Abstract
To analyze the orientation in target cell membranes of the pediocin-like bacteriocin (antimicrobial peptide) curvacin A, 55 variants were generated by site-directed mutagenesis and their potencies against four different target cells determined. The result suggest that the somewhat hydrophilic short central helix (residues 19 to 24), along with the N-terminal β-sheet-like structure (residues 1 to 16), inserts in the interface region of the target cell membrane, with Ala22 close to the hydrophobic core of the membrane. The following hinge region, with Gly28 as an important residue, may then form a turn wherein Gly28 becomes positioned near the border between the interface and the hydrophobic regions, thus permitting the longer and more-hydrophobic C-terminal helix (residues 29 to 41) to insert into the hydrophobic core of the membrane. This helix contains three glycine residues (G33, G37, and G40) that form a putative helix-helix-interacting GxxxGxxG motif. The replacement of any of these glycines with a larger residue was very detrimental, suggesting their possible involvement in helix-helix interactions with a membrane-embedded receptor protein.
Ribosomally synthesized antimicrobial peptides (AMPs) are produced by a wide variety of organisms, from bacteria to humans. They are usually cationic and membrane permeabilizing and contain between 25 and 60 residues (42, 44). AMPs produced by gram-positive bacteria are generally termed bacteriocins. Bacteriocins produced by “food grade” lactic acid bacteria (LAB) have been the focus of extensive studies because of their potential application as nontoxic food preservatives and therapeutic agents for gastrointestinal infections. Nisin is an example of a LAB AMP that is approved as a biopreservative in several countries (10, 12). The results of recent studies also show that oral intake of bacteriocin-producing LAB protects mice from lethal doses of Listeria monocytogenes (11).
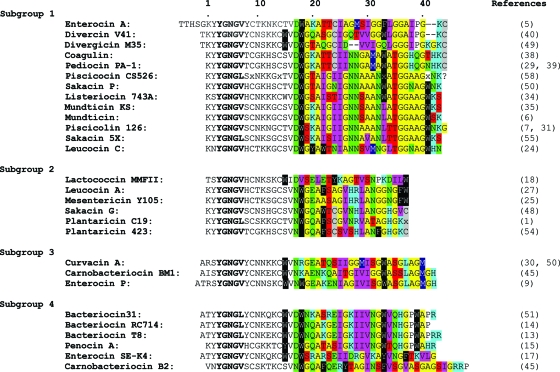
There are two main classes of LAB AMPs: the lanthionine-containing (class I) bacteriocins and the non-lanthionine-containing (class II) bacteriocins (12). Class II bacteriocins are heterogeneous and may be further divided into four groups: (i) the pediocin-like bacteriocins, (ii) the two-peptide bacteriocins, (iii) the cyclic peptides, and (iv) the nonpediocin one-peptide linear bacteriocins (12). Pediocin-like bacteriocins have similar sequences, especially in their N-terminal half, which contains a disulfide bridge and a common YGNGV/L “pediocin box” motif (22). These bacteriocins have been further divided into three or four subgroups according to sequence similarities in the more-diverse C-terminal half (Fig. 1) (19, 41).
FIG. 1.
Multiple sequence alignment of the pediocin-like bacteriocins (1, 5-7, 9, 13-15, 17, 18, 24, 25, 27, 29-31, 34, 35, 38-40, 45, 48, 49-51, 54, 55, 58). The common YGNGV/L (“pediocin box”) motif is shown in bold. The residues in the C-terminal half are colored as follows: Asn, Asp, Gln, and Glu are in green; Ile, Leu, and Val are in purple; Trp, Tyr, and Phe are in black; Cys is in gray; Ala and Gly are in yellow; Arg, Lys, and His in blue; Ser and Thr are in red; Pro is in white; and Met is in dark blue. Note that in numbering the residues (as indicated above the sequences), residue number 2 before the well-conserved YGNGV motif is in all cases referred to as residue 1, since this residue is the first residue in most—but not all—of the peptides.
The three-dimensional structures of the following peptides from each subgroup of pediocin-like bacteriocins have been analyzed by nuclear magnetic resonance spectroscopy: sakacin P and a mutant of sakacin P (subgroup 1) (53), leucocin A (subgroup 2) (26), curvacin A (subgroup 3) (28), and carnobacteriocin B2 (subgroup 4) (57). These bacteriocins are unstructured in water but become structured when exposed to membrane-mimicking environments (26, 28, 53, 57). Their well-conserved cationic N-terminal half then forms a disulfide bridge-stabilized, three-stranded, antiparallel β-sheet-like structure which is separated from the more-hydrophobic/amphiphilic helix-containing C-terminal half by a flexible hinge. The cationic N-terminal β-sheet-like half appears to mediate binding to the target cell surface through electrostatic interactions (8, 36), whereas the helix-containing C-terminal half penetrates into the hydrophobic part of target cell membranes (19, 33). The hinge apparently provides the structural flexibility that enables the C-terminal half to dip into the hydrophobic part of membranes (19). The C-terminal half defines the antimicrobial spectrum of these bacteriocins (33) and is recognized (possibly indirectly) by the cognate immunity protein (32, 33), and its structure is thus of special interest.
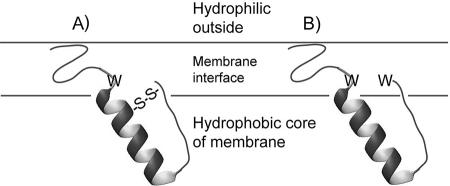
In subgroup 1 and 2 bacteriocins, the C-terminal half forms a hairpin-like structure consisting of a central α-helix followed by a somewhat extended C-terminal tail that folds back on the helix (26, 53). This hairpin-like structure is stabilized either by a disulfide bridge between a C-terminal cysteine residue and a cysteine residue in the middle of the α-helix (Fig. 2A) or by two tryptophan residues (one in the middle of the peptide and one near the C-terminal end) that are positioned in the membrane interface (Fig. 2B) (19, 21). The C-terminal half of the subgroup 4 bacteriocin carnobacteriocin B2 contains an amphiphilic α-helix stretching about 21 residues from the middle of the peptide toward its C terminus (57), while the C-terminal half of the subgroup 3 bacteriocin curvacin A forms a helix-hinge-helix structure (28). The first helix is short (residues 19 to 24) and hydrophilic, whereas the second helix is amphiphilic/hydrophobic and stretches from residue 29 to the C terminus (28).
FIG. 2.
A cartoon depiction of the structure and orientation in membranes of subgroup 1 and 2 pediocin-like bacteriocins, in which the C-terminal hairpin structure is stabilized by either a disulfide bridge (A) or an interface-localized tryptophan residue near the C-terminal end of the bacteriocin (B).
The subgroup 3 bacteriocins lack both the C-terminal tryptophan residue and the C-terminal disulfide bridge present in subgroup 1 and 2 bacteriocins (Fig. 1). It is thus not clear how the C-terminal helix-hinge-helix structure in subgroup 3 bacteriocins is positioned in target membranes. To gain insight into how the structure is positioned, we have in this study investigated the effect on antimicrobial activity of altering residues in the C-terminal helix-hinge-helix of curvacin A by in vitro site-directed mutagenesis.
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli DH5α was used for the production and isolation of the plasmids before transferring plasmids to Lactobacillus sake Lb790. The E. coli strain was grown at 37°C in LB medium either with vigorous agitation in liquid medium or on agar plates solidified by adding 2% (wt/vol) agar. Except for Carnobacterium piscicola UI 49, all other LAB were grown at 30°C in MRS medium (Oxoid) without agitation in liquid medium or on agar plates solidified by adding 1.5% (wt/vol) agar. C. piscicola UI 49 was grown in GM17 medium (M17 medium [Oxoid] supplemented with 0.4% glucose and 0.1% Tween 80) at 30°C. Antibiotic concentrations of 150 μg/ml erythromycin were used for E. coli; for L. sake Lb790 transformants, erythromycin concentrations of 2 μg/ml or 10 μg/ml together with chloramphenicol concentrations of 5 μg/ml or 10 μg/ml were used for initial growth or growth in culture medium, respectively.
Two-plasmid expression system for production of bacteriocins.
All bacteriocins were produced by using a previously developed system for heterologous bacteriocin expression in the bacteriocin-deficient strain L. sake Lb790 (4). The system is based on two plasmids, pSAK20 and a pLPV111-based (4) E. coli-Lactobacillus shuttle vector in which a bacteriocin gene and its cognate immunity gene have been placed under the control of a bacteriocin-specific promoter derived from the sakacin A producer L. sake Lb706 (see reference 3 for details). In this study, we combined pSAK20 with a new pLPV111-derived plasmid, pCurA (encoding the curvacin A structural gene and the curvacin A immunity gene), enabling the production of curvacin A. For the construction of pCurA, the curvacin A gene and curvacin A immunity gene were amplified by the use of PCR and Taq polymerase (Fermentas) on DNA preparations isolated from the natural curvacin A producer Lactobacillus curvatus LTH1174 (50). The following primers were used in the PCR: CurAF (ATAACGCGTGAATTCCCCTGTTTAGGAATGATTTCTGTAGG) and CurAR (ATAATCGATCTAATGGATTACTCCAAATTCC). The PCR product was subcloned into a T vector using a pGEM-T Easy vector kit from Promega. Subsequently, the curvacin A and immunity-encoding genes were cut out of the T vector with restriction enzymes BstXI and PaeI (Fermentas) and ligated into the pLPV111 vector digested with the same restriction enzymes, yielding pCurA. The other plasmid of the expression system, pSAK20, is a pVS2-based (56) plasmid that contains the orf4-sapKRTE operon from L. sake Lb706 (3, 4). The products of orf4 and the sapK and sapR genes are necessary for the activation of the bacteriocin-specific promoters on pLPV111-derived bacteriocin-encoding plasmids, whereas the products of the sapT and sapE genes ensure the processing and secretion of the prebacteriocins encoded by these plasmids (3, 4). This system permitted the routine production of correctly processed and secreted (mutant) bacteriocins, which could be purified by using well-established methods for the purification of these peptides from LAB (see below).
Plasmid isolation, preparation of competent cells, and cell transformation.
Plasmids were isolated from E. coli DH5α and LAB by using a Nucleo Spin plasmid kit (Macherey-Nagel). To ensure the lysis of LAB, lysozyme was added to resuspension buffer A1 included in the Nucleo Spin plasmid kit to a final concentration of 5 mg/ml. For the preparation of competent E. coli DH5α cells, the cells were cultured in LB medium to an optical density at 600 nm (OD600) of about 0.3. The culture was cooled on ice for 10 min, and the cells were then washed with ice-cold 0.1 M CaCl2 and suspended in ice-cold 0.1 M CaCl2 containing 15% (vol/vol) glycerol. The cells were ready for transformation after incubation on ice for 45 min and were transformed by the use of heat shock (42°C for 90 s). L. sake Lb790/pSAK20 cells were made competent as described in reference 2. Briefly, the cells were cultured to an OD600 of between 0.5 and 0.6 in MRS broth containing 10 μg/ml chloramphenicol and 2% (wt/vol) glycine and thereafter washed with 1 mM MgCl2 and then with 30% (wt/vol) polyethylene glycol 1500 (molecular weight range, 1,300 to 1,600). The cells were then transformed by electroporation by using a Gene Pulser and Pulse Controller unit (Bio-Rad Laboratories) as described previously (2).
Site-directed mutagenesis and DNA sequencing.
Mutations in the curvacin A gene encoded by pCurA were made by using a QuickChange site-directed mutagenesis kit (Stratagene). The PCRs were run on an MJ Research PTC-200 Peltier thermal cycler (MJ Research, Inc.) using Pfu Turbo DNA polymerase (Stratagene). The 50-μl reaction mixtures contained about 40 ng of plasmid template, 125 ng of each oligonucleotide primer (Eurogentec), deoxynucleoside triphosphates (Stratagene) to a final concentration of 0.05 mM for each nucleotide, and 2.5 units of Pfu Turbo DNA polymerase. After a 2-min hot start at 95°C, 18 cycles of the following program were run: denaturation (45 s at 95°C), primer annealing (1 min at 48°C), and polymerization (12 min at 68°C). The PCR product was digested for 1 h at 37°C with restriction enzyme DpnI (Stratagene) to eliminate the original template and thereby increase mutation efficiency. The resulting mutated plasmid contained a nick which was sealed by DNA repair systems in E. coli DH5α after transformation. The DNA sequence of mutant plasmids was verified by automated DNA sequence determination, using an ABI Prism 377 DNA sequencer and an ABI Prism dye terminator cycle sequencing ready reaction kit (Perkin-Elmer).
Purification of curvacin A and mutant bacteriocins.
Wild-type and mutant bacteriocins were purified to homogeneity from 500-ml cultures by applying the bacterial culture directly to a cation exchanger, followed by reverse-phase chromatography, as described previously (52). Briefly, the cells in 500-ml overnight cultures were applied to a 5- to 6-ml SP Sepharose fast flow (GE Healthcare) cation exchange column that had been equilibrated with 50 ml 20 mM sodium phosphate buffer, pH 6. The column was then washed with 100 ml of the sodium phosphate buffer, and the peptides subsequently eluted with 40 ml of the sodium phosphate buffer supplemented with NaCl to a final concentration of 1 M. Trifluoroacetic acid (TFA) and 2-propanol were added to the eluent to final concentrations of 0.1% and 5% (vol/vol), respectively, and the eluent was then applied to a reverse-phase column (Resource RPC; GE Healthcare). The peptides were eluted from the reverse-phase column (equilibrated with distilled water containing 0.1% [vol/vol] TFA) with a linear 2-propanol (containing 0.1% [vol/vol] TFA) gradient. To confirm that the recombinant lactobacilli had correctly produced and processed the bacteriocins, the molecular masses of the isolated peptides were determined by mass spectrometry, using a matrix-assisted laser desorption ionization-time-of-flight Voyager-DERP mass spectrometer (PerSeptive Biosystems) with α-cyano-4-hydroxy-cinnamic acid as matrix. The purity of the bacteriocins was verified to be greater than 80% by analytical reverse-phase chromatography using a μRPC SC 2.1/10 C2/C18 column (GE Healthcare) on a Smart chromatography system (GE Healthcare) or Äkta chromatography system (GE Healthcare). The concentration of purified bacteriocins was determined by measuring UV absorption at 280 nm, which was converted to protein concentration by using molecular extinction coefficients calculated from the contributions of individual amino acid residues.
Bacteriocin assay.
Bacteriocin activity was measured by using a microtiter plate assay system essentially as described previously (43). Each well of a microtiter plate contained 200 μl of culture medium with bacteriocin fractions at twofold dilutions and the indicator strain at an OD600 of about 0.01. The following strains were used as indicator strains: C. piscicola UI 49 (LMGT 2332), Lactobacillus coryniformis subsp. torquens (NCDO 2740), Enterococcus faecalis (NCDO 581), and L. sake (NCDO 2714). The microtiter plate cultures were incubated overnight (14 to 16 h) at 30°C for all strains except LMGT 2332, which was incubated for approximately 8 h, after which the growth of the indicator strain was measured spectrophotometrically at 600 nm with a microtiter plate reader. The MIC was defined as the concentration of bacteriocin that inhibited the growth of the indicator strain by 50%. The MICs presented are the results of at least three independent measurements.
RESULTS AND DISCUSSION
A total of 55 curvacin A mutants, derived by altering residues in the C-terminal half (starting with residue Trp16), were produced, purified, and assayed. The activities of all purified mutants were measured using four different indicator strains, and the activity relative to that of wild-type curvacin A was calculated (Tables 1 and 2). Four different strains were used in order to detect general trends in mutational effects, as such effects may in some cases be indicator strain dependent (21, 36).
TABLE 1.
Relative MICa values of mutant peptides that have been altered in the central helix and hinge region, assayed against four different indicator strains
| Mutated peptide | Fold change in activity against each indicator strain with indicated peptide substitutionb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gln | Leu | Ala | Ser | Trp | His | Phe | Tyr | Asn | Glu | Asp | |
| Trp16 | >200 | >300, 400, 100, 90 | 30, 30, 30, 20 | 60, 50, 70, 20 | |||||||
| Arg19 | 80, 60, 200, 100 | 50, 3, 70, 30 | |||||||||
| Gly20 | 1, 1, 2, 2 | 1, 1, 2, 1 | |||||||||
| Glu21 | 20, 20, 10, 10 | 90, 70, 60, 20 | |||||||||
| Ala22 | 2, 1, 2, 1 | 40, 40, 30, 8 | |||||||||
| Thr23 | 70, 50, 100, 100 | 40, 40, 30, 30 | |||||||||
| Gln24 | 10, 10, 9, 6 | 10, 20, 20, 9 | |||||||||
| Ser25 | 60, 50, 80, 50 | 80, 200, 200, 100 | |||||||||
| Gly28 | ≥400 | >400 | 10, 10, 40, 8 | ≥300 | |||||||
The relative MICs are the MIC of the mutant peptide divided by the MIC of wild-type curvacin A. The MIC is defined as the amount of bacteriocin that inhibited the growth by 50%. Four different indicator strains, C. piscicola UI 49 (LMGT 2332), L. coryniformis subsp. torquens (NCDO 2740), E. faecalis (NCDO 581), and L. sake (NCDO 2714), were used. MIC for wild-type curvacin A was 0.05 nM when assayed against C. piscicola UI 49; 0.07 nM when assayed against E. faecalis; and 0.08 nM when assayed against L. coryniformis subsp. torquens and L. sake. Standard deviations were less than 30% of the MIC for 50% of the measurements, less than 50% for 80% of the measurements, and less than 75% for 97% of the measurements.
Where four relative MICs are reported, these values represent results obtained with the following indicator strains, C. piscicola UI 49, L. coryniformis subsp. torquens, E. faecalis, and L. sake, respectively. Where only one relative MIC value is reported, this value represents results for all four indicator strains.
TABLE 2.
Relative MICa values of mutant peptides that have been altered in the C-terminal region, assayed against four different indicator strains
| Mutated peptide | Fold change in activity against each indicator strain with indicated peptide substitutionb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gln | Leu | Ala | Ser | His | Trp | Phe | Tyr | Gly | |
| Gly29 | 9, 7, 6, 7 | 7, 1, 4, 3 | 7, 8, 9, 4 | 40, 30, 70, 30 | |||||
| Met30 | 50, 60, 60, 20 | >200, >300, >300, 100 | |||||||
| Ile31 | >200, >400, >300, >90 | ||||||||
| Ser32 | 9, 20, 5, 5 | 10, <1, 3, 1 | 20, 6, 20, 20 | 20, 10, 20, 30 | |||||
| Gly33 | ≥400 | >400 | 50, 90, 30, 20 | ||||||
| Trp34 | >400 | 100, 400, >200, 30 | >400 | 10, 10, 30, 3 | 90, 100, 90, 20 | ||||
| Ala35c | >400 | ||||||||
| Ser36c | 40, 70, 10, 40 | ||||||||
| Gly37 | >400 | >400 | 80, 200, 300, 90 | 20, 20, 10, 20 | |||||
| Leu38 | 200, 400, >200, 30 | >200 | |||||||
| Ala39c | >300 | ||||||||
| Gly40 | 60, 40, 30, 50 | 60, 20, 20, 60 | |||||||
| Met41 | >400 | 10, 10, 20, 6 | 6, 10, 8, 5 | ||||||
The relative MICs are the MIC of the mutant peptide divided by the MIC of wild-type curvacin A. The MIC is defined as the amount of bacteriocin that inhibited the growth by 50%. Four different indicator strains, C. piscicola UI 49 (LMGT 2332), L. coryniformis subsp. torquens (NCDO 2740), E. faecalis (NCDO 581), and L. sake (NCDO 2714), were used. The MIC for wild-type curvacin A was 0.05 nM when assayed against C. piscicola UI 49; 0.07 nM when assayed against E. faecalis; and 0.08 nM when assayed against L. coryniformis subsp. torquens and L. sake. Standard deviations were less than 30% of the MIC for 50% of the measurements, less than 50% for 80% of the measurements, and less than 75% for 97% of the measurements.
Where four relative MICs are reported, these values represent results obtained with the following indicator strains: C. piscicola UI 49, L. coryniformis subsp. torquens, E. faecalis, and L. sake. Where only one relative MIC value is reported, this value represents results for all four indicator strains.
Other mutations were introduced in the curvacin A gene, but production of the protein could not be detected. These mutations were Ala35 to Leu, Ser36 to Gln, and Ala39 to Leu.
Replacement of Trp16.
In integrated membrane proteins, aromatic residues such as Trp and Tyr, but not hydrophobic residues such as Leu, are often found in the interface region of membranes (37). To investigate whether Trp16 is positioned in the membrane interface or in the hydrophobic core of the membrane, this residue was replaced with other aromatic residues (Phe and Tyr), a hydrophobic residue (Leu), and a hydrophilic positively charged residue (His). Replacement with Tyr or Phe reduced the activity of the mutant by factors of 20 to 70, while replacement with a His residue reduced the activity by factors of 90 to 400, and replacement with a Leu residue inactivated the bacteriocin (activity reduced more than 200-fold) (Table 1). The very detrimental effect of replacing Trp16 with Leu or His compared to that of replacing it with aromatic residues suggests that Trp16 is not in the hydrophobic core of the membrane nor in the hydrophilic exterior but rather in the interface region. This interpretation is consistent with earlier results that indicate that the N-terminal domain of the pediocin-like bacteriocins remains outside the membrane core and most likely positions itself in the membrane interface region (23) (Fig. 3).
FIG. 3.
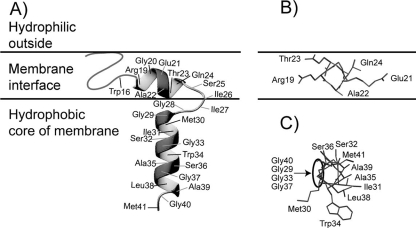
(A) Cartoon depiction of the proposed orientation in membranes of the subgroup 3 bacteriocin curvacin A. The N-terminal β-sheet-like region and the central polar helix are both positioned in the interface region of the membrane, whereas the hinge region with Gly28 as the central residue enables the C-terminal hydrophobic helix to penetrate into the hydrophobic core of the membrane. (B) Position of the central helix in the membrane viewed down the helical axis, with Ala22 positioned close to the hydrophobic part of the membrane. (C) The C-terminal helix viewed down the helical axis. The GxxxG-like motif created by Gly33, Gly37, and Gly40 (with Gly29 positioned between Gly40 and Gly33), forming a putative site for helix-helix interactions with a membrane-embedded receptor protein, is circled.
Residues in the central helix.
In general, alterations in the central helix of curvacin A affected the activity to a lesser extent than alterations in the more-hydrophobic C-terminal helix (Table 1 and 2). In curvacin A, Arg19 occupies a position where sakacin P has a tryptophan (Trp18) that is positioned in the membrane interface (19, 23). Arginine is a basic amino acid and thus expected to be in a hydrophilic environment. Replacing Arg19 with a hydrophobic residue (Leu) reduced the activity by factors of 60 to 200, while replacement with an aromatic Trp residue reduced the activity by factors of 3 to 70 (Table 1). Arg19 is clearly not in a hydrophobic environment but more likely positioned in the interface region of the membrane along with Trp16 (Fig. 3), especially taking into account that its replacement with Trp reduced the activity only threefold when assayed against C. piscicola UI 49 (LMGT 2332) (Table 1).
Replacing Gly20 with either an alanine or a polar serine residue was well tolerated, as it resulted in activity as good as that of wild-type curvacin A (Table 1), consistent with Gly20 also being near or in the more-hydrophilic part of the membrane (Fig. 3). Glu21 to Gln and Asp replacements were relatively detrimental (10- to 20-fold and 20- to 90-fold reduction in activity) (Table 1) considering the conservative nature of these mutations. Thus, both the negative charge and its position are of importance, and Glu21 seems therefore to be involved in a specific interaction with another protein or with polar lipid head groups.
Ala22 is clearly in a hydrophobic environment, as its replacement with a small hydrophilic residue reduced the activity of the mutant 8- to 40-fold, whereas replacement with a large, hydrophobic Leu residue resulted in wild-type activity (Table 1). This indicates that position 22 is not sterically restricted and is close to the hydrophobic core of the membrane (Fig. 3).
The replacement of the hydrophilic Thr23 residue with either a hydrophilic Gln or a hydrophobic Leu residue reduced the activities of the mutants by factors of 50 to 100 and 30 to 40, respectively (Table 1). Shortening the side chain of Gln24 to an Asn residue reduced the activity about 10-fold. Moreover, its polar amide group could not be replaced by a negatively charged carboxyl group, since replacement with a Glu residue reduced the activity about 10- to 20-fold (Table 1). As seems to be the case for Glu21, Thr23 and Gln24 appear to be sterically restricted, and both might specifically interact either with polar lipid head groups or a protein.
Residues in the hinge region.
Ser25 is the first residue of the hinge region between the short central helix and the long C-terminal helix. The small polar side chain of Ser25 is important for activity, as its replacement with a large hydrophilic Gln residue reduced the activity of the mutant 50- to 80-fold and replacement with a large hydrophobic Leu residue reduced the activity about 80- to 200-fold (Table 1). The polarity of Ser25 suggests that the residue is in the membrane interface and not buried in the hydrophobic core (Fig. 3).
The Ile residues at positions 26 and 27 were not mutated. Ile26 is conserved in the two other subgroup 3 bacteriocins, enterocin P and carnobacteriocin BM1, indicating that a large hydrophobic residue is important in this position. In a position near the hydrophobic core of the membrane, the long side chain of Ile26 might penetrate into the hydrophobic core. Ile27 is not conserved and can presumably be replaced with a small hydrophobic Ala residue, as in enterocin P, or a small hydrophilic Thr residue, as in carnobacteriocin BM1, suggesting that Ile27 is close to the membrane interface, as indicated in Fig. 3A.
All mutations to the “hinge residue” Gly28 reduced the bacteriocin activity (Table 1). Mutations to a large hydrophobic (Leu) or a small or large hydrophilic (Ser or Gln) residue resulted in more than a 300-fold reduction in antimicrobial activity, while mutations to a small hydrophobic residue (Ala) reduced the activity about 10 to 40 times. The residue at position 28 must therefore be small and nonpolar, suggesting that the Gly28 provides the flexibility of the hinge needed to properly position the C-terminal part of the molecule correctly in the core of the membrane.
Residues in the C-terminal helix.
The replacement of Gly29, the first residue in the C-terminal helix, with a large hydrophobic residue (Leu) was relatively well tolerated, as it resulted in one- to sevenfold reduction in activity (Table 2). Replacement with a large hydrophilic residue (Gln) reduced the activity only 6- to 9-fold, about the same as replacement with a small hydrophobic Ala residue (4- to 9-fold reduction), while a small hydrophilic Ser residue reduced the activity 30- to 70-fold (Table 2). Position 29 is thus not very sensitive to changes in side chain structure. The results may be explained if Gly29 is in the hydrophobic phase of the membrane but close to the interface region, as indicated in Fig. 3A. This location of Gly29 would permit its replacement with hydrophobic Ala and Leu residues and might allow hydrophilic Gln, but not Ser with its short side chain, to snorkel toward the more-hydrophilic interface region.
Large hydrophobic residues are favored in positions 30 and 31, as replacing Met30 with Ala and Ser reduced the activities of the mutants 20 to 60 times and at least 100 to 300 times, respectively, and replacing Ile31 with an Ala residue reduced the activity at least 90 to 400 times (Table 2). Thus, both Met30 and Ile31 are in a hydrophobic environment. Large hydrophobic residues are also present at these positions in the two other subgroup 3 bacteriocins, enterocin P and carnobacteriocin BM1 (Fig. 1), further supporting the evidence that large hydrophobic residues are important in these positions.
Similarly to the results for Gly29, Ser32 could be replaced with a large hydrophobic residue (Leu; 1- to 10-fold reduction in activity) and with a large hydrophilic residue (Gln; 5- to 20-fold reduction in activity) or a small residue (Ala; 6- to 20-fold reduction in activity) (Table 2). As is the case for position 29, position 32 is thus not very sensitive to side-chain structure and seems to be in the hydrophobic part of the membrane but close to the interface region.
Neither Gly33 nor Gly37 could be replaced with a large hydrophobic (Leu) or hydrophilic (Gln) residue without complete loss of activity (more than 300-fold reduction in activity) (Table 2). Even the replacement of Gly with a small Ala residue in positions 33 and 37 reduced the activities of the mutants 20- to 90-fold and 80- to 300-fold, respectively. The replacement of Gly40 with a Leu or Gln residue was also detrimental (20- to 60-fold reduction in activity), but not to the same extent as in positions 33 and 37. These three residues (Gly33, Gly37, and Gly40) are on the same side of the C-terminal helix (Fig. 3A and C) and form a strip down the helix that lacks large side chains. The lack of side chains along this strip may allow close helix-helix interaction on this side of the helix.
The replacement of Trp34 indicates that both hydrophobicity and aromaticity are important at this position, as its replacement with an aromatic and hydrophobic Phe residue reduced the activity of the mutant 3- to 30-fold, replacement with an aromatic and somewhat hydrophilic Tyr residue reduced it 20- to 100-fold, and replacement with a hydrophobic Leu residue reduced it 20- to 400-fold, while replacement with hydrophilic residues (His or Gln) was highly deleterious (more than 400-fold reduction in activity) (Table 2). These results suggest that Trp34 is in a hydrophobic environment and possibly engaged in a specific interaction (Fig. 3A).
No production of mutant peptides could be detected upon introducing a Ser-to-Gln mutation at position 36 or Ala-to-Leu mutations at positions 35 and 39. Mutant peptides with Ala-to-Gln replacement at either position 35 or 39 were, however, produced. These mutant peptides had greatly reduced activities (more than 300-fold reduction) (Table 2), indicating that small and/or hydrophobic residues are required at these positions. A mutant peptide with a Ser-to-Leu replacement at position 36 was also produced and had a 10- to 70-fold reduction in activity (Table 2).
The replacement of the large and hydrophobic Leu residue at position 38 with an Ala residue resulted in a 30- to 400-fold reduction in activity, while replacement with a Ser residue resulted in more than a 200-fold reduction (Table 2). Evidently, a large and hydrophobic side chain is important in this position. The replacement of the last residue of curvacin A, Met41, with an aromatic (Trp) or a hydrophobic (Leu) residue resulted in a 10- to 20-fold reduction in activity, whereas its replacement with a hydrophilic (Gln) residue was detrimental, resulting in more than a 400-fold reduction in activity, indicating that Met41 is not sterically restricted and is in a hydrophobic environment.
Conclusion
The mutagenesis results are overall consistent with a model where the somewhat hydrophilic short central helix (residues 19 to 24) is inserted in the interface region of membranes, with the side chain of Ala22 close to the hydrophobic membrane core and Glu21, Gln24, and possibly Thr23 involved in specific interactions with lipid head groups or proteins (Fig. 3A and B). The hinge region, with Gly28 as a central residue, may then form a turn-like segment where Gly28 is positioned near the border between the interface and hydrophobic regions of the membrane, thus allowing the longer and more-hydrophobic C-terminal helix to penetrate into the hydrophobic core (Fig. 3A).
The helix-hinge-helix structure and its orientation in membranes is probably shared among all three subgroup 3 bacteriocins (curvacin A, enterocin P, and carnobacteriocin BM1), in view of their very similar amino acid sequences (Fig. 1). This structure is somewhat different from the hairpin-like structure of the subgroup 1 and 2 bacteriocins (Fig. 2). However, for all of these bacteriocins, a helix in the C-terminal half is inserted into the hydrophobic membrane core, and this half defines the antimicrobial spectrum of these bacteriocins (33) and is recognized (indirectly) by their cognate immunity proteins (32, 33). Interestingly, the effect of some mutations, such as S32L, W34L, W34F, and L38A, in the more-hydrophobic C-terminal helix was especially dependent on the indicator strain used when assaying bacteriocin activity. These mutations were at least 10 times more detrimental when assayed with some indicator strains than with others (S32L, W34L, W34F, and L38A) (Table 2), suggesting that residues in this helix are indeed involved in specifying the antimicrobial spectrum. The results of a recent study revealed that pediocin-like bacteriocins interact with the subunits of the mannose phosphotransferase system permease that are embedded in target cell membranes and that immunity proteins that protect cells from being killed by these bacteriocins bind to the bacteriocin-permease complex and thereby prevent bacteriocin-induced killing (16). Interactions in the membrane between these bacteriocins and the mannose phosphotransferase permease thus apparently alter the conformation of the permease in a manner that results in membrane leakage. Interestingly, 15-mer fragments covering most of the helical region of the pediocin-like bacteriocins inhibit the bacteriocins in a specific manner (20, 46, 59), suggesting that the membrane-penetrating helix in the C-terminal part of the pediocin-like bacteriocins is involved in helix-helix interactions with the mannose phosphotransferase permease. In this connection, it should be noted that three glycine residues (Gly33, Gly37, and Gly40) in the C-terminal helix in curvacin A form a putative helix-helix-interacting GxxxGxxG motif (47) that is conserved in the three subgroup 3 bacteriocins (S instead of G at position 37 in carnobacteriocin BM1) (Fig. 1). Moreover, the replacement of any of these glycine residues with larger residues reduced the activity drastically, as expected if they are in fact involved in helix-helix interactions with a membrane-embedded protein, such as the mannose phosphotransferase permease.
Acknowledgments
EMBIO (steering board for research in molecular biology, biotechnology, and bioinformatics at the University of Oslo) is acknowledged for financial support of Per Eugen Kristiansen.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Atrih, A., N. Rekhif, A. J. Moir, A. Lebrihi, and G. Lefebvre. 2001. Mode of action, purification and amino acid sequence of plantaricin C19, an anti-Listeria bacteriocin produced by Lactobacillus plantarum C19. Int. J. Food Microbiol. 68:93-104. [DOI] [PubMed] [Google Scholar]
- 2.Aukrust, T. W., M. B. Brurberg, and I. F. Nes. 1995. Transformation of Lactobacillus by electroporation. Methods Mol. Biol. 47:201-208. [DOI] [PubMed] [Google Scholar]
- 3.Axelsson, L., and A. Holck. 1995. The genes involved in production of and immunity to sakacin A, a bacteriocin from Lactobacillus sake Lb706. J. Bacteriol. 177:2125-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelsson, L., T. Katla, M. Bjornslett, V. G. Eijsink, and A. Holck. 1998. A system for heterologous expression of bacteriocins in Lactobacillus sake. FEMS Microbiol. Lett. 168:137-143. [DOI] [PubMed] [Google Scholar]
- 5.Aymerich, T., H. Holo, L. S. Havarstein, M. Hugas, M. Garriga, and I. F. Nes. 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62:1676-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennik, M. H. J., B. Vanloo, R. Brasseur, L. G. M. Gorris, and E. J. Smid. 1998. A novel bacteriocin with a YGNGV motif from vegetable-associated Enterococcus mundtii; full characterization and interaction with target organisms. Biochim. Biophys. Acta 1373:47-58. [DOI] [PubMed] [Google Scholar]
- 7.Bhugaloo-Vial, P., X. Dousset, A. Métivier, O. Sorokine, P. Anglade, P. Boyaval, and D. Marion. 1996. Purification and amino acid sequence of piscicocins V1a and V1b that display significantly different levels of specific inhibitory activity. Appl. Environ. Microbiol. 62:4410-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y., R. D. Ludescher, and T. J. Montville. 1997. Electrostatic interactions, but not the YNGNV consensus motif, govern the binding of pediocin PA-1 and its fragments of phospholipid vesicles. Appl. Environ. Microbiol. 63:4770-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cintas, L. M., P. Casaus, L. S. Havarstein, P. E. Hernandez, and I. F. Nes. 1997. Biochemical and genetic characterization of enterocin P, a novel sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl. Environ. Microbiol. 63:4321-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 11.Corr, S. C., Y. Li, C. U. Riedel, P. W. O'Toole, C. Hill, and C. G. Gahan. 2007. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 104:7617-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 13.De Kwaadsteniet, M., T. Fraser, C. A. Van Reenen, and L. M. Dicks. 2006. Bacteriocin T8, a novel class IIa sec-dependent bacteriocin produced by Enterococcus faecium T8, isolated from vaginal secretions of children infected with human immunodeficiency virus. Appl. Environ. Microbiol. 72:4761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Campo, R., C. Tenorio, R. Jimenez-Diaz, C. Rubio, R. Gomez-Lus, F. Baquero, and C. Torres. 2001. Bacteriocin production in vancomycin-resistant and vancomycin-susceptible Enterococcus isolates of different origins. Antimicrob. Agents Chemother. 45:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diep, D. B., L. Godager, D. Brede, and I. F. Nes. 2006. Data mining and characterization of a novel pediocin-like bacteriocin system from the genome of Pediococcus pentosaceus ATCC 25745. Microbiology 152:1649-1659. [DOI] [PubMed] [Google Scholar]
- 16.Diep, D. B., M. Skaugen, Z. Salehian, H. Holo, and I. F. Nes. 2007. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. USA 104:2384-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eguchi, T., K. Kaminaka, J. Shima, S. Kawamoto, K. Mori, S. H. Choi, K. Doi, S. Ohmomo, and S. Ogata. 2001. Isolation and characterization of enterocin SE-K4 produced by thermophilic enterococci, Enterococcus faecalis K-4. Biosci. Biotechnol. Biochem. 65:247-253. [DOI] [PubMed] [Google Scholar]
- 18.Ferchichi, M., J. Frere, K. Mabrouk, and M. Manai. 2001. Lactococcin MMFII, a novel class IIa bacteriocin produced by Lactococcus lactis MMFII, isolated from a Tunisian dairy product. FEMS Microbiol. Lett. 205:49-55. [DOI] [PubMed] [Google Scholar]
- 19.Fimland, G., V. Eijsink, and J. Nissen-Meyer. 2002. Mutational analysis of the role of tryptophan residues in an antimicrobial peptide. Biochemistry 41:9508-9515. [DOI] [PubMed] [Google Scholar]
- 20.Fimland, G., R. Jack, G. Jung, I. F. Nes, and J. Nissen-Meyer. 1998. The bactericidal activity of pediocin PA-1 is specifically inhibited by a 15-mer fragment that spans the bacteriocin from the center toward the C terminus. Appl. Environ. Microbiol. 64:5057-5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fimland, G., L. Johnsen, L. Axelsson, M. B. Brurberg, I. F. Nes, V. Eijsink, and J. Nissen-Meyer. 2000. A C-terminal disulfide bridge in pediocin-like bacteriocins renders bacteriocin activity less temperature dependent and is a major determinant of the antimicrobial spectrum. J. Bacteriol. 182:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fimland, G., L. Johnsen, B. Dalhus, and J. Nissen-Meyer. 2005. Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. J. Pept. Sci. 11:688-696. [DOI] [PubMed] [Google Scholar]
- 23.Fimland, G., J. Pirneskoski, J. Kaewsrichan, A. Jutila, P. E. Kristiansen, P. K. Kinnunen, and J. Nissen-Meyer. 2006. Mutational analysis and membrane interactions of the beta-sheet-like N-terminal domain of the pediocin-like antimicrobial peptide sakacin P. Biochim. Biophys. Acta 1764:1132-1140. [DOI] [PubMed] [Google Scholar]
- 24.Fimland, G., K. Sletten, and J. Nissen-Meyer. 2002. The complete amino acid sequence of the pediocin-like antimicrobial peptide leucocin C. Biochem. Biophys. Res. Commun. 295:826-827. [DOI] [PubMed] [Google Scholar]
- 25.Fleury, Y., M. Abdel Dayem, J. J. Montagne, E. Chaboisseau, J. P. Le Caer, P. Nicolas, and A. Delfour. 1996. Covalent structure, synthesis, and structure-function studies of mesentericin Y 10537, a defensive peptide from Gram-positive bacteria Leconostoc mesenteroides. J. Biol. Chem. 271:14421-14429. [DOI] [PubMed] [Google Scholar]
- 26.Fregeau Gallagher, N. L., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 27.Hastings, J. W., M. Sailer, K. Johnson, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 173:7491-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haugen, H. S., G. Fimland, J. Nissen-Meyer, and P. E. Kristiansen. 2005. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide curvacin A. Biochemistry 44:16149-16157. [DOI] [PubMed] [Google Scholar]
- 29.Henderson, J. T., A. L. Chopko, and P. D. van Wassenaar. 1992. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 295:5-12. [DOI] [PubMed] [Google Scholar]
- 30.Holck, A., L. Axelsson, S. E. Birkeland, T. Aukrust, and H. Blom. 1992. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. J. Gen. Microbiol. 138:2715-2720. [DOI] [PubMed] [Google Scholar]
- 31.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnsen, L., G. Fimland, D. Mantzilas, and J. Nissen-Meyer. 2004. Structure-function analysis of immunity proteins of pediocin-like bacteriocins: C-terminal parts of immunity proteins are involved in specific recognition of cognate bacteriocins. Appl. Environ. Microbiol. 70:2647-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnsen, L., G. Fimland, and J. Nissen-Meyer. 2005. The C-terminal domain of pediocin-like antimicrobial peptides (class IIa bacteriocins) is involved in specific recognition of the C-terminal part of cognate immunity proteins and in determining the antimicrobial spectrum. J. Biol. Chem. 280:9243-9250. [DOI] [PubMed] [Google Scholar]
- 34.Kalmokoff, M. L., S. K. Banerjee, T. Cyr, M. A. Hefford, and T. Gleeson. 2001. Identification of a new plasmid-encoded sec-dependent bacteriocin produced by Listeria innocua 743. Appl. Environ. Microbiol. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawamoto, S., J. Shima, R. Sato, T. Eguchi, S. Ohmomo, J. Shibato, N. Horikoshi, K. Takeshita, and T. Sameshima. 2002. Biochemical and genetic characterization of mundticin KS, an antilisterial peptide produced by Enterococcus mundtii NFRI 7393. Appl. Environ. Microbiol. 68:3830-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazazic, M., J. Nissen-Meyer, and G. Fimland. 2002. Mutational analysis of the role of charged residues in target-cell binding, potency and specificity of the pediocin-like bacteriocin sakacin P. Microbiology 148:2019-2027. [DOI] [PubMed] [Google Scholar]
- 37.Killian, J. A., and G. von Heijne. 2000. How proteins adapt to a membrane-water interface. Trends Biochem. Sci. 25:429-434. [DOI] [PubMed] [Google Scholar]
- 38.Le Marrec, C., B. Hyronimus, P. Bressollier, B. Verneuil, and M. C. Urdaci. 2000. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I(4). Appl. Environ. Microbiol. 66:5213-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marugg, J. D., C. F. Gonzalez, B. S. Kunka, A. M. Ledeboer, M. J. Pucci, M. Y. Toonen, S. A. Walker, L. C. Zoetmulder, and P. A. Vandenbergh. 1992. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, and bacteriocin from Pediococcus acidilactici PAC1.0. Appl. Environ. Microbiol. 58:2360-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metivier, A., M. F. Pilet, X. Dousset, O. Sorokine, P. Anglade, M. Zagorec, J. C. Piard, D. Marion, Y. Cenatiempo, and C. Fremaux. 1998. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: primary structure and genomic organization. Microbiology 144:2837-2844. [DOI] [PubMed] [Google Scholar]
- 41.Morisset, D., J. M. Berjeaud, D. Marion, C. Lacombe, and J. Frere. 2004. Mutational analysis of mesentericin Y105, an anti-Listeria bacteriocin, for determination of impact on bactericidal activity, in vitro secondary structure, and membrane interaction. Appl. Environ. Microbiol. 70:4672-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nes, I. F., H. Holo, G. Fimland, H. H. Hauge, and J. Nissen-Meyer. 2001. Unmodified peptide-bactericins (class II) produced by lactic acid bacteria, p. 81-116. In H. Dutton, M. Haxwell, H. McArthur, and R. G. Wax (ed.), Peptide antibiotics: discovery, modes of action and application. Marcel Decker, New York, NY.
- 43.Nissen-Meyer, J., H. Holo, L. S. Håvarstein, K. Sletten, and I. F. Nes. 1992. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J. Bacteriol. 174:5686-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nissen-Meyer, J., and I. F. Nes. 1997. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch. Microbiol. 167:67-77. [PubMed] [Google Scholar]
- 45.Quadri, L. E., M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 46.Saavedra, L., C. Minahk, A. P. D. Holgado, and F. Sesma. 2004. Enhancement of the enterocin CRL35 activity by a synthetic peptide derived from the NH2-terminal sequence. Antimicrob. Agents Chemother. 48:2778-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Senes, A., D. E. Engel, and W. F. DeGrado. 2004. Folding of helical membrane proteins: the role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 14:465-479. [DOI] [PubMed] [Google Scholar]
- 48.Simon, L., C. Fremaux, Y. Cenatiempo, and J. M. Berjeaud. 2002. Sakacin G, a new type of antilisterial bacteriocin. Appl. Environ. Microbiol. 68:6416-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tahiri, I., M. Desbiens, R. Benech, E. Kheadr, C. Lacroix, S. Thibault, D. Ouellet, and I. Fliss. 2004. Purification, characterization and amino acid sequencing of divergicin M35: a novel class IIa bacteriocin produced by Carnobacterium divergens M35. Int. J. Food Microbiol. 97:123-136. [DOI] [PubMed] [Google Scholar]
- 50.Tichaczek, P. S., J. Nissen-Meyer, I. F. Nes, R. F. Vogel, and W. P. Hammes. 1992. Characterization of the bacteriocins curvacin A and sakacin P produced by Lactobacillus curvatus LTH 1174 and L. sake LTH673. Syst. Appl. Microbiol. 15:460-468. [Google Scholar]
- 51.Tomita, H., S. Fujimoto, K. Tanimoto, and Y. Ike. 1996. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pYI17. J. Bacteriol. 178:3585-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uteng, M., H. H. Hauge, I. Brondz, J. Nissen-Meyer, and G. Fimland. 2002. Rapid two-step procedure for large-scale purification of pediocin-like bacteriocins and other cationic antimicrobial peptides from complex culture medium. Appl. Environ. Microbiol. 68:952-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uteng, M., H. H. Hauge, P. R. L. Markciwck, G. Fimland, D. Mantzilas, J. Nissen-Meyer, and C. Muhle-Goll. 2003. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417-11426. [DOI] [PubMed] [Google Scholar]
- 54.Van Reenen, C. A., M. L. Chikindas, W. H. Van Zyl, and L. M. Dicks. 2003. Characterization and heterologous expression of a class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum 423, in Saccharomyces cerevisiae. Int. J. Food Microbiol. 81:29-40. [DOI] [PubMed] [Google Scholar]
- 55.Vaughan, A., V. G. Eijsink, T. F. O'Sullivan, K. O'Hanlon, and D. van Sinderen. 2001. An analysis of bacteriocins produced by lactic acid bacteria isolated from malted barley. J. Appl. Microbiol. 91:131-138. [DOI] [PubMed] [Google Scholar]
- 56.von Wright, A., S. Tynkkynen, and M. Suominen. 1987. Cloning of a Streptococcus lactis subsp. lactis chromosomal fragment associated with the ability to grow in milk. Appl. Environ. Microbiol. 53:1584-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, Y., M. E. Henz, N. L. Fregeau Gallagher, S. Chai, A. C. Gibbs, L. Z. Yan, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 1999. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 38:15438-15447. [DOI] [PubMed] [Google Scholar]
- 58.Yamazaki, K., M. Suzuki, Y. Kawai, N. Inoue, and T. J. Montville. 2005. Purification and characterization of a novel class IIa bacteriocin, piscicocin CS526, from surimi-associated Carnobacterium piscicola CS526. Appl. Environ. Microbiol. 71:554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan, L. Z., A. C. Gibbs, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 2000. Analogues of bacteriocins: antimicrobial specificity and interactions of leucocin A with its enantiomer, carnobacteriocin B2, and truncated derivatives. J. Med. Chem. 43:4579-4581. [DOI] [PubMed] [Google Scholar]