Abstract
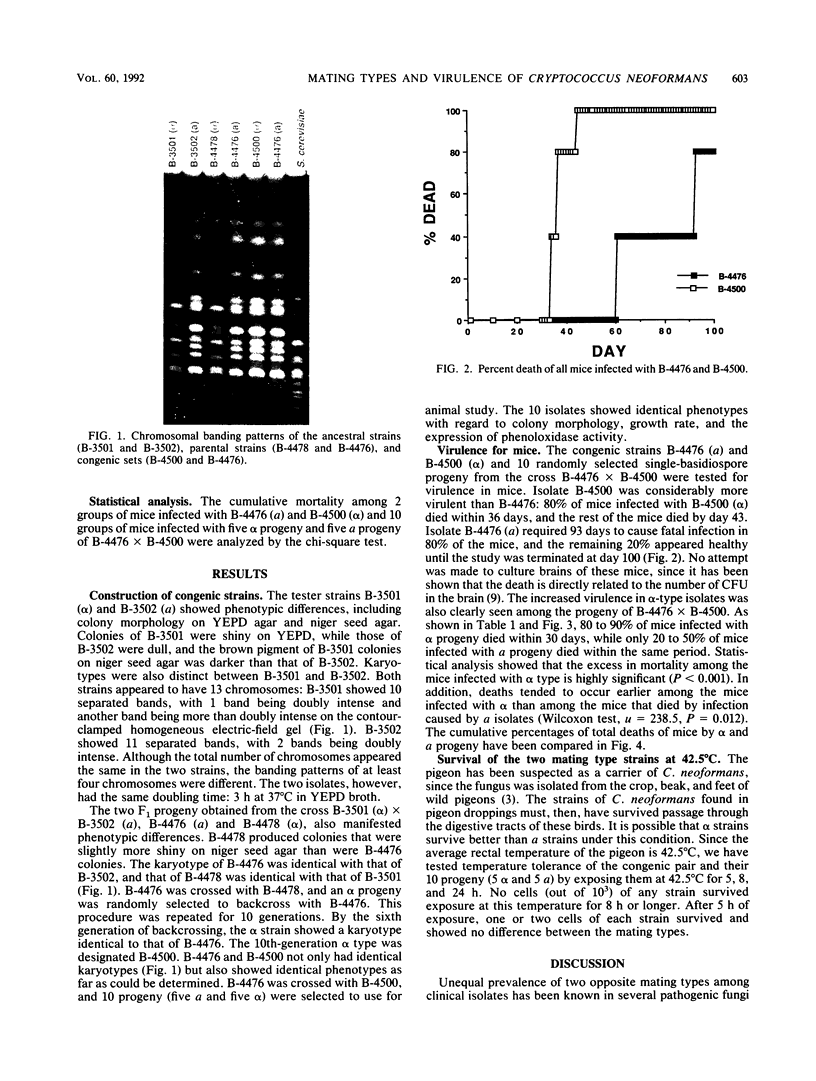
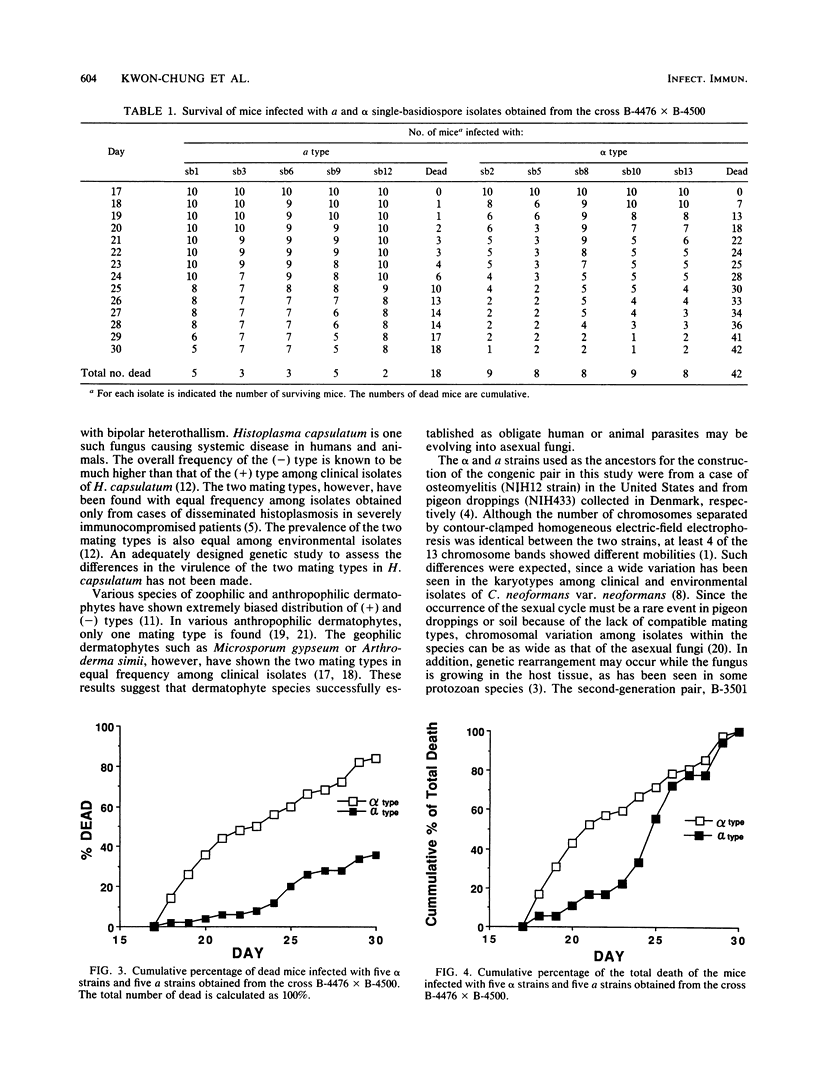
A pair of congenic Cryptococcus neoformans var. neoformans strains, B-4476 (a mating type) and B-4500 (alpha mating type), that presumably differ only in mating type was constructed. This pair and their progeny, five alpha type and five a type, were tested for virulence in mice. In the parent strains as well as the progeny, alpha type was clearly more virulent than a type. In addition, death tended to occur earlier among the alpha-strain-infected mice that died than among the mice that died by infection caused by a strains. These data strongly suggest the genetic association of virulence with mating type in this human fungal pathogen.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edman J. C., Kwon-Chung K. J. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol Cell Biol. 1990 Sep;10(9):4538–4544. doi: 10.1128/mcb.10.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson L., Specht C. A., Milgrim C., Novotny C. P., Ullrich R. C. Cloning and comparison of A alpha mating-type alleles of the Basidiomycete Schizophyllum commune. Mol Gen Genet. 1989 Jul;218(1):72–77. doi: 10.1007/BF00330567. [DOI] [PubMed] [Google Scholar]
- Janse C. J., Boorsma E. G., Ramesar J., van Vianen P., van der Meer R., Zenobi P., Casaglia O., Mons B., van der Berg F. M. Plasmodium berghei: gametocyte production, DNA content, and chromosome-size polymorphisms during asexual multiplication in vivo. Exp Parasitol. 1989 Apr;68(3):274–282. doi: 10.1016/0014-4894(89)90109-4. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bartlett M. S., Wheat L. J. Distribution of the two mating types among Histoplasma capsulatum isolates obtained from an urban histoplasmosis outbreak. Sabouraudia. 1984;22(2):155–157. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Distribution of alpha and alpha mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978 Oct;108(4):337–340. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E., Rhodes J. C. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Van Leeuwenhoek. 1982;48(1):25–38. doi: 10.1007/BF00399484. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Rhodes J. C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986 Jan;51(1):218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Weeks R. J., Larsh H. W. Studies on Emmonsiella capsulata (Histoplasma capsulatum). II. Distribution of the two mating types in 13 endemic states of the United States. Am J Epidemiol. 1974 Jan;99(1):44–49. doi: 10.1093/oxfordjournals.aje.a121583. [DOI] [PubMed] [Google Scholar]
- Littman M. L., Borok R. Relation of the pigeon to cryptococcosis: natural carrier state, heat resistance and survival of Cryptococcus neoformans. Mycopathol Mycol Appl. 1968 Oct 14;35(3):329–345. doi: 10.1007/BF02050749. [DOI] [PubMed] [Google Scholar]
- May G., Le Chevanton L., Pukkila P. J. Molecular analysis of the Coprinus cinereus mating type A factor demonstrates an unexpectedly complex structure. Genetics. 1991 Jul;128(3):529–538. doi: 10.1093/genetics/128.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staib F. Pflanzen als Nährsubstrat für Cryptococcus neoformans. Zentralbl Bakteriol Orig A. 1971 Dec;218(4):486–495. [PubMed] [Google Scholar]
- Still C. N., Jacobson E. S. Recombinational mapping of capsule mutations in Cryptococcus neoformans. J Bacteriol. 1983 Oct;156(1):460–462. doi: 10.1128/jb.156.1.460-462.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale P. M., Mackenzie D. W., Austwick P. K. Arthroderma simii sp. nov., the perfect state of Trichophyton simii (Pinoy) comb. nov. Sabouraudia. 1965 Jun;4(2):112–123. [PubMed] [Google Scholar]
- Stockdale P. M. The Microsporum gypseum complex (Nannizzia incurvata Stockd., N. gypsea (Nann.) comb. nov., N. fulva sp. nov.). Sabouraudia. 1963 Oct;3(1):114–126. [PubMed] [Google Scholar]
- Wickes B. L., Golin J. E., Kwon-Chung K. J. Chromosomal rearrangement in Candida stellatoidea results in a positive effect on phenotype. Infect Immun. 1991 May;59(5):1762–1771. doi: 10.1128/iai.59.5.1762-1771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. N. Pseudo-cleistothecia in Trichophyton rubrum. Sabouraudia. 1968 Feb;6(2):160–162. [PubMed] [Google Scholar]




