Abstract
Extracting fungal mRNA from ectomycorrhizas (ECMs) and forest soil samples for monitoring in situ metabolic activities is a significant challenge when studying the role of ECMs in biogeochemical cycles. A robust, simple, rapid, and effective method was developed for extracting RNA from rhizospheric soil and ECMs by adapting previous grinding and lysis methods. The quality and yield of the extracted RNA were sufficient to be used for reverse transcription. RNA extracted from ECMs of Lactarius quietus in a 100-year-old oak stand was used to construct a cDNA library and sequence expressed sequence tags. The transcripts of many genes involved in primary metabolism and in the degradation of organic matter were found. The transcription levels of four targeted fungal genes (glutamine synthase, a general amino acid transporter, a tyrosinase, and N-acetylhexosaminidase) were measured by quantitative reverse transcription-PCR in ECMs and in the ectomycorrhizospheric soil (the soil surrounding the ECMs containing the extraradical mycelium) in forest samples. On average, levels of gene expression for the L. quietus ECM root tips were similar to those for the extraradical mycelium, although gene expression varied up to 10-fold among the samples. This study demonstrates that gene expression from ECMs and soil can be analyzed. These results provide new perspectives for investigating the role of ectomycorrhizal fungi in the functioning of forest ecosystems.
In boreal and temperate forests, almost all fine roots are associated with symbiotic fungi, forming composite organs called ectomycorrhizas (ECMs) (53). ECMs are aggregated in the uppermost 20 cm of soil, where nutrient turnover is high (35, 52). ECM fungal communities are species rich, and hundreds of different fungal symbionts in a forest stand can be identified by DNA-based molecular methods (16, 20, 32, 33, 54). ECM fungi establish a series of hyphal networks with different physiological activities, comprising the mycorrhizal mantle; the intraradical Hartig net; the extraradical mycelium, which colonizes the soil; and the hyphal aggregates, which colonize decaying organic matter. ECMs also efficiently take up water as well as organic and inorganic nutrients from the soil via the extraradical mycelium and translocate them to colonized tree roots, where they receive host carbohydrates in return (41). The soil ECM hyphal web has a central position at the soil-tree interface and can produce extracellular enzymes that hydrolyze polysaccharides, proteins, and chitin, although to a lesser extent than litter decomposers (1, 7, 10, 14, 40; P. E. Courty, P. J. Hoegger, S. Kilaru, A. Kohler, M. Buée, J. Garbaye, F. Martin, and U. Kües, unpublished data). The ECM fungi play a crucial role in forest tree health by enhancing nutrient acquisition, drought tolerance, and soil pathogen resistance of their hosts (43).
The genome sequence of the ectomycorrhizal basidiomycete Laccaria bicolor has been published (40), and the gene expression patterns of ECM symbionts have been well characterized using transcript profiling in several ectomycorrhizal systems grown in vitro and in soil microcosms (19, 26, 34, 55). Gene expression has also been studied separately in the ECM root tip and in the extraradical mycelium in the Paxillus involutus-Betula pendula association (45, 57). However, the transcriptome of environmental ECM samples has not yet been investigated. Although technically challenging, gene profiling of ECMs collected in situ would provide potential molecular markers for studying biogeochemical cycles related to the adaptation and the resilience of ECM communities subjected to environmental constraints (3, 21, 42, 49). In the work described here, we sought to extract RNA from ectomycorrhizas and soil collected in the forest and to demonstrate the reliability of the results.
In the present pilot study, we identified the major transcripts expressed in Lactarius quietus-Quercus petraea ECMs and in the surrounding soil containing the extraradical mycelium. L. quietus is the most abundant ECM species associated with oak forests in northeastern France (13).
MATERIALS AND METHODS
Site and forest stand.
The experimental site is a 100-year-old oak forest with a continuous canopy and a hornbeam [Quercus petraea (Mattuschka) Liebl., Quercus robur Ehrh., and Carpinus betulus L.] understory, located in the Champenoux State Forest in northeastern France (48°75′N, 6°35′E; altitude, 250 m). The luvic cambisol (pH [H2O] 4.6) has a loamy texture in the A1 (0 to 5 cm) (P, 0.3 g kg−1, according to the method described previously by Duchaufour and Bonneau [18]; total N, 1.9 g kg−1; C/N ratio, 14.6) and A2 (P, 0.4 g kg−1; total N, 2.35 g kg−1; C/N ratio, 13.1) horizons. The forest floor is flat, with scarce vegetation (oak seedlings, Convallaria majalis L., and Deschampsia cespitosa L.) (14).
Pure culture of Lactarius quietus isolate S24.
Pure cultures of L. quietus isolate S24, obtained from an ECM sampled at the experimental site, were grown and maintained on Pachlewski medium agar plates (47). For gene expression, the mycelium was transferred onto cellophane membrane-covered agar plates containing low-sugar (5 g liter−1 glucose) agar Pachlewski medium and grown for 4 weeks before the proliferating hyphal tips at the edge of the colony were harvested. Total RNA was isolated from snap-frozen (liquid nitrogen) and ground fungal tissues using the extraction protocol developed for ECMs described below. This condition was considered to be the control of the experiment.
Sampling and identification of L. quietus ECMs.
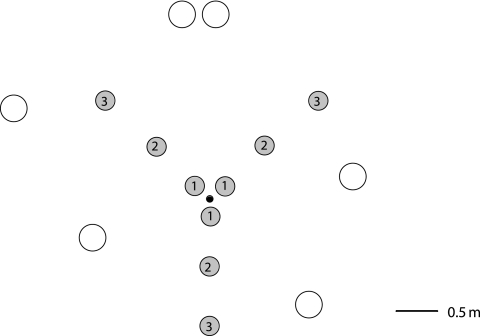
In order to construct a cDNA library from L. quietus ectomycorrhizas, six soil cores (4 cm in diameter and 10 cm deep) were randomly sampled monthly from March to June 2006 over a wide range of pedoclimatic and phenological conditions including the bud break period (12). In March 2007, nine soil cores (4 cm in diameter and 10 cm deep) were harvested from an initial point at increasing intervals (0.04, 0.5, and 1 m) along three diverging axes (i.e., three transects) forming 120° angles (see Fig. 1 for the oak tree position) to measure the transcription level of selected genes in L. quietus ECMs and in the surrounding soil. Soil cores were immediately transported to the laboratory and processed within 1 h of harvesting. The top of each soil core (0 to 5 cm), corresponding to the A1 horizon, enriched in organic matter and containing densely packed fine roots, was observed with a stereomicroscope (magnification, ×40). L. quietus ECMs were identified according to methods described previously by Agerer (2). Twenty to 60 ECM tips coming from different ECM clusters (depending on the soil core) were sampled using forceps; cleaned of soil, organic matter, or litter fragments; and immediately frozen in liquid nitrogen to prevent the degradation of RNA. One representative sample of L. quietus ECM per soil core was genotyped by internal transcribed spacer sequencing of the fungal tissues (8, 14) to confirm the fungal species. The 5 mm of soil surrounding L. quietus ECMs, where the extraradical mycelium is located, was also sampled and frozen in liquid nitrogen.
FIG. 1.
Distribution of the nine soil cores (gray circles, 4 cm in diameter and 20 cm deep) sampled from an initial point (black dot) at increasing intervals (0.04, 0.5, and 1 m) along three diverging axes (i.e., three transects) forming 120° angles in the Champenoux oak forest in March 2007. White circles correspond to oak trees. The numbers in the gray circles correspond to samples collected at the same interval from the central point: 1, 0.04 m; 2, 0.5 m; 3, 1 m.
RNA extraction from L. quietus ECMs.
Total RNA was extracted from snap-frozen (liquid nitrogen) ECMs of L. quietus sampled in situ by using the hot phenol procedure (9, 51). Tissues (100 mg) were ground in a mortar with liquid nitrogen, 0.5 g of glass beads (106 μm, catalog number G4649; Sigma, France), and 200 μl of 3% diatomaceous earth suspension (catalog number D3877; Sigma, France). The resulting powder was homogenized in a 746-μl mixture of extraction buffer (100 mM Tris-HCl [pH 8], 20 mM EDTA, 0.5 M NaCl, 0.5% sodium dodecyl sulfate, 0.1 M 2-β-mercaptoethanol) and phenol (aquaphenol, 5:1 [vol/vol]) (catalog number 130181; Appligene, France), followed by incubation at 65°C for 10 min. After the addition of chloroform (1:2 [vol/vol]), the extract (1,222 μl) was maintained on ice for 15 min and then centrifuged at 9,000 × g for 10 min at 4°C. The upper layer of the supernatant (≈700 μl) was collected, avoiding the interface, which contained most of the whole genomic DNA. RNA was then precipitated by the addition of 8 M LiCl to a final concentration of 2 M and incubated on ice for 60 min. After centrifugation at 9,000 × g for 10 min at 4°C, the RNA pellet was resuspended in 20 μl of Tris-EDTA buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 7.5]) and purified twice by the phenol-chloroform procedure. RNA was finally precipitated overnight at −20°C in 3 M sodium acetate (1:3 [vol/vol], pH 5.2) and 100% ethanol (2 V). The solution was centrifuged at 9,000 × g for 10 min at 4°C, and the RNA pellet was washed twice in 70% ethanol and resuspended in 20 μl diethyl pyrocarbonate (DEPC)-treated water (0.02%). To ensure that the RNA solution was clear of DNA, the RNase-free DNase set (catalog number 79254; Qiagen, France) was used to digest DNA and to clean the solution.
RNA extraction from the soil surrounding L. quietus ECMs.
One gram of soil from the L. quietus ectomycorrhizosphere containing the extraradical mycelium was sampled in the A1 horizon. Four aliquots (0.25 g) were then suspended in 250 μl of DEPC-treated water and incubated at −80°C for 60 min. A solution containing 0.5 g of glass beads (106 μm; Sigma), 33.3 μl of 20% sodium dodecyl sulfate, 167 μl of 3% diatomaceous earth (Sigma, France), 583 μl of phenol solution (catalog number 77607; Fluka, Germany), and 0.2% (vol/vol) 2-β-mercaptoethanol was then added to the frozen aliquots before grinding in a mortar for 4 min. After centrifugation at 14,000 × g for 15 min at 4°C, the supernatants were transferred into new tubes, mixed with 49 μl of 3 M sodium acetate and 637 μl of 100% ethanol, and incubated overnight at −20°C. The nucleic acid pellets obtained after centrifugation (15 min at 14,000 × g at 4°C) were washed with a 70% ethanol solution, dried at room temperature, and dissolved in 25 μl of DEPC-treated water. The four nucleic acid solutions were pooled, and the total RNA was separated from the DNA using the RNA/DNA Mini kit (catalog number 4123; Qiagen, France) (10) as recommended by the manufacturer. Before starting the purification of the extracted RNA with the RNeasy Plant Mini kit (Qiagen, France) according to the manual instructions, 2.5 mg/ml of active charcoal (Sigma) was added to the washing buffer.
RNA extraction was performed under three conditions: (i) 100 mg of pure L. quietus mycelium was extracted with the RNeasy Plant Mini kit (catalog number 74904; Qiagen, France), (ii) 1 g of forest soil was extracted with the protocol described above, and (iii) 1 g of forest soil was mixed with 100 mg of L. quietus mycelium and extracted with our protocol. The quality and total RNA concentration for each of these preparations were evaluated using the Experion automated RNA electrophoresis system (RNA HighSens chips; Bio-Rad, France). The extraction yield of L. quietus RNA from the mix of L. quietus mycelium and forest soil was 80.6% ± 2.8% (n = 3) of the RNA quantity obtained with the RNA Plant Mini kit from pure culture mycelium.
Construction of cDNA library, DNA sequencing, and generation and analysis of cDNA arrays.
A full-length cDNA library of L. quietus-Q. petraea ECMs was constructed from 1 μg of total RNA using the Smart cDNA synthesis kit in λTriplEx2 (Clontech, Palo Alto, CA). The resulting cDNA was packaged into λ phages using the Gigapack III Gold packaging kit (Stratagene, La Jolla, CA). From the initial plating, the library was estimated to contain 1 × 109 recombinant clones. The pTriplEx2 phagemid clones in Escherichia coli were obtained by using the mass in vivo excision protocol according to the manufacturer's instructions (Clontech). cDNA inserts from 960 bacterial clones derived from the cDNA library were amplified, and inserts longer than 500 bp were single-pass sequenced from the 5′ end using primer FORNAT (5′-AAGCGCGCCATTGTGTTGGTACCC-3′) with a CEQ 8000XL sequencer (Beckman Coulter) (29).
Sequence processing and annotation.
Raw sequence data from 469 clones were edited using the CEQ sequence analysis program (Beckman Coulter). All sequence outputs obtained from the automated sequencer were scanned visually to confirm peak shape and correspondence with base calls. Sequence data were then uploaded in the SEQUENCHER (version 4.1.2) program for Macintosh (Gene Codes Corporation, Ann Arbor, MI). Leading vector, trailing vector, polylinker sequences, and sequence ends with more than 3% ambiguous base calls were removed. Edited sequences were exported as FASTA text files for further processing (29). Each expressed sequence tag (EST) was compared with data in the NCBI Database (ftp://ftp.ncbi.nlm.nih.gov/blast/db/), the KOG Database (http://genome.jgi-psf.org/help/kogclass.html), the KEGG Database (ftp://ftp.genome.jp/pub/kegg/tarfiles/), the Conserved Domain Database (ftp://ftp.ncbi.nih.gov/pub/mmdb/cdd/), and Gene Ontology (http://www.geneontology.org). ESTs from Lactarius quietus were compared with ESTs from Pisolithus microcarpus strain 441 in the Ectomycorrhiza Database (http://mycor.nancy.inra.fr/ectomycorrhizadb/index.html) and with genes or ESTs from Laccaria bicolor present on the genome database of the DOE Joint Genome Institute (http://genome.jgi-psf.org/Lacbi1/Lacbi1.home.html) (40).
Quantitative PCR analysis.
L. quietus gene expression in L. quietus-Q. petraea ECMs and in the surrounding soil of ECMs was performed using a two-step quantitative reverse transcription-PCR (qRT-PCR) procedure. RNA was quantified with the Experion automated RNA electrophoresis system (Bio-Rad, France) and then reverse transcribed (80 ng per reaction) using the iScript cDNA synthesis kit (catalog number 170-8891; Bio-Rad, France). cDNAs were used as templates in real-time quantitative PCRs with gene-specific primers (Table 1) designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and Amplify 3.1 (http://engels.genetics.wisc.edu/amplify). The following criteria were used: product size between 100 and 400 bp, a melting temperature of 60°C ± 1°C, and a %GC content of >50%. Four genes were selected from the L. quietus cDNA library for their potential biological relevance in primary metabolism (an amino acid transporter, glutamine synthase), in the mobilization of carbon and nitrogen from organic matter (N-acetylhexosaminidase), and in the oxidation of polyphenols (tyrosinase) (Table 1). Target gene expression was normalized to eukaryotic initiation factor 4A (eIF-4A). Reactions of quantitative PCR were run using the MJ-opticon2 DNA real-time PCR system (Bio-Rad, Hercules, CA). The following cycling parameters were applied: 95°C for 3 min and then 40 cycles of 95°C for 30 s, 60°C for 1 min, and 72°C for 30 s. A negative control was run for each primer pair. For data analysis, the geometric mean of the biological replicates (n = 3 to 9) for ECM and soil was calculated, respectively. The primer efficiency was between 90% and 110%. Differences were calculated by using the threshold cycle (ΔΔCT) method (37).
TABLE 1.
Lactarius quietus genes selected and primer pairs designed for qRT-PCR analysis on the basis of their putative functions
| Clone | Description | Primer sequence
|
|
|---|---|---|---|
| Forward | Reverse | ||
| Lq-P03F04 | Glutamine synthetase | ACTATCGTGCCTGTCTTTATGCT | ACCTTTACACCCCACTGCTCT |
| Lq-P05F09 | Amino acid transporter | GGTTGGTGGGCTGTGATG | CAGTCCCTTTGCCTTGAGTG |
| Lq-P02D05 | N-Acetylhexosaminidase | ACGCGAGGTGCTACACAGA | GCAGACGAGATCCACACCA |
| Lq-P05H06 | Tyrosinase | TGGATAGGCTTACGTCGCTTG | GTCCGTGGGTGATGTGGT |
| Lq-P08H10 | Translation initiation factor eIF-4A | CGCACCGACACCATCAATA | GCGGAGAGAAGGACGACTT |
Nucleotide sequence accession numbers.
ESTs described here are available at the NCBI GenBank database under accession numbers FK857662 to FK858114.
RESULTS
Overall distribution of sequence categories.
We postulated that genes expressed in ECMs sampled in environmental conditions would include genes involved in symbiotic metabolism and in environmental functions related to nutrient acquisition and interactions with other rhizospheric organisms. Gene profiling, based on the sequencing of ESTs, was thus carried out on a population of L. quietus-Q. petraea ECMs sampled in situ in the A1 horizon of a 100-year-old oak forest. cDNA inserts from 960 bacterial clones derived from a cDNA library of ECMs were PCR amplified. The inserts had an average size of 580 bp and a size range of between 50 and 1,400 bp. Four hundred sixty-nine cDNA inserts longer than 500 bp were sequenced from the 5′ end. Upon assembling the ESTs using the SEQUENCHER program, we were left with 406 nonredundant singletons and 23 tentative consensus sequences (TCs). The number of ESTs in TCs ranged between 2 and 9. The larger TCs showed a strong homology with an unknown protein and a metallothionein-like protein of Quercus robur.
To identify potential homologues to known genes, ESTs were compared to sequences deposited in the NCBI databases using the BLASTN and BLASTX algorithms (4). Among these ESTs, 296 (48%) were similar to known plant or fungal genes, including genes of known function, putative open reading frames, and ESTs (Table 2). This result is in agreement with results obtained for other ECM EST projects: Pisolithus microcarpus-Eucalyptus globulus (47, 55), Laccaria bicolor-Pseudotsuga menziesii (47), and Paxillus involutus-Betula pendula (26, 45). Finally, the remaining ESTs showed no significant similarity to any other sequences in the NCBI databases, suggesting that these genes might be expressed only in Q. robur or L. quietus or only in samples from environmental conditions or that these ESTs corresponded to very rare transcripts that have not been found in previous EST projects.
TABLE 2.
Fungal and plant ESTs obtained from the environmental Lactarius quietus-Quercus petraea ECM librarya
| Clone and category | GenBank accession no. | GenBank accession no. of best BLASTX hit | No. of ESTs | Best BLASTX hit | Species | BLASTX E value |
|---|---|---|---|---|---|---|
| Annotated | ||||||
| Lq-P03F04 | FK857726 | EDR12882 | 1 | Glutamine synthetase | Laccaria bicolor | 2e−79 |
| Lq-P10D11 | FK858087 | EDR10917 | 1 | Transketolase | Laccaria bicolor | 6e−79 |
| Lq-P09F06 | FK858000 | EDR14846 | 1 | Alpha-glucan synthase | Laccaria bicolor | 3e−78 |
| Lq-P05F09 | FK857824 | EDR15065 | 1 | Amino acid transporter | Laccaria bicolor | 8e−75 |
| Lq-P10E01 | FK858089 | EDR09370 | 1 | Malate dehydrogenase, NAD dependent | Laccaria bicolor | 3e−71 |
| Lq-P04H03 | FK857781 | CAB96110 | 1 | Chitin synthase | Agaricus bisporus | 5e−69 |
| Lq-P03G05 | FK857733 | P78571 | 1 | 40S ribosomal protein S13 | Agaricus bisporus | 1e−66 |
| Lq-P08C11 | FK857936 | EDR15577 | 1 | Glutamine amidotransferase | Laccaria bicolor | 4e−66 |
| Lq-P06E09 | FK857870 | EDR14747 | 1 | Transport protein Sec22 | Laccaria bicolor | 1e−60 |
| Lq-P04E02 | FK857762 | EDR09864 | 1 | Transaldolase | Laccaria bicolor | 5e−58 |
| Lq-P04D03 | FK857756 | ABR88135 | 1 | Trehalose phosphorylase | Pleurotus pulmonarius | 6e−54 |
| Lq-P01A08 | FK858017 | XP_964223 | 1 | Mitochondrial cytochrome c peroxidase | Neurospora crassa | 5e−51 |
| Lq-P05H06 | FK857833 | BAB71736 | 1 | Tyrosinase | Lentinula edodes | 2e−43 |
| Lq-P08H10 | FK857969 | EDR11618 | 1 | Translation initiation factor eIF-4A | Laccaria bicolor | 1e−42 |
| Lq-P04H09 | FK857784 | EDR07307 | 1 | Glutaredoxin | Laccaria bicolor | 2e−34 |
| Lq-P06C06 | FK857857 | EDR03895 | 1 | Thioredoxin | Laccaria bicolor | 3e−34 |
| Lq-P02D05 | FK857674 | EDR13643 | 1 | N-Acetylhexosaminidase | Laccaria bicolor | 4e−27 |
| Lq-P06E01 | FK857865 | CAE12162 | 6 | Metallothionein-like protein | Quercus robur | 1e−24 |
| Lq-P05G03 | FK857827 | EDR05211 | 1 | Endo-1,3,1,4-beta-D-glucanase | Laccaria bicolor | 9e−23 |
| Lq-P01B11 | FK858026 | AAR01249 | 1 | Laccase | Laccaria bicolor | 1e−22 |
| Lq-P05H05 | FK857832 | ABD61576 | 1 | Copper radical oxidase | Phanerochaete chrysosporium | 1e−21 |
| Lq-P10B02 | FK858071 | AAN76524 | 2 | Heat shock protein 90 | Cryptococcus bacillisporus | 5e−18 |
| Lq-P02E05 | FK857678 | BAD11071 | 2 | Hin1-like protein | Capsicum chinense | 2e−13 |
| Lq-P09F03 | FK857997 | CAC84735 | 2 | Iron transport protein 2 | Ricinus communis | 3e−08 |
| Lq-P09D09 | FK857989 | EDR10921 | 2 | Glutamate decarboxylase | Laccaria bicolor | 5e−07 |
| Unknown function | ||||||
| Lq-P03F09 | FK857729 | EAU91321 | 1 | Coprinopsis cinerea | 4e−79 | |
| Lq-P03D11 | FK857719 | EAU90318 | 1 | Coprinopsis cinerea | 2e−78 | |
| Lq-P08D12 | FK857943 | EDR15024 | 1 | Laccaria bicolor | 3e−78 | |
| Lq-P09A10 | FK857976 | EAU91748 | 1 | Coprinopsis cinerea | 1e−75 | |
| Lq-P05H04 | FK857831 | EDR10963 | 1 | Laccaria bicolor | 6e−70 | |
| Lq-P08C12 | FK857937 | EDR15585 | 1 | Laccaria bicolor | 2e−69 | |
| Lq-P03E06 | FK857722 | EDR11244 | 1 | Laccaria bicolor | 7e−65 | |
| Lq-P09H07 | FK858012 | EDR13752 | 1 | Laccaria bicolor | 4e−59 | |
| Lq-P02H08 | FK857691 | EAU91434 | 1 | Coprinopsis cinerea | 2e−54 | |
| Lq-P06C02 | FK857854 | EAL20762 | 1 | Laccaria bicolor | 6e−54 | |
| No homology | ||||||
| Lq-P06H04 | FK857888 | 5 | ||||
| Lq-P02B05 | FK857667 | 2 | ||||
| Lq-P02E04 | FK857677 | 2 | ||||
| Lq-P08E09 | FK857949 | 2 |
Sequences were classified into four categories. “Annotated” corresponds to sequences showing significant matches with protein sequences with identified functions in data banks. “Unknown function” corresponds to sequences showing significant matches (E value of <1.0e−3) and homology to a protein with no identified function. “No homology” groups sequences for which the E value was >1.0e−3 or for which no match was observed in data banks. Clones in boldface type correspond to genes for which transcription was studied by qRT-PCR.
Forty-two percent of the ESTs were similar to fungal sequences (i.e., Laccaria bicolor and Coprinopsis cinerea), 48% were similar to plant sequences (i.e., Quercus robur and Populus trichocarpa), and the remaining 10% were similar to bacteria and other organisms. The largest category (18%) of identified sequences corresponded to genes coding for the gene/protein expression machinery (Gene Ontology number 5840, cellular component), which includes transcripts such as those coding for ribosomal proteins, translational regulatory proteins, elongation factors, and the ubiquitin/proteasome pathway components. About 10% of the ESTs coded for primary metabolism, secondary metabolism, and transport mechanism components (e.g., amino acid transporter, glutamine synthetase, and malate dehydrogenase). These ESTs included cell wall or secreted proteins involved in the degradation of organic matter (e.g., N-acetylhexosaminidase and beta-glucanase) (Table 2).
Comparison of L. quietus gene expression patterns in L. quietus-Q. petraea ECMs and in the surrounding soil.
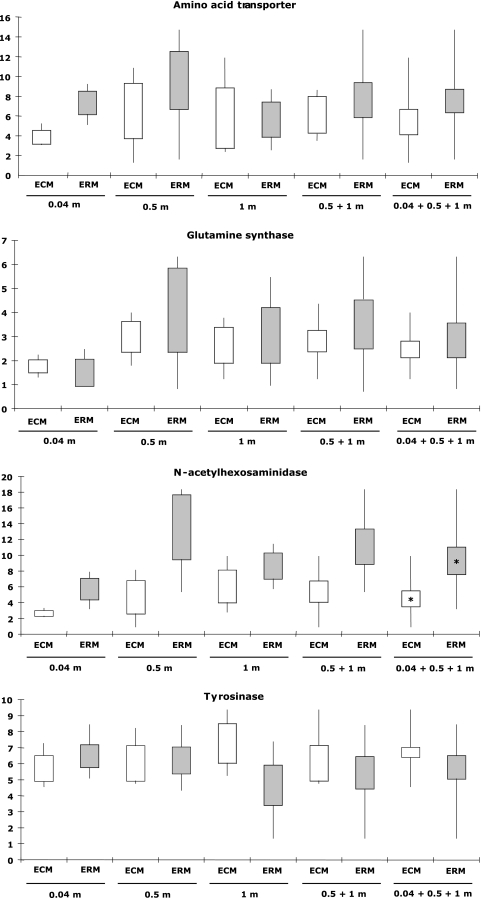
To test the possibility of measuring gene expression by qRT-PCR in ECMs and in the surrounding soil, four transcripts were selected on the basis of their putative involvement in primary metabolism, carbon/nitrogen mobilization from soil organic matter, and oxidation of polyphenols: the glutamine synthase (GS) transcript, the tyrosinase (Tyr) transcript, the N-acetylhexosaminidase (Nah) transcript, and a general amino acid transporter (Aat) transcript. We used the eIF-4A gene, which is expressed both in ECM and soil mycelium, as the internal control and analyzed the level of expression of these selected genes. The corresponding transcripts were detected in soil and ECM samples. In most cases, these genes were expressed at a much higher level in ECMs or soil extraradical mycelium than in the mycelium of L. quietus from pure culture (Fig. 2). Nevertheless, the expression of these four genes was within the same order of magnitude in ECMs and extraradical mycelium from the same soil core. In ECMs, the transcript levels ranged between 1.3 and 11.9 for the Aat transcript, 1.2 and 3.9 for the GS transcript, 0.9 and 9.9 for the Nah transcript, and 4.5 and 9.4 for the Tyr transcript (Fig. 2). In the soil, the gene transcription levels were between 1.6 and 14.7 for the Aat transcript, 0.8 and 6.3 for the GS transcript, 3.2 and 18.3 for the Nah transcript, and 1.3 and 8.4 for the Tyr transcript (Fig. 2).
FIG. 2.
Quantification by qRT-PCR of the level of transcripts coding for glutamine synthase, amino acid transporter, N-acetylhexosaminidase, and tyrosinase in Lactarius quietus-Quercus petraea ECMs sampled in situ and in the extraradical mycelium surrounding soil (ERM). Raw values have been normalized using eIF-4 as a reference transcript. Data are expressed as the transcript level with respect to the control sample (mycelium grown on agar medium), the expression of which is set at 1. Values are the means of 3, 6, or 9 replicates: 0.04 m, 0.5 m, and 1 m (n = 3); metric variability (0.5 m plus 1 m) (n = 6); and metric and centimetric variability (0.04 m plus 0.5 m plus 1 m) (n = 9). The boxes (white, ECMs; gray, ERM) correspond to the standard deviation and the line to the maximum-minimum values of gene transcription. A t test was done for each transcript between transcript levels in ECMs and those in the extraradical mycelium surrounding soil. An asterisk indicates a P value of <0.05.
Transcript concentration in ECMs or soil mycelium varied somewhat between samples separated by more than 4 cm (soil core diameter) (Fig. 2). Among ECM or extraradical mycelium sampled along the three transects at 0.04 m, 0.5 m, and 1 m from the central point (n = 3), differences in transcript concentrations were not significant due to the high variability between samples. However, for the Nah gene, we noted that the transcript concentration in ECMs or extraradical mycelium sampled at 0.04 m was lower than those sampled at 0.5 m and 1 m. We note that the only significant difference in gene transcription between ECMs and the extraradical mycelium from surrounding soil was found for Nah gene expression (n = 9), which was higher in the extraradical mycelium than in the ECM root tips.
DISCUSSION
Transcriptomics of Lactarius quietus ECMs sampled in situ.
The construction of a cDNA library followed by EST sequencing is an important step in characterizing the transcriptome of an organism. EST sequencing has already been reported for ECM fungi such as Laccaria bicolor and Pisolithus microcarpus (47), Hebeloma cylindrosporum (31) and Tuber borchii (30) in axenic cultures, and Tuber borchii (48) and Paxillus involutus (26, 45, 57) in soil microcosms. Here, ESTs isolated directly from Lactarius quietus ECMs sampled in situ provided a way to access the environmental transcriptome of this ECM basidiomycete. A promising application of environmental transcriptomics is to retrieve gene sequences coding for activities having relevance for ecological studies that are weakly expressed under axenic conditions with nutrient-rich agar medium. In L. quietus ECMs sampled in situ, we found mainly ESTs involved in primary and secondary metabolism (e.g., trehalose phosphorylase and glutamine synthase) or in stress and defense reactions (e.g., glutaredoxin and thioredoxin). In forest soil, L. quietus ECMs are directly affected by rapid modifications of biotic factors such as the host root carbohydrate concentration, the quality of organic matter, or the presence of numerous pathogens and by abiotic factors such as soil moisture and soil temperature. Interestingly, this in situ EST approach detected several genes involved in the degradation of organic matter (e.g., N-acetylhexosaminidase and beta-glucanase) or the detoxication of the degradation products (e.g., laccase and tyrosinase).
Usually, the detection of functional genes expressed in natural environments relies on sets of PCR primers designed from known DNA sequences retrieved from databases such as GenBank; their contents are heavily biased toward model fungi (38, 44). These PCR primers are rarely tested against a comprehensive collection of environmental fungal species. It is thus difficult to develop a truly quantitative approach with these degenerate primers, whose annealing conditions depend on gene sequences specific to the fungal species. In contrast, sequencing of environmental transcript libraries can reduce this problem by providing site-specific functional gene sequences (15, 23, 49, 50).
From a methodological point of view, one question is the extent to which gene expression in the extraradical mycelium in the ectomycorrhizosphere can be attributed to L. quietus rather than to other soil organisms. This bias has been limited for the following reasons: (i) when the primers used to quantitate transcript levels by quantitative PCR were tested with pure cultures of L. quietus, Lactarius subdulcis, and Laccaria bicolor, amplification was successful with L. quietus owing only to the annealing-stringent conditions, and (ii) when studying the fungal species diversity throughout the sampling site (data not shown), the three other occurring Lactarius species were much less frequent and less abundant than L. quietus. Furthermore, ECMs established by Lactarius species other than L. quietus were never found in the soil cores analyzed. Another concern is that gene expression patterns may change in response to sample processing prior to RNA extraction. We addressed this issue by minimizing the delay between sample collection and fixation in liquid nitrogen (see Materials and Methods).
Sampling pattern.
Another important aspect in environmental transcriptomics is the number of biological replicates necessary to handle ecological and technical sources of variation. The cost of a qRT-PCR analysis calls for moderation in the number of samples. The objective, therefore, is to find the smallest number of samples that still provides results that are of a good-enough quality (27) and that allow the identification of regulated genes by the commonly used t tests. In the oak forest, no significant differences were found for the Nah transcript between L. quietus ECMs and the extraradical mycelium from the surrounding soil when there were three or six samples. A statistically significant variation was found only when we took into account the variability at the cm scale (n = 3; 0.04 m from the central point) and at the metric scale (n = 6; 0.5 and 1 m from the central point). As a consequence, in order to obtain a valuable assessment of how gene expression varies between ecological conditions (i.e., comparison of soil-ECM and temporal variation), the number of replicates should be higher than 6 but limited to 10 samples for cost considerations. Despite the known spatial heterogeneity of forest soils, taking different samples at a cm scale is not necessary.
Transcriptome and ecology of ECMs.
Levels of Nah transcripts were significantly higher in the extraradical mycelium surrounding soil than in the ECM root tip. In the Paxillus involutus-Betula pendula association, differential gene expression between the extraradical mycelium and the ECM root tips in microcosms has been characterized (34, 45, 57). These results confirmed the functional specialization of tissues forming ECM associations (Hartig net, mantle, rhizomorphs, and extraradical mycelium). In the oak forest of Champenoux, measurements of activities of enzymes secreted by L. quietus ECM root tips showed temporal and soil horizon variations (13, 14). In L. quietus ECM root tips, the secretion of hydrolytic enzymes, likely involved in the catabolism of compounds released by the degradation of organic matter, showed striking variations correlated with host phenological steps such as vessel formation, radial growth, and leaf expansion of the host tree (12).
We observed that genes coding for glutamine synthetase, N-acetylhexosaminidase, tyrosinase, and an amino acid transporter in ECM root tips and in the extraradical mycelium were highly expressed in comparison with mycelium grown on nutrient-rich agar medium. In temperate forest soils, nitrogen is a limiting factor in tree growth. More than 95% of soil nitrogen is present in an organic form, whereas trees take up principally inorganic nitrogen (NH4+, NO3−), which is present in low concentrations (10 to 100 μM) (39). ECM fungi assimilate soil nitrogen in inorganic and organic forms for their own nutrition or for host tree nutrition. In forest soils, ECM and saprotrophic fungi are spatially distributed (35). Ectomycorrhizal fungi such as L. quietus dominated in the decomposed humus and litter, where they apparently mobilized nitrogen. On the other hand, saprotrophic fungi were predominant in the shed litter components on the surface of the forest floor, where organic carbon is mineralized (35). This suggests that saprotrophic fungi mobilize mostly carbon, and ectomycorrhizal fungi mobilize mainly nitrogen during litter decomposition (24). The extraradical mycelium of ECM fungi, which plays a key role in nitrogen nutrition, is considered the absorption structure of ectomycorrhizal symbiosis. Previous studies done with pure fungal cultures under axenic conditions showed that the enzymes studied here are regulated by nitrogen compounds. The glutamine synthetase gene is a central enzyme of nitrogen metabolism that allows the assimilation of nitrogen and the biosynthesis of glutamine. The glutamine synthase activity of the extraradical hyphae in Glomus intraradices or in the mycelium of Hebeloma cylindrosporum was considerably modulated in response to different nitrogen sources (6, 25). An amino acid transporter of Amanita muscaria (AmAAP1), to which the Lq-P05F09 EST is similar, was overexpressed in the absence of a nitrogen source utilized by the fungus. This transporter is involved in the uptake of amino acids from soil for fungal nutrition or in preventing amino acid loss from hyphal leakage in the absence of a suitable nitrogen source (46). Chitin constitutes a potentially important nitrogen source in soil. The expression of the N-acetylhexosaminidase gene indicates that fungi have the potential to exploit polymers of amino sugars as a source of nitrogen for themselves and their host plants (36). In Trichoderma atroviride, N-acetylhexosaminidases are produced in response to a shortage of nitrogen even when glucose is provided in excess (17). Tyrosinases are involved in the pigmentation of mycelia by the oxidation of phenols but also in defense and virulence mechanisms (11, 28, 56).
In this paper, we extracted RNA from ECM root tips and extraradical mycelium from an oak forest soil. The yield was very high, and the quality of RNA was good enough for cDNA synthesis (library construction and qRT-PCR). This step allowed us to construct a cDNA library to identify ECM genes of ecological relevance (i.e., laccase and glutamine synthetase). The high levels of expression of these genes arose presumably because ECMs in forest soils are starved of nutrients, particularly nitrogen. With the ability to measure gene expression in ECMs and ectomycorrhizosphere soil under field conditions, the next step is to set up high-throughput approaches to determine the metatranscriptome of forest soils (5, 22).
Acknowledgments
The Ph.D. scholarship of P.E.C. was funded by grants of the French Ministry of Ecology and Sustainable Development; part of this research has been supported by the Biological Invasions program of the same ministry. This project was supported by the Network of Excellence EVOLTREE.
We are grateful to the Office National des Forêts for permitting sampling in the Champenoux State Forest. The research used the DNA sequencing facilities at INRA-Nancy, supported by INRA and the Lorraine Region.
Footnotes
Published ahead of print on 12 September 2008.
REFERENCES
- 1.Abuzinadah, R. A., and D. J. Read. 1986. The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. I. Utilization of peptides and proteins by ectomycorrhizal fungi. New Phytol. 103:481-493. [Google Scholar]
- 2.Agerer, R. 1987-1998. Colour atlas of ectomycorrhizae. Einhorn-Verlag Eduard Dietenberger, Munich, Germany.
- 3.Allen, E. E., and J. F. Banfield. 2005. Community genomics in microbial ecology and evolution. Nat. Rev. Microbiol. 3:489-498. [DOI] [PubMed] [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baily, J., L. Fraissinet-Tachet, M. C. Verner, J. C. Debaud, M. Lemaire, M. Wésolowski-Louvel, and R. Marmeisse. 2007. Soil eukaryotic functional diversity, a metatranscriptomic approach. ISME J. 1:632-642. [DOI] [PubMed] [Google Scholar]
- 6.Breuninger, M., C. G. Trujillo, E. Serrano, R. Fischer, and N. Requena. 2004. Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet. Biol. 41:542-552. [DOI] [PubMed] [Google Scholar]
- 7.Buée, M., P. E. Courty, D. Mignot, and J. Garbaye. 2007. Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal community. Soil Biol. Biochem. 39:1947-1955. [Google Scholar]
- 8.Buée, M., D. Vairelles, and J. Garbaye. 2005. Year-round monitoring of diversity and potential metabolic activity of the ectomycorrhizal community in a beech forest subjected to two thinning regimes. Mycorrhiza 15:235-245. [DOI] [PubMed] [Google Scholar]
- 9.Bugos, R. C., V. L. Chiang, X. H. Zhang, E. R. Campbell, G. K. Podila, and W. H. Campbell. 1995. RNA isolation from plant tissues recalcitrant to extraction in guanidine. BioTechniques 19:734-737. [PubMed] [Google Scholar]
- 10.Burke, R. M., and J. W. G. Cairney. 1998. Carbohydrate oxidases in ericoid and ectomycorrhizal fungi: a possible source of Fenton radicals during degradation of lignocellulose. New Phytol. 139:637-645. [Google Scholar]
- 11.Cairney, J. W. G., and R. M. Burke. 1998. Do ecto- and ericoid mycorrhizal fungi produce peroxidase activity? Mycorrhiza 8:61-65. [Google Scholar]
- 12.Courty, P. E., N. Bréda, and J. Garbaye. 2007. Relation between oak tree phenology and the secretion of organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Biol. Biochem. 39:1635-1663. [Google Scholar]
- 13.Courty, P. E., R. Pouysegur, M. Buée, and J. Garbaye. 2006. Laccase and phosphatase activities of the dominant ectomycorrhizal types in a lowland oak forest. Soil Biol. Biochem. 38:1219-1222. [Google Scholar]
- 14.Courty, P. E., K. Pritsch, M. Schloter, A. Hartmann, and J. Garbaye. 2005. Activity profiling of ectomycorrhiza communities in two forest soils using multiple enzymatic tests. New Phytol. 167:309-319. [DOI] [PubMed] [Google Scholar]
- 15.Daniel, R. 2005. The metagenomics of soil. Nat. Rev. Microbiol. 3:470-478. [DOI] [PubMed] [Google Scholar]
- 16.Dickie, I. A., B. Xu, and R. T. Koide. 2002. Vertical niche differentiation of ectomycorrhizal hyphae in soil as shown by T-RFLP analysis. New Phytol. 156:527-535. [DOI] [PubMed] [Google Scholar]
- 17.Donzelli, B. G. G., and G. E. Harman. 2001. Interaction of ammonium, glucose, and chitin regulates the expression of cell wall-degrading enzymes in Trichoderma atroviride strain P1. Appl. Environ. Microbiol. 67:5643-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duchaufour, P., and M. Bonneau. 1959. Une nouvelle méthode de dosage du phosphore assimilable dans les sols forestiers. Bull. AFES 41:193-198. [Google Scholar]
- 19.Duplessis, S., P. E. Courty, D. Tagu, and F. Martin. 2005. Transcript patterns associated with ectomycorrhiza development in Eucalyptus globulus-Pisolithus microcarpus. New Phytol. 165:599-611. [DOI] [PubMed] [Google Scholar]
- 20.Gardes, M., and T. D. Bruns. 1996. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: above- and below-ground views. Can. J. Bot. 74:1572-1583. [Google Scholar]
- 21.Giovannoni, S. J., and U. Stingl. 2005. Molecular diversity and ecology of microbial plankton. Nature 437:343-348. [DOI] [PubMed] [Google Scholar]
- 22.Grant, S., W. D. Grant, D. A. Cowan, B. E. Jones, Y. Ma, A. Ventosa, and S. Heaphy. 2006. Identification of eukaryotic open reading frames in metagenomic cDNA libraries made from environmental samples. Appl. Environ. Microbiol. 72:135-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handelsman, J., M. R. Rondon, S. F. Brady, J. Clardy, and R. M. Goodman. 1998. Molecular biological access to the chemistry of unknown soil microbes: a new frontier for natural products. Chem. Biol. 5:245-249. [DOI] [PubMed] [Google Scholar]
- 24.Hobbie, E. A., and T. R. Horton. 2007. Evidence that saprotrophic fungi mobilise carbon and mycorrhizal fungi mobilise nitrogen during litter decomposition. New Phytol. 173:447-449. [DOI] [PubMed] [Google Scholar]
- 25.Javelle, A., M. Morel, B. R. Rodriguez-Pastrana, B. Botton, B. André, A. M. Marini, A. Brun, and M. Chalot. 2003. Molecular characterization, function and regulation of ammonium transporters (Amt) and ammonium-metabolizing enzymes (GS, NADP-GDH) in the ectomycorrhizal fungus Hebeloma cylindrosporum. Mol. Microbiol. 47:411-430. [DOI] [PubMed] [Google Scholar]
- 26.Johansson, T., A. Le Quéré, D. Ahren, B. Söderström, R. Erlandsson, J. Lundeberg, M. Uhlen, and A. Tunlid. 2004. Transcriptional responses of Paxillus involutus and Betula pendula during formation of ectomycorrhizal root tissue. Mol. Plant-Microbe Interact. 17:202-215. [DOI] [PubMed] [Google Scholar]
- 27.Jorstad, T. S., M. Langass, and A. M. Bones. 2007. Understanding sample size: what determines the required number of microarrays for an experiment? Trends Plant Sci. 12:46-50. [DOI] [PubMed] [Google Scholar]
- 28.Kanda, K., T. Sato, S. Ishii, H. Enei, and S. I. Ejiri. 1996. Purification and properties of tyrosinase isozymes from the gill of Lentinus edodes fruiting body. Biosci. Biotechnol. Biochem. 60:1273-1278. [DOI] [PubMed] [Google Scholar]
- 29.Kohler, A., C. Delaruelle, D. Martin, N. Encelot, and F. Martin. 2003. The poplar root transcriptome: analysis of 7000 expressed sequence tags. FEBS Lett. 542:37-41. [DOI] [PubMed] [Google Scholar]
- 30.Lacourt, I., S. Duplessis, S. Abbà, P. Bonfante, and F. Martin. 2002. Isolation and characterization of differentially expressed genes in the mycelium and fruit body of Tuber borchii. Appl. Environ. Microbiol. 68:4574-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambilliotte, R., R. Cooke, D. Samson, C. Fizames, F. Gaymard, C. Plassard, M. V. Tatry, C. Berger, M. Laudié, F. Legeai, E. Karsenty, M. Delseny, S. Zimmermann, and H. Sentenac. 2004. Large-scale identification of genes in the fungus Hebeloma cylindrosporum paves the way to molecular analyses of ectomycorrhizal symbiosis. New Phytol. 164:505-512. [Google Scholar]
- 32.Landeweert, R., P. Leeflang, E. Smit, and T. Kuyper. 2005. Diversity of an ectomycorrhizal fungal community studied by a root tip and total soil DNA approach. Mycorrhiza 15:1-6. [DOI] [PubMed] [Google Scholar]
- 33.Landeweert, R., P. Leeflang, T. W. Kuyper, E. Hoffland, A. Rosling, K. Wernars, and E. Smit. 2003. Molecular identification of ectomycorrhizal mycelium in soil horizons. Appl. Environ. Microbiol. 69:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Quéré, A., D. Wright, B. Söderström, A. Tunlid, and T. Johansson. 2005. Global patterns of gene regulation associated with the development of ectomycorrhiza between birch (Betula pendula Roth.) and Paxillus involutus (Batsch) Fr. Mol. Plant-Microbe Interact. 18:659-673. [DOI] [PubMed] [Google Scholar]
- 35.Lindahl, B. D., K. Ihrmark, J. Boberg, S. E. Trumbore, P. Högberg, J. Stenlid, and R. D. Finlay. 2007. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 173:611-620. [DOI] [PubMed] [Google Scholar]
- 36.Lindahl, B. D., and A. F. S. Taylor. 2004. Occurrence of N-acetylhexosaminidase-encoding genes in ectomycorrhizal basidiomycetes. New Phytol. 164:193-199. [DOI] [PubMed] [Google Scholar]
- 37.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 38.Luis, P., G. Walther, H. Kellner, F. Martin, and F. Buscot. 2004. Diversity of laccase genes from basidiomycetes in a forest soil. Soil Biol. Biochem. 36:1025-1036. [Google Scholar]
- 39.Marschner, B., and B. Dell. 1994. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159:89-102. [Google Scholar]
- 40.Martin, F., A. Aerts, D. Ahrén, A. Brun, E. G. J. Danchin, F. Duchaussoy, J. Gibon, A. Kohler, E. Lindquist, V. Pereda, A. Salamov, H. J. Shapiro, J. Wuyts, D. Blaudez, M. Buée, P. Brokstein, B. Canbäck, D. Cohen, P. E. Courty, P. M. Coutinho, C. Delaruelle, J. C. Detter, A. Deveau, S. DiFazio, S. Duplessis, L. Fraissinet-Tachet, E. Lucic, P. Frey-Klett, C. Fourrey, I. Feussner, G. Gay, J. Grimwood, P. J. Hoegger, P. Jain, S. Kilaru, J. Labbé, Y. C. Lin, V. Leguée, F. Le Tacon, R. Marmeisse, D. Melayah, B. Montanini, M. Muratet, U. Nehls, H. Niculita-Hirzel, M. P. Oudot-Le Secq, M. Peter, H. Quesneville, B. Rajashekar, M. Reich, N. Rouhier, J. Schmutz, T. Yin, M. Chalot, B. Henrissat, U. Kües, S. Lucas, Y. Van de Peer, G. K. Podila, A. Polle, P. J. Pukkila, P. M. Richardson, P. Rouzé, I. R. Sanders, J. E. Stajich, A. Tunlid, G. Tuskan, and I. V. Grigoriev. 2008. The genome sequence of the basidiomycete fungus Laccaria bicolor provides insights into the mycorrhizal symbiosis. Nature 452:88-92. [DOI] [PubMed] [Google Scholar]
- 41.Martin, F., A. Kohler, and S. Duplessis. 2007. Living in harmony in the wood underground: ectomycorrhizal genomics. Curr. Opin. Plant Biol. 10:204-210. [DOI] [PubMed] [Google Scholar]
- 42.Martin, F. 2001. Frontiers in molecular mycorrhizal research—genes, loci, dots and spins. New Phytol. 150:499-505. [Google Scholar]
- 43.Marx, D. H. 1972. Ectomycorrhizae as biological deterrents to pathogenic root infections. Annu. Rev. Phytopathol. 10:429-454. [DOI] [PubMed] [Google Scholar]
- 44.Morel, M., M. Buée, M. Chalot, and A. Brun. 2006. NADP-dependent glutamate dehydrogenase: a dispensable function in ectomycorrhizal fungi. New Phytol. 169:179-190. [DOI] [PubMed] [Google Scholar]
- 45.Morel, M., C. Jacob, A. Kohler, T. Johansson, F. Martin, M. Chalot, and A. Brun. 2005. Identification of genes differentially expressed in extraradical mycelium and ectomycorrhizal roots during Paxillus involutus-Betula pendula ectomycorrhizal symbiosis. Appl. Environ. Microbiol. 71:382-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nehls, U., R. Kleber, J. Wiese, and R. Hampp. 1999. Isolation and characterization of a general amino acid permease from the ectomycorrhizal fungus Amanita muscaria. New Phytol. 144:343-349. [Google Scholar]
- 47.Peter, M., P. E. Courty, A. Kohler, C. Delaruelle, D. Martin, D. Tagu, P. Frey-Klett, S. Duplessis, M. Chalot, G. Podila, and F. Martin. 2003. Analysis of expressed sequence tags from the ectomycorrhizal basidiomycetes Laccaria bicolor and Pisolithus microcarpus. New Phytol. 159:117-129. [DOI] [PubMed] [Google Scholar]
- 48.Polidori, E., D. Agostini, S. Zeppa, L. Potenza, F. Palma, D. Sisti, and V. Stocchi. 2002. Identification of differentially expressed cDNA clones in Tilia platyphyllos-Tuber borchii ectomycorrhizae using a differential screening approach. Mol. Genet. Genomics 266:858-864. [DOI] [PubMed] [Google Scholar]
- 49.Poretsky, R. S., N. Bano, A. Buchan, G. LeCleir, J. Kleikemper, M. Pickering, W. M. Pate, M. A. Moran, and T. Hollibaugh. 2005. Analysis of microbial gene transcripts in environmental samples. Appl. Environ. Microbiol. 71:4121-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rondon, M. R., P. R. August, A. D. Bettermann, S. F. Brady, T. H. Grossman, M. R. Liles, K. A. Loiacono, B. A. Lynch, I. A. MacNeil, C. Minor, C. L. Tiong, M. Gilman, M. S. Osburne, J. Clardy, J. Handelsman, and R. M. Goodman. 2000. Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Appl. Environ. Microbiol. 66:2541-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 52.Schenk, H. J., and R. B. Jackson. 2002. Rooting depths, lateral root spreads, and belowground/aboveground allometries of plants in water-limited environments. J. Ecol. 90:480-494. [Google Scholar]
- 53.Taylor, A. F. S. 2002. Fungal diversity in ectomycorrhizal communities: sampling effort and species detection. Plant Soil 244:19-28. [Google Scholar]
- 54.Taylor, D. L., and T. B. Bruns. 1999. Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol. Ecol. 8:1837-1850. [DOI] [PubMed] [Google Scholar]
- 55.Voiblet, C., S. Duplessis, N. Encelot, and F. Martin. 2001. Identification of symbiosis-regulated genes in Eucalyptus globulus-Pisolithus tinctorius ectomycorrhiza by differential hybridization of arrayed cDNAs. Plant J. 25:181-191. [DOI] [PubMed] [Google Scholar]
- 56.Wichers, H. J., K. Recourt, M. Hendriks, C. E. Ebbelaar, G. Biancone, F. A. Hoeberichts, H. Mooibroek, and C. Soler-Rivas. 2003. Cloning, expression, and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl. Microbiol. Biotechnol. 61:336-341. [DOI] [PubMed] [Google Scholar]
- 57.Wright, D. P., T. Johansson, A. Le Quéré, B. Söderström, and A. Tunlid. 2005. Spatial patterns of gene expression in the extramatrical mycelium and mycorrhizal root tips formed by the ectomycorrhizal fungus Paxillus involutus in association with birch (Betula pendula) seedlings in soil microcosms. New Phytol. 167:579-596. [DOI] [PubMed] [Google Scholar]