Abstract
Even though at least 400 Listeria phages have been isolated from various sources, limited information is available on phages from the food processing plant environment. Phages in the processing plant environment may play critical roles in determining the Listeria population that becomes established in the plant. In this study, we pursued the isolation of Listeria-specific phages from environmental samples from four turkey processing plants in the United States. These environmental samples were also utilized to isolate Listeria spp. Twelve phages were isolated and classified into three groups in terms of their host range. Of these, nine (group 1) showed a wide host range, including multiple serotypes of Listeria monocytogenes, as well as other Listeria spp. (L. innocua, L. welshimeri, L. seeligeri, and L. ivanovii). The remaining phages mostly infected L. monocytogenes serotype 4b as well as L. innocua, L. ivanovii, and/or L. welshimeri. All but one of the strains of the serotype 4b complex (4b, 4d, 4e) from the processing plant environment could be readily infected by the wide-host-range phages isolated from the environment of the processing plants. However, many strains of other serotypes (1/2a [or 3a] and 1/2b [or 3b]), which represented the majority of L. monocytogenes strains isolated from the environmental samples, were resistant to infection by these phages. Experiments with two phage-resistant strains showed reduced phage adsorption onto the host cells. These findings suggest that phage resistance may be an important component of the ecology of L. monocytogenes in the turkey processing plants.
Listeria monocytogenes is an important food-borne pathogen responsible for listeriosis, an illness with severe symptoms and relatively high mortality rates (20 to 30%). Individuals at risk are primarily pregnant women and their fetuses, immunocompromised patients, and the elderly. Most cases of human listeriosis involve bacteria of serotypes 1/2a, 1/2b, and 4b. The food processing plant environment is of key importance for the contamination of ready-to-eat foods by L. monocytogenes (15, 16). However, attributes of the bacteria that determine their distribution, prevalence, and persistence in the processing plant environment remain poorly characterized.
The ability of L. monocytogenes to form biofilms and to resist disinfectants commonly employed for processing plant sanitation has been postulated to be an important determinant of the organism's ecology in the processing plant environment (16). Differences in ability to form biofilms and to tolerate disinfectants used in processing plants may contribute to the higher relative prevalence of certain serotypes. In several studies, strains of serotype 1/2a were significantly more frequently isolated from environmental samples of processing plants than were strains of serotype 4b (17, 27, 30, 31). There is evidence that serotype 1/2a strains were more likely to form biofilms and to be resistant to the disinfectant benzalkonium chloride (BC) than were strains of serotype 4b (4, 24, 25).
Another potentially important determinant of Listeria's ecology in the processing plant (and other) environments would be expected to be the susceptibility of the organisms to Listeria-specific bacteriophage (listeriaphage). The first listeriaphage report was published in 1945 (28), and to date, at least 400 phages have been isolated from various sources, including foods, sewage, silage, and lysogenic strains (21). The differential susceptibility of Listeria strains to selected phages has been utilized extensively as a strain-typing tool in epidemiological studies (22). Recent studies suggest the potential of phages as biological control agents for Listeria in foods and in the processing plants (13, 18, 19), and in 2006, the U.S. FDA approved the application of a listeriaphage mixture on the surface of ready-to-eat meat and poultry products (11). GRAS (generally regarded as safe) status has been granted to listeriaphage P100, isolated from a sewage effluent sample of a dairy processing plant in Germany (5). P100 is a virulent, wide-host-range phage capable of infecting Listeria strains of various species and serotypes, similar to the previously characterized phage A511 (5, 23).
In spite of the evidence for listeriaphage in many environments, we currently have a limited understanding of the potential roles of phage in affecting the ecology and population structure of the pathogen in the processing plant environment. In this study, listeriaphages were isolated from environmental samples obtained from turkey processing plants in the United States. Listeria isolates from these processing plants were characterized in terms of their susceptibility to selected phages with wide host range to further understand the impact of phage on the ecology of L. monocytogenes in the processing plant.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Turkey processing plant environmental strains of L. monocytogenes used in this study are listed in Table 1. Several of these strains of the serotype 4b complex (serotype 4b and the closely related serotypes 4d and 4e) were described earlier (9, 10). Strains of the serotype 4b complex included several harboring genetic markers characteristic for epidemic clone I (ECI) and epidemic clone II (ECII), determined as described previously (7, 9). The strains were a subset of those characterized for resistance to heavy metals and to BC (25) and were chosen so as to represent different strain types (genomic fingerprints determined by pulsed-field gel electrophoresis by AscI and ApaI). The BC resistance phenotypes of the organisms were determined earlier (25) and are also indicated in Table 1. The isolates were obtained from August 2003 to March 2006 following USDA-FSIS isolation procedures (14) from five (A, B, C, D, and F) turkey processing plants in the United States, which were operated by different companies and were located in different, nonadjoining states. Putative serotypic designations were determined by means of multiplex PCR, with serotypic designations being 1/2a (or 3a), 1/2b (or 3b), 1/2c (or 3c), and the serotype 4b complex (4b, 4d, 4e) (8). A complete description of L. monocytogenes (including serotypes and genomic fingerprints) and other Listeria strains isolated from these plants is being prepared for publication elsewhere. In addition, a panel of strains representing different serotypes was used to determine the host range of the listeriaphage. These strains, along with representatives of other Listeria species, are part of our laboratory's Listeria strain collection and are listed in Table 2. Six of these strains were used as indicators to screen environmental samples for the presence of phage. These six strains were L. monocytogenes F2365 (serotype 4b; ECI), H7550 (serotype 4b; ECII), 4b1 (serotype 4b; sporadic), F6854 (serotype 1/2a), G3978 (serotype 1/2b), and WSLC 1001 (serotype 1/2c) (Table 2). Listeria strains were routinely grown in brain heart infusion (BHI; Difco, Sparks, MD) broth or BHI agar (BHI broth supplemented with 1.5% agar; Difco) at 37°C and preserved at −80°C in BHI broth with 20% glycerol.
TABLE 1.
Phage susceptibility of L. monocytogenes isolates from turkey processing plants
| Isolate no. (alternative designation) | Date (mo/yr) | Source or reference | Genetic markersa | BCb | Phage susceptibility toc:
|
||
|---|---|---|---|---|---|---|---|
| 20422-1 | 805405-1 | A511 | |||||
| Serotype 4b (n = 17) | |||||||
| 1493 (L0226) | 08/03 | Overhead pipe, plant B (9) | ECII | − | + | + | + |
| 532 | 09/03 | Floor, plant A | ND | − | + | + | + |
| 1106 | 09/03 | Floor drain, plant A | ECII | − | + | + | + |
| 1117d (34-6a) | 09/03 | Floor drain, plant A (9) | ECII | + | − | − | − |
| 1495 (L0315) | 10/03 | Floor, plant B (9) | ECII | − | + | + | + |
| 1497 (L0328) | 10/03 | Air-conditioning unit, plant B (9) | ECI | + | + | + | + |
| 1277 (82-2a) | 12/03 | Floor drain, plant A (9) | ND | − | + | + | + |
| 1501 (L0617) | 02/04 | Raw product, plant B (9) | ECI | − | + | + | + |
| 1498 (L0603) | 02/04 | Dip tank, plant B (9) | ECII | − | + | + | + |
| 80 (171A) | 04/04 | Floor drain, plant A (9) | ND | − | + | + | + |
| 1506 (L0704) | 05/04 | Sink and hose, plant B (9) | ECII | − | + | + | + |
| 1513 (L0719) | 05/04 | Floor, plant B (9) | ECII | − | + | + | + |
| 900 | 10/04 | Floor drain, plant A | ND | − | + | + | + |
| 1157 | 04/05 | Floor drain, plant A | ND | − | + | + | + |
| 2509 | 03/06 | Cage dumper slide, plant D | ND | − | + | + | + |
| 2688 | 03/06 | Conveyor belt, plant D | ND | − | + | + | + |
| 2616 | 03/06 | Table, plant A | ND | − | + | + | + |
| Total susceptibility | 16/17 (94%) | 16/17 (94%) | 16/17 (94%) | ||||
| Serotype 1/2a or 3a (n = 24) | |||||||
| 175 | 09/03 | Drain, plant A | − | + | + | + | |
| 483 | 04/04 | Roast drain, plant A | + | − | − | − | |
| 513 | 04/04 | Floor, plant A | − | − | − | − | |
| 10 | 04/04 | Floor drain, plant D | + | + | + | + | |
| 90 | 06/04 | Chiller rework drain, plant A | − | − | − | − | |
| 93 | 06/04 | Chiller drain, plant A | + | + | + | − | |
| 162 | 06/04 | Floor drain, plant A | + | + | + | − | |
| 627 | 06/04 | Ground-meat floor, plant A | + | − | − | − | |
| 653 | 06/04 | Boots, plant A | + | − | − | − | |
| 720 | 08/04 | Floor drain, plant A | + | − | − | − | |
| 884 | 10/04 | Chiller drain, plant A | + | + | + | + | |
| 1559 | 10/04 | Floor fecal, plant F | − | + | + | + | |
| 1566 | 10/04 | Raw product, plant F | − | + | + | + | |
| 1096 | 12/04 | Ground-meat floor, plant A | + | − | − | − | |
| 1637 | 01/05 | Raw product, plant B | − | + | + | + | |
| 1747 | 03/05 | Cart wheels, plant C | − | + | + | + | |
| 1845 | 07/05 | Raw product, plant B | − | + | + | + | |
| 1907 | 06/05 | Drains, plant F | + | + | + | + | |
| 2622 | 03/06 | Bucket, plant A | + | − | − | − | |
| 2627 | 03/06 | Rehang drain, plant A | − | − | − | − | |
| 2506 | 03/06 | Cages (live birds), plant D | − | + | + | + | |
| 2507 | 03/06 | Cages (live birds), plant D | − | − | − | + | |
| 2508 | 03/06 | CO2 table, plant D | − | + | + | + | |
| 2662 | 03/06 | Carcass (before wash), plant D | − | + | + | + | |
| Total susceptibility | 14/24 (58%) | 14/24 (58%) | 13/24 (54%) | ||||
| Serotype 1/2b or 3b (n = 16) | |||||||
| 1491 | 08/03 | Cart, plant B | − | + | + | + | |
| 174 | 09/03 | Floor, plant A | − | − | − | − | |
| 1104 | 09/03 | Drain, plant A | + | − | − | − | |
| 1499 | 02/04 | Cart, plant B | − | − | − | − | |
| 83 | 04/04 | Roast drain, plant A | + | − | − | − | |
| 523 | 04/04 | Floor drain, plant A | − | − | − | − | |
| 27 | 04/04 | Chiller, plant D | − | + | + | − | |
| 1505 | 05/04 | Floor drain, plant B | − | − | − | − | |
| 1507 | 05/04 | Floor drain, plant B | − | − | − | − | |
| 597 | 06/04 | Floor drain, plant A | + | − | − | − | |
| 172 | 06/04 | Boots, plant A | + | − | − | − | |
| 706 | 08/04 | Table, plant A | − | − | − | − | |
| 731 | 08/04 | Rehang catwalk, plant A | − | + | + | − | |
| 854 | 10/04 | Live hang sink, plant A | + | + | + | − | |
| 197 | 12/04 | Floor drain, plant D | + | + | + | − | |
| 1830 | 05/05 | Raw product, plant B | − | + | + | + | |
| Total susceptibility | 6/16 (37%) | 6/16 (37%) | 2/16 (12.5%) | ||||
| Serotype 1/2c or 3c (n = 3) | |||||||
| 130d | 07/04 | Raw product, plant D | + | − | − | − | |
| 961 | 10/04 | Boots, plant A | + | − | − | − | |
| 2642 | 03/06 | Floor drain, plant A | + | − | − | − | |
| Total susceptibility | 0/3 (0%) | 0/3 (0%) | 0/3 (0%) | ||||
Genetic markers for ECI and ECII were determined as described previously (7, 9). ND; not determined.
Resistance to benzalkonium chloride (BC) was described previously (25). +, BC resistant; −, BC sensitive.
+, phage sensitive; −, phage resistant.
In these strains, infections with high-titer preparations of 20422-1 and 805405-1 occasionally resulted in very opaque plaques which were difficult to detect unambiguously and could not be accurately enumerated. For this reason, strains 1117 and 130 were designated as phage resistant.
TABLE 2.
Host-range spectrum of the listeriaphage from the turkey processing plants
| Listeria strain | Genomic markersa | Listeriaphage susceptibility to indicated phage group based on host rangeb
|
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| L. monocytogenes serotype 4b | ||||
| F2381 | ECI | + | + | + |
| 4b1 | ND | + | + | + |
| 18-1a | ND | + | + | + |
| F2365 | ECI | + | + | + |
| G4021 | ECI | + | − | − |
| G3982 | ECI | + | + | + |
| G3992 | ECI | + | − | − |
| G4030 | ECI | + | − | − |
| G3987 | ECI | + | + | + |
| 2003-332 | ECI | + | − | − |
| 2004-287 | ECI | + | + | + |
| NC0065706 | ECI | + | + | + |
| H7550 | ECII | + | − | − |
| H7738 | ECII | + | − | − |
| 1106 | ECII | + | + | − |
| 1493 (L0226) | ECII | + | − | − |
| 1495 (L0315) | ECII | + | − | − |
| 1498 (L0603) | ECII | + | − | − |
| 1506 (L0704) | ECII | + | − | − |
| J1735 | ECII | + | − | − |
| J1815 | ECII | + | − | − |
| J1925 | ECII | + | − | − |
| Serotype 1/2a | ||||
| F6854 | ECIII | + | − | − |
| G3979 | ND | + | − | − |
| L. monocytogenes serotype 1/2b | ||||
| G3978 | ND | + | − | − |
| G4027 | ND | + | − | − |
| L. monocytogenes serotype 1/2c | ||||
| WSLC 1001 | + | − | + | |
| L. innocua | ||||
| L1307a | + | + | + | |
| L. welshimeri | ||||
| L1225 | + | + | − | |
| L. seeligeri | ||||
| SK2795 | + | − | − | |
| L. grayi | ||||
| SK2796 | − | − | − | |
| L. ivanovii | ||||
| SK2797 | + | + | + | |
ECIII, epidemic clone III; ND, not determined. Genetic markers and epidemiological backgrounds for ECI and ECII were determined as described (7, 9). L. monocytogenes F6854 was included as the reference strain for ECIII (26).
+, susceptibility; −, resistance. Group 1 includes phages 20119-1, 20125-1, 20131-1, 20422-1, 30208, 30210, 30211, 805405-1, and 820320-1; group 2 includes 30102-1 and 31056-1; group 3 includes 904401.
Listeriaphage isolation.
For listeriaphage isolations, we screened 113 environmental samples that were obtained from four turkey slaughter and processing plants in the United States (plants A, B, C, and H). Sites within the processing plants included floor drains, floor surfaces, surfaces of equipment and fixtures in the plants (sinks, fans, doors, and air-conditioning units), and boots and aprons of employees. The sites yielding phage-positive samples have been listed in Table 3. Samples from plant A (n = 49) were obtained, as described previously (9), using SpongeSicle swabs (Biotrace International BioProducts, WA) over six visits (at approximately 2-month intervals) from June 2004 to June 2005. Samples from plant B (n = 44) were obtained in two visits in May (n = 30) and June (n = 14) of 2005, and plant C and H provided samples at only one visit each—plant C in June 2005 (n = 11) and plant H in October 2004 (n = 9). The sampling sponges were immediately placed on ice and transported to the laboratory (overnight transport on wet ice was employed for samples that were collected from plants from states other than NC). Samples were typically processed for listeriaphage and/or Listeria within 48 h of collection.
TABLE 3.
Phage, L. monocytogenes, and Listeria spp. recovered from phage-positive environmental samples
| Sample collection date (mo/yr) | Source | Phage(s) | Listeria group |
|---|---|---|---|
| 06/04 | Floor drain 1, plant A | 20119-1, 20125-1, 20131-1 | L. monocytogenes (serotype 1/2a or 3a and serotype 1/2b or 3b) |
| 06/04 | Floor drain 2, plant A | 20422-1 | Listeria spp. |
| 08/04 | Boots 1, Plant A | 30102-1 | None |
| 08/04 | Floor drain, plant A | 30208, 30210, 30211 | L. monocytogenes (serotype 1/2b or 3b) |
| 08/04 | Boots 2, plant A | 31056-1 | Listeria spp. |
| 05/05 | Conveyor belt, plant B | 805405-1 | None |
| 05/05 | Aprons, plant B | 820320-1 | None |
| 06/05 | Floor drain, plant B | 904401 | Listeria spp. |
To isolate phage from environmental samples, the sampling sponges were immersed in 10 ml BHI broth; then, each sponge was repeatedly squeezed with forceps, and the tubes were vortexed for 5 min at room temperature. The suspension was filtered through a 0.22-μm filter (Millipore, Bedford, MA), and 100 μl of each filtrate was added to 3 ml BHI broth containing 10 mM CaCl2, along with 30 μl of an overnight broth culture of an indicator L. monocytogenes strain; each filtrate was tested in a separate tube with each of the six indicator strains. After incubation at 37°C overnight, each culture was centrifuged (13,000 rpm, 2 min) and filtered (0.22-μm filter), and this filtrate was used in a plaque assay with the same strain. If plaques were detected, phage was purified from a single plaque, in two consecutive infections, as described previously (1). Phage enumerations were performed as described previously (1), following incubations of the phage-bacterium mixtures at 37°C for 24 to 36 h.
Determination of phage susceptibility.
To determine phage susceptibility of the strains, a host-phage mixture spot assay was used first to screen the strains. Phage suspensions (45 μl BHI broth, 10 mM CaCl2, 5 μl phage solution with a titer of 107 PFU/ml) were prepared in each well of a 96-well microtiter plate, and 1 μl of the host culture (grown overnight at 37°C in BHI broth) was added to each well. After 30 min of incubation at 37°C, 5 μl of each phage-bacterium mixture was spotted onto Luria-Bertani (LB; Difco) agar (1.5%) supplemented with CaCl2 (final concentration, 10 mM); the agar plates were air dried in a laminar flow cabinet for 30 min immediately prior to use. The plates were incubated at 37°C overnight and evaluated for evidence of plaques or lack of growth within each spot. To confirm the results and enumerate plaques, the standard plaque assay was used, as described previously (1). Briefly, the host strains were grown overnight at 37°C in BHI broth, and 200 μl of the culture (108 CFU/ml) was mixed with 100 μl phage filtrate (107 PFU/ml) and CaCl2 (10 mM) in 3 ml LB soft agar (LB broth with 0.75% agar). The mixture was poured onto regular agar plates (LB broth with 1.5% agar) containing CaCl2 (10 mM). All experiments were repeated at least three times with phages 20422-1, 805405-1, and A511 (kindly provided by M. J. Loessner).
Statistical analysis.
Statistical analysis was performed with SAS version 9.1.3 (Cary, NC). Chi-square tests were utilized to determine the correlation between phage resistance and BC resistance.
Phage propagation and adsorption assays.
Phage lysates containing approximately 6 × 107 PFU/ml were prepared using L. monocytogenes DP-L862 (serotype 1/2a) as a host. This strain has been reported to not harbor inducible prophages and is highly effective for phage propagation (R. Calendar, personal communication). For phage propagation, an overnight culture of DP-L862 was diluted (1:100) into fresh BHI broth (10 ml), incubated at 37°C for 2 h (A600, 0.1 to 0.2) with shaking (120 rpm), and mixed with 200 μl phage suspension (107 PFU/ml) and CaCl2 (final concentration, 10 mM). After 6 hours of further incubation at 37°C (without shaking), a phage lysate was obtained by centrifugation (8,000 rpm for 10 min at 4°C) and filtration (0.22-μm filter). Phage titers were determined following infection of DP-L862 and enumeration of plaques. For phage adsorption assays, host cells were infected as described above and at specific times after infection (0, 0.5, 1.5, 3.5, 6, and 10 h); culture supernatant (150 μl) was obtained by centrifugation and filtered as described above. Unadsorbed phage remaining in this filtrate was enumerated as described above, using L. monocytogenes F2365 as the host.
Nucleotide sequence determinations of genomic fragments of phages 20422-1 and 805405-1.
Phage 20422-1 DNA was purified from phage isolated following infections of L. monocytogenes DP-L862 using the Lambda miniprep phage extraction kit (Qiagen, Valencia, CA). The DNA was digested with NheI (New England Biolabs, Waverly, MA) and cloned into pUC19 (Promega, Madison, WI) digested with XbaI (New England Biolabs). The recombinant plasmids were transformed into competent Escherichia coli DH5α cells, and transformants were selected using ampicillin (100 μg/ml) and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Inserts from 16 randomly chosen white colonies were amplified using M13 forward (5′-GTAAAACGACGGCCAGT-3′) and reverse (5′-GTCCTTTGTCGATACTG-3′) primers, and insert sizes were estimated following electrophoresis in agarose gels. The three largest amplicons (ca. 1.2 kb, detected in two transformants; ca. 1.5 kb, detected in four transformants; and ca. 2 kb, detected in three transformants) were purified (gel extraction kit; Qiagen) and sequenced (Davis Sequencing, Davis, CA) with M13 forward and reverse primers. Following analysis of the sequence data that revealed strong homology with P100, additional sequence information of 20422-1 and 805405-1 was obtained from eight amplicons from each phage, using primer pairs corresponding to different locations on the annotated P100 genome (GenBank accession number DQ004855), specifically gp10, gp17, gp68, gp89, gp108, gp165-tRNA-pro, gp173-tRNA-Cys, and gp170 (primer sequences available on request). Nucleotide and deduced polypeptide sequences were analyzed using BLAST (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi).
Restriction analysis of 20422-1 and 805405-1.
Phage DNA isolated as described above was digested with SacI (New England Biolabs) under conditions suggested by the vendor and electrophoresed in 1% agarose (90 mM Tris-borate, 2 mM EDTA [pH 8.0]). SacI-digested DNA of P100 (kindly provided by Steven Hagens) was used for comparisons.
Sequence of gtcA in phage-sensitive and -resistant strains.
The cell wall teichoic acid glycosylation protein gene (gtcA) was amplified from genomic DNA of several L. monocytogenes isolates (serotypes 1/2a and 1/2b) that were sensitive or resistant to infection by 20422-1, 805405-1, and A511. DNA was extracted as described previously (6) using the DNeasy tissue kit (Qiagen). The primers were VCpNP95_1/2F (5′-ATAAGCGGCCGCATTAATTTGGCTCTTGAAGGAATTAC-3′) (designed for this study) and VCpNP95R (5′-ATAACCCGGGGTACTCAGGATGAATTCCAG-3′) (6). The PCR was programmed to run at 95°C for 3 min, followed by 34 cycles of 94°C for 30 s, 52°C for 1 min, and 72°C for 90 s, with a final 7-min extension at 72°C. PCR amplicons (871 bp) were purified as described above and sequenced (Davis Sequencing) using primer VCpNP95_1/2F. Nucleotide sequence alignments with gtcA of L. monocytogenes EGD-e (serotype 1/2a; GenBank accession number AL591824) were done with BioEdit (http://www.mbio.ncsu.edu/BioEdit/BioEdit.html).
Nucleotide sequence accession numbers.
The nucleotide sequences of the sequenced genomic fragments from phages 20422-1 and 805405-1 have been deposited in GenBank under accession numbers FJ147488 to FJ147506.
RESULTS
Listeriaphage isolation.
Twelve phage isolates were recovered from 8 of the 113 environmental samples that were analyzed (7.1% phage recovery frequency). Of the eight phage-positive samples, five were from plant A (representing 10.2% of the 49 samples tested from that plant) and three from plant B (6.8% of the 44 samples that were screened). The plant A samples that yielded phage were from two of the six visits, in June and August 2004. Two of the phage-positive samples from plant A, both from floor drains, also yielded L. monocytogenes with serotypes 1/2a (or 3a) and/or 1/2b (or 3b) (Table 3). The other three plant A samples were either negative for Listeria or yielded Listeria spp. other than L. monocytogenes. None of the three phage-positive samples from plant B yielded L. monocytogenes, and only one was positive for other Listeria spp. (Table 3). L. monocytogenes and other Listeria spp. were isolated from 12.4% and 23.9% of the 113 samples, respectively, with 4.4% of the samples yielding both L. monocytogenes and isolates of other Listeria spp. (Table 4). Neither phage nor L. monocytogenes were isolated from any of the samples from plants C and H; however, some of these samples yielded other Listeria spp. (Table 4).
TABLE 4.
Recovery of Listeria spp. and L. monocytogenes from samples screened for listeriaphage
| Plant | No. of samples | No. (%) of samples positive for:
|
|||
|---|---|---|---|---|---|
| Phage | L. monocytogenesa | Other Listeria spp.b | Listeria complexc | ||
| A | 49 | 5 (10.2) | 13 (26.5) | 10 (20.4) | 3 (6.1) |
| B | 44 | 3 (6.8) | 1 (2.3) | 13 (30) | 2 (4.5) |
| C | 11 | 0 | 0 | 1 (9.0) | 0 |
| H | 9 | 0 | 0 | 3 (33.3) | 0 |
| Total | 113 | 8 (7.1) | 14 (12.4) | 27 (23.9) | 5 (4.4) |
Samples yielded only L. monocytogenes.
Samples yielded only other Listeria spp. (no L. monocytogenes).
Samples yielded Listeria complex, including both L. monocytogenes and other Listeria spp.
On the basis of their host range against a panel of Listeria strains representing different species and serotypes, the 12 phages were classified into three groups (Table 2). The majority of the phages (n = 9) belonged to group 1 and had broad host range, being able to infect L. monocytogenes strains of multiple serotypes, as well as strains representing other Listeria spp., except for Listeria grayi. Phages in this group were isolated from samples derived from both plant A and plant B (Tables 2 and 3). The phages of groups 2 and 3 infected only a subset of L. monocytogenes, primarily of the serotype 4b complex, as well as L. innocua, L. ivanovii, and/or L. welshimeri (Table 2).
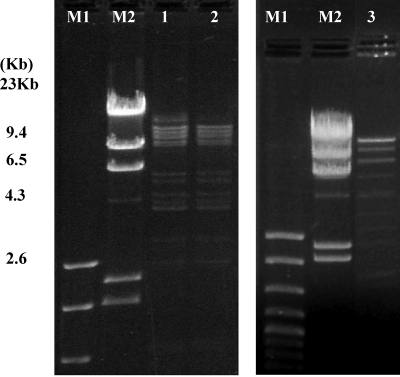
Two wide-host-range phages, 20422-1 and 805405-1, that had identical host ranges but were obtained at different times and from two different plants (plants A and B, respectively) (Table 3) were chosen for further characterization. Nucleotide sequences of 11 different fragments of the phage 20422-1 genome revealed high homology with fragments of the genome of the broad-host-range virulent listeriaphages P100 and A511 (93 to 100% and 90 to 99%, respectively) (Table 5). In addition, we determined the nucleotide sequences of eight of these fragments in the genome of phage 805405-1. The nucleotide sequences of all eight fragments were identical between phages 20422-1 and 805405-1 (Table 5). The two phages also yielded identical DNA fingerprints following digestion with SacI, whereas no similarity was detected with the SacI profile of P100 (Fig. 1) or A511 (as predicted by the distribution of SacI sites in the A511 genome) (data not shown).
TABLE 5.
Sequence similarities in selected genomic fragments of 20422-1, 805405-1, A511, and P100a
| Genomic fragment | Size (kb) | % Identity with:
|
% Identity between 20422-1 and 805405-1d | |||
|---|---|---|---|---|---|---|
| P100
|
A511
|
|||||
| DNA | A.A | DNA | A.A | |||
| gp05-06 | 1.6 | 97 | 100 | 97 | 100 | ND |
| gp28-29 | 2.2b | 97/98 | 99/99 | 99/98 | 99/97 | ND |
| gp29-30 | 1.2 | 100 | 100 | 99 | 100 | ND |
| gp10 | 0.9 | 98 | 99 | 98 | 99 | 100 |
| Cps | 1.0 | 97 | 100 | 98 | 100 | 100 |
| gp68 | 0.6 | 97 | 99 | 97 | 99 | 100 |
| gp89 | 0.7 | 96 | 97 | 96 | 96 | 100 |
| gp108 | 0.32 | 93 | 94 | 90 | 90 | 100 |
| gp165-tRNA-pro | 1.0 | 97 | 95 | 97c | 97 | 100 |
| tRNA-cys-gp173 | 1.0 | 97 | 96 | 97 | 96 | 100 |
| gp170 | 0.27 | 94 | 93 | 94 | 93 | 100 |
| Average | 96.7 | 97.5 | 96.6 | 97.1 | 100 | |
gp05-06, gp28-29, and gp29-30 fragments were obtained from the 20422-1 plasmid library; the other eight were obtained by PCR. A.A, amino acid.
Sequence was determined for 1,966 nucleotides (nt) of this fragment (988 and 978 nt at each of the ends).
In the genome of A511, the corresponding gene (A511_gp176) lacks an internal stretch of 38 nt (present in P100, 20422-1, and 805405-1).
ND, not determined. The sequences of the gp05-06, gp28-29, and gp29-30 fragments were determined only from 20422-1.
FIG. 1.
Genomic profiles of DNA from 20422-1, 805405-1, and P100 following digestion with SacI. Lanes M1 and M2, size markers exACTGene (Fisher Scientific, Fairlawn, NJ) and DNA molecular-weight marker II (Roche, Indianapolis, IN), respectively; lanes 1 to 3, SacI-digested DNA from 20422-1, 805405-1, and P100, respectively.
L. monocytogenes serotypes 1/2a (or 3a) and 1/2b (or 3b) from environmental samples were often resistant to the broad-host-range phages.
To determine whether the broad-host-range phages isolated here could also infect isolates of L. monocytogenes from samples similar to those that yielded phage, we investigated the susceptibilities of selected environmental strains. We determined the ability of phages 20422-1 and 805405-1 to infect a panel of L. monocytogenes strains from turkey processing plants, including plants A and B, representing different serotypes and genomic fingerprints. Noticeable differences in susceptibility profiles were noted depending on the serotype of the isolates. With one exception (strain 1117), strains of the serotype 4b complex (including those harboring ECI and ECII genetic markers) were susceptible to phages 20422-1 and 805405-1 (Table 1). In contrast, 10 (42%) and 10 (63%) of the strains of serotype 1/2a (or 3a) and serotype 1/2b (or 3b), respectively, were resistant to both phages (20422-1 and 805405-1), failing to form plaques upon infection. All three available environmental strains of serotype 1/2c (or 3c) were resistant to the phages. All strains were found to have identical susceptibility profiles with phages 20422-1 and 805405-1 (Table 1). The prevalence of resistance to the phages in strains of different serotypes is also summarized in Table 6. Efficiency of plaque formation did not vary markedly among the susceptible strains, with the exception of those harboring the ECII genetic markers; these strains had ca. 2- to 102-fold lower efficiency of plaque formation than other strains (data not shown).
TABLE 6.
Prevalence of phage-resistant strains among different serotypes of L. monocytogenes from the turkey processing plant environment
| Serotype (no. of strains) | No. (%) of strains resistant to phage:
|
||
|---|---|---|---|
| 20422-1 | 805405-1 | A511 | |
| 4b complex (17) | 1 (6) | 1 (6) | 1 (6) |
| 1/2a or 3a (24) | 10 (42) | 10 (42) | 11 (46) |
| 1/2b or 3b (16) | 10 (63) | 10 (63) | 14 (87.5) |
| 1/2c or 3c (3) | 3 (100) | 3 (100) | 3 (100) |
To determine whether strains resistant to these phages were also resistant to other broad-host-range listeriaphage, the bacteria were also infected with A511. Of the 10 serotype 1/2a (or 3a) strains that were resistant to 20422-1 and 805405-1, 9 were also resistant to A511. Two strains were susceptible to 20422-1 and 805405-1 but resistant to A511 (Table 1). In the case of serotype 1/2b (or 3b) strains, all 10 that were resistant to 20422-1 and 805405-1 were also resistant to A511. Four strains were resistant to A511 but susceptible to 20422-1 and 805405-1. Thus, only 2 of the 16 tested strains of serotype 1/2b (or 3b) were susceptible to all three broad-host-range phages that were tested. Phage susceptibilities of serotype 1/2c (or 3c) isolates and of serotype 4b isolates yielded consistent results with 20422-1, 805405-1, and A511 (Table 1). The serotype-associated susceptibility data have been summarized in Table 6. Serotype 4b isolates with ECII genetic markers had a lower efficiency of plaque formation with A511 (data not shown), as was also observed (and described above) with 20422-1 and 805405-1.
Phages failed to adsorb on resistant isolates of serotypes 1/2a (or 3a) and 1/2b (or 3b).
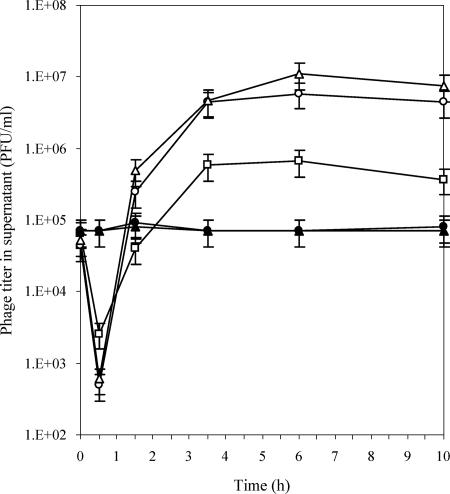
To determine whether the observed resistance of certain serotype 1/2a (or 3a) or 1/2b (or 3b) isolates to 20422-1 and 805405-1 was associated with failure of the phage to adsorb onto the host cells, phage adsorption assays were employed with the phage-resistant strains 513 (serotype 1/2a or 3a, plant A) and 597 (serotype 1/2b or 3b, plant A). The phage-susceptible strains 1845 (1/2a or 3a, plant B) and 27 (1/2b or 3b, plant D) were used as controls, along with L. monocytogenes F2365 (serotype 4b) (Fig. 2). Enumeration of phage particles remaining in the supernatants 30 min after infection indicated that the phage failed to adsorb onto the resistant strains 513 and 597; in contrast, assays with the susceptible strains indicated that phage adsorption took place, as expected (Fig. 2). When the supernatants were monitored at subsequent time points (up to 10 h), it was noted that phage was amplified when phage-susceptible isolates were used as hosts, as expected; in contrast, infections of L. monocytogenes 513 and 597 did not result in detectable amplification of the phage, in agreement with the observed phage resistance of these organisms (Fig. 2).
FIG. 2.
Assay for adsorption of phage 20422-1 on phage-susceptible and phage-resistant L. monocytogenes strains. Open and filled symbols indicate phage-susceptible and -resistant strains, respectively. Phage-susceptible strains 1845 (serotype 1/2a; open triangles) and 27 (serotype 1/2b; open squares) and phage-resistant strains 513 (serotype 1/2a; closed circles) and 597 (serotype 1/2b; closed triangles) were infected with phage 20422-1 propagated in L. monocytogenes DP-L862. L. monocytogenes F2365 (serotype 4b; open circles) was used as the reference strain. Phage titer (PFU/ml) in the supernatant was determined at the indicated times as described in Materials and Methods.
gtcA sequences did not differ between phage-susceptible and -resistant isolates of serotypes 1/2a (or 3a) and 1/2b (or 3b).
In L. monocytogenes EGD-e (serotype 1/2a), inactivation of gtcA resulted in resistance to phage LMUP121, which could infect strains of serotypes 1/2a, 1/2b, and 4b (3, 12). Phage 20422-1 was also unable to infect a gtcA mutant of L. monocytogenes EGD-e (data not shown). To determine whether phage resistance among our isolates was associated with mutations in gtcA, the gene was amplified and sequenced from three phage-resistant L. monocytogenes environmental strains, including two (720 and 513) of serotype 1/2a (or 3a) and one (1499) of serotype 1/2b (or 3b). The gtcA sequences were also determined for three phage-susceptible strains: L. monocytogenes strains 175 and 10 of serotype 1/2a (or 3a) and strain 1491 of serotype 1/2b (or 3b). Sequence alignments revealed that gtcA of all four serotype 1/2a (or 3a) strains had 100% identity with gtcA of L. monocytogenes EGD-e (serotype 1/2a), regardless of whether the strains were resistant or susceptible to the phage (data not shown). The gtcA sequence of the phage-resistant serotype 1/2b (or 3b) strain 1499 had 98% identity at the nucleotide sequence level with gtcA of L. monocytogenes EGD-e, but the deduced polypeptides had 100% sequence identity. Only one amino acid substitution was detected among the six strains (in the phage-susceptible strain 1491).
BC resistance among phage-resistant strains.
An earlier report indicated that L. monocytogenes strains resistant to quaternary ammonium compounds were more likely to be phage resistant than other strains (24). In our study, we found that the majority (8/12 [67%]) of the serotype 1/2a (or 3a) isolates that were resistant to two or more of the phages were also BC resistant. The inverse was found for serotype 1/2a (or 3a) strains that were susceptible to all three phages; 9/12 (75%) of these strains were susceptible to BC (Table 1). The difference in prevalence of BC resistance among phage-resistant versus phage-susceptible strains of serotype 1/2a (or 3a) was statistically significant (P < 0.05). In the case of serotype 1/2b (or 3b) strains, 6/14 (43%) of those that were phage resistant were BC resistant as well. However, associations between phage resistance and BC resistance were hard to ascertain among strains of this serotype, since only two were susceptible to all three phages (both were also BC susceptible) (Table 1). Prevalence of phage resistance among strains of the serotype 4b complex was too low to determine any correlations with BC resistance (Table 1).
DISCUSSION
Even though numerous listeriaphage have been isolated from sources such as silage, sewage, and Listeria lysogens (2, 12, 20, 22), reports on phages from the environment of food processing plants have been lacking. In this study, the majority of the phage isolates from the environment of turkey processing plants were Listeria genus-specific, being able to infect L. monocytogenes strains of different serotypes and strains of other Listeria spp., except for L. grayi. The wide host range of the phages was similar to those of A511 and P100, both of which were previously isolated in Europe (5, 23). Interestingly, 20422-1 and 805405-1 were isolated from processing plants located in two different (nonadjoining) states in the United States, but they featured exactly the same host-range spectrum. Furthermore, analysis of selected fragments of the genome of 20422-1 and 805405-1 revealed that the sequences were identical between these two phages, as were their SacI restriction profiles. The nucleotide sequences from 20422-1 and 805405-1 were highly conserved (overall 90 to 100% identity) with homologous DNA sequences of P100 and A511. Such findings suggest the widespread presence of a family of genetically closely related wide-host-range phages capable of infecting strains of different L. monocytogenes serotypes and different Listeria species. Isolation and characterization of additional Listeria genus-specific phages from other locations and habitats will be required to adequately assess the extent of genomic diversity among such phages.
Another important finding of this study was that, even though 20422-1 and 805405-1 infected most L. monocytogenes strains in the original screening panel, a significant fraction of strains from the processing plant environment were resistant to these phages. This was especially the case for strains of serotype 1/2a (or 3a), 42% of which were resistant to both 20422-1 and 805405-1, and for strains of serotype 1/2b (or 3b), the majority of which (63%) were resistant. Furthermore, the majority of the strains resistant to 20422-1 and 805405-1 were also resistant to A511. However, six strains were susceptible to 20422-1 and 805405-1 but resistant to A511, and the reverse was observed for one strain. These differences in the host-range spectrum suggest that 20422-1 and 805405-1 are distinct from A511 in terms of their potential roles in the ecology of Listeria, in spite of the similarity suggested by the available nucleotide sequence data.
The observed serotype-associated differences in prevalence of resistance to phages 20422-1 and 805405-1 (as well as A511) among the environmental strains investigated here suggest an important ecological role for wide-host-range phage in the processing plant environment. We have found that strains of serotype 1/2a (or 3a) were the majority of L. monocytogenes isolates (48%) in the turkey processing plants that we had surveyed, followed by strains of serotype 1/2b (or 3b) (39%) (R. M. Siletzky and S. Kathariou, unpublished findings). Several other investigations of L. monocytogenes from the environment of food processing plants have also shown that strains of serotypes 1/2a and 1/2b were markedly more predominant than strains of serotype 4b (17, 27, 30, 31). One may speculate that strains of serotypes with a higher prevalence of resistance to wide-host-range phages (such as the wide-host-range phages investigated here) would be at an advantage in the processing plant environment and would tend to predominate, thus contributing to the observed differential prevalence of different serotypes.
In our study, strains of the serotype 4b complex were typically susceptible to all three phages, with only one strain, 1117, found to be resistant. A study of L. monocytogenes samples from ready-to-eat foods indicated that five of the seven tested strains of the serotype 4b complex were resistant to a phage cocktail, whereas 14 of 34 (41.2%) serotype 1/2a (or 3a) strains and 11 of 51 (20%) serotype 1/2b (or 3b) strains were resistant (29). Several reasons may account for the differences between these findings and those from our study, including the different origins of the organisms (processing plant environment versus ready-to-eat foods), different strains, and different phages.
Listeria ecology in the processing plant environment is complex and influenced by several attributes. Analysis of 192 isolates from the processing plant environment, including those analyzed here for phage susceptibility, revealed that resistance to the heavy metal cadmium and to the quaternary ammonium disinfectant BC was noticeably higher among strains of serotypes 1/2a (or 3a) and 1/2b (or 3b) than among those of the serotype 4b complex (25). Disinfectant resistance, phage resistance, and other adaptations (e.g., biofilm formation) may all contribute to the observed prevalence of certain serotypes of L. monocytogenes in the processing plant environment and to the extensively documented persistence of certain strains (15, 16).
Several diverse mechanisms have been identified as being responsible for phage resistance in bacteria, including failure of the phage to adsorb, inhibition of phage DNA injection, restriction/modification systems, lysogeny with a similar phage, and abortive infections (32). Our findings with two phage-resistant strains (513 and 597) indicated that the phage failed to adsorb, suggesting that these strains lacked phage receptors. Sequencing of gtcA, previously implicated in phage adsorption in L. monocytogenes (3, 6), failed to identify differences between phage-sensitive and -resistant strains. The receptor for phage A511 has been reported to be peptidoglycan (33), whereas that for 20422-1 may be different and remains unknown (6). Further studies are needed to elucidate possible cell wall alterations in the phage-resistant strains investigated here and also to determine whether alternative mechanisms also contribute to resistance in such strains.
In an earlier study, an association was reported between resistance to quaternary ammonium compounds, such as BC, among serotype 1/2a and 1/2c isolates of environmental or food origin and phage nontypability of the isolates (suggesting resistance to the phages used in the phage typing panel) (24). Examination of BC resistance among our strains suggested that such resistance was indeed more prevalent among phage-resistant strains of serotype 1/2a (or 3a) than among those that were susceptible to all three phages that were tested. Preliminary data from our laboratory indicate that BC resistance in these strains is associated with a plasmid-borne efflux system (10); however, these phage-resistant strains may harbor additional attributes contributing to BC resistance, e.g., cell surface changes such as those proposed earlier (24).
In conclusion, we have provided evidence for the presence of Listeria genus-specific phages in the processing plant ecological system and documented the frequent occurrence of phage resistance among strains of serotypes which are prevalent in the processing plant environment, specifically serotypes 1/2a (or 3a) and 1/2b (or 3b). Such data indicate that strains of these serotypes may pose special challenges to the use of phage as biocontrol in the processing plant environment. Further studies are needed to characterize the ecological role of these phages in the processing plant environment and to elucidate mechanisms underlying the apparent scarcity of resistance among strains of the serotype 4b complex, which have been responsible for the majority of food-borne outbreaks of listeriosis.
Acknowledgments
This project was partially funded by USDA grant 2006-35201-17377. Isolation and serotyping of the L. monocytogenes isolates from the processing plants were done in association with a project funded by the USDA National Alliance for Food Safety and Security (NAFSS) as a cooperative agreement with USDA-ARS. The NAFSS project involved collaborations among S. Kathariou and L.-A. Jaykus (North Carolina State University), J. Eifert (Virginia Tech), E. Ryser (Michigan State University), R. Meinersmann (USDA-ARS, Athens, GA), and M. Berrang (USDA-ARS, Athens, GA).
We are grateful to Steven Hagens for the gift of phage P100 for comparative analysis of SacI restriction profiles of 20422-1, 805405-1, and P100 and to Martin Loessner for the gift of phage A511. We thank both S. Hagens and M. Loessner for critical feedback on the manuscript. We are grateful to Driss Elhanafi for assistance in the cloning of phage DNA fragments, to S. Romine and S. Kernodle for plant sampling and Listeria isolates, to S. Mullapudi for sharing of genome fingerprinting data, and to all members of our laboratory for discussions, encouragement, and support in the course of this project.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Adams, M. H. 1959. Methods of study of bacterial viruses, p. 443-457. In M. H. Adams (ed.), Bacteriophages. Interscience Publishers, Inc., New York, NY.
- 2.Audurier, A., and C. Martin. 1989. Phage typing of Listeria monocytogenes. Int. J. Food Microbiol. 8:251-257. [DOI] [PubMed] [Google Scholar]
- 3.Autret, N., I. Dubail, P. Trieu-Cuot, P. Berche, and A. Charbit. 2001. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69:2054-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borucki, M. K., J. D. Peppin, D. White, F. Loge, and D. R. Call. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl. Environ. Microbiol. 69:7336-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlton, R. M., W. H. Noordman, B. Biswas, E. D. de Meester, and M. J. Loessner. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43:301-312. [DOI] [PubMed] [Google Scholar]
- 6.Cheng, Y., N. Promadej, J. W. Kim, and S. Kathariou. 2008. Teichoic acid glycosylation mediated by gtcA is required for phage adsorption and susceptibility of Listeria monocytogenes serotype 4b. Appl. Environ. Microbiol. 74:1653-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, Y., R. M. Siletzky, and S. Kathariou. 2008. Genomic divisions/lineages, epidemic clones, and population structure, p. 337-358. In D. Liu (ed.), Handbook of Listeria monocytogenes. CRC Press, Boca Raton, FL.
- 8.Doumith, M., C. Buchrieser, P. Glaser, C. Jacquet, and P. Martin. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 42:3819-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eifert, J. D., P. A. Curtis, M. C. Bazaco, R. J. Meinersmann, M. E. Berrang, S. Kernodle, C. Stam, L. A. Jaykus, and S. Kathariou. 2005. Molecular characterization of Listeria monocytogenes of the serotype 4b complex (4b, 4d, 4e) from two turkey processing plants. Foodborne Pathog. Dis. 2:192-200. [DOI] [PubMed] [Google Scholar]
- 10.Elhanafi, D., and S. Kathariou. 2007. Genetic characterization of benzalkonium chloride resistance mechanism in the food-borne pathogen Listeria monocytogenes, abstr. P73. International Symposium on Problems of Listeriosis XVI, Savannah, GA.
- 11.FDA. 2006. Food additives permitted for direct addition to food for human consumption; bacteriophage preparation. Fed. Regist. 71:47729-47732. [PubMed] [Google Scholar]
- 12.Hodgson, D. A. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312-323. [DOI] [PubMed] [Google Scholar]
- 13.Hudson, J. A., C. Billington, G. Carey-Smith, and G. Greening. 2005. Bacteriophages as biocontrol agents in food. J. Food Prot. 68:426-437. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, J. 1998. Isolation and identification of Listeria monocytogenes from meat, poultry, and egg products. In U.S. Department of Agriculture Food Safety and Inspection Service microbiology laboratory guidebook, 3rd ed. U.S. Department of Agriculture Food Safety and Inspection Service, Washington, DC.
- 15.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 16.Kornacki, J. L., and J. B. Gurtler. 2007. Incidence and control of Listeria in food processing facilities, p. 681-766. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis and food safety, 3rd ed. CRC Press, Boca Raton, FL.
- 17.Lawrence, L. M., and A. Gilmour. 1995. Characterization of Listeria monocytogenes isolated from poultry products and from the poultry-processing environment by random amplification of polymorphic DNA and multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 61:2139-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leverentz, B., W. S. Conway, M. J. Camp, W. J. Janisiewicz, T. Abuladze, M. Yang, R. Saftner, and A. Sulakvelidze. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leverentz, B., W. S. Conway, W. Janisiewicz, and M. J. Camp. 2004. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Prot. 67:1682-1686. [DOI] [PubMed] [Google Scholar]
- 20.Loessner, M. J. 1991. Improved procedure for bacteriophage typing of Listeria strains and evaluation of new phages. Appl. Environ. Microbiol. 57:882-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loessner, M. J., and C. E. D. Rees. 2005. Listeria phages: basics and applications, p. 362-379. In M. K. Waldor, D. I. Friedman, and S. L. Adhya (ed.), Phages: their role in bacterial pathogenesis and biotechnology, 1st ed. ASM Press, Washington, DC.
- 22.Loessner, M. J., and M. Busse. 1990. Bacteriophage typing of Listeria species. Appl. Environ. Microbiol. 56:1912-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loessner, M. J., L. A. Estela, R. Zink, and S. Scherer. 1994. Taxonomical classification of 20 newly isolated Listeria bacteriophages by electron microscopy and protein analysis. Intervirology 37:31-35. [DOI] [PubMed] [Google Scholar]
- 24.Mereghetti, L., R. Quentin, N. Marquet-Van Der Mee, and A. Audurier. 2000. Low sensitivity of Listeria monocytogenes to quaternary ammonium compounds. Appl. Environ. Microbiol. 66:5083-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mullapudi, S., R. M. Siletzky, and S. Kathariou. 2008. Heavy-metal and benzalkonium chloride resistance of Listeria monocytogenes isolates from the environment of turkey-processing plants. Appl. Environ. Microbiol. 74:1464-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson, K. E., D. E. Fouts, E. F. Mongodin, J. Ravel, R. T. DeBoy, J. F. Kolonay, D. A. Rasko, S. V. Angiuoli, S. R. Gill, I. T. Paulsen, J. Peterson, O. White, W. C. Nelson, W. Nierman, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, D. H. Haft, J. Selengut, S. Van Aken, H. Khouri, N. Fedorova, H. Forberger, B. Tran, S. Kathariou, L. D. Wonderling, G. A. Uhlich, D. O. Bayles, J. B. Luchansky, and C. M. Fraser. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32:2386-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojeniyi, B., H. C. Wegener, N. E. Jensen, and M. Bisgaard. 1996. Listeria monocytogenes in poultry and poultry products: epidemiological investigations in seven Danish abattoirs. J. Appl. Bacteriol. 80:395-401. [DOI] [PubMed] [Google Scholar]
- 28.Schultz, E. W. 1945. Listerella infections: a review. Stanford Med. Bull. 3:135-151. [Google Scholar]
- 29.Shen, Y., Y. Liu, Y. Zhang, J. Cripe, W. Conway, J. Meng, G. Hall, and A. A. Bhagwat. 2006. Isolation and characterization of Listeria monocytogenes isolates from ready-to-eat foods in Florida. Appl. Environ. Microbiol. 72:5073-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soumet, C., C. Ragimbeau, and P. Maris. 2005. Screening of benzalkonium chloride resistance in Listeria monocytogenes strains isolated during cold smoked fish production. Lett. Appl. Microbiol. 41:291-296. [DOI] [PubMed] [Google Scholar]
- 31.Thevenot, D., M. L. Delignette-Muller, S. Christieans, S. Leroy, A. Kodjo, and C. Vernozy-Rozand. 2006. Serological and molecular ecology of Listeria monocytogenes isolates collected from 13 French pork meat salting-curing plants and their products. Int. J. Food Microbiol. 112:153-161. [DOI] [PubMed] [Google Scholar]
- 32.Walker, S. A., and T. R. Klaenhammer. 2003. The genetics of phage resistance in Lactococcus lactis, p. 291-315. In B. J. B. Wood and P. J. Warner (ed.), Genetics of lactic acid bacteria, 1st ed. Kluwer Academic/Plenum Publishers, New York, NY.
- 33.Wendlinger, G., M. J. Loessner, and S. Scherer. 1996. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142:985-992. [DOI] [PubMed] [Google Scholar]