Abstract
Buruli ulcer or Mycobacterium ulcerans disease occurs mainly in areas in proximity to standing or slowly running freshwater, habitats in which free-living amoebae occur. For this reason, a possible link between the habitat of M. ulcerans and free-living amoebae was investigated. Free-living amoebae and mycobacteria were isolated from water and biofilm specimens taken from protected and unprotected sources of water in villages known to have either high or low endemicity for Buruli ulcer in Benin. Amoebae were isolated from 78.8% of samples. A greater proportion of water bodies in areas of high endemicity had amoebae than in areas of low endemicity (83.3% versus 66.7%). Protected sources of water were significantly more likely to contain amoebae in areas of high endemicity than in areas of low endemicity (88.0% versus 11.1%). Several pathogenic free-living amoebae and mycobacteria were isolated. However, no M. ulcerans was isolated and no specimen was positive for IS2404 PCR. Our results show that the study area has a water hygiene problem, which is greater in areas of high Buruli ulcer endemicity than in areas of low endemicity. Our observations indicate that additional studies are required to explore the possible link between free-living amoebae and mycobacteria.
Buruli ulcer (BU) is a disease caused by Mycobacterium ulcerans. The infection severely affects skin and bone and most commonly touches rural communities in West and Central Africa and, to a lesser extent, in Australia (20, 55).
Mycobacteria are frequently found in water and mud of swamps (34). These mycobacteria may be mechanically concentrated by small water-filtering organisms (e.g., microphagous fish, mosquito larvae, small crustaceans or molluscs, or even some protozoa such as amoebae), which may be ingested by water-dwelling predators (like aquatic hemiptera) or even by some nonmicrophagous fish (16). These water-dwelling predators thereby may become passive reservoirs of environmental mycobacteria. Some of these aquatic predators may bite animals or humans and mechanically introduce environmental mycobacteria into the skin or deposit the mycobacteria on the surface of the skin (39).
This scenario may be true for M. ulcerans as well, a mycobacterium of which the environmental reservoir and the mode(s) of transmission are not fully understood. There are, however, indications of a reservoir linked to aquatic environments (54), with some evidence of the involvement of insects (16, 26, 27, 38, 40, 47). Until now, only one well-characterized pure culture of M. ulcerans has been isolated from an aquatic insect after culture in a liquid medium followed by several passages in mouse footpads (40).
It has been hypothesized that M. ulcerans cannot exist saprophytically as a free organism. Compared to other mycobacteria, the organism is fragile. Researchers have suggested that M. ulcerans may persist in the environment as a commensal, associated with another organism that protects the bacilli against unfavorable physical parameters of the environment and changes in these parameters (39). Moreover, analysis of the genome of M. ulcerans shows an adaptation to a specific ecological niche (51).
Because of the association with freshwater, it has been suggested that freshwater zooplanktons may act as reservoirs for M. ulcerans or may even facilitate the multiplication of the bacteria (39). For that reason, in this study, we hypothesize that one of the hosts of M. ulcerans might be a planktonic organism, such as a protozoan. A number of studies have demonstrated the intracellular survival or multiplication of mycobacteria within protozoan hosts (reviewed in reference 53). Mycobacteria have also been found living as endosymbionts of Acanthamoeba (57). Protozoa may protect the bacilli against adverse environmental conditions (29, 39), and there have been reports of coinfections with mycobacteria and Acanthamoeba for both immunocompromised and immunocompetent patients (49, 56).
A finding that free-living amoebae can be one of the environmental hosts of M. ulcerans would be very important, since amoebae are common in freshwater environments and they have been found to be resistant to conditions of extreme temperature, pH, and osmolarity while encysted (43).
The objective of the present study was to determine the nature and distribution of free-living amoebae in areas of Benin with low and high endemicities for BU.
MATERIALS AND METHODS
Collection of specimens in Benin.
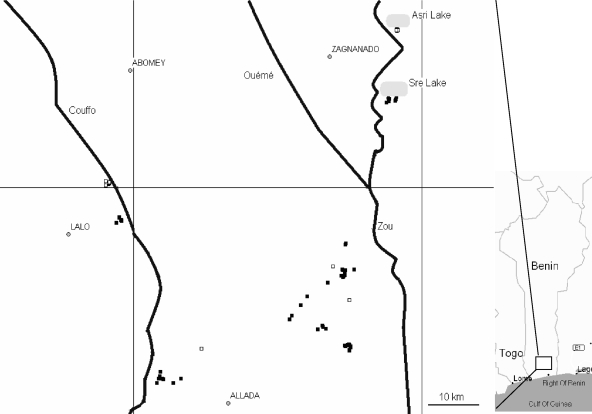
The study area (Fig. 1) comprised a number of villages in the valleys of the Zou, Ouémé, and Couffo rivers in southern Benin. Villages were selected on the basis that they were known to have either high or low incidence of BU.
FIG. 1.
Map of the sampling area with major towns, rivers, and sampling sites indicated. Symbols: ▪, high BU endemicity; □, low BU endemicity.
Sampling was carried out during the dry season, in February 2005 and February 2006. Forty-four water (150-ml) and 52 biofilm samples (14 were sampled using sterile swabs [in triplicate], while for 38 sterile sponges were used) were collected in villages in Benin with high numbers of BU patients. In addition, 3 water and 33 biofilm samples (2 swabs and 31 sponges) were taken in villages with low endemicity for BU. For biofilm samples, swabs and sponges (VWR, Leuven, Belgium) were wiped on the sampled surfaces of stones, leaves, and pieces of floating wood or the interiors of taps.
Samples were classified as having come from a “protected source” (rain tanks, boreholes, concrete wells, and water towers) or an “unprotected source” (rivers, swamps, and natural wells).
The position of each sampling site was registered using the Global Positioning System.
Isolation of free-living amoebae.
A 50-ml aliquot of each water specimen was processed for the isolation of free-living amoebae on the day of collection. The specimens were centrifuged at 500 × g for 5 min and the sediment and a small volume of supernatant inoculated on the centers of 1.5% nonnutrient agar plates covered with Escherichia coli (31).
The first swab of each set was smeared on the same medium, while for sponges a 1-cm3 cube was cut using sterile scissors and placed on the agar plates.
The plates were incubated at room temperature (±20°C) and examined daily using the 10× objective of a light microscope. If amoebae were observed, they were transferred to a new agar plate by lifting a small portion of the agar and placing it upside down on the new agar plate (2). To get rid of fungal contamination, the cultures were transferred successively to fresh plates. The dense amoebic growth was sampled as it advanced beyond the area of bacterial or fungal contamination.
Identification of free-living amoebae.
To identify amoebae, a small portion of each agar plate was transferred to an Eppendorf tube and transported to Belgium at ambient temperature. The amoebae on the pieces of agar were brought in culture and morphologically identified up to the genus or family level (31).
Vahlkampfiidae and Acanthamoeba spp. were identified using “fast” molecular methods: the migrating ring of amoebae was scraped from the agar plates by use of an inoculation loop and distilled water. The resulting suspension of amoebae was centrifuged at 1,000 × g for 10 min and the supernatant discarded. DNA was extracted using the UNSET method (19). For other genera of amoebae, such fast molecular methods are not yet available.
For Vahlkampfiidae of the genus Naegleria, the internal transcribed spacers (ITS), including 5.8S ribosomal DNA, were amplified with the primers ITSfw and ITSrv as previously described (10, 12). For other genera of Vahlkampfiidae, the DNA was amplified using more general primers, JITSfw and JITSrv (13).
For Acanthamoeba, the DNA was amplified using the primers JDP1 and JDP2, which produce an amplimer designated DF3 (diagnostic fragment 3). The sequence of this DF3 within ASA.S1 allows the identification of the 15 genotypes of the genus Acanthamoeba (6, 45).
The PCR products of Naegleria and other members of the Vahlkampfiidae were sequenced at ICP (Brussels, Belgium) using the respective PCR primers (10, 13). For Acanthamoeba, the amplified products were sequenced by the VIB Genetic Service Facility (Antwerp, Belgium) using the internal sequencing primer 892c (45).
Sequences were aligned with those published using the software CLC Workbench (CLCbio, Aarhus, Denmark).
Detection of mycobacteria.
A further 50-ml aliquot of each water specimen was processed for the detection of mycobacteria. Each aliquot was centrifuged at 2,000 × g for 20 min in Benin. The pellet was stored at −20°C and transported to Belgium.
The second swab of each set was stored at −20°C and transported without further processing to Belgium. Because sponges were stored at 4°C for too long, it was not possible to use them for the cultivation of mycobacteria. Instead, in Belgium, they were macerated for 1 min in a stomacher in 50 ml of distilled water. The resulting suspension was centrifuged at 3,500 × g for 20 min and processed for molecular analysis.
Specimens from water samples and swabs were decontaminated using the reversed Petroff method (32), centrifuged at 3,500 × g for 20 min, and inoculated on Löwenstein-Jensen (LJ) medium tubes for the isolation of mycobacteria. The tubes were incubated at 30°C and read monthly up to 1 year.
Mycobacterial DNA extraction was done using the modified method of Boom (5, 40). Briefly, 250 μl of the suspension was treated with equal volumes of lysis buffer L6 followed by 50 μl of proteinase K (20 mg/ml). This mixture was incubated overnight at 60°C with gentle shaking. To capture DNA, 40 μl of diatomaceous earth stock solution (prepared by mixing 10 g of diatomaceous earth obtained from Sigma Aldrich Chemie GmbH [Steinheim, Germany] in 50 ml of water containing 500 μl of 37% HCl) was added to the suspension and placed in a shaker incubator at 37°C for 2 h. The pellets were washed with 900 μl of L2 buffer followed by 900 μl of 70% ethanol and 900 μl of acetone. The pellet was then dried at 55°C and resuspended in 90 μl TE (10 mM Tris, 1 mM EDTA, pH 8). Tubes were centrifuged and 50 μl of the DNA extract was transferred to a new tube.
Detection of M. ulcerans DNA and of mycobacterial DNA from the specimens was done using PCR for IS2404 (18, 44), an insertion sequence specific for M. ulcerans and some other mycolactone-producing mycobacteria (41), and for the 16S rRNA (37), respectively.
Identification of mycobacteria.
Mycobacteria were identified up to the species level based on phenotypic characteristics (55a) and sequencing of the 16S rRNA gene (4, 22). When amplification was observed using 16S rRNA PCR, the 16S rRNA gene was sequenced to identify the mycobacterium detected.
Isolation of intracellular mycobacteria.
The remaining 50 ml of the water specimens and the third swab of each set were processed for the isolation of intracellular mycobacteria. Streptomycin (10 μg/ml) and rifampin (5 μg/ml) were added to remove extracellular mycobacteria. The specimens were incubated overnight at room temperature and then centrifuged at 500 × g for 5 min. The pellet was suspended in phosphate-buffered saline, and 0.5% sodium dodecyl sulfate was added to lyse free-living amoebae. The resulting suspension was centrifuged at 2,000 × g for 20 min in Benin, and the pellet was stored at −20°C and transported to Belgium.
Specimens were decontaminated using the reversed Petroff method (32), centrifuged at 3,500 × g for 20 min, and inoculated onto LJ medium. The tubes were incubated at 30°C and read monthly up to 1 year.
Statistical analysis.
Data were statistically analyzed using Epi Info (version 3.3.2; Centers for Disease Control, Atlanta, GA) and SPSS (version 15.0; SPSS Inc., Chicago, IL) software. Pearson chi-square and Fisher's exact tests were used to compare proportions. The odds ratios (OR) and 95% confidence intervals (CI) were calculated to express the power of a correlation.
RESULTS
Isolation of free-living amoebae.
An overview of the data relating to amoebae in the samples collected during the study is provided in Table 1. Amoebae were isolated from 104 samples out of 132 (78.8%). The frequency of samples that were positive for amoebae was similar whether the sample came from a “protected” or an “unprotected” source (67.6% [23/34] versus 82.7% [81/98], respectively; P = 0.07). However, amoebae were more frequently isolated from biofilms from unprotected sources than from biofilms from protected sources (80.3% [57/71] versus 35.7% [5/14], respectively; P = 0.001; OR = 7.33 [CI, 2.12 to 25.32]).
TABLE 1.
Overview of collected specimens and proportions of specimens from which amoebae were isolated in areas of high versus low BU endemicity
| Type of specimen | No. of amoebal cultures/no. of specimens (%) in areas of:
|
Total | |||||
|---|---|---|---|---|---|---|---|
| High BU endemicity
|
Low BU endemicity
|
||||||
| Unprotected water body | Protected water body | Total | Unprotected water body | Protected water body | Total | ||
| Biofilm | 36/46 (78.3) | 4/6 (66.7) | 40/52 (76.9) | 21/25 (84.0) | 1/8 (12.5) | 22/33 (66.7) | 62/85 (72.9)c |
| Water | 22/25 (88.0) | 18/19 (94.7) | 40/44 (90.9) | 2/2 (100.0) | 0/1 (0) | 2/3 (66.7) | 42/47 (89.4)c |
| Total | 58/71 (81.7) | 22/25 (88.0)b | 80/96 (83.3)a | 23/27 (85.2) | 1/9 (11.1)b | 24/36 (66.7)a | 104/132 (78.8) |
P = 0.037; OR = 2.50 (CI, 1.04 to 6.01).
P < 0.0001; OR = 58.67 (CI, 5.30 to 648.98).
P = 0.027; OR = 3.12 (CI, 1.10 to 8.85).
Amoebae were isolated significantly more often from water than from biofilm samples (89.4% versus 72.9%, respectively; P = 0.027; OR = 3.12 [CI, 1.10 to 8.85]).
Water bodies in areas of high endemicity contained significantly more amoebae than those in areas of low endemicity (83.3% versus 66.7%, respectively; P = 0.037; OR = 2.50 [CI, 1.04 to 6.01]). This difference was significant only for protected sources, which contained more amoebae in areas of high endemicity than in areas of low endemicity (88.0% [22/25] versus 11.1% [1/9]; P < 0.0001; OR = 58.67 [CI, 5.30 to 648.98]). In both areas of low and areas of high endemicity, unprotected water bodies frequently contained amoebae (85.2% versus 81.7%, respectively; P = 0.77).
From several specimens, more than one genus of free-living amoebae grew. Only members of Vahlkampfiidae, including Naegleria, and acanthamoebae were identified with molecular methods (Table 2). The following species were molecularly identified: Naegleria philippinensis, N. angularis, a Naegleria sp. with ITS sequences identical to those of strain NG583 (J. F. De Jonckheere, unpublished data) and to strain RNG305 from Japan (EMBL accession no. AB332199), Tetramitus jugosus, and T. entericus. Acanthamoeba was the most frequently isolated genus. Although we did not specifically try to isolate pathogenic amoebae, all Acanthamoeba genotypes isolated were opportunistic pathogens.
TABLE 2.
Morphological and molecular identification of amoebae isolated from water bodies
| Morphological identification (genus [no. of isolates]) | Molecular identification
|
|||
|---|---|---|---|---|
| Species (Vahlkampfiidae) or genotype (Acanthamoeba) | No. of isolates | Reported pathogenicitya | Growth at 37°Cb | |
| Acanthamoeba (39) | Acanthamoeba T4 | 22 | Established (AK, GAE, DAI) | NT |
| Acanthamoeba T5 | 3 | Established (AK, DAI) | NT | |
| Acanthamoeba T6 | 3 | Established (AK) | NT | |
| Acanthamoeba T11 | 3 | Established (AK) | NT | |
| Naegleria (26) | Naegleria angularis | 1 | Unknown | + |
| Naegleria philippinensis | 1 | Established (PAM) | + | |
| Naegleria sp. strain NG583 | 1 | Unknown | − | |
| Vahlkampfiidae other than Naegleria (18) | Tetramitus entericus | 3 | Unknown | +* |
| Tetramitus jugosus | 6 | Unknown | +° | |
| Hartmannella-like (24) | ||||
| Mayorella-like (5) | ||||
| Vexillifera-like (7) | ||||
GAE, granulomatous Acanthamoeba encephalitis; PAM, primary amoebic meningoencephalitis.
NT, not tested; *, two out of three; ○, one out of six.
The other genera were morphologically identified. Fast molecular identification methods are available only for the specified groups of free-living amoebae, since common human pathogens exist in Acanthamoeba and Naegleria.
Detection of mycobacteria.
Mycobacteria were found only in areas of high BU endemicity (Table 3). Of the 96 specimens originating from areas of high endemicity, 13 (13.5%) showed mycobacterial growth or mycobacterial DNA. This was significantly more than in areas of low endemicity, where no mycobacteria were found (P = 0.019). There was no difference in the mycobacterial detection rates between protected and unprotected waters (16% versus 12.7%, respectively; P = 0.74).
TABLE 3.
Overview of collected specimens and proportions of specimens in which mycobacteria were detected in areas of high versus low BU endemicity
| Type of specimen | No. of specimens positive for mycobacteria (by culture and/or PCR)/ total no. of specimens (%)
|
Total | |||||
|---|---|---|---|---|---|---|---|
| High BU endemicity
|
Low BU endemicity
|
||||||
| Unprotected water body | Protected water body | Total | Unprotected water body | Protected water body | Total | ||
| Biofilm | 3/46 (6.5) | 1/6 (16.7) | 4/52 (7.7) | 0/25 (0) | 0/8 (0) | 0/33 (0) | 4/85 (4.7) |
| Water | 6/25 (24.0) | 3/19 (15.8) | 9/44 (20.5) | 0/2 (0) | 0/1 (0) | 0/3 (0) | 9/47 (19.1) |
| Total | 9/71 (12.7) | 4/25 (16.0) | 13/96 (13.5)a | 0/27 (0) | 0/9 (0) | 0/36 (0)a | 13/132 (9.8) |
P = 0.019.
No significant difference between biofilm and water was observed for protected water bodies, while for unprotected waters, mycobacteria were more frequently detected in water than in biofilm (24.0% versus 6.5%; P = 0.04).
No significant difference was found in mycobacterial detection between specimens showing growth of amoebae or not (8.7% [9/104] versus 14.3% [4/28]; P = 0.47). Neither was there a link between areas of high or areas of low endemicity, protected or unprotected waters, or water or biofilm specimens (data not shown).
From the 13 specimens positive for mycobacteria, 4 were positive for mycobacterial DNA by 16S rRNA PCR, while from 10 specimens mycobacterial isolates were obtained (Table 4). Nine mycobacterial species were identified, while three specimens contained mycobacteria that could not be identified up to the species level. They were therefore named Mycobacterium sp.
TABLE 4.
Mycobacterial species detected from water and biofilm specimens from water bodies in areas with high BU endemicity
| Species | No. of specimens | Sampling site
|
Type of specimen | Detection method | |
|---|---|---|---|---|---|
| Endemicity | Water body | ||||
| Mycobacterium arupense | 1 | High | Unprotected | Water | Culture |
| Mycobacterium asiaticum | 1 | High | Protected | Water | Culture |
| Mycobacterium flavescens | 1 | High | Unprotected | Water | Culture |
| Mycobacterium fortuitum | 1 | High | Unprotected | Water | PCR |
| Mycobacterium gordonae | 2 | High | Protected | 1 biofilm, 1 water | 1 culture, 1 PCR and culture |
| Mycobacterium malmoense | 1 | High | Unprotected | Biofilm | Culture |
| Mycobacterium nebraskense-like | 1 | High | Unprotected | Water | Culture |
| Mycobacterium scrofulaceum-like | 1 | High | Unprotected | Biofilm | Culture |
| Mycobacterium simiae | 1 | High | Unprotected | Water | Culture |
| Mycobacterium sp. | 3 | High | 2 unprotected, 1 protected | 2 water, 1 biofilm | 1 culture, 2 PCR |
No specimen was positive for IS2404.
Isolation of intracellular mycobacteria.
From the 16 biofilm specimens treated, 2 (12.5%), namely, M. malmoense (from a swamp) and M. gordonae (from a rainwater tank), showed mycobacterial growth. Both of these biofilm specimens were obtained from areas of high BU endemicity.
No growth of intracellular mycobacteria was obtained from the 42 water specimens that were positive for amoebae.
DISCUSSION
Free-living amoebae of the genera Naegleria, Acanthamoeba, and Balamuthia are amphizoïc protozoa that are ubiquitous in nature (48). The present study confirms the presence of amphizoïc amoebae in the environment in Benin.
Our results demonstrate that the study area has a serious water hygiene problem. Both protected and unprotected waters were frequently found to contain potentially pathogenic free-living amoebae.
Free-living amoebae were found more frequently in areas of high BU endemicity than in areas of low BU endemicity (Table 2). This is significant only for protected water bodies. This is a strong indication that so-called protected water sources in areas of BU endemicity are not safe.
Previous studies have demonstrated that populations that use unprotected sources of water for domestic purposes have prevalence rates of BU higher than those who use protected sources (1, 21, 30, 54). The preferable habitat of M. ulcerans is probably unprotected waters, since the presumed reservoir and/or vector(s) of M. ulcerans probably occurs more frequently in unprotected waters (39). One study demonstrated an association between better water hygiene and lower incidence of BU (30). However, our study has revealed that in our study area, the presumably more hygienic water (i.e., that coming from protected sources) contains disturbingly high levels of free-living, potentially pathogenic amoebae. Whether this would have an influence on BU endemicity is not clear and should be further studied. Similarly, the reasons for isolating more free-living amoebae in protected water bodies in areas of high BU endemicity than from those in areas of low BU endemicity are not clear. This indicates that the water sources that we consider “protected” for the purpose of our study, namely, rain tanks, boreholes, concrete wells, and water towers, do not prevent amoebae from entering the system. Concrete wells, for example, were sampled only in areas of high endemicity: these wells tend to collect surface water and so may be prone to contamination.
The observation that mycobacteria were isolated only from water bodies in areas of high BU endemicity might indicate that in those sites the environment is more suitable for the survival of mycobacteria.
A study by our group on the physicochemical characteristics of water bodies in areas of high and areas of low BU endemicity as well as the level of fecal contamination is ongoing and may explain some of the observations in this study.
We identified three Naegleria spp. and two Tetramitus spp. (Table 2). N. philippinensis was originally described as an isolate from human cerebrospinal fluid, but the pathogenicity of the other vahlkampfiids is unknown. Strains that do not grow at 37°C are in principle not pathogenic to humans.
Two of the species detected in this study have rarely been identified before. N. angularis has been isolated only once before from a water sample collected in Peru (14), and the Naegleria sp. corresponding to NG583 is a one-strain species (J. F. De Jonckheere, unpublished data), although it was also recently detected in Japan.
Several species of Acanthamoeba are pathogenic opportunistic free-living amoebae that may cause encephalitis, skin infections, and keratitis (25). The four genotypes of Acanthamoeba (T4, T5, T6, and T11) isolated in Benin are established pathogens (Table 2). They can cause Acanthamoeba keratitis (AK), granulomatous Acanthamoeba encephalitis, and disseminated Acanthamoeba infections (DAI). In developed countries, AK infections are usually associated with contact lens wearing (11). In developing nations, however, most cases of AK result from ocular trauma (46).
All acanthamoebae isolated during this study have been implicated as causes of serious human disease, in particular AK (Table 2). The T4 genotype of Acanthamoeba was the most frequently isolated in this study (Table 2). Several studies indicate that genotype T4 of Acanthamoeba is the predominant agent of AK as well as nonkeratitis infections (6), presumably because they are the most frequent genotype observed in the environment. However, it has also been suggested that this genotype is well adapted to culture conditions and emerges from an initial mixture of different genotypes as the predominant genotype. T5, which represents the species A. lenticulata, was also isolated. Although genotype T5 is highly pathogenic in experimental animals, only two human cases of Acanthamoeba infections have been due to genotype T5, an AK (50) and a DAI (3).
The time between isolation of the amoebae and isolation of the DNA was extensive (up to 1 year) and as a result several of the culture plates had dried. Vahlkampfiidae cysts are more sensitive to desiccation than those of the genus Acanthamoeba. This may explain why many more acanthamoebae could be genotyped than vahlkampfiids.
A significant association between the presence of amoebae and the presence of mycobacteria in a hospital water network has previously been observed (52). In that study, 46.7% of specimens from which amoebae were isolated showed mycobacterial growth; specimens from which no amoebae were recovered showed less mycobacterial growth (18.4%; P = 0.009). In our study, specimens from which amoebae were isolated were less likely to contain mycobacteria (9/104; 8.7%) than specimens from which no amoebae were recovered (4/28; 14.3%), although this difference was not significant (P = 0.4). An explanation for the discrepancy between our results and those of Thomas et al. (52) could be that in artificial environments such as the hospital water network studied by Thomas et al. (52), biofilm formation is the only way for mycobacteria to survive in an adverse environment where, for example, chlorination is used for sterilization (36).
The following mycobacterial species detected in our study are known environmental mycobacteria (36): M. gordonae, M. fortuitum, M. flavescens, M. asiaticum, M. scrofulaceum, and M. simiae. The recently described species M. arupense (8), considered as clinically relevant (28), has never before been isolated from the environment. In a recent study, however, M. arupense was identified in an African rodent (Mastomys natalensis) captured in Tanzania (15). The present study confirms the presence of this species in the African environment.
The number of samples that tested positive for mycobacteria in this study was surprisingly low. Only 24.0% (Table 3) of unprotected water specimens were positive for mycobacteria. In a previous study performed in areas of high BU endemicity in the Democratic Republic of Congo (Lower-Congo and Maniema provinces), 80.6% (233 specimens out of 289) were culture positive for mycobacteria. One possible explanation for this discrepancy is the difference between the centrifuges used. In Benin, a centrifuge with a maximum centrifugal force of 2,000 × g was available, while in the study in the Democratic Republic of Congo, a centrifuge with a centrifugal force of 3,000 × g was used (34). A relative centrifugal force of at least 3,000 × g is recommended for the concentration of M. tuberculosis in sputum specimens (42). At a relative centrifugation force of 3,000 × g for 20 min, only 4% of the mycobacteria will remain suspended, while at 2,000 × g for 20 min, approximately 9% will remain suspended (42).
The detection limit of the PCR assay for mycobacteria in general is 40 acid-fast bacilli/sample (L. Rigouts, unpublished data), while for in vitro culture the detection limit is 100 bacilli/sample (9).
In the present study, none of the specimens were positive for IS2404. The IS2404 PCR on DNA extracted by the modified Boom method is very sensitive, detecting as few as five acid-fast bacilli in a sample (33). In previous studies, 10 to 20% of aquatic insects or fish collected in areas of high BU endemicity in Benin or Ghana during the dry season were positive for IS2404 by PCR (16, 23, 38). However, water specimens collected in the same areas were never found positive for IS2404 (F. Portaels, unpublished data).
Intracellular mycobacteria were isolated only from biofilm specimens. The cultivation of M. malmoense from amoebae present in a biofilm is in agreement with a previous study (35) which demonstrated the presence of M. malmoense in the environment in the Democratic Republic of Congo. M. malmoense is a slow-growing opportunistic pathogen causing pulmonary disease. Our study supports the hypothesis of an environmental source of infection.
Amoebae have not been observed to use mycobacteria as a source of food, so it is likely that mycobacteria are concentrated passively within amoebae (M. Eddyani, unpublished data).
Mycobacteria are highly hydrophobic; therefore, they easily attach to surfaces or are phagocytized by macrophages and protozoa (17). Thus, free-living amoebae may play a role as a reservoir for mycobacteria, potentially explaining the discrepancy between the fastidious nature of the bacteria and their widespread presence in the environment.
Preliminary experiments in our laboratory have shown that M. ulcerans is rapidly phagocytized by Acanthamoeba polyphaga but survives and persists inside these amoebae for up to 14 days without disturbing the growth of the amoebae (M. Eddyani, unpublished data).
This study demonstrates a clear relationship between the occurrence of free-living amoebae in protected water sources and BU endemicity. This might reflect the difference in socioeconomic status between the areas of high and of low BU endemicity studied. However, the actual mechanism linking BU endemicity and the occurrence of free-living amoebae is not clear and warrants further study.
Acknowledgments
We greatly acknowledge Joe Falkinham III for help with the design of the method for isolation of intracellular mycobacteria, Christian Roch Johnson and Ghislain Sopoh for facilitation of the environmental sampling, Cécile Uwizeye for technical assistance, and Martine Debacker for help with the statistical analyses. Conor Cahill, a professional freelance writer, provided writing support.
This work was partly supported by grant G.0375.05 of the Fund for Scientific Research of Flanders (Brussels, Belgium), the Damien Foundation (Brussels, Belgium), and the European Commission (project INCO CT-2005-051476-BURULICO).
Footnotes
Published ahead of print on 5 September 2008.
REFERENCES
- 1.Aiga, H., T. Amano, S. Cairncross, J. A. Domako, O.-K. Nanas, and S. Coleman. 2004. Assessing water-related risk factors for Buruli ulcer: a case-control study in Ghana. Am. J. Trop. Med. Hyg. 71:387-392. [PubMed] [Google Scholar]
- 2.Ash, L. R., and T. C. Orihel. 1987. Parasites: a guide to laboratory procedures and identification. American Society of Clinical Pathologists Press, Chicago, IL.
- 3.Barete, S., A. Combes, J. F. De Jonckheere, A. Datry, S. Varnous, V. Martinez, E. Caumes, D. Capron, C. Francès, C. Gibert, and O. Chosidow. 2007. Fatal disseminated Acanthamoeba lenticulata infection in a heart transplant patient. Emerg. Infect. Dis. 13:736-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böddinghaus, B., T. Rogall, T. Flohr, H. Blöcker, and E. C. Böttger. 1990. Detection and identification of mycobacteria by amplification of rRNA. J. Clin. Microbiol. 28:1751-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booton, G. C., D. J. Kelly, Y.-W. Chu, D. V. Seal, E. Houang, D. S. C. Lam, T. J. Byers, and P. A. Fuerst. 2002. 18S ribosomal DNA typing and tracking of Acanthamoeba species isolates from corneal scrape specimens, contact lenses, lens cases, and home water supplies of Acanthamoeba keratitis patients in Hong Kong. J. Clin. Microbiol. 40:1621-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booton, G. C., G. S. Visvesvara, T. J. Byers, D. J. Kelly, and P. A. Fuerst. 2005. Identification and distribution of Acanthamoeba species genotypes associated with nonkeratitis infections. J. Clin. Microbiol. 43:1689-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloud, J. L., J. J. Meyer, J. I. Pounder, K. C. Jost, A. Sweeney, K. C. Carroll, and G. L. Woods. 2006. Mycobacterium arupense sp. nov., a non-chromogenic bacterium isolated from clinical specimens. Int. J. Syst. Evol. Microbiol. 56:1413-1418. [DOI] [PubMed] [Google Scholar]
- 9.Collins, C. H., J. M. Grange, and M. D. Yates. 1997. Tuberculosis bacteriology: organization and practice. Butterworth-Heinemann, Oxford, United Kingdom.
- 10.De Jonckheere, J. F. 1998. Sequence variation in the ribosomal internal transcribed spacer, including 5.8S, of Naegleria spp. Protist 149:221-228. [DOI] [PubMed] [Google Scholar]
- 11.De Jonckheere, J. F. 2003. Epidemiological typing of Acanthamoeba strains isolated from keratitis cases in Belgium. Bull. Soc. Belge Ophthalmol. 287:27-33. [PubMed] [Google Scholar]
- 12.De Jonckheere, J. F. 2004. Molecular definition and the ubiquity of species in the genus Naegleria. Protist 155:89-103. [DOI] [PubMed] [Google Scholar]
- 13.De Jonckheere, J. F., and S. Brown. 2005. The identification of vahlkampfiid amoebae by ITS sequencing. Protist 156:89-96. [DOI] [PubMed] [Google Scholar]
- 14.De Jonckheere, J. F., and S. Brown. 2005. Description of a new species with a remarkable cyst structure in the genus Naegleria: Naegleria angularis n. sp. Acta Protozool. 44:61-65. [Google Scholar]
- 15.Durnez, L., M. Eddyani, G. F. Mgode, A. Katakweba, C. R. Katholi, R. R. Machang'u, R. R. Kazwala, F. Portaels, and H. Leirs. 2008. First detection of mycobacteria in African rodents and insectivores, using stratified pool screening. Appl. Environ. Microbiol. 74:768-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddyani, M., D. Ofori-Adjei, G. Teugels, D. De Weirdt, D. Boakye, W. M. Meyers, and F. Portaels. 2004. Potential role for fish in transmission of Mycobacterium ulcerans disease (Buruli ulcer): an environmental study. Appl. Environ. Microbiol. 70:5679-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkinham, J. O., G. Nichols, J. Bartram, A. Dufour, and F. Portaels. 2004. Natural ecology and survival in water of mycobacteria of potential public health significance, p. 15-25. In S. Pedley, J. Bartram, G. Rees, A. Dufour, and J. A. Cotruvo (ed.), Pathogenic mycobacteria in water: a guide to public health consequences, monitoring and management. IWA Publishing, London, United Kingdom.
- 18.Guimaraes-Peres, A., F. Portaels, P. de Rijk, K. Fissette, S. R. Pattyn, J. P. van Vooren, and P. A. Fonteyne. 1999. Comparison of two PCRs for detection of Mycobacterium ulcerans. J. Clin. Microbiol. 37:206-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugo, E. R., V. J. Stewart, R. J. Gast, and T. J. Byers. 1992. Purification of amoeba mtDNA using the UNSET procedure, p. D-7.1-D-7.2. In J. J. Lee and A. T. Soldo (ed.), Protocols in protozoology. Allen Press, Lawrence, KS.
- 20.Johnson, P. D., T. Stinear, P. L. Small, G. Pluschke, R. W. Merritt, F. Portaels, K. Huygen, J. A. Hayman, and K. Asiedu. 2005. Buruli ulcer (Mycobacterium ulcerans infection): new insights, new hope for disease control. PLoS Med. 2:e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, R. C., M. Makoutodé, G. E. Sopoh, P. Elsen, J. Gbovi, L. H. Pouteau, W. M. Meyers, M. Boko, and F. Portaels. 2005. Buruli ulcer distribution in Benin. Emerg. Infect. Dis. 3:500-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirschner, P., B. Springer, U. Vogel, A. Meier, A. Wrede, M. Kiekenbeck, F.-C. Bange, and E. C. Böttger. 1993. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J. Clin. Microbiol. 31:2882-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotlowski, R., A. Martin, A. Ablordey, K. Chemlal, P. A. Fonteyne, and F. Portaels. 2004. One-tube cell lysis and DNA extraction procedure for PCR-based detection of Mycobacterium ulcerans in aquatic insects, molluscs and fish. J. Med. Microbiol. 53:927-933. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted.
- 25.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsollier, L., R. Robert, J. Aubry, J. P. Saint André, H. Kouakou, P. Legras, A. L. Manceau, C. Mahaza, and B. Carbonnelle. 2002. Aquatic insects as a vector for Mycobacterium ulcerans. Appl. Environ. Microbiol. 68:4623-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsollier, L., E. Deniaux, P. Brodin, A. Marot, C. M. Wondje, J. P. Saint-Andre, A. Chauty, C. Johnson, F. Tekaia, E. Yeramian, P. Legras, B. Carbonnelle, G. Reysset, S. Eyangoh, G. Milon, S. T. Cole, and J. Aubry. 2007. Protection against Mycobacterium ulcerans lesion development by exposure to aquatic insect saliva. PLoS Med. 4:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masaki, T., K. Ohkusu, H. Hata, N. Fujiwara, H. Iihara, M. Yamada-Noda, P. H. Nhung, M. Hayashi, Y. Asano, Y. Kawamura, and T. Ezaki. 2006. Mycobacterium kumamotonense sp. nov. recovered from clinical specimen and the first isolation report of Mycobacterium arupense in Japan: novel slowly growing, nonchromogrenic clinical isolates related to Mycobacterium terrae complex. Microbiol. Immunol. 50:889-897. [DOI] [PubMed] [Google Scholar]
- 29.Miltner, E. C., and L. E. Bermudez. 2000. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 44:1990-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nackers, F., R. C. Johnson, J. R. Glynn, C. Zinsou, R. Tonglet, and F. Portaels. 2007. Environmental and health-related risk factors for Mycobacterium ulcerans disease (Buruli ulcer) in Benin. Am. J. Trop. Med. Hyg. 77:834-836. [PubMed] [Google Scholar]
- 31.Page, F. C. 1976. An illustrated key to freshwater and soil amoebae. Freshwater Biological Association, Ambleside, United Kingdom.
- 32.Palomino, J. C., and F. Portaels. 1998. Effects of decontamination methods and culture conditions on viability of Mycobacterium ulcerans in the BACTEC system. J. Clin. Microbiol. 36:402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips, R., C. Horsfield, S. Kuijper, A. Lartey, I. Tettey, S. Etuaful, B. Nyamekye, P. Awuah, K. M. Nyarko, F. Osei-Sarpong, S. Lucas, A. H. J. Kolk, and M. Wansbrough-Jones. 2005. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an assay using punch biopsy specimens for diagnosis of Buruli ulcer. J. Clin. Microbiol. 43:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portaels, F. 1978. Etude d'actinomycétales isolées de l'homme et de son environnement en Afrique Centrale. Ph.D. thesis. Université Libre de Bruxelles, Brussels, Belgium.
- 35.Portaels, F., L. Larsson, and P. A. Jenkins. 1995. Isolation of Mycobacterium malmoense from the environment in Zaire. Tuber. Lung Dis. 76:160-162. [DOI] [PubMed] [Google Scholar]
- 36.Portaels, F. 1995. Epidemiology of mycobacterial diseases. Clin. Dermatol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 37.Portaels, F., P. A. Fonteyne, H. de Beenhouwer, P. de Rijk, A. Guédénon, J. Hayman, and W. M. Meyers. 1996. Variability in the 3′ end of 16S rRNA sequences of Mycobacterium ulcerans is related to geographic origin of isolates. J. Clin. Microbiol. 34:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Portaels, F., P. Elsen, A. Guimaraes-Peres, P. A. Fonteyne, and W. M. Meyers. 1999. Insects in the transmission of Mycobacterium ulcerans infection (Buruli ulcer). Lancet 353:986. [DOI] [PubMed] [Google Scholar]
- 39.Portaels, F., K. Chemlal, P. Elsen, P. D. R. Johnson, J. A. Hayman, J. Hibble, R. Kirkwood, and W. M. Meyers. 2001. Mycobacterium ulcerans in wild animals. Rev. Sci. Tech. 20:252-264. [DOI] [PubMed] [Google Scholar]
- 40.Portaels, F., W. M. Meyers, A. Ablordey, A. Castro, K. Chemlal, P. de Rijk, P. Elsen, K. Fissette, A. Fraga, R. Lee, E. Mahrous, P. Small, P. Stragier, E. Torrado, A. Van Aerde, M. T. da Silva, and J. Pedrosa. 2008. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl. Trop. Dis. 2:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranger, B. S., E. A. Mahrous, L. Mosi, S. Adusumilli, R. E. Lee, A. Colorni, M. Rhodes, and P. L. C. Small. 2006. Globally distributed mycobacterial fish pathogens produce a novel plasmid-encoded toxic macrolide, mycolactone F. Infect. Immun. 74:6037-6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieder, H. L., T. M. Chonde, H. Myking, R. Urbanczik, A. Laszlo, S. J. Kim, A. Van Deun, and A. Trébucq. 1998. The public health service national tuberculosis reference laboratory and the national laboratory network. Minimum requirements, role and operation in a low-income country. International Union Against Tuberculosis and Lung Disease, Paris, France.
- 43.Rodríguez-Zaragoza, S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 44.Ross, B. C., L. Marino, F. Oppedisano, R. Edwards, R. M. Robins-Browne, and D. R. Johnson. 1997. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. J. Clin. Microbiol. 7:1696-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroeder, J. M., G. C. Booton, J. Hay, I. A. Niszl, D. V. Seal, M. B. Markus, P. A. Fuerst, and T. J. Byers. 2001. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J. Clin. Microbiol. 39:1903-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma, S., M. Srinivasan, and C. George. 1990. Acanthamoeba keratitis in non-contact lens wearers. Arch. Ophthalmol. 108:676-678. [DOI] [PubMed] [Google Scholar]
- 47.Silva, M. T., F. Portaels, and J. Pedrosa. 2007. Aquatic insects and Mycobacterium ulcerans: an association relevant to Buruli ulcer control. PLoS Med. 4:229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siripanth, C. 2005. Amphizoic amoebae: pathogenic free-living protozoa; review of the literature and review of cases in Thailand. J. Med. Assoc. Thai. 88:701-707. [PubMed] [Google Scholar]
- 49.Sison, J. P., C. A. Kemper, M. Loveless, D. McShane, G. S. Visvesvara, and S. C. Deresinski. 1995. Disseminated Acanthamoeba infection in patients with AIDS: case reports and review. Clin. Infect. Dis. 20:1207-1216. [DOI] [PubMed] [Google Scholar]
- 50.Spanakos, G., K. Tzanetou, D. Miltsakakis, E. Patsoula, E. Malamou-Lada, and N. C. Vakalis. 2006. Genotyping of pathogenic Acanthamoebae isolated from clinical samples in Greece—report of a clinical isolate presenting T5 genotype. Parasitol. Int. 55:147-149. [DOI] [PubMed] [Google Scholar]
- 51.Stinear, T., T. Seemann, S. Pidot, W. Frigui, G. Reysset, T. Garnier, G. Meurice, D. Simon, C. Bouchier, L. Ma, M. Tichit, J. L. Porter, J. Ryan, P. D. Johnson, J. K. Davies, G. A. Jenkin, P. L. Small, L. M. Jones, F. Tekaia, F. Laval, M. Daffé, J. Parkhill, and S. T. Cole. 2007. Reductive evolution and niche adaptation inferred from the genome of Mycobacterium ulcerans, the causative agent of Buruli ulcer. Genome Res. 17:192-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas, V., K. Herrera-Rimann, D. S. Blanc, and G. Greub. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl. Environ. Microbiol. 72:2428-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas, V., and G. McDonnell. 2007. Relationship between mycobacteria and amoebae: ecological and epidemiological concerns. Lett. Appl. Microbiol. 45:349-357. [DOI] [PubMed] [Google Scholar]
- 54.Uganda Buruli Group. 1971. Epidemiology of Mycobacterium ulcerans infection (Buruli ulcer) at Kinyara, Uganda. Trans. R. Soc. Trop. Med. Hyg. 65:763-775. [DOI] [PubMed] [Google Scholar]
- 55.van der Werf, T. S., Y. Stienstra, R. C. Johnson, R. Phillips, O. Adjei, B. Fleischer, M. H. Wansbrough-Jones, P. D. Johnson, F. Portaels, W. T. van der Graaf, and K. Asiedu. 2005. Mycobacterium ulcerans disease. Bull. W. H. O. 83:785-791. [PMC free article] [PubMed] [Google Scholar]
- 55a.Vincent Lévy-Frébault, Véronique, and F. Portaels. 1992. Proposed minimal standards for the genus Mycobacterium and for description of new slowly growing Mycobacterium species. Int. J. Syst. Bacteriol. 42:315-323. [DOI] [PubMed] [Google Scholar]
- 56.Walochnik, J., A. Aichelburg, O. Assadian, A. Steuer, G. Visvesvara, N. Vetter, and H. Aspöck. 2008. Granulomatous amoebic encephalitis caused by Acanthamoeba amoebae of genotype T2 in a human immunodeficiency virus-negative patient. J. Clin. Microbiol. 46:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu, H. S., H. J. Jeong, Y. C. Hong, S. Y Seol, D. I. Chung, and H. H. Kong. 2007. Natural occurrence of Mycobacterium as an endosymbiont of Acanthamoeba isolated from a contact lens storage case. Korean J. Parasitol. 45:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]