Abstract
To examine whether there is a relationship between the degree of Campylobacter contamination observed in product lots of retail Icelandic broiler chicken carcasses and the incidence of human disease, 1,617 isolates from 327 individual product lots were genetically matched (using the flaA short variable region [SVR[) to 289 isolates from cases of human campylobacteriosis whose onset was within approximately 2 weeks from the date of processing. When there was genetic identity between broiler isolates and human isolates within the appropriate time frame, a retail product lot was classified as implicated in human disease. According to the results of this analysis, there were multiple clusters of human disease linked to the same process lot or lots. Implicated and nonimplicated retail product lots were compared for four lot descriptors: lot size, prevalence, mean contamination, and maximum contamination (as characterized by direct rinse plating). For retail product distributed fresh, Mann-Whitney U tests showed that implicated product lots had significantly (P = 0.0055) higher mean contamination than nonimplicated lots. The corresponding median values were 3.56 log CFU/carcass for implicated lots and 2.72 log CFU/carcass for nonimplicated lots. For frozen retail product, implicated lots were significantly (P = 0.0281) larger than nonimplicated lots. When the time frame was removed, retail product lots containing Campylobacter flaA SVR genotypes also seen in human disease had significantly higher mean and maximum contamination numbers than lots containing no genotypes seen in human disease for both fresh and frozen product. Our results suggest that cases of broiler-borne campylobacteriosis may occur in clusters and that the differences in mean contamination levels may provide a basis for regulatory action that is more specific than a presence-absence standard.
Campylobacter jejuni and its close relatives are the most common causes of bacterial food-borne infection in the developed world (21) and as such present a serious target for interventions to reduce human disease. Since most cases of campylobacteriosis are associated with raw or undercooked poultry meat (21), interventions that reduce the prevalence of C. jejuni within poultry flocks may have a major impact on human health. However, in terms of food safety, the high prevalence seen among lots in many countries (see, e.g., reference 5) makes a presence-absence standard untenable as a regulatory mechanism.
Iceland regulates Campylobacter contamination in retail broilers through preslaughter testing. Approximately 5 to 7 days (from January 2000 through October 2001) or 2 to 4 days (after October 2001) before slaughter, fecal samples have been collected and cultured for the presence of Campylobacter spp. If test results are negative, a flock is routinely processed, and most of the product is distributed fresh. If test results are positive, the retail product is frozen or further processed before distribution, and the date of processing is altered to best fit the market conditions. Because the processing companies want to ensure availability of adequate fresh product at all times, the processing of Campylobacter-positive flocks is often delayed past the planned date of slaughter. In addition, some portion of Campylobacter-negative processing lots is often frozen and stored for later distribution, and some retail broilers from both Campylobacter-positive and -negative lots are sent for further processing to be distributed as cooked product. Because of the greater value of fresh product, cooked product primarily comes from Campylobacter-positive product lots, which would otherwise be distributed frozen. This regulatory scheme has proved extremely effective, as shown by the drop in the number of cases of campylobacteriosis in Iceland after its implementation (17). Unfortunately, this regulatory scheme would prove prohibitively complex and costly for countries with larger broiler industries. Therefore, there is a need for a scientifically based approach to identify broiler lots most likely to cause human disease without preslaughter testing of every production flock.
Rosenquist et al. (16) divided sources of consumer risk from the presence of broiler-borne Campylobacter spp. into three general areas: kitchen hygiene, prevalence, and contamination. While kitchen hygiene is an area best addressed through education, prevalence and contamination are valid targets for food safety regulation. In their model, Rosenquist et al. (16) used “prevalence” to indicate presence or absence among flocks and found that higher levels of between-flock prevalence posed a greater risk for consumers. The between-flock prevalence is identical to the prevalence of contaminated product in the marketplace when flocks are assumed to be of equal size. Unfortunately, since it treats flock contamination as a presence-absence characteristic, this method is unsuitable as a regulatory metric when Campylobacter prevalence is high. Because the frequency with which a consumers encounter contaminated product from a particular lot is a function of lot size and within-lot prevalence, these descriptors may provide a better regulatory target for individual lots.
When partitioning risk, Rosenquist et al. (16) considered the level of contamination of individual carcasses and found that carcasses with more contamination presented a greater risk to the consumer. While it would be impossible for any regulatory agency to sample all retail product before its distribution, contamination can be used as a lot descriptor for regulatory purposes. In this study, we used mean contamination and maximum contamination data to score each lot for the contamination observed for sampled carcasses.
In order to assess the significance of these four lot descriptors (lot size, within-lot prevalence, mean contamination, and maximum contamination), it is necessary to know whether any product lots caused disease. Absent a study where large numbers of consumers are given carcasses of known contamination, it is impossible to know for certain that a specific lot gave rise to a particular case of human campylobacteriosis: there is always a possibility that some unsampled source or unconsidered pathway was the actual cause. However, through the comparison of genetic types found in each lot with those found in cases of human disease and the knowledge of temporal relationships between the processing of those lots and the appearance of symptoms, particular product lots can be identified as being more likely to have caused disease. Any similarities in the lots thus identified can then provide the basis for food safety regulations.
MATERIALS AND METHODS
Sample collection.
For each case of human campylobacteriosis, fecal samples were sent to the laboratory on modified Cary-Blair medium (3). Samples were then plated onto Campylobacter charcoal differential agar and incubated at 37°C for 48 h in a microaerobic environment (5% O2, 10% CO2, 85% N2). Colonies with typical Campylobacter morphology were characterized for oxidase and catalase production, motility, and Gram staining. Isolates identified as representing Campylobacter spp. were shipped on Wang's medium (20) to Athens, Georgia. A total of 326 isolates from cases of human illness were collected from January 2001 through December 2004; of these, 298 were typed as described below. The remaining 28 isolates were either nontypeable or arrived in an uncultivable state prior to August 2002. Of the typed isolates, 10 were collected before the sampling of retail product lots, and 11 were collected after October 2004 and did not match any of our sampled product lots according to the rules described below.
Cecal samples from the processing stage of production and postprocessing retail product samples were collected from May 2001 through September 2004 as a part of Iceland's National Campylobacter Surveillance Program. Three of four companies producing broilers initially collaborated with this study. For production lots from those three producers, four pooled samples of 10 cecal samples were collected at the start of processing; after processing, up to 20 carcasses were saved for enumeration. For the fourth producer, samples initially consisted of preprocessing cecal samples through July 2004; following this date, samples were provided as described for the other three producers. Cecal samples were processed as described by Stern et al. (18), and postprocessing broiler carcasses were sampled as described by Georgsson et al. (4). A total of 387 processing lots from all broiler production companies gave Campylobacter-positive results during the sampling period.
For both cecal and retail carcass samples, one confirmed Campylobacter colony per positive sample result was restreaked, placed in Wang's transport medium, and shipped to the USDA-ARS in Athens, Georgia, for molecular typing. Upon arrival in Georgia, isolates were streaked onto Campy-Cefex agar and stored in glycerol at −80°C. Prior to August 2002, unrecoverable isolates that arrived in Georgia were discarded; after that date, the transport tubes of unrecoverable isolates were stored at 4°C for later molecular analysis.
Molecular typing.
DNA was prepared from frozen isolates by placing 10 μl of glycerol stock in 50 μl of sterile, distilled water. Cells were lysed at 100°C for 5 min. Microcentrifuge tubes containing DNA samples were spun briefly to precipitate cellular debris (6). DNA was prepared for unrecoverable isolates by pipetting 40 μl of Wang's transport medium into 200 μl of sterile distilled water, boiling these samples at 100°C for 5 min, and spinning to precipitate cellular debris (2). For both preprocessing and postprocessing samples, the flaA short variable region (SVR; a hypervariable part of the flagellin gene) was then amplified using the primers Fla4F (5′-GGA TTT CGT ATT AAC ACA AAT GGT GC-3′) (12) and FlaA625RU (5′-CAA GWC CTG TTC CWA CTG AAG-3′) (10) with a reaction mixture containing 2 mM MgCl2, 0.125 μM each primer, 0.8 mM each deoxynucleoside triphosphate, and 2.5 U of AmpliTaq in a 100-μl reaction volume. Tubes were subjected to 35 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 1 min, followed by a 5-min extension at 72°C. Sequencing was performed using degenerate primers Fla106F (5′-GAY GAT GCT TCW GGK ATG-3′) and FlaA625RU by use of BigDye Terminator 3.1 chemistry (Applied Biosystems). Sequence data were obtained using a model 3730 DNA analyzer (Applied Biosystems). The 359-nucleotide region between primers FlaA242FU and FlaA625RU was used for allelic comparisons. In all, 298 isolates from cases of human disease were sequenced, and 1,617 isolates from 327 broiler production lots were sequenced. The sequences of unique genotypes can be found in GenBank under accession numbers DQ335538 to DQ335542, DQ335545 to DQ335548, DQ335552 to DQ335554, DQ335556, DQ335557, DQ335559, DQ335561, DQ335564 and EU431225 to EU431320.
Phylogenetic analysis.
Phylogenetic analysis was performed using the PHYLIP 3.65 suite of programs (J. Felsenstein, Department of Genome Sciences, University of Washington, Seattle [http://evolution.genetics.washington.edu/phylip.html]), following alignment of unique genotypes by use of CLUSTAL W software (19). SEQBOOT software was used to generate a data set of 1,000 bootstrap replicates from the alignment of unique genotypes, and DNAML software was used to generate trees from these data replicates. CONSENSE software was used to create a consensus tree and calculate the bootstrap support for each branch. Data from this tree were then reinput into DNAML as a user tree with the original sequence data in order to assign maximum-likelihood branch lengths to the tree previously determined through bootstrapping. Branches with less than 50% bootstrap support or with branch lengths that were not significantly different from 0 were collapsed.
Sample comparisons.
An arbitrary time period was developed for the temporal comparison of human and broiler isolates. For a given contaminated product lot, the soonest a person might show symptoms would be 2 days after carcass processing; we considered all cases of human campylobacteriosis that occurred between 2 and 16 days postprocessing for those cases where we had a date for the onset of symptoms. For those cases of campylobacteriosis where the onset date was unknown, we added the means ± the standard deviations of time between symptom onset and laboratory receipt of the sample to this range of 2 to 16 days and used this broader range of dates to temporally link the day of processing with the date of laboratory receipt.
Statistical analysis.
Mean contamination (the mean of the log CFU per carcass) and maximum contamination (the highest observed log CFU) were determined for each processing lot by the use of Excel 2004 software (Microsoft Corporation, Bellevue, WA). Linear correlations were used to study the relationships among the results showing the prevalence of Campylobacter contamination within a process lot to the mean contamination and maximum contamination results for that lot by the use of the VassarStats website (http://faculty.vassar.edu/lowry/VassarStats.html). One-tailed Mann-Whitney U tests (VassarStats) were used to test whether implicated lots had significantly larger values for prevalence, mean contamination, and maximum contamination than nonimplicated lots for both fresh and frozen retail product lots. For the correlations and U tests described above, prevalence, mean contamination, and maximum contamination were used as descriptors for processing lots, as discussed below. VassarStats software was also used to perform one-tailed Mann-Whitney U tests to determine whether lot sizes (the number of carcasses processed at one time from a single broiler house) were significantly larger for implicated lots than nonimplicated lots for both fresh- and frozen-product lots. Lots matching only poultry farm workers, slaughterhouse workers, or children younger than 2 years living on farms were excluded from the analysis: in these cases, alternate routes of Campylobacter infection are at least as likely as food-borne transmission.
U tests were also used to determine whether product lots associated with at least one isolate with a genotype seen in any of the human cases (regardless of timing) were significantly larger or had significantly higher values for prevalence, mean contamination, or maximum contamination than product lots containing no isolates with genotypes seen in human cases. Product lots containing isolates with both genotypes seen in and genotypes absent from human cases were grouped with lots containing only isolates with genotypes seen in human cases. As described above, product lots distributed fresh were tested separately from those distributed frozen. Because these tests were performed posteriorly to the tests on implicated and nonimplicated product lots, we used a Bonferroni correction for the α: tests were considered significant when the P value was less than or equal to 0.025.
RESULTS
A total of 59 genotypes were seen among the human isolates, and 81 genotypes were observed for retail isolates. Thirty genotypes were seen in both human and retail isolates. In all, 250 (84%) of the 298 sequenced human isolates had genotypes also seen in broiler flocks. As shown in Fig. 1, an unrooted phylogenetic tree resulting from the comparison of all unique genotypes, there are no obvious phylogenetic patterns either for the genotypes seen in only one sample type or for the genotypes seen in both sample types.
FIG. 1.
Unrooted maximum likelihood tree of Campylobacter flaA SVR alleles. Alleles present in human isolates are marked with a filled circle. Alleles present in broiler samples are marked with an open square. Numbers above branches indicate bootstrap support (out of 1,000 bootstrap replicates) for the nodes. The bar along the bottom indicates branch length as substitutions per site.
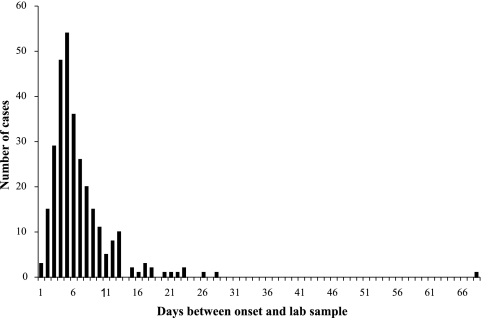
In order to compare human isolates with possible sources among broiler-processing lots, we considered any lot that was processed between 2 days before the onset of symptoms and 16 days before onset a temporal match. We used an arbitrary 2-week window to account for variations in the number of days postprocessing that a carcass might be purchased, the number of days that a consumer might store the retail product before consumption, and the number of days between exposure and onset of symptoms. For human cases without onset information (n = 51), the time between the onset of symptoms and the receipt of the sample in the diagnostic laboratory was plotted for all cases where both dates were known (Fig. 2). Because this distribution was skewed to the right, we analyzed the data as a log-normal distribution. The 5th (1 day) and 95th (11 days) percentiles were used to determine a window of exposure and infection for potential source processing lots that accounted for most observations. Those isolates from cases in which the date of onset was unknown were compared to isolates from retail product lots processed at least 3 days and no more than 27 days prior to the receipt of the laboratory sample.
FIG. 2.
Frequency distribution of time between onset of symptoms and receipt of the fecal sample in the reference laboratory for cases of human campylobacteriosis.
When we examine the human and broiler isolates by use of the above-described criteria, a total of 79 human isolates matched isolates in at least one processing lot, representing 27% of sequenced human isolates for which the above-described comparisons were possible. It should be stressed that this is not evidence that these particular cases of campylobacteriosis were the result of broiler-borne transmission. However, if a particular processing lot was a source for human disease through a food-borne route, it seems reasonable to infer that such a temporal and genetic relationship would be a prerequisite.
Table 1 shows groups in which more than one human isolate matched isolates in the same processing lot or lots. A complete list of all matches among human and broiler isolates is available (see Table S1 in the supplemental material). In these tables, each human campylobacteriosis sample is listed once, but a processing lot may be listed multiple times. Of the implicated processing lots, 26 were distributed fresh, for a maximum of 146,904 carcasses (because of market need, a proportion was distributed as frozen or cooked product). An additional 28 implicated processing lots were frozen prior to distribution; this represents 192,053 carcasses, but an unknown number of these went to further processing rather than being directly distributed to the consumer. Of these 26 fresh and 28 frozen implicated lots, we have prevalence and enumeration data for 21 fresh and 15 frozen processing lots.
TABLE 1.
Exact matches between Campylobacter isolates from humans and isolates associated with retail broiler producta
| Group | Genotype | Human isolate
|
Implicated broiler retail lot
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Date | Identifier | Processing date | Product state | Size (no. of broilers) | Prevalence (%) | Mean contamination (log CFU/carcass) | Maximum contamination (log CFU/carcass) | ||
| A10922 | AO-035 | 21 July 2001 (O) | EA2-T002a | 16 July 2001 | Fresh | 2,722 | 100 | 3.17 ± 0.18 | 3.52 | |
| AO-040 | 22 July 2001 (O) | EA2-T002b | 17 July 2001 | Fresh | 1,291 | 100 | 3.36 ± 0.27 | 3.80 | ||
| AO-045 | 23 July 2001 (O) | EA2-O006 | 19 July 2001 | Fresh | 4,150 | No data | No data | No data | ||
| AO-047 | 27 July 2001 (O) | |||||||||
| AO-048 | 30 July 2001 (O) | |||||||||
| AO-053 | 1 August 2001 (O) | |||||||||
| AO-054 | 1 August 2001 (O) | |||||||||
| 2 | A10922 | AO-055 | 3 August 2001 (O) | EA2-O006 | 19 July 2001 | Fresh | 4,150 | No data | No data | No data |
| OA2-U001b | 1 August 2001 | Frozen | 1,455 | No data | No data | No data | ||||
| 3 | A09798 | AO-060 | 4 August 2001 (O) | EA3-A022 | 1 August 2001 | Fresh | 6,082 | 100 | 2.52 ± 0.49 | 3.33 |
| AO-061 | 5 August 2001 (O) | |||||||||
| 4 | A10922 | AL-056 | 6 August 2001 (L) | EA2-T002a | 16 July 2001 | Fresh | 2,722 | 100 | 3.17 ± 0.18 | 3.52 |
| EA2-T002b | 17 July 2001 | Fresh | 1,291 | 100 | 3.36 ± 0.27 | 3.80 | ||||
| EA2-O006 | 19 July 2001 | Fresh | 4,150 | No data | No data | No data | ||||
| OA2-U001b | 1 August 2001 | Frozen | 1,455 | No data | No data | No data | ||||
| 5 | A09798 | AL-067 | 14 August 2001 (L) | EA3-A022 | 1 August 2001 | Fresh | 6,082 | 100 | 2.52 ± 0.49 | 3.33 |
| EA3-A023 | 7 August 2001 | Fresh | 6,700 | 100 | 1.67 ± 0.58 | 2.46 | ||||
| 7 | A06678 | AO-098 | 3 September 2001 (O) | OA1-A026 | 27 August 2001 | Frozen | 10,382 | 100 | No data | No data |
| EA1-K009 | 27 August 2001 | Fresh | 5,699 | 100 | 3.37 ± 0.28 | 4.11 | ||||
| EA1-A029 | 29 August 2001 | Fresh | 8,984 | 60 | No data | No data | ||||
| 8 | A06678 | AO-087 | 9 September 2001 (O) | OA1-A026 | 27 August 2001 | Frozen | 10,382 | 100 | No data | No data |
| AO-089 | 9 September 2001 (O) | EA1-K009 | 27 August 2001 | Fresh | 5,699 | 100 | 3.37 ± 0.28 | 4.11 | ||
| AO-085 | 10 September 2001 (O) | EA1-A029 | 29 August 2001 | Fresh | 8,984 | 60 | No data | No data | ||
| AO-086 | 10 September 2001 (O) | EA1-A030 | 5 September 2001 | Fresh | 10,397 | 100 | 3.71 ± 0.21 | 3.97 | ||
| AO-088 | 10 September 2001 (O) | |||||||||
| AO-090 | 11 September 2001 (O) | |||||||||
| AO-096 | 11 September 2001 (O) | |||||||||
| AO-092 | 12 September 2001 (O) | |||||||||
| AL-091 | 14 September 2001 (L) | |||||||||
| AL-095 | 17 September 2001 (L) | |||||||||
| 9 | A06678 | AO-097 | 13 September 2001 (O) | EA1-A029 | 29 August 2001 | Fresh | 8,984 | 60 | No data | No data |
| AO-093 | 14 September 2001 (O) | EA1-A030 | 5 September 2001 | Fresh | 10,397 | 100 | 3.71 ± 0.21 | 3.97 | ||
| AO-094 | 14 September 2001 (O) | |||||||||
| AO-101 | 14 September 2001 (O) | |||||||||
| 10 | A06678 | AO-099 | 18 September 2001 (O) | EA1-A030 | 5 September 2001 | Fresh | 10,397 | 100 | 3.71 ± 0.21 | 3.97 |
| AO-100 | 20 September 2001 (O) | |||||||||
| 11 | A06678 | AO-106 | 29 September 2001 (O) | EA1-G035 | 24 September 2001 | Fresh | 6,998 | 100 | 3.81 ± 0.28 | 4.29 |
| AO-104 | 30 September 2001 (O) | OA1-G036 | 26 September 2001 | Frozen | 7,006 | 100 | No data | No data | ||
| AO-108 | 2 October 2001 (O) | OA1-A034 | 27 September 2001 | Frozen | 11,659 | 100 | No data | No data | ||
| 12 | A06678 | AO-110 | 4 October 2001 (O) | EA1-G035 | 24 September 2001 | Fresh | 6,998 | 100 | 3.81 ± 0.28 | 4.29 |
| AO-112 | 7 October 2001 (O) | OA1-G036 | 26 September 2001 | Frozen | 7,006 | 100 | No data | No data | ||
| AL-113 | 19 October 2001 (L) | OA1-A034 | 27 September 2001 | Frozen | 11,659 | 100 | No data | No data | ||
| OA1-G040 | 2 October 2001 | Frozen | 6,336 | 95 | No data | No data | ||||
| 14 | A06678 | AO-114 | 15 October 2001 (O) | OA1-G040 | 2 October 2001 | Frozen | 6,336 | 95 | No data | No data |
| 18 | A05089 | BL-051 | 31 July 2002 (L) | EB1-G046 | 25 July 2002 | Fresh | 7,495 | 100 | 3.56 ± 0.30 | 4.24 |
| 20 | A05089 | BL-059 | 10 August 2002 (L) | EB1-G046 | 25 July 2002 | Fresh | 7,495 | 100 | 3.56 ± 0.30 | 4.24 |
| OB1-G047 | 25 July 2002 | Frozen | 7,482 | 90 | 2.54 ± 0.52 | 3.47 | ||||
| 24 | A13669 | CO-019 | 22 July 2003 (O) | EC1-G059c | 16 July 2003 | Fresh | 6,906 | 100 | 4.17 ± 0.55 | 5.14 |
| CO-021 | 24 July 2003 (O) | |||||||||
| CO-023 | 24 July 2003 (O) | |||||||||
| 25 | A10922 | CO-020 | 22 July 2003 (O) | EC1-G060 | 18 July 2003 | Fresh | 7,099 | 100 | 3.41 ± 0.46 | 4.57 |
| CO-022 | 22 July 2003 (O) | |||||||||
| 27 | A13669 | CO-032b | 14 August 2003 (O) | OC1-G066a | 12 August 2003 | Frozen | 11,709 | 100 | 2.88 ± 0.44 | 4.34 |
| 29 | A13669 | CO-038b | 24 August 2003 (O) | OC1-G066a | 12 August 2003 | Frozen | 11,709 | 100 | 2.88 ± 0.44 | 4.34 |
| OC1-G066b | 14 August 2003 | Frozen | 6,189 | 100 | 3.10 ± 0.37 | 3.70 | ||||
| OC1-G068a | 19 August 2003 | Frozen | 7,065 | 97 | 2.72 ± 0.58 | 4.26 | ||||
| OC1-G068b | 20 August 2003 | Frozen | 6,782 | 95 | 1.88 ± 0.40 | 2.70 | ||||
| 31 | A04101 | CO-042 | 27 September 2003 (O) | EC1-L018 | 22 September 2003 | Fresh | 6,875 | 100 | 2.92 ± 0.67 | 4.11 |
| CO-043 | 30 September 2003 (O) | |||||||||
| 33 | A09884 | CO-051c | 17 October 2003 (O) | EC2-V011b | 13 October 2003 | Fresh | 1,633 | 100 | 4.10 ± 0.38 | 4.80 |
| EC2-O029a | 14 October 2003 | Fresh | 3,553 | 75 | 1.09 ± 0.37 | 2.11 | ||||
| 34 | A14226 | CO-047 | 23 October 2003 (O) | EC2-V011b | 13 October 2003 | Fresh | 1,633 | 100 | 4.10 ± 0.38 | 4.80 |
| EC2-O029a | 14 October 2003 | Fresh | 3,553 | 75 | 1.09 ± 0.37 | 2.11 | ||||
| 39 | A04741 | DO-068 | 25 August 2004 (O) | OD3-A043 | 18 August 2004 | Frozen | 9,621 | 90 | 1.53 ± 0.52 | 2.86 |
| DO-069 | 25 August 2004 (O) | |||||||||
| DL-071 | 30 August 2004 (L) | |||||||||
| 43 | A08616 | DO-075b | 5 September 2004 (O) | OD1-G065a | 1 September 2004 | Frozen | 6,490 | 100 | 2.89 ± 0.57 | 4.08 |
| DO-078b | 13 September 2004 (O) | OD1-G065b | 2 September 2004 | Frozen | 6,116 | 100 | 2.82 ± 0.41 | 3.60 | ||
For the isolates from humans, “Date” refers to the date of onset of symptoms (“O”) where we have the information or to the date the sample was received in the laboratory (“L”) where information about onset isn't available.
Patient worked on a poultry farm or in a poultry abattoir.
Patient was a child under 2 living on a farm.
During the study period, there were a number of apparent Campylobacter temporal clusters associated with broiler-borne transmission. For example, in July and August 2001, nine human isolates in groups 1, 2, and 4 in Table 1 had the A10922 genotype. These isolates potentially implicated four processing lots. In September and October, there were two apparent Campylobacter temporal clusters with the A06678 genotype. The first apparent cluster consisted of 17 cases of campylobacteriosis (groups 7 to 10) that genetically and temporally matched four retail lots processed at the end of August and the beginning of September. The second apparent cluster of Campylobacter isolates with the A06678 genotype involved seven cases of campylobacteriosis (groups 11, 12, and 14) matching four retail lots processed at the end of September and the beginning of October. It should be kept in mind that even in the apparent clusters, transmission to humans may have occurred through a route other than broiler-borne contamination and that some sharing of genotypes in a temporally appropriate manner may have been due to transmission to humans and broilers from some common third source.
In addition to these larger apparent clusters, there were numerous examples of multiple human isolates temporally matching the same broiler isolates. In August 2001, three human A09798 isolates (groups 3 and 5) matched isolates from two processing lots. Three human A13669 isolates (group 24) matched isolates from one retail lot in July 2003, and three A04741 isolates (group 39) matched isolates from one retail lot in August 2004. In addition, there were three incidents (groups 18 and 20; group 25; and group 31) where the Campylobacter isolates from two members of the general public matched isolates from the same one or two retail lots. There were also two possible clusters (groups 27 and 29 and group 43) among poultry farm or slaughterhouse workers where the isolates from human disease matched at least one of the same retail product lot isolates. Finally, there was an example of two human isolates (groups 33 and 34) with different genotypes (A09884 and A14226) that matched isolates from the same two retail lots, both of which produced Campylobacter isolates with the two genotypes.
When the criteria are expanded to include genotypes that differ by one substitution as matches, there are only a few additional matches (see Table S2 in the supplemental material). There are eight additional matches between particular human isolates and isolates from broiler lots; of these, three human isolates are better explained by exact matches to isolates from other broiler lots. In addition, isolates from 3 of the 11 processing lots shown in Table S1 in the supplemental material already matched human isolates exactly. If genotypes differing by one mutation were counted as a match, isolates from each of these retail lots would then have two human isolate matches. Taking these findings as a whole, though, there seem to be few compelling reasons to relax the standards of a genetic match from complete identity between genotypes to situations where these genotypes differ by one mutation.
In terms of Campylobacter contamination, retail lots can be described by the prevalence of Campylobacter-positive carcasses within the lot, by mean contamination, and by maximum contamination. While there is an obvious relationship between mean contamination and maximum log contamination, there was a significant correlation between prevalence and mean log contamination (r2 = 0.42 [P < 0.0001]) and between prevalence and maximum log contamination (r2 = 0.54 [P < 0.00001]; data not shown). Nonetheless, since they are not completely correlated, these retail lot descriptors should be considered separately for their utility in distinguishing implicated from nonimplicated lots.
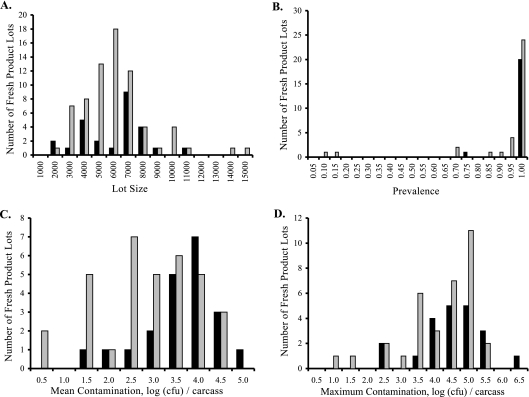
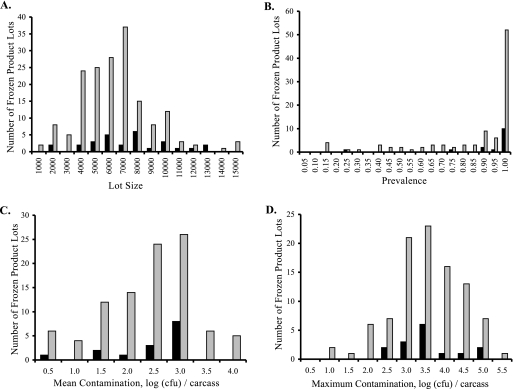
When we consider implicated versus nonimplicated lots for retail product distributed fresh (Fig. 3), we can see overlapping distributions for prevalence, mean contamination, and maximum contamination data. For each of these three lot descriptors, implicated lots have distributions shifted to higher prevalence or degree of contamination. The same pattern is seen for retail product that was frozen after processing (Fig. 4). When the significance of these descriptors was tested using a Mann-Whitney U test (Table 2), implicated lots had significantly (P = 0.0055) higher mean contamination than nonimplicated lots but insignificant differences in lot sizes (P = 0.2297), prevalences (P = 0.0668), and maximum contamination levels (P = 0.1515) for retail lots distributed fresh (Table 3). When lots frozen after processing were considered, implicated lots were larger (P = 0.0281) than nonimplicated lots but had insignificant differences with respect to prevalence (P = 0.1977), mean contamination (P = 0.33), and maximum contamination (P = 0.4325).
FIG. 3.
Comparison of fresh-product lots implicated in human disease to those not implicated in human disease. Black bars show the numbers of implicated lots. Gray bars show the numbers of nonimplicated lots. (A) Lot size. (B) Prevalence. (C) Mean contamination. (D) Maximum contamination.
FIG. 4.
Comparison of frozen product lots implicated in human disease to those not implicated in human disease. Black bars show the numbers of implicated lots. Gray bars show the numbers of nonimplicated lots. (A) Lot size. (B) Prevalence. (C) Mean contamination. (D) Maximum contamination.
TABLE 2.
Results of Mann-Whitney U tests for comparison of implicated and nonimplicated product for fresh and frozen retail product lots
| Product state |
P
|
|||
|---|---|---|---|---|
| Lot size | Prevalence | Mean contamination | Maximum contamination | |
| Fresh | 0.2297 | 0.0668 | 0.0055 | 0.1515 |
| Frozen | 0.0281 | 0.1977 | 0.33 | 0.4325 |
TABLE 3.
Mann-Whitney U test comparisons of lot size, prevalence, mean contamination, and maximum contamination results for product lots with and without alleles observed in isolates from humans for fresh and frozen retail product lotsa
| Product state |
P
|
|||
|---|---|---|---|---|
| Lot size | Prevalence | Mean contamination | Maximum contamination | |
| Fresh | 0.2946 | 0.0708 | 0.0009 | 0.0233 |
| Frozen | 0.4168 | 0.0005 | <0.0001 | 0.0002 |
Because this test was performed a posteriori, the critical value (α) is 0.025.
While median values are not used for testing significance in the Mann-Whitney U test, they are illustrative of the differences between implicated and nonimplicated lots in these comparisons. For fresh-product lots, the median values for the descriptor of mean contamination were 3.56 log CFU/carcass for implicated lots and 2.72 log CFU for nonimplicated lots. For frozen product, the corresponding medians were 2.53 log CFU and 2.30 log CFU for implicated and nonimplicated lots, respectively. For the descriptor of maximum contamination, the median values were 4.24 log CFU (implicated) and 4.23 log CFU (nonimplicated) for fresh product and 3.21 log CFU (implicated) and 3.24 (nonimplicated) for frozen product lots. The median prevalence was 100% for implicated and nonimplicated lots for both fresh and frozen product. The median lot sizes were 6,565 (implicated) and 5,244 (nonimplicated) for fresh product and 6,894 (implicated) and 5,863 (nonimplicated) for frozen product.
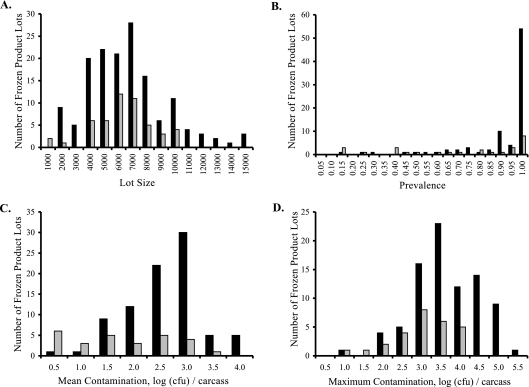
Subsequent to the analysis presented above, we examined whether processing lots containing isolates with genotypes also seen in isolates from cases of human disease (“WITH” processing lots) differed from lots whose isolates had only genotypes not seen in cases of human disease (“WITHOUT” lots). Figure 5 shows the distributions of lot size, prevalence, mean contamination, and maximum contamination results for WITH and WITHOUT lots for product distributed fresh, while Fig. 6 shows the same distributions for product distributed frozen. For both fresh and frozen retail product, the prevalence, mean contamination, and maximum contamination values appear to be higher for WITH lots than for WITHOUT lots. However, it is also apparent that for both product types, retail lots containing at least one genotype also seen in human isolates greatly outnumber lots with genotypes never seen in human isolates. Whether any of these descriptors were significantly larger in WITH than WITHOUT lots was tested using one-tailed Mann-Whitney U tests (Table 3). Lot size was not significant for either product type (fresh, P = 0.2946; frozen, P = 0.4168). For fresh product, WITH lots had significantly higher results for mean contamination (P = 0.0009) and maximum contamination (P = 0.0233). Prevalence was not significantly greater in WITH lots (P = 0.0708). For frozen lots, WITH lots had significant higher results for prevalence (P = 0.0005), mean contamination (P < 0.0001), and maximum contamination (P = 0.0002) than WITHOUT lots.
FIG. 5.
Comparison of fresh-product lots containing Campylobacter flaA SVR alleles also seen in human disease (“WITH”) to lots containing no alleles seen in human disease (“WITHOUT”). Black bars show the numbers of “WITH” lots. Gray bars show the numbers of “WITHOUT” lots. (A) Lot size. (B) Prevalence. (C) Mean contamination. (D) Maximum contamination.
FIG. 6.
Comparison of frozen product lots containing Campylobacter flaA SVR alleles also seen in human disease (“WITH”) to lots containing no alleles seen in human disease (“WITHOUT”). Black bars show the numbers of “WITH” lots. Gray bars show the numbers of “WITHOUT” lots. (A) Lot size. (B) Prevalence. (C) Mean contamination. (D) Maximum contamination.
As shown above, the median values for these lot descriptors are illustrative. For mean contamination results, the median values for fresh product were 3.29 log CFU and 1.92 log CFU for WITH and WITHOUT lots, respectively. For frozen product, these medians were 2.44 log CFU and 1.30 log CFU for WITH and WITHOUT lots. For maximum contamination, the medians were 4.29 log CFU (WITH) and 3.35 log CFU (WITHOUT) for fresh product and 3.31 log CFU (WITH) and 2.70 log CFU (WITHOUT) for frozen product. The median prevalence was 100% for both types of fresh product and for frozen WITH lots. The median prevalence of frozen WITHOUT lots was 80%. Finally, the median sizes of fresh-product lots were 5,246 for WITH lots and 5,413 for WITHOUT lots, and the median sizes of frozen product lots were 5,919 and 5,911 for WITH and WITHOUT lots, respectively.
DISCUSSION
When Campylobacter isolates from broiler flocks were compared to those from cases of human disease, a large number of flaA SVR genotypes were seen for both sample types. Of the 59 unique genotypes seen among human isolates, 30 were also seen in broiler flocks. In all, 84% of human isolates had genotypes seen in broiler-associated isolates collected during processing and postprocessing. A similar study performed in Quebec, Canada, found 20% of human isolates shared pulsed-field gel electrophoresis profiles with isolates from poultry flocks (13). The greater level of genotype sharing seen in Iceland might be due to the restriction of our study to cases of human campylobacteriosis acquired domestically along with our ability to sample a much higher percentage of product lots because of the smaller size and closed nature of the broiler production industry in Iceland: we were able to type isolates from 84.5% of all Campylobacter-positive retail broiler flocks produced and sold in Iceland during the study period. The difference also might be due to an overall lower level of genetic diversity of Campylobacter strains within Iceland, due to the smaller size of the poultry industry and the isolating nature of islands.
When an attempt is made to match product lots with individual cases of campylobacteriosis, one interesting observation emerged: there were a number of temporal clusters of cases of campylobacteriosis with identical flaA SVR genotypes. These clusters implicated a limited number of product lots through genetics and temporal relationships. While broiler-borne campylobacteriosis is usually described as a sporadic disease (1), our results were more similar to those seen by Pearson et al. (15), who used serotyping to trace an outbreak of Campylobacter infection to preparation and consumption of broilers from a specific wholesaler. Of course, timing and genetics data do not prove that any particular case of campylobacteriosis was acquired through the consumption of broilers, but broiler-borne transmission would require such a relationship.
When an arbitrary but reasonable time frame was used to screen isolates from processed broiler lots with flaA SVR genotypes identical to those of Campylobacter isolates from cases of human disease, different results were seen with product lots distributed fresh versus those distributed frozen. Among fresh-product lots, those implicated in human disease—i.e., those matched both genetically and temporally to human cases—exhibited significantly higher mean contamination levels than lots that were not implicated. The differences seen in lot size and maximum contamination results were not significant, and prevalence was nearly significant (P = 0.0668).
For product frozen before distribution, implicated lots were significantly larger than nonimplicated lots, but there were no significant differences in prevalence, mean contamination, or maximum contamination. That lot size was significant for frozen product but not for fresh product may reflect differences in the ways in which large and small frozen lots are handled, if smaller lots are sent on at a higher rate to further processing into cooked product or if smaller lots are preferentially stored for longer periods of time.
The storage for frozen lots may account for the differences in the significance of mean contamination results between fresh and frozen product as well. Our time frame uses the processing dates to match individual product lots with cases of campylobacteriosis, under the assumption that producers will minimize storage costs by distributing product soon after processing. Frozen product, clearly, can be stored far longer than our 2-week window for temporal matching, and thus, lots that may have actually caused disease would be classified as nonimplicated, blurring the relationships between disease and the four lot descriptors. This is true to a lesser extent for fresh-product lots, since portions of these lots may be frozen for later distribution when market conditions demand it.
This is not the only manner in which the relationship between disease and the incidence of Campylobacter-positive lots is complicated. There are a number of reasons that lots that were actual sources of broiler-borne campylobacteriosis may be classified here as nonimplicated. In addition to the complicating factor of storage time between processing and distribution, sampling within lots may have missed genotypes that were the cause of human disease; if there are any differences in virulence among Campylobacter strains (9), low-frequency genotypes might cause disease yet be missed during the genotyping process. Perhaps more importantly, many people with campylobacteriosis are never seen by the health care system (8, 11), and had those isolates been included in this study, some of the nonimplicated lots might have been implicated.
There are also a number of reasons why implicated lots might not have actually caused human disease. At the most basic level, many human isolates implicate multiple product lots. While it is possible for individuals to have acquired a Campylobacter infection from multiple sources, it is highly unlikely that this occurs often enough to account for the data shown in Table 1 and in Table S1 in the supplemental material. Second, it is possible that where there is a genetic match, both human and broiler flock could have acquired a Campylobacter infection from some third source, or the patient could have acquired a Campylobacter infection from a processing lot for which we have no sequence data. Third, it is possible that even if a particular broiler flock had been the source of a Campylobacter infection that sickened a specific person, the transmission of bacteria might have been environmental rather than food-borne. For these reasons it is important to bear in mind that “implicated” is not synonymous with “proven source of disease through broiler-borne transmission.”
An interesting result occurred when the genetic matches among isolates from human disease and product lots were examined without the time frame data. For product distributed frozen, prevalence, mean contamination, and maximum contamination results were all highly significant. For fresh-product lots, mean contamination was highly significant, and maximum contamination was just barely significant. Lot size was not significant for either product type. For each significant descriptor mentioned above, lots with genotypes seen in human campylobacteriosis had higher values of contamination or prevalence than did lots lacking genotypes seen in human disease.
The differences between the results when a time frame is included versus when only genetic matches are considered could have resulted from a number of causes. First, the time frame may be wrong; as discussed above for frozen lots, the chosen time frame might have little bearing on frozen product simply because of the ability of producers to store frozen product for later distribution. Since portions of fresh-product lots are frozen or cooked prior to distribution, extended storage times may be a factor for these lots as well. Even disregarding storage by producers, it is possible that consumers could also increase the time frame for infection from a particular product lot by longer-term storage of uncooked product (for example, in home freezers). Cross-contamination of other foods (e.g., apples or other refrigerator-friendly produce) might also account for the increased level of infection seen with longer storage compared to that seen with fresh, uncooked poultry.
Second, higher prevalence or contamination within broiler flocks may lead to increased environmental transmission of Campylobacter spp. Higher numbers of Campylobacter cells (resulting from greater within-flock prevalence, higher within-gut contamination, or both) may increase the chance that Campylobacter bacteria may escape from the broiler house and survive in the environment long enough to cause human disease. In this case, Campylobacter spp. from a flock might infect humans before the flock is processed. After processing, the spread of pathogens from the litter into drinking water from surface sources might occur, but the appropriate time frame might be days to weeks (if a heavy rain is required to liberate the Campylobacter spp.) after processing occurs.
Third, Campylobacter strains with genotypes seen in humans may also be better at replicating within broilers. There may be genetic differences in replication rates: higher replication rates would lead to increased density whenever growth time within the flock is limited by slaughter. Such life history variation has been observed previously for other bacteria (7). This explanation may be especially true of fresh product, where fecal samples from the flock gave Campylobacter-negative results 2 to 7 days before slaughter. There might also be genetic differences in the ability to grow to higher densities, which may indicate a better ability to compete against other members of the gut microflora. The ability to replicate to higher levels (through faster growth rate or increased tolerance for higher density) might influence whether infection of a human gut by a given strain leads to a virulent enough outcome for the patient to seek medical attention. Since we measured prevalence and contamination postprocessing, this assumes that higher levels of intestinal Campylobacter spp. in a broiler preprocessing are correlated with higher levels on the carcass postprocessing.
Fourth, some genotypes might be better at surviving processing. Any strain better able to survive during processing will appear to have higher prevalence and levels of contamination when measured for the retail product than strains with less resistance. These strains may also be better able to survive preparation and consumption to infect humans through a food-borne route, or they might better survive in the environment to infect individuals through drinking water. For example, a better tolerance for desiccation may increase survival in an air chiller during processing, on surfaces during food preparation, or in litter after the broiler house is cleaned. A better tolerance to osmotic stress may enhance survival during a wash process, during casual cleaning of food preparation surfaces in the kitchen (for example, a quick rinse rather than use of bleach or soap), or during immersion in fresh water. Likewise, tolerance of oxidative stress may confer better survival in an air chiller; greater viability in packaging, on surfaces, and on cross-contaminated product; and longer survival in surface water. Such differences in stress tolerance have been found among Campylobacter species and among Campylobacter jejuni strains (11a).
That we see stronger associations between human disease and product lots when our arbitrary time frame is removed raises questions as to whether our data are still applicable to food-borne transmission. It is almost certain that both broiler-borne transmission and environmental transmission resulted in cases of campylobacteriosis during this study. As long as some transmission of Campylobacter species from broiler flocks to humans occurred through the consumption of broilers, the significance of these descriptors is likely to be applicable to broiler-borne transmission. Given the confounding factors described above, the fact that mean contamination was significantly higher in implicated fresh lots than in nonimplicated lots despite the probability that some implicated lots did not cause disease and some nonimplicated lots were the sources of disease indicates that there is very likely a real relationship between mean contamination in a product lot and its potential risk to consumers.
For any individual Campylobacter cell, the commonalities in stressors between production and environmental pathways may create similarities in the significance of these lot descriptors for both modes of transmission. For example, high levels of contamination may increase the chances of Campylobacter spp. being transferred through cross-contamination in the kitchen. High contamination may also increase the likelihood of Campylobacter cells getting into fresh water and may increase the likelihood of infection.
Where food safety regulation is concerned, our data show some promise for alternatives to presence-absence regulation of retail broilers. In an ideal world, we could compare product lots that were the cause of human campylobacteriosis through broiler-borne transmission to those that caused no disease, but to get such a data set—one that eliminated all other possible routes of transmission yet still managed to accurately reflect the dynamics of pathogen transfer in the home kitchen—would be next to impossible and prohibitively expensive. We have chosen instead to compare lots that are implicated in human disease through timing and genetics to lots that are not implicated. While “implicated” lots are not identical to “disease-causing” lots, the overlap is likely great enough to apply the results of our data to food safety regulations.
For our four lot descriptors, mean contamination, maximum contamination, and prevalence were correlated in our sample. However, within-lot prevalence was not significant for fresh product, and lot size was not significant for either product type in the comparison of lots with and without genotypes seen in human disease. This suggests that market prevalence may not be a significant factor in broiler-borne transmission, but further research needs to be done to test this more directly. While within-lot prevalence can be determined more easily than contamination in a regulatory environment, the lack of significance and the similarity of the distributions for implicated and nonimplicated lots indicate that it is not a good target for food regulation.
Mean contamination results were more significant than maximum contamination or prevalence results, including the results obtained for the implicated versus nonimplicated comparison of fresh product. The differences in median values for mean contamination (e.g., 3.56 log CFU for implicated fresh lots versus 2.72 for nonimplicated fresh lots) indicates that there may be differences between the distributions of implicated and nonimplicated product that could serve as a basis for regulation. Maximum contamination was more significant than prevalence, but the utility of maximum contamination as a regulatory standard is lessened by its greater sensitivity to sample size compared to mean contamination. Mean contamination also provides a better, more fine-grained approach to regulation than is possible when the lot is judged on a presence-absence basis for Campylobacter infection analysis.
These results are remarkably similar to the findings of Nauta and Havelaar (14), who used a quantitative risk model calibrated to values of Campylobacter contamination and prevalence observed for commercial broilers in The Netherlands. Their model predicts a strong correlation between the contamination of individual carcasses and the probability of causing human disease. Their model also shows that simple tests with which higher levels of contamination lead to higher probability of detection can be effective in reducing risk to consumers. That both their theoretical study and our observational study point to similar conclusions indicates that mean contamination can serve as a practical regulatory criterion for decreasing campylobacteriosis from broilers.
Supplementary Material
Acknowledgments
This work was supported by National Research Initiative grant 2002-35212-12369 from the Epidemiological Approaches to Food Safety program of the USDA Cooperative State Research, Education, and Extension Service (awarded to N.J.S., R. Lowman, and K.L.H.) and by USDA Agricultural Research Service grant CRIS 6612-32000-034-00.
This work was performed as part of the Campy-on-Ice Consortium, which consists of Haraldur Briem and Gudrún Sigmundsdóttir (Directorate of Health, Reykjavik, Iceland); Hjördís Harðardóttir and Karl Kristinsson (Landspitali National University Hospital, Reykjavik, Iceland); Vala Friðriksdóttir and Eggert Gunnarsson (Institute of Experimental Pathology, Reykjavik, Iceland); Franklín Georgsson (Food Laboratory, The Environmental and Food Agency of Iceland, Reykjavik, Iceland); Jarle Reiersen (The Icelandic Food and Veterinary Authority, Selfoss, Iceland); Eva Berndtson (Swe-Chick, Kristianstad, Sweden); Jean-Robert Bisaillon and Ruff Lowman (Canadian Food Inspection Agency, Ottawa, Ontario, Canada); Aamir Fazil and Pascal Michel (Laboratory for Food-borne Zoonoses, Public Health Agency of Canada, St. Hyacinthe, Quebec, Canada); Greg Paoli (Decisionalysis Risk Consultants, Inc., Ottawa, Ontario, Canada); and Kenneth Callicott, Kelli Hiett, and Norman Stern (USDA-Agricultural Research Service, Poultry Microbiological Safety Research Unit, Athens, GA).
We thank Susan Brooks, Tabitha Mashburn, and Latoya Wiggins of the USDA Agricultural Research Service for their technical assistance.
The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Footnotes
Published ahead of print on 12 September 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni: an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callicott, K. A., N. J. Stern, K. L. Hiett, and the Campy on Ice Consortium. 2005. Isolation of DNA for PCR assays from noncultivable Campylobacter jejuni isolates. Poult. Sci. 84:1530-1532. [DOI] [PubMed] [Google Scholar]
- 3.Cary, S. G., and E. B. Blair. 1964. New transport medium for shipments of clinical specimens: I. Fecal specimens. J. Bacteriol. 88:96-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgsson, F., A. E. Þorkelsson, M. Geirsdóttir, and N. J. Stern. 2006. The influence of freezing and duration of storage on campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 23:677-683. [DOI] [PubMed] [Google Scholar]
- 5.Hiett, K. L., N. J. Stern, P. Fedorka-Cray, N. A. Cox, M. T. Musgrove, and S. Ladely. 2002. Molecular subtype analysis of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 68:6220-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiett, K. L., N. A. Cox, R. J. Buhr, and N. J. Stern. 2002. Genotype analysis of Campylobacter isolated from distinct segments of the reproductive tracts of broiler breeder hens. Curr. Microbiol. 45:400-404. [DOI] [PubMed] [Google Scholar]
- 7.King, T., A. Ishihama, A. Kori, and T. Ferenci. 2004. A regulatory trade-off as a source of strain variation in the species Escherichia coli. J. Bacteriol. 186:5614-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Majowicz, S. E., V. L. Edge, A. Fazil, W. B. McNab, K. A. Dore, P. N. Sockett, J. A. Flint, D. Middleton, S. A. McEwen, and J. B. Wilson. 2005. Estimating the under-reporting rate for infectious gastrointestinal illness in Ontario. Can. J. Public Health 96:178-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik-Kale, P., B. H. Raphael, C. T. Parker, L. A. Joens, J. D. Klena, B. Quiñones, A. M. Keech, and M. E. Konkel. 2007. Characterization of genetically matched isolates of Campylobacter jejuni reveals that mutations in genes involved in flagellar biosynthesis alter the organism's virulence potential. Appl. Environ. Microbiol. 73:3123-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meinersmann, R. J., L. O. Helsel, P. I. Fields, and K. L. Hiett. 1997. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J. Clin. Microbiol. 35:2810-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michel, P., J. B. Wilson, S. W. Martin, R. C. Clarke, S. A. McEwen, and C. L. Gyles. 2000. Estimation of the under-reporting rate for the surveillance of Escherichia coli O157:H7 cases in Ontario, Canada. Epidemiol. Infect. 125:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Murphy, C., C. Carroll, and K. N. Jordan. 2006. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 100:623-632. [DOI] [PubMed] [Google Scholar]
- 12.Nachamkin, I., K. Bohachick, and C. M. Patton. 1993. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 31:1531-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadeau, E., S. Messier, and S. Quessy. 2002. Prevalence and comparison of genetic profiles of Campylobacter strains isolated from poultry and sporadic cases of campylobacteriosis in humans. J. Food Prot. 65:73-78. [DOI] [PubMed] [Google Scholar]
- 14.Nauta, M. J., and A. H. Havelaar. 2008. Risk-based standards for Campylobacter in the broiler meat chain. Food Control 19:372-381. [Google Scholar]
- 15.Pearson, A. D., M. H. Greenwood, J. Donaldson, T. D. Healing, D. M. Jones, M. Shahamat, R. K. A. Feltham, and R. R. Colwell. 2000. Continuous source outbreak of campylobacteriosis traced to chicken. J. Food Prot. 63:309-314. [DOI] [PubMed] [Google Scholar]
- 16.Rosenquist, H., N. L. Nielsen, H. M. Sommer, B. Nørrun, and B. B. Christensen. 2003. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chicken. Int. J. Food Microbiol. 83:87-103. [DOI] [PubMed] [Google Scholar]
- 17.Stern, N. J., K. L. Hiett, G. A. Alfredsson, K. G. Kristinsson, J. Reiersen, H. Harðardottir, H. Briem, E. Gunnersson, F. Georgsson, R. Lowman, E. Berndtson, A. M. Lammerding, G. M. Paoli, and M. T. Musgrove. 2003. Campylobacter spp. in Icelandic poultry operations and human disease. Epidemiol. Infect. 130:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern, N. J., J. Reiersen, R. Lowman, J.-R. Bisaillon, V. Friðriksdottir, E. Gunnarsson, and K. L. Hiett. 2005. Occurrence of Campylobacter spp. in cecal contents among commercial broilers in Iceland. Foodborne Pathog. Dis. 2:82-89. [DOI] [PubMed] [Google Scholar]
- 19.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, W.-L., N. W. Luechtefeld, L. B. Reller, and M. J. Blaser. 1980. Enriched brucella medium for storage and transport of cultures of Campylobacter fetus subsp. jejuni. J. Clin. Microbiol. 12:479-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. 2002. The increasing incidence of human campylobacteriosis. Report and proceedings of a W.H.O. consultation of experts, Copenhagen, Denmark, 21-25 November 2000. WHO/CDS/CSR/APH publication 2001.7. World Health Organization, Geneva, Switzerland.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.