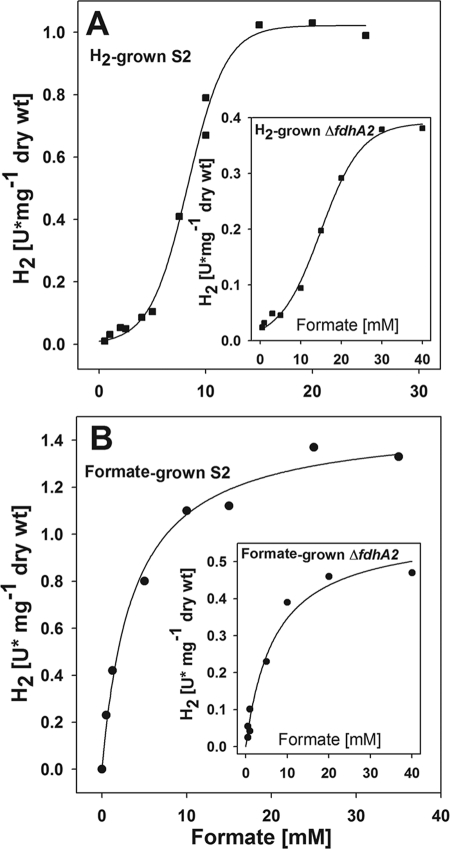
The kinetics depended on the growth substrates of the cells. For incubations of formate-grown cells, H2 production followed Michaelis-Menten kinetics. The Kf, or concentration of formate required for half-maximal activity, was 3.6 ± 0.5 mM (mean ± 1 standard deviation), and the Vf, or the maximal specific activity, was 1.5 ± 0.1 U·mg−1 (dry weight) (Fig. 5). For H2-grown cells, H2 production followed sigmoidal kinetics, and the kinetic values were 9 ± 1 mM and 1.1 ± 0.2 U·mg−1 (dry weight), respectively. The sigmoidal kinetics for the H2-grown cells were not due to the presence of two isozymes in the wild-type cells, because the ΔfdhA2 mutant possessed similar kinetics (Fig. 5). Instead, the biphasic kinetics suggested that the formate transporter FdhC plays a significant role in the kinetics of H2 production. Expression of fdhC is reduced in H2-grown cells (33). Thus, at low formate concentrations, low levels of the transporter may limit the rate of formate uptake and H2 production. High formate concentrations would then compensate for low levels of the transporter. In contrast, the levels of the transporter may be sufficient in the formate-grown cells so that uptake is no longer rate limiting at millimolar formate concentrations.
FIG. 5.
Kinetics of formate-dependent H2 production by resting cells of the wild-type strain S2 previously grown either with H2 (A) or formate (B). The kinetics of the ΔfdhA2 mutant are shown in the insets. The S2 and ΔfdhA2 mutant strains were grown to absorbances of about 0.8 and 0.6, respectively.
Pathways of H2 production.
Given the requirement for Fdh, two pathways of H2 production were likely (Fig. 1). In selenium-grown cells, the major pathway was expected to include the oxidation of Fdh-generated F420H2 by the F420-dependent [Ni-Fe] hydrogenase Fru. The selenium-independent isozyme Frc was not expected to play a role, because it would not be expressed under these conditions (27). In extracts of cells grown with both selenium and formate, the specific activities of MV-dependent Fdh and Fru were 18 and 2.1 U·mg−1 of the total protein. Assuming that 60% of the cell was protein, the corresponding cellular specific activities were 10.8 and 1.3 U·mg−1 (dry weight), values that were consistent with a role in whole-cell H2 production. To examine this point further, the H2 production activities of a series of in-frame deletion mutants in the F420-dependent hydrogenases, ΔfruA, ΔfrcA, and ΔfruA-ΔfrcA, were examined. The activity of the ΔfruA-ΔfrcA mutant was severely reduced (Table 3). The ΔfruA mutant possessed nearly identical activity as the double mutant, suggesting the FruA played an important role (data not shown). In contrast, the activity of the ΔfrcA mutant was identical to the wild type (data not shown), as expected if this enzyme is not produced in the presence of selenium. These observations clearly suggested that F420 is an intermediate in the process and provided evidence for an F420-dependent formate-hydrogen lyase system in M. maripaludis. Because the ΔfruA-ΔfrcA mutant retained about 10% of the wild-type H2 production activity (Table 3), an additional pathway must be present. Deletions of either mtd or hmd reduced the activity by about 30 to 40%, which indicated that the second system utilized the H4MPT-dependent pathway (Fig. 1).
TABLE 3.
Activities of formate-dependent H2 and CH4 production for hydrogenase and methylene-H4MPT dehydrogenase mutant strainsa
| Genotype | Sp act (U·mg−1 [dry wt])
|
|
|---|---|---|
| H2 | CH4 | |
| Wild type | 0.56 ± 0.10 | 0.36 ± 0.03 |
| ΔfruA-ΔfrcA | 0.06 ± 0.02 | 0.20 ± 0.04 |
| Δhmd | 0.40 ± 0.08 | 0.12 ± 0.02 |
| Δmtd | 0.35 ± 0.05 | 0.13 ± 0.02 |
Cells were grown with formate to an absorbance of about 0.6, except for Δmtd, for which the absorbance was about 0.4. H2 and CH4 production levels were measured using cells from the same culture. The values are means ± 1 standard deviation of triplicate measurements from each of two independently grown cultures.
Is H2 an intermediate of methanogenesis with formate?
The rates of H2 generation were comparable to the rates of methanogenesis, which suggested that H2 was produced in sufficient amounts to be an intermediate during methanogenesis with formate. During hydrogenotrophic growth, H2 is proposed to be the electron donor for the Eha-dependent reduction of CO2 to formylmethanofuran as well as the heterodisulfide reductase, the first and last steps of methanogenesis, respectively (for a review see reference 10). During growth with formate, F420 is initially reduced. If methanogenesis proceeds in a fashion similar to hydrogenotrophic growth, H2 could be generated from F420H2 to produce the reductant for the first and last steps of methanogenesis. To test this hypothesis, rates of formate-dependent H2 and CH4 production were measured in cells derived from the same cultures in order to reduce the variability caused by growth and handling (Table 3). In these experiments, there was little correlation between the rates of H2 and CH4 production in the wild type and ΔfruA-ΔfrcA, Δhmd, and Δmtd mutants. In fact, for the ΔfruA-ΔfrcA mutant, the rate of methanogenesis exceeded the rate of H2 production, which seemed to preclude the possibility that H2 could be an obligate intermediate of methanogenesis.
Methanogenesis is very O2 sensitive, and it is possible that the activity may have been damaged during preparation of the cell suspensions used in this experiment. Therefore, the rates of methanogenesis were also determined during growth of the wild type and mutants without preparation of resting cells. For the wild type, the specific activity for methanogenesis was low except during the exponential and linear growth phases (Fig. 6). For the ΔfruA-ΔfrcA and Δhmd mutants, growth and methanogenesis were nearly the same as the wild type. Even though the growth and CH4 production were delayed for the Δmtd mutant (Fig. 6), the maximum rate of methanogenesis during the exponential growth phase was nearly the same as that of the wild type. For this mutant, H2 must first accumulate in the medium to allow for activity of the low-affinity Hmd before growth commences (19). In conclusion, while the rate of H2 production measured with a H2 probe is too low to be an obligatory intermediate for methanogenesis from formate, it is still formally possible that a H2 cycle could still exist within the cell. In this case, the cellular H2 levels would not equilibrate with the bulk H2 dissolved in the medium. However, this possibility seems unlikely, given that inactivation of each of the major pathways of H2 production, either in the ΔfruA-ΔfrcA or the Δhmd and Δmtd mutants, had little effect on the cellular rate of methanogenesis from formate.
FIG. 6.
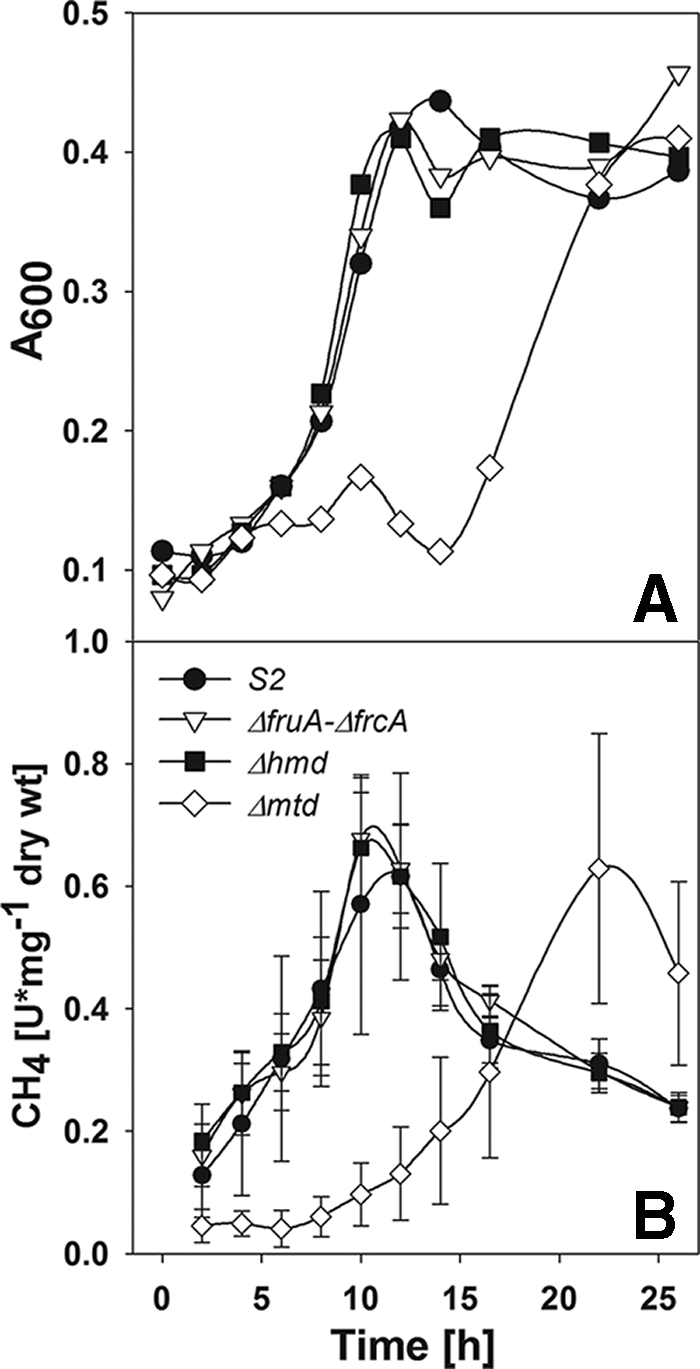
Growth (A) and specific activity of CH4 production (B) by the wild-type S2 (•), ΔfruA-ΔfrcA (▿), Δhmd, (▪), and Δmtd (⋄) mutant strains grown with formate (100 mM). Each point represents the mean value of three replicates, and error bars in panel B represent 1 standard deviation. Similar curves were obtained in a replicate experiment.
Recently, a novel hypothesis for energy conservation by the hydrogenotrophic methanogens was proposed (30). This model predicts that the exergonic H2-dependent reduction of the heterodisulfide is not membrane associated and does not generate a proton or sodium motive force. Instead, this exergonic reaction is directly coupled to the endergonic reduction of the low-potential ferredoxin required for CO2 reduction by flavin-mediated electron bifurcation. According to this model, one low-potential electron from H2 oxidation partially reduces the ferredoxin, while a high-potential electron partially reduces the heterodisulfide. An additional cycle is required to fully reduce both the ferredoxin and heterodisulfide (30). This model avoids the necessity of an energy-conserving hydrogenase, such as Eha, to reduce the low-potential ferredoxin. While the observation that H2 is not an obligate intermediate during growth with formate supports this model, it remains unclear how F420H2 generated from formate donates electrons for the flavin-mediated electron bifurcation. If H2 is not an intermediate, an enzymatic complex with the function of F420H2:flavin oxidoreductase would be required. At present, a candidate for this enzyme has not been identified, either from biochemical or genome analyses.
Possible applications.
The growing energy demand, environmental concerns, and limited resources of fossil fuels draw attention to H2, which is a clean and efficient energy source. Microbial H2 production has involved a range of approaches (8, 13, 14, 24). Methanogens are very active H2 consumers; if these systems could be used for H2 production, high rates would be possible. To test this concept, H2 production from formate by resting cells of methanococci was evaluated. Rates of formate-dependent H2 production have been previously examined in a few bacteria. The methylotrophs Methylomonas albus and Methylosinus trichosporium produced H2 at rates of 1.6 and 0.4 mU·mg−1 (dry weight), respectively, after 5 hours of incubation under anaerobic conditions (21). Similar low rates were observed for Shewanella oneidensis MR-1 (23) and Alcaligenes eutrophus (22). The highest rates of 1.7 to 4.2 U·mg−1 (dry weight) were obtained with genetically engineered Escherichia coli strains (35). The rates obtained from wild-type methanococci, up to 1.4 U·mg−1 (dry weight), were comparable. However, because of the equilibrium constant, high concentrations of H2 cannot be produced from formate regardless of the catalyst employed. Therefore, biotechnological applications would require an efficient means of harvesting H2 at low levels. Because F420H2 is a key intermediate in methanococcal H2 production, one might speculate that the substrate range could be increased by genetically engineering methanococci to couple the reduction of F420 to the oxidation of substrates other than formate.
While our studies were focused on M. maripaludis, other methanogens also possess many of the activities required for formate-dependent H2 production and could be candidates for biotechnological applications. Thus, use of thermophilic or freshwater species might extend this application to a much broader range of conditions. The use of formate-utilizing methanogens appears to be the optimal solution. In this approach the growth and H2 production are decoupled. In such bioreactors, formate first is used to obtain the cell mass. Then, by the continuous supply of this substrate, the cell mass could be an efficient catalyst for H2 production. For wild-type cells, the rates of formate-dependent H2 production are among the highest reported for prokaryotes. Further optimization of the growth and reaction conditions as well as genetic engineering could potentially increase these rates yet again. These observations open the possibilities for the use of methanococci in bioreactors for the generation of H2 from the relatively inexpensive chemical, which can be derived from biomass (35).
Acknowledgments
This work was supported by grant DE-FG02-05ER15709 from the U.S. Department of Energy Office of Basic Energy Sciences, Basic Research for the Hydrogen Fuel Initiative, to W.B.W. and J.A.L.
We thank Robert Maier and Stephane Benoit, Department of Microbiology, University of Georgia, for help with the H2 probe and Juergen Wiegel, of the same department, for valuable discussions. We also thank David Hong Phan for assistance with H2 measurements and Magdalena Sieprawska-Lupa for excellent assistance with F420 purification.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Afting, C., A. Hochheimer, and R. K. Thauer. 1998. Function of H2-forming methylenetetrahydromethanopterin dehydrogenase from Methanobacterium thermoautotrophicum in coenzyme F420 reduction with H2. Arch. Microbiol. 169:206-210. [DOI] [PubMed] [Google Scholar]
- 2.Afting, C., E. Kremmer, C. Brucker, A. Hochheimer, and R. K. Thauer. 2000. Regulation of the synthesis of H2-forming methylenetetrahydromethanopterin dehydrogenase (Hmd) and of HmdII and HmdIII in Methanothermobacter marburgensis. Arch. Microbiol. 174:225-232. [DOI] [PubMed] [Google Scholar]
- 3.Balch, W. E., G. E. Fox, L. J. Magrum, C. R. Woese, and R. S. Wolfe. 1979. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 43:260-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balch, W. E., and R. S. Wolfe. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron, S. F., and J. G. Ferry. 1989. Reconstitution and properties of a coenzyme F420-mediated formate hydrogenlyase system in Methanobacterium formicicum. J. Bacteriol. 171:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belay, N., R. Sparling, and L. Daniels. 1986. Relationship of formate to growth and methanogenesis by Methanococcus thermolithotrophicus. Appl. Environ. Microbiol. 52:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bleicher, K., and J. Winter. 1994. Formate production and utilization by methanogens and by sewage sludge consortia: interference with the concept of interspecies formate transfer. Appl. Microbiol. Biotechnol. 40:910-915. [Google Scholar]
- 8.Dutta, D., D. De, S. Chaudhuri, and S. K. Bhattacharya. 2005. Hydrogen production by cyanobacteria. Microb. Cell. Fact. 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eirich, L. D., G. D. Vogels, and R. S. Wolfe. 1979. Distribution of coenzyme F420 and properties of its hydrolytic fragments. J. Bacteriol. 140:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferry, J. G., and K. A. Kastead. 2007. Methanogenesis, p. 288-314. In R. Cavicchioli (ed.), Archaea: molecular and cellular biology. ASM Press, Washington, DC.
- 11.Ferry, J. G., and R. S. Wolfe. 1977. Nutritional and biochemical characterization of Methanospirillum hungatii. Appl. Environ. Microbiol. 34:371-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin, M. J., W. J. Wiebe, and W. B. Whitman. 1988. Populations of methanogenic bacteria in a Georgia salt marsh. Appl. Environ. Microbiol. 54:1151-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallenbeck, P. C. 2005. Fundamentals of the fermentative production of hydrogen. Water Sci. Technol. 52:21-29. [PubMed] [Google Scholar]
- 14.Hallenbeck, P. C., and J. R. Benemann. 2002. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 27:1185-1193. [Google Scholar]
- 15.Hanus, F. J., K. R. Carter, and H. J. Evans. 1980. Techniques for measurement of hydrogen evolution by nodules. Methods Enzymol. 69:731-737. [Google Scholar]
- 16.Hedderich, R. 2004. Energy-converting [NiFe] hydrogenases from archaea and extremophiles: ancestors of complex I. J. Bioenerg. Biomembr. 36:65-75. [DOI] [PubMed] [Google Scholar]
- 17.Hendrickson, E. L., A. K. Haydock, B. C. Moore, W. B. Whitman, and J. A. Leigh. 2007. Functionally distinct genes regulated by hydrogen limitation and growth rate in methanogenic Archaea. Proc. Natl. Acad. Sci. USA 104:8930-8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrickson, E. L., R. Kaul, Y. Zhou, D. Bovee, P. Chapman, J. Chung, E. Conway de Macario, J. A. Dodsworth, W. Gillett, D. E. Graham, M. Hackett, A. K. Haydock, A. Kang, M. L. Land, R. Levy, T. J. Lie, T. A. Major, B. C. Moore, I. Porat, A. Palmeiri, G. Rouse, C. Saenphimmachak, D. Soll, S. Van Dien, T. Wang, W. B. Whitman, Q. Xia, Y. Zhang, F. W. Larimer, M. V. Olson, and J. A. Leigh. 2004. Complete genome sequence of the genetically tractable hydrogenotrophic methanogen Methanococcus maripaludis. J. Bacteriol. 186:6956-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrickson, E. L., and J. A. Leigh. 2008. Roles of coenzyme F420-reducing hydrogenases and hydrogen- and F420-dependent methylenetetrahydromethanopterin dehydrogenases in reduction of F420 and production of hydrogen during methanogenesis. J. Bacteriol. 190:4818-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, J. B., and T. C. Stadtman. 1980. Reconstitution of a formate-NADP+ oxidoreductase from formate dehydrogenase and a 5-deazaflavin-linked NADP+ reductase isolated from Methanococcus vannielii. J. Biol. Chem. 255:1049-1053. [PubMed] [Google Scholar]
- 21.Kawamura, S., J. G. O′Neil, and J. F. Wilkinson. 1983. Hydrogen production by methylotrophs under anaerobic conditions. J. Ferment. Technol. 61:151-156. [Google Scholar]
- 22.Klibanov, A. M., B. N. Alberti, and S. E. Zale. 1982. Enzymatic synthesis of formic acid from hydrogen and carbon dioxide and production of hydrogen from formic acid. Biotechnol. Bioeng. 24:25-36. [DOI] [PubMed] [Google Scholar]
- 23.Meshulam-Simon, G., S. Behrens, A. D. Choo, and A. M. Spormann. 2007. Hydrogen metabolism in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 73:1153-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandi, R., and S. Sengupta. 1998. Microbial production of hydrogen: an overview. Crit. Rev. Microbiol. 24:61-84. [DOI] [PubMed] [Google Scholar]
- 25.Pennings, J. L., P. Vermeij, L. M. de Poorter, J. T. Keltjens, and G. D. Vogels. 2000. Adaptation of methane formation and enzyme contents during growth of Methanobacterium thermoautotrophicum (strain ΔH) in a fed-batch fermentor. Antonie van Leeuwenhoek 77:281-291. [DOI] [PubMed] [Google Scholar]
- 26.Porat, I., W. Kim, E. L. Hendrickson, Q. Xia, Y. Zhang, T. Wang, F. Taub, B. C. Moore, I. J. Anderson, M. Hackett, J. A. Leigh, and W. B. Whitman. 2006. Disruption of the operon encoding Ehb hydrogenase limits anabolic CO2 assimilation in the archaeon Methanococcus maripaludis. J. Bacteriol. 188:1373-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rother, M., I. Mathes, F. Lottspeich, and A. Bock. 2003. Inactivation of the selB gene in Methanococcus maripaludis: effect on synthesis of selenoproteins and their sulfur-containing homologs. J. Bacteriol. 185:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schauer, N. L., and J. G. Ferry. 1980. Metabolism of formate in Methanobacterium formicicum. J. Bacteriol. 142:800-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thauer, R. K. 1998. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Microbiology 144:2377-2406. [DOI] [PubMed] [Google Scholar]
- 30.Thauer, R. K., A. K. Kaster, H. Seedorf, W. Buckel, and R. Hedderich. 2008. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6:579-591. [DOI] [PubMed] [Google Scholar]
- 31.Wang, R. T. 1980. Amperometric hydrogen electrode. Methods Enzymol. 69:409-413. [Google Scholar]
- 32.Whitman, W. B., J. Shieh, S. Sohn, D. S. Caras, and U. Premachandran. 1986. Isolation and characterization of 22 mesophilic methanococci. Syst. Appl. Microbiol. 7:235-240. [Google Scholar]
- 33.Wood, G. E., A. K. Haydock, and J. A. Leigh. 2003. Function and regulation of the formate dehydrogenase genes of the methanogenic archaeon Methanococcus maripaludis. J. Bacteriol. 185:2548-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki, S. 1982. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J. Biol. Chem. 257:7926-7929. [PubMed] [Google Scholar]
- 35.Yoshida, A., T. Nishimura, H. Kawaguchi, M. Inui, and H. Yukawa. 2005. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl. Environ. Microbiol. 71:6762-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]