Abstract
MreB, a homolog of eukaryotic actin, participates in morphogenesis, cell division, cell polarity, and chromosome segregation in prokaryotes. In this study, a yellow fluorescent protein conjugate (YFP-MreBVp) was generated to investigate the behavior of MreB in merodiploid strain SC9 of the enteropathogen Vibrio parahaemolyticus. Under normal growth conditions, YFP-MreBVp formed helical filaments with a pitch of 0.64 ± 0.09 μm in about 85% of exponential-phase cells, and different clusters, relaxed coils, and ring configurations were observed in a small proportion of the cells. Overexpression of YFP-MreBVp substantially altered the structure of the MreB cytoskeleton and resulted in swollen and pleomorphic cells. Disturbing the activities of penicillin-binding proteins or adding magnesium suppressed the morphological distortions. These results indicate that mislocalization of cell wall-synthesizing machinery was responsible for morphological abnormality. By expressing YFP-MreBVp in the ectopic host bacterium Escherichia coli, shrinkage, fragmentation, and annealing of MreBVp filaments were directly observed. This work revealed the dynamic pattern of the localization of YFP-MreBVp in V. parahaemolyticus and its relationship to cell morphogenesis, and the YFP-MreBVp-E. coli system may be used to investigate the dynamic spatial structures of the MreB cytoskeleton in vivo.
MreB, a prokaryotic actin homolog, is found in almost all nonspherical bacteria (33). In most mreB-bearing bacteria, mreB is essential and located in the mre locus, which also contains mreC and mreD. Some bacterial species have additional MreB-like proteins, such as the Bacillus subtilis Mbl (MreB-like) and MreBH (MreB homolog) proteins (4). MreB and related proteins assemble into helical filaments beneath the cell membrane in B. subtilis (6), Caulobacter crescentus (10), and Escherichia coli (32). MreB also assembles into a single ring at a putative division site (33, 35).
MreB homologs have been implicated in morphogenesis, cell polarity, chromosome segregation, and sporulation (33). Analogous to eukaryotic actin, MreB filaments may serve as a structural brace and directly control cell shape (9). Alternatively, MreB may direct the localization of cell wall-synthesizing machinery and modify the cell wall in a manner similar to that postulated for actin in yeast (19). The work of Daniel and Errington with B. subtilis (5) supports the latter notion. These workers used a fluorescent derivative of vancomycin as a probe to label nascent peptidoglycan in gram-positive bacteria. Their results suggest that cylindrical wall synthesis in B. subtilis occurs in a helical pattern directed by the Mbl filaments. In B. subtilis, MreBH colocalizes with the two other MreB isoforms and defines the helical localization of the LytE cell wall hydrolase, suggesting that controlled elongation of B. subtilis depends on the coordination of cell wall synthesis and hydrolysis in helical tracts that have been established by MreB proteins (4). MreC and MreD act as a bridge between the MreB cytoskeleton and the cell wall-synthesizing machinery (33).
Vibrio parahaemolyticus is a human enteropathogen that naturally inhabits marine and estuarine environments. It contains all three of the homologs of eukaryotic cytoskeletal proteins, MreB, FtsZ, and CreS. However, no investigation of the cytoskeleton of this pathogen has been described previously. In this study, a yellow fluorescent protein (YFP) conjugate, YFP-MreBVp, was generated to investigate the behavior of MreB in merodiploid strain SC9 of V. parahaemolyticus and also in the ectopic host bacterium E. coli. The effect of overexpression of YFP-MreBVp on cell morphology was also examined. This conjugate fluorescent protein was used to tag and visualize the normal MreB cytoskeleton. The expression of YFP-MreBVp was controlled at low level, whereas the normal morphology of V. parahaemolyticus and E. coli and the normal function of the native MreB cytoskeleton were not influenced.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
V. parahaemolyticus strain 1137 (Kanagawa phenomenon positive, serotype O3:K6) was isolated in Taiwan from a clinical specimen (42). E. coli strain XL1-Blue was used for cloning, E. coli SM10λ-pir was used for conjugation with V. parahaemolyticus strain 1137, and both E. coli strains were used to express YFP-MreBVp or YFP-MreBEc. V. parahaemolyticus strains were grown in tryptic soy broth (Difco, Becton-Dickinson Diagnostic Systems, Sparks, MD) supplemented with 3% sodium chloride. Chloramphenicol was added at a concentration of 5 μg/ml to the medium to culture recombinant V. parahaemolyticus strains. E. coli strains were grown in Luria-Bertani (LB) broth (Difco). To culture strains XL1-Blue and SM10λ-pir, tetracycline (15 μg/ml) and kanamycin (50 μg/ml), respectively, were added to the growth medium. Strains of V. parahaemolyticus and E. coli were grown at 37°C unless indicated otherwise.
Bioinformatic analysis.
Open reading frames flanking mreB were analyzed using Region View provided by Comprehensive Microbial Resource (http://www.tigr.org/tigr-scripts/CMR2/CMRHomePage.spl) (28). The DNA sequences 500 bp upstream of mreB were analyzed using BPROM (http://www.softberry.com/berry.phtml?topic=bprom&group=programs&subgroup=gfindb) to predict putative promoters. Stem-loop structures, indicating putative ρ-independent terminators, were predicted using FindTerm (http://www.softberry.com/berry.phtml?topic=findterm&group=programs&subgroup=gfindb).
Construction of yfp-mreB mreB merodiploid strain.
To construct plasmid pSC9 (Plac-yfp::mreBVp), the V. parahaemolyticus mreB gene was obtained via PCR from chromosomal DNA of strain 1137 using primers mreBVp1F (5′-ACTCGTCTAGATTTAAGAAACTTCGTGGCATGTTT-3′) and mreBVp1R (5′-AGCTGAAGCTTTTATTCTTCAGAGAACAGATCGCC-3′); XbaI and HindIII sites were incorporated upstream and downstream of the mreB amplicon, respectively. The XbaI/HindIII-digested PCR product was then ligated into the large XbaI/HindIII fragment of pYLS68 (obtained from L. Rothfield, Department of Microbiology, University of Connecticut Health Center) (31), which yielded an in-frame yfp::mreBVp fusion. In the fusion protein, a Ser-Arg spacer was inserted between YFP and MreB to replace the start codon in mreB. pSC10 (Plac-yfp::mreBVp Cmr mobRP4) was constructed by ligating the large HindIII fragment that contained the cat gene and the mobRP4 region from pDS132 (obtained from D. Schneider, Laboratoire Adaptation et Pathogenie des Microorganismes, CNRS UMR 5163, Universite Joseph Fourier Grenoble, Grenoble, France) (29) into HindIII-digested pSC9. The YFP that fused to the N terminus of MreB did not contain the stop codon. The presence of plasmid pSC10, which contained the cat gene, was verified by resistance to chloramphenicol. The resulting strain, V. parahaemolyticus SC9, contained yfp that was fused to V. parahaemolyticus mreB in addition to the wild-type mreB chromosomal locus. An E. coli merodiploid strain was constructed using similar procedures (Table 1).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant features | Reference |
|---|---|---|
| E. coli strains | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tcr)] | 1 |
| SM10λ-pir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λ pirR6K, Kmr | 34 |
| SC7 | XL1-Blue containing pSC15 | This study |
| SC16 | XL1-Blue containing pSC10 | This study |
| SC17 | SM10λ-pir containing pSC10 | This study |
| V. parahaemolyticus strains | ||
| 1137 | Wild type, serotype O3:K6, KP+, clinical isolate | 42 |
| SC9 | 1137 containing pSC10 | This study |
| Plasmids | ||
| pYLS68 | Plac-yfp::minD minE::cfp, Apr | 31 |
| pDS132 | R6K ori mobRP4 sacB, Cmr | 29 |
| pSC9 | Plac-yfp::mreBVplacIqMB1 ori, Apr | This study |
| pSC10 | Plac-yfp::mreBVplacIqMB1 ori mobRP4, Apr Cmr | This study |
| pSC15 | Plac-yfp::mreBEclacIqMB1 ori mobRP4, Apr Cmr | This study |
Isopropyl-β-d-thiogalactopyranoside (IPTG) was used at a final concentration of 10 μM to the culture medium for 1 to 1.5 h to induce the expression of YFP-MreBVp in V. parahaemolyticus.
Microscopy and image analysis.
FM4-64 (Molecular Probes, Carlsbad, CA) was added to a sample at a final concentration of 1 μg/ml, and the sample was incubated at 25°C in the dark for 10 min for membrane staining (30). The cell cycle was monitored by observing the length and constriction or septation of the cells that were stained using the membrane stain FM4-64 to demarcate the boundaries of the cells (30). To examine the integrity of the membrane, the cells were incubated with propidium iodide (Molecular Probes) at a final concentration of 30 μM at 25°C in the dark for 20 min. Cells were immobilized on agarose pads or poly-l-lysine-coated slides (Sigma Co., St. Louis, MO) before microscopic examination. Agarose pads were made by adding 1.5% of low-melting-point agarose to tryptic soy broth supplemented with 3% sodium chloride or to LB medium. Antibiotics and IPTG were added to the culture medium when they were needed. Three microliters of a sample was placed on top of the agarose pad or poly-l-lysine-coated slides.
Immobilized cells were examined with a Nikon Eclipse E800 fluorescence microscope equipped with a Nikon Plan Fluor 100× 1.30 N.A. oil immersion objective at room temperature. Images were obtained using an Evolution VF charge-coupled device camera (Media Cybernetics, Inc., Silver Spring, MD) and Image-Pro Express software (Media Cybernetics, Inc.). The same exposure times (300 ms for phase-contrast microscopy and 500 to 800 ms for fluorescence microscopy) and other acquisition parameters were used for all samples in all sets of experiments. YFP images were obtained using a filter set that comprised a 450- to 490-nm excitation filter, a 505-nm longpass dichroic mirror, and a 520-nm bandpass emission filter. YFP is actually a “red-shifted” green fluorescent protein (GFP), and the emission spectra of YFP and GFP are very similar. Indeed, YFP appeared green with the microscopic parameters used. FM4-64 images were obtained using a filter set that comprised a 510- to 560-nm excitation filter, a 575-nm longpass dichroic mirror, and a 590-nm bandpass emission filter. Images were captured as 12-bit images, converted to the TIFF format, and analyzed using VayTek Image (VayTek, Inc., Fairfield, IA) or ImageJ (National Institutes of Health, Bethesda, MD). Five to seven images of optical sections of fluorescence images were obtained for a cell with a spacing of about 140 nm. Bacteria were simultaneously immobilized perpendicular to the slide plane to obtain fluorescence images of cell cross sections without using deconvolution or confocal microscopy. The method developed by Sun and Margolin was used (38).
RESULTS AND DISCUSSION
mre locus in Vibrio species.
The genome of V. parahaemolyticus strain RIMD2210633 has been sequenced (21), and strain 1137, which was used here, and strain RIMD2210633 both belong to the group containing the genetically closely related pandemic O3:K6 strains (42). Open reading frames that flanked mreB in various species were analyzed. Remarkably, in bacteria that belong to the Vibrionaceae (Photobacterium profundum, Vibrio cholerae, Vibrio fischeri, V. parahaemolyticus, and Vibrio vulnificus), mreBCD, maf, and cafA are always present together, and therefore they are jointly referred to as the “mre cluster” (Fig. 1). This organization is conserved in many gammaproteobacteria, and only the mre cluster of E. coli is shown in Fig. 1. Maf is highly conserved in bacteria and is required for growth in the presence of reducing agents. Overexpression of Maf results in extensive filamentation in B. subtilis (2). CafA (cytoplasmic axial filament A) forms long axial filaments that run through the cells. Overproduction of CafA leads to the formation of chains of cells and minicells (27). The presence of such a highly conserved protein that has cytoskeletal protein-like properties and whose gene is located just downstream of mreB is of great interest. In members of the Vibrionaceae, a large region called the mannose-sensitive hemagglutinin locus is located upstream of the mre cluster, between yhdA and mreB (22). This arrangement supports the hypothesis that the mannose-sensitive hemagglutinin locus was probably acquired as a mobile genetic element (22).
FIG. 1.
Conserved organization of the mre cluster in Vibrio spp. and E. coli. Each unit represents a single gene (open reading frame). Open reading frames in the mre cluster are indicated by filled boxes. Arrows indicate the putative promoters that were either identified here (filled arrows) or reported previously (open arrows) (22, 41). Stem-loop structures indicate the putative ρ-independent terminators. MSHA, mannose-sensitive hemagglutinin.
Further bioinformatic analysis predicted two putative promoters within a 500-bp region upstream of mreB in V. parahaemolyticus, V. vulnificus, and V. cholerae. In V. parahaemolyticus, the sequences of the downstream and upstream promoters are TTGCTG (−35) and TGGTACATT (−10) and the sequences of the upstream promoters are CTGATG (−35) and TGTTACACT (−10). A ρ-independent terminator sequence is present between mreB and mreC in V. parahaemolyticus and E. coli (Fig. 1). The presence of these promoter and terminator sequences in V. parahaemolyticus strain 1137 used in this work was confirmed by nucleotide sequencing (data not shown), and these sequences may be important in regulating the expression of these genes. We reasoned that the terminator is used to control the relative levels of MreB and MreC/MreD. Consistent with this, the level of mreB expression is much higher than the level of mreCD expression in E. coli (41). Furthermore, plasmids that contain the mreBCD fragment, but not plasmids with mreB alone, can complement ΔmreB mutants (17).
Localization of YFP-MreBVp in V. parahaemolyticus cells.
In this study, the dynamic behavior of MreB in V. parahaemolyticus was monitored using a fluorescent protein-tagged MreB homolog. The functionality of fluorescent protein-tagged MreB homologs varies among species or even within the same species (11, 32, 35). Changing the length of the linker between MreB and the fluorescent protein partner changes its functionality (11). To avoid potential trouble, the MreB cytoskeleton in a merodiploid strain was studied (32). In this study, the expression of the YFP-MreBVp protein was controlled by the lac promoter and lacIq repressor on a low-copy-number plasmid. Expression of YFP-MreBVp, induced by IPTG at concentrations of 10 to 50 μM, resulted in the formation of rod-shaped cells, which were indistinguishable from wild-type cells. A concentration of 10 μM was used here. We reasoned that YFP-MreBVp and native MreB in strain SC9 cells assembled into the same cytoskeletal structure and formed hybrid filaments (32).
The localization of YFP-MreBVp in mid-exponential-phase V. parahaemolyticus SC9 cells was determined. Fluorescence microscopy revealed that most cells contained MreB helical structures that extended along the long axis of the cell, with two to three turns per cell (Fig. 2A). The helical pitch was estimated to be 0.64 ± 0.09 μm based on measurements of 93 clearly defined structures. The helical pitches of MreB homologs in other species are 0.46 ± 0.08 μm (17) or 0.64 ± 0.4 μm (15) in E. coli, 0.3 ± 0.1 μm in V. cholerae (37), and 0.73 ± 0.12 μm in B. subtilis (14).
FIG. 2.
Various configurations of the MreB cytoskeleton in mid-exponential-phase V. parahaemolyticus cells examined by fluorescence microscopy. Strain SC9 cells were grown at 37°C in the presence of 10 μM IPTG until they entered the mid-exponential phase. (A) Extended helix; (B) single ring; (C) two separate clusters of helical filaments at opposite poles; (D) single cluster of helical filaments near one pole; (E) irregular structure; (F) relaxed helix; (G) single helix with a compressed end and a loose end; (H) two clusters at opposite poles linked by a thin helix; (I) cross sections of MreB cytoskeleton in cells immobilized with their long axes perpendicular to the coverslip. In panels C and D, the dotted parentheses indicate the range of a cluster, and the solid parenthesis indicates the length of the cell, as observed in a corresponding phase-contrast image. Some of the panels contain schematic diagrams. Scale bar = 1 μm.
The number of helical turns increased with cell length, while the helical pitch was constant. This observation revealed that the MreB filaments grew as the cells elongated. The behavior of the MreB filament in different stages of the cell cycle was examined further. Various configurations of the MreB filament were observed in newborn cells, indicating the flexibility of the MreB cytoskeleton. About 85% of the newborn cells contained a helical MreB filament that extended throughout the cell (Fig. 2A). A ringlike MreB arrangement was observed in fewer than 1% of these cells (Fig. 2B). Approximately 7% of the cells contained clustered MreB helical cables. Some of these cables formed two separate clusters located near the two opposite poles of the cell (Fig. 2C), while other cells contained only a single cluster of helical filaments close to one pole (Fig. 2D). MreB filaments sometimes packed more tightly at one end of the cell and were looser at the other end (Fig. 2G). The rest (7%) of the cells contained irregular relaxed MreB coils (Fig. 2E and 2F). However, the morphology of these cells was normal. Notably, cells with irregular cytoskeletal structures frequently had more than one MreB filament that was curved, and these filaments were sometimes wrapped around each other (Fig. 2E). The patterns of MreB localization in elongating cells were similar to those in newborn cells. However, in a few cells, a thin filament linked the two clusters of MreB filaments located at opposite poles (Fig. 2H), suggesting that a single filament formed the two clusters. The decrease in fluorescence in the central region was not caused by the difference between the focal planes used to capture the images. The overall pattern of MreB localization in V. parahaemolyticus was similar to that observed in E. coli (32). The three-dimensional configuration of the MreB cytoskeleton of V. parahaemolyticus was verified by observing cells that were immobilized perpendicular to the coverslip using a conventional fluorescence microscope (38). A loop that was bright on one side but gradually lost fluorescence on the other side was probably a helix (Fig. 2I, lower image). In some cases, the coiled MreB helix was clearly in a slanted position (Fig. 2I, upper image).
The presence of different structures of the MreB cytoskeleton in V. parahaemolyticus in the exponential phase (Fig. 2) suggests that MreB was highly dynamic. The relaxed portion of the MreB cytoskeleton probably disassembled and released free monomers or oligomers to assemble new filaments elsewhere (Fig. 2). By repeating the disassembly-assembly process, the MreB cytoskeleton oscillates between the two poles, and its behavior thus resembles that of MinCDE (32). The disassembly-assembly process may also participate in the partitioning of the MreB cytoskeleton into daughter cells, and the ringlike structure in Fig. 2B may represent one of the steps of the cell division process (39).
Effects of YFP-MreBVp overexpression in V. parahaemolyticus.
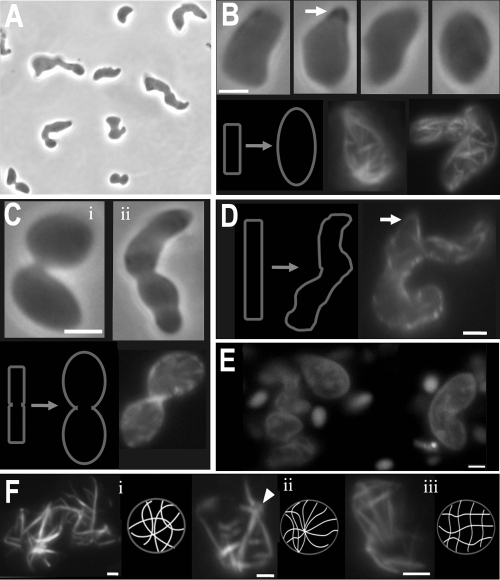
To determine the optimal expression level of YFP-MreBVp, various concentrations of IPTG were used to induce YFP-MreBVp expression. When YFP-MreBVp was overexpressed with 1 mM IPTG in V. parahaemolyticus, the bacillus cells were markedly changed and were swollen and pleomorphic (Fig. 3A). The pleomorphic effects may have been caused by disturbance of cell division and the MreB function, as shown by a time-lapse study. Newborn cells bulged into oval shapes, sometimes with protuberances (Fig. 3B). Cell division was inhibited when YFP-MreBVp was overexpressed, so that the dividing cells swelled and formed two linked ovals (Fig. 3C, panel i). Two unseparated cells that contained several knobs were also observed (Fig. 3C, panel ii). The filamentous cells twisted, and each cell typically had ectopic poles (Fig. 3D). In this work, more than 99% of these cells were viable, as revealed by propidium iodide staining even after 24 h of YFP-MreBVp induction (Fig. 3E).
FIG. 3.
Cell morphology and MreB cytoskeleton of YFP-MreBVp-overexpressing V. parahaemolyticus cells. Strain SC9 cells were grown at 37°C for 3 h in the presence of 1 mM IPTG and then examined using phase-contrast and fluorescent microscopy. (A) Pleomorphic morphological abnormality; (B) newborn cells; (C) dividing cells; (D) elongated cells; (E) viability of YFP-MreBVp-overexpressing cells; (F) MreB cytoskeleton in YFP-MreBVp-overexpressing cells. The viability of cells in panel E was determined using propidium iodide staining for 20 min before examination with a fluorescence microscope. Schematic diagrams of morphological consequences and fluorescence images of YFP-MreBVp are included in some panels. The arrows in panels B and D indicate the protuberance or ectopic pole, while the arrowhead in panel F indicates the center of the MreB radiate network. Scale bars = 2 μm.
The overexpression of YFP-MreBVp in V. parahaemolyticus yielded multiple filaments which were relatively straight and did not have a helical structure (Fig. 3B, C, D, and F). Three different patterns of the MreB cytoskeletal networks were recognized: irregular (Fig. 3F, panel i), radiate (Fig. 3F, panel ii), and reticular (Fig. 3F, panel iii). Optical sectioning verified the presence of multiple MreB filaments in these cells (data not shown). Carballido-Lopez and Errington also reported that altering the expression level of the fusion protein affects the helical pitch of GFP-Mbl filaments. Moreover, the overexpression of Mbl also results in shape abnormalities, such as cell branching (3). Cell division is inhibited and morphological abnormality also occurs in E. coli and Streptomyces coelicolor when MreB is overexpressed (23, 40). The proportion of the phenotypic change that was associated with the YFP tag in this study was unclear.
Overexpression of actin is lethal in eukaryotes. In Saccharomyces cerevisiae, two- to fourfold overexpression of actin results in the formation of large swollen cells, and the maintenance of cell polarity, cell division, and bud formation are impaired (26). Similar effects, except cell lethality (3), were observed in MreB-overexpressing V. parahaemolyticus (Fig. 3) and in other bacteria. Overexpression of MreB does not influence cell viability in E. coli (40) and V. parahaemolyticus (Fig. 3), whereas it is lethal in C. crescentus (13).
MreB overexpression disturbs the configuration and organization of the MreB cytoskeleton and may account for the inhibition of cell division (39). Excess MreB may also cause imbalances in protein complexes that are formed by MreB and its interacting proteins (e.g., MreC/MreD), and it ultimately affects morphogenesis, cell division, and the subcellular architecture (including the cell polarity, the localization of other molecules, and the structure of the nucleoid) of bacterial cells. Peptidoglycan synthesis during altered polarized growth, directed by the MreB cytoskeleton (14), is probably responsible for the change in cell morphology in MreB-overexpressing cells. In MreBVp-overexpressing cells, the sprawling MreB filaments presumably recruit elongation-specific cell wall-synthesizing machinery and change the peptidoglycan synthesis pattern from helical to diffusive. In MreBVp-overexpressing cells, excess MreB may generate extra filaments, and the resulting forked MreB filaments may disturb the regular straight axis of cell elongation, resulting in divergent, twisty, or branched growth patterns (Fig. 3).
Amelioration of morphological abnormality caused by YFP-MreBVp overexpression.
In this study, addition of chemicals that interfere with peptidoglycan synthesis (ampicillin) or stabilization of the cell wall (Mg2+) reduced the morphological abnormality of the cells with overexpressed YFP-MreBVp (Fig. 4). In the presence of a sublethal concentration of beta-lactam antibiotics, bacteria typically form spheroid cells (7). The level of ampicillin (500 μg/ml) used here was low and did not influence the morphology of V. parahaemolyticus SC9 cells with normal MreB expression. However, swelling of the cells with overexpressed YFP-MreBVp was significantly inhibited by adding 500 μg/ml ampicillin (Fig. 4B and C), but the cells remained filamentous and twisted and many long cells (length, about 70 μm) were frequently observed. The cells were also slightly wider than the control cells (Fig. 4A). MreB filaments did not have the helical configuration and were distributed randomly in YFP-MreBVp-overexpressing cells that were grown in the presence of ampicillin (Fig. 4B and C). Ampicillin added to the YFP-MreBVp-overexpressing cells probably interacted directly with penicillin-binding proteins and indirectly with MreB, MreC, FtsZ, or other related proteins to restore the normal growth polarity but with defects in cell division, although no normal helical MreB was observed (Fig. 4). Details concerning the mechanism by which morphological abnormality is reduced by beta-lactam antibiotics in this case are not known.
FIG. 4.
Amelioration of morphological abnormality of YFP-MreBVp-overexpressing V. parahaemolyticus cells with ampicillin and magnesium. YFP-MreBVp-overexpressing strain SC9 cells were obtained by induction with 1 mM IPTG. Amelioration was accomplished by adding 500 μg/ml ampicillin or 25 mM MgSO4 or MgCl2. (A) Strain SC9 cells grown in the presence of ampicillin and in the absence of IPTG; (B and C) cell morphology and MreB cytoskeleton of YFP-MreBVp-overexpressing cells grown in the presence of ampicillin; (D) morphology of YFP-MreBVp-overexpressing cells grown in the presence of magnesium. Scale bars = 2 μm.
Formstone and Errington (11) found that almost normal growth and morphology are restored in cells of the mreB null mutant and cells of B. subtilis with MreB depleted in the presence of high concentrations of magnesium. Magnesium also restores peptidoglycan synthesis and cell proliferation in mutants with mutations in mreC and mreD (18) or penicillin-binding protein ponA genes (25). However, normal morphology is not restored in these cytoskeleton mutants (18). In this study, adding 25 mM MgSO4 or MgCl2 completely restored the normal morphology in most of the YFP-MreBVp-overexpressing cells of V. parahaemolyticus (Fig. 4D, panel i). Cell division defects occurred in a few cells, resulting in long cell chains (Fig. 4D, panels ii and iii). Several MreB filaments without specific configurations were present in these normal-shaped, YFP-MreBVp-overexpressing cells (data not shown). These observations further suggest that MreB influences cell shape via spatial regulation of the cell wall-synthesizing machinery, and the MreB cytoskeleton may not act as a mechanical brace.
Expression of YFP-tagged V. parahaemolyticus MreB in E. coli.
In a comparative study, a merodiploid strain of E. coli SC7 (yfp-mreB mreB) expressing YFP-MreBEc was also constructed. The patterns of localization of YFP-MreBEc in E. coli in exponential phase were equivalent to those of V. parahaemolyticus SC9 (data not shown). Overexpression of YFP-MreBEc also resulted in multiple MreB filaments and swelling of E. coli cells (data not shown). These results suggest that the functions and regulation of the MreB cytoskeleton are similar in E. coli and V. parahaemolyticus.
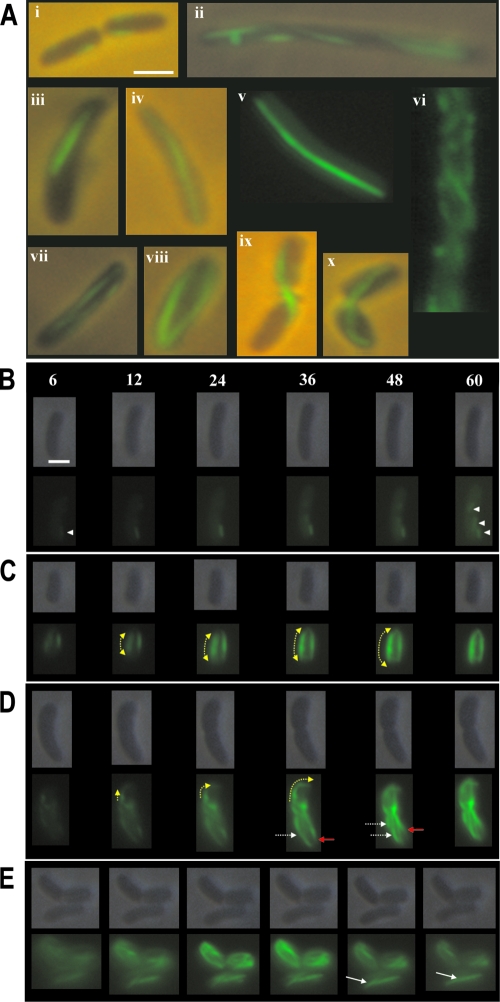
MreBVp shares 91.6% identity and 96.3% similarity with MreBEc. We examined whether YFP-MreBVp copolymerizes with MreBEc in E. coli. Despite the high level of homology between MreBVp and MreBEc, YFP-MreBVp formed independent filaments that were probably attached to the cytoplasmic membrane in E. coli (Fig. 5A). When different E. coli strains (merodiploid strains SC16 and SC17 with native mreBEc) were induced to produce YFP-MreBVp with 10 μM IPTG, a slightly curved filament that was composed of YFP-MreBVp extended along the cytoplasm in most cells (Fig. 5A, panels iv and v). Since the linear YFP-MreBVp filaments did not disturb the morphology of the E. coli hosts (Fig. 5), they could polymerize with themselves but not with MreBEc in E. coli. Accordingly, they could not access the cell wall-synthesizing machinery of the hosts. Some cells contained randomly distributed short fragments or filaments (Fig. 5A, panels i to iii). Two hours of induction increased the number of cells that contained more than one filament (Fig. 5A, panels vii and viii). The YFP-MreBVp filaments were not cleaved after septation and blocked cell division after constriction (Fig. 5A, panels ix and x). Helical YFP-MreBVp filaments were not present in E. coli cells, although curved filaments were wound around each other in a few cells (Fig. 5A, panel vi).
FIG. 5.
Expression of YFP-MreBVp in an ectopic E. coli host. (A) Structures of YFP-MreBVp filaments expressed in E. coli cells. Panels v and vi show results of fluorescence microscopy, while the other panels show combinations of phase-contrast and fluorescence images. (B to E) Time-lapse images of E. coli cells that expressed YFP-MreBVp. The cells were immobilized on an LB agarose pad and examined at 25°C. Each panel shows phase-contrast images and corresponding fluorescence images. The numbers at the top in panel B are times (in min) after IPTG induction. Arrowheads indicate the emergence of short fragments; dashed yellow arrows indicate the growth of filaments; dashed white arrows indicate breaking of filaments; solid white arrows indicate annealing of filaments; and red arrows indicate shrinking of filaments. Scale bars = 2 μm.
The formation of YFP-MreBVp filaments in E. coli was monitored by time-lapse microscopy after addition of 10 μM IPTG. YFP fluorescence occurred after 6 min of induction (Fig. 5B). Several short filaments beneath the cytoplasmic membrane appeared 12 min after induction (Fig. 5B). These short fragments emerged and grew independently (Fig. 5B) unidirectionally or bidirectionally (Fig. 5C and D). Filaments broke into shorter fragments (Fig. 5D) or pairs of small parts of fragments joined (Fig. 5E). Shrinkage of the filaments was also observed (Fig. 5D), which may have represented filament fragmentation or a burst of rapid depolymerization. The fluorescence intensity of YFP-MreBVp filaments increased with time. These results suggest that the single MreB filaments observed with a fluorescence microscope were bundles of several protofilaments. These bundles were continually remodeled by annealing and fragmentation. These short filaments were much shorter than the cells, and the direction of their polarized assembly was independent of the overall cellular polarity.
MreBVp expressed in E. coli assembled into linear filaments that grew unidirectionally or bidirectionally and underwent annealing and fragmentation (Fig. 5). To the best of our knowledge, this study is the first study in which shrinkage, fragmentation, and annealing of MreB filaments were directly observed in bacteria (Fig. 5B to E). Kim et al. (16) indicated that the MreB helices comprise several short protofilaments, which organize randomly to form a helical structure without uniform polarity (16). Using electron microscopy, Esue et al. demonstrated that MreB spontaneously forms filamentous bundles in vitro (8). Short bundles are present in early stages of assembly and elongate with time. Mbl also grows without preferential polarity in B. subtilis (3).
The configuration and localization of YFP-MreBVp filaments in the ectopic hosts (Fig. 5A) resembled the configuration and localization of a mutant of plasmid partitioning protein SopA (20) and mutants of another prokaryotic actin homolog, ParM, that is deficient in ATPase activity (24). Wild-type ParM and SopA both form dynamic structures in vivo and in vitro (20, 24). Garner et al. proposed that the helical and bidirectional ParM filaments spontaneously nucleate and elongate throughout the cell (12). ATPase-deficient mutants of ParM form static, straight filaments that attach to the cytoplasmic membrane and do not separate following septation (24). A SopA mutant can also incorporate wild-type SopA-GFP to form stable, straight filaments that are as long as the cell (20). These filaments cannot be severed following septation, and the cells form long chains that appear to be linked by the filaments. The similarities between filaments of ParM/SopA mutants and ectopically expressed YFP-MreBVp suggest that the ATPase activity of YFP-MreBVp may be interrupted in E. coli.
Two strategies may be used by bacteria to maintain the specific configuration of the dynamic but relatively stable MreB helices. First, there may be some MreB-interacting proteins that help organize and stabilize the MreB helices. Second, MreB may target a preexisting track to accommodate a particular helical configuration. The observation that YFP-MreBVp filaments did not adopt a helical configuration in ectopic E. coli hosts (Fig. 5) provided both evidence for and the opportunity to investigate these hypotheses. YFP-MreBVp can be coexpressed with other proteins of V. parahaemolyticus in E. coli to identify auxiliary proteins that affect the in vivo behavior and the helical configuration of the MreB cytoskeleton. The few differences between the sequences of MreBVp and MreBEc also facilitate the choice of targets for mutagenesis to elucidate the molecular bases of the protein-protein interactions between MreB and putative MreB-interacting proteins. Accordingly, the YFP-MreBVp-E. coli system or any other similar system may provide a platform for clarifying the structure of the MreB cytoskeleton in vivo. Srinivasan et al. (36) used MreBEc with the fission yeast Schizosaccharomyces pombe to investigate the mechanism of assembly and organization of the MreB cytoskeleton (36). They observed that MreBEc forms relatively straight filaments in the ectopic yeast host and that these filaments can bundle together.
In conclusion, this work revealed the dynamic pattern of localization of MreB in V. parahaemolyticus and its relationship to cell morphogenesis. The YFP-MreBVp-E. coli system described here may provide a model for investigating the dynamic structures of the MreB cytoskeleton in vivo.
Acknowledgments
We thank the National Science Council of Taiwan, Republic of China, for financially supporting this research under contracts NSC 94-2313-B-031-003 and NSC 95-2313-B-031-002.
L. I. Hor, L. Rothfield and D. Schneider are thanked for kindly providing bacterial strains and plasmids, and K. B. Choo is thanked for providing advice and equipment. Ted Knoy is thanked for editorial assistance.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Bullock, W. O., J. M. Fernandez, and J. M. Short. 1987. XL1-Blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. Bio/Technology 5:376-378. [Google Scholar]
- 2.Butler, Y. X., Y. Abhayawardhane, and G. C. Stewart. 1993. Amplification of the Bacillus subtilis maf gene results in arrested septum formation. J. Bacteriol. 175:3139-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carballido-Lopez, R., and J. Errington. 2003. The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev. Cell 4:19-28. [DOI] [PubMed] [Google Scholar]
- 4.Carballido-Lopez, R., A. Formstone, Y. Li, S. D. Ehrlich, P. Noirot, and J. Errington. 2006. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev. Cell 11:399-409. [DOI] [PubMed] [Google Scholar]
- 5.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 6.Defeu Soufo, H. J., and P. L. Graumann. 2004. Dynamic movement of actin-like proteins within bacterial cells. EMBO Rep. 5:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLoney, C. R., and N. L. Schiller. 1999. Competition of various beta-lactam antibiotics for the major penicillin-binding proteins of Helicobacter pylori: antibacterial activity and effects on bacterial morphology. Antimicrob. Agents Chemother. 43:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esue, O., M. Cordero, D. Wirtz, and Y. Tseng. 2005. The assembly of MreB, a prokaryotic homolog of actin. J. Biol. Chem. 280:2628-2635. [DOI] [PubMed] [Google Scholar]
- 9.Esue, O., D. Wirtz, and Y. Tseng. 2006. GTPase activity, structure, and mechanical properties of filaments assembled from bacterial cytoskeleton protein MreB. J. Bacteriol. 188:968-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figge, R. M., A. V. Divakaruni, and J. W. Gober. 2004. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol. Microbiol. 51:1321-1332. [DOI] [PubMed] [Google Scholar]
- 11.Formstone, A., and J. Errington. 2005. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Mol. Microbiol. 55:1646-1657. [DOI] [PubMed] [Google Scholar]
- 12.Garner, E. C., C. S. Campbell, and R. D. Mullins. 2004. Dynamic instability in a DNA-segregating prokaryotic actin homolog. Science 306:1021-1025. [DOI] [PubMed] [Google Scholar]
- 13.Gitai, Z., N. Dye, and L. Shapiro. 2004. An actin-like gene can determine cell polarity in bacteria. Proc. Natl. Acad. Sci. USA 101:8643-8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, L. J., R. Carballido-Lopez, and J. Errington. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913-922. [DOI] [PubMed] [Google Scholar]
- 15.Karczmarek, A., R. Martinez-Arteaga, S. Alexeeva, F. G. Hansen, M. Vicente, N. Nanninga, and T. den Blaauwen. 2007. DNA and origin region segregation are not affected by the transition from rod to sphere after inhibition of Escherichia coli MreB by A22. Mol. Microbiol. 65:51-63. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S. Y., Z. Gitai, A. Kinkhabwala, L. Shapiro, and W. E. Moerner. 2006. Single molecules of the bacterial actin MreB undergo directed treadmilling motion in Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 103:10929-10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruse, T., J. Moller-Jensen, A. Lobner-Olesen, and K. Gerdes. 2003. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 22:5283-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leaver, M., and J. Errington. 2005. Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis. Mol. Microbiol. 57:1196-1209. [DOI] [PubMed] [Google Scholar]
- 19.Lesage, G., and H. Bussey. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:317-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim, G. E., A. I. Derman, and J. Pogliano. 2005. Bacterial DNA segregation by dynamic SopA polymers. Proc. Natl. Acad. Sci. USA 102:17658-17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 22.Marsh, J. W., and R. K. Taylor. 1999. Genetic and transcriptional analyses of the Vibrio cholerae mannose-sensitive hemagglutinin type 4 pilus gene locus. J. Bacteriol. 181:1110-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazza, P., E. E. Noens, K. Schirner, N. Grantcharova, A. M. Mommaas, H. K. Koerten, G. Muth, K. Flardh, G. P. van Wezel, and W. Wohlleben. 2006. MreB of Streptomyces coelicolor is not essential for vegetative growth but is required for the integrity of aerial hyphae and spores. Mol. Microbiol. 60:838-852. [DOI] [PubMed] [Google Scholar]
- 24.Moller-Jensen, J., R. B. Jensen, J. Lowe, and K. Gerdes. 2002. Prokaryotic DNA segregation by an actin-like filament. EMBO J. 21:3119-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray, T., D. L. Popham, and P. Setlow. 1998. Bacillus subtilis cells lacking penicillin-binding protein 1 require increased levels of divalent cations for growth. J. Bacteriol. 180:4555-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norden, C., D. Liakopoulos, and Y. Barral. 2004. Dissection of septin actin interactions using actin overexpression in Saccharomyces cerevisiae. Mol. Microbiol. 53:469-483. [DOI] [PubMed] [Google Scholar]
- 27.Okada, Y., M. Wachi, A. Hirata, K. Suzuki, K. Nagai, and M. Matsuhashi. 1994. Cytoplasmic axial filaments in Escherichia coli cells: possible function in the mechanism of chromosome segregation and cell division. J. Bacteriol. 176:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson, J. D., L. A. Umayam, T. Dickinson, E. K. Hickey, and O. White. 2001. The comprehensive microbial resource. Nucleic Acids Res. 29:123-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippe, N., J. P. Alcaraz, E. Coursange, J. Geiselmann, and D. Schneider. 2004. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51:246-255. [DOI] [PubMed] [Google Scholar]
- 30.Pogliano, J., N. Osborne, M. D. Sharp, A. Abanes-De Mello, A. Perez, Y. L. Sun, and K. Pogliano. 1999. A vital stain for studying membrane dynamics in bacteria: a novel mechanism controlling septation during Bacillus subtilis sporulation. Mol. Microbiol. 31:1149-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shih, Y. L., X. Fu, G. F. King, T. Le, and L. Rothfield. 2002. Division site placement in E. coli: mutations that prevent formation of the MinE ring lead to loss of the normal midcell arrest of growth of polar MinD membrane domains. EMBO J. 21:3347-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih, Y. L., T. Le, and L. Rothfield. 2003. Division site selection in Escherichia coli involves dynamic redistribution of Min proteins within coiled structures that extend between the two cell poles. Proc. Natl. Acad. Sci. USA 100:7865-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shih, Y. L., and L. Rothfield. 2006. The bacterial cytoskeleton. Microbiol. Mol. Biol. Rev. 70:729-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Slovak, P. M., G. H. Wadhams, and J. P. Armitage. 2005. Localization of MreB in Rhodobacter sphaeroides under conditions causing changes in cell shape and membrane structure. J. Bacteriol. 187:54-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan, R., M. Mishra, M. Murata-Hori, and M. K. Balasubramanian. 2007. Filament formation of the Escherichia coli actin-related protein, MreB, in fission yeast. Curr. Biol. 17:266-272. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava, P., G. Demarre, T. S. Karpova, J. McNally, and D. K. Chattoraj. 2007. Changes in nucleoid morphology and origin localization upon inhibition or alteration of the actin homolog, MreB, of Vibrio cholerae. J. Bacteriol. 189:7450-7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, Q., and W. Margolin. 1998. FtsZ dynamics during the division cycle of live Escherichia coli cells. J. Bacteriol. 180:2050-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vats, P., and L. Rothfield. 2007. Duplication and segregation of the actin (MreB) cytoskeleton during the prokaryotic cell cycle. Proc. Natl. Acad. Sci. USA 104:17795-17800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wachi, M., and M. Matsuhashi. 1989. Negative control of cell division by mreB, a gene that functions in determining the rod shape of Escherichia coli cells. J. Bacteriol. 171:3123-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachi, M., K. Osaka, T. Kohama, K. Sasaki, I. Ohtsu, N. Iwai, A. Takada, and K. Nagai. 2006. Transcriptional analysis of the Escherichia coli mreBCD genes responsible for morphogenesis and chromosome segregation. Biosci. Biotechnol. Biochem. 70:2712-2719. [DOI] [PubMed] [Google Scholar]
- 42.Wong, H. C., S. H. Liu, T. K. Wang, C. L. Lee, C. S. Chiou, D. P. Liu, M. Nishibuchi, and B. K. Lee. 2000. Characteristics of Vibrio parahaemolyticus O3:K6 from Asia. Appl. Environ. Microbiol. 66:3981-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]